Investigation of Ternary CuZnM (M = Cr, Ce, Zr, Al) Catalysts in CO2 Hydrogenation for Methanol Synthesis
Abstract
:1. Introduction
2. Results
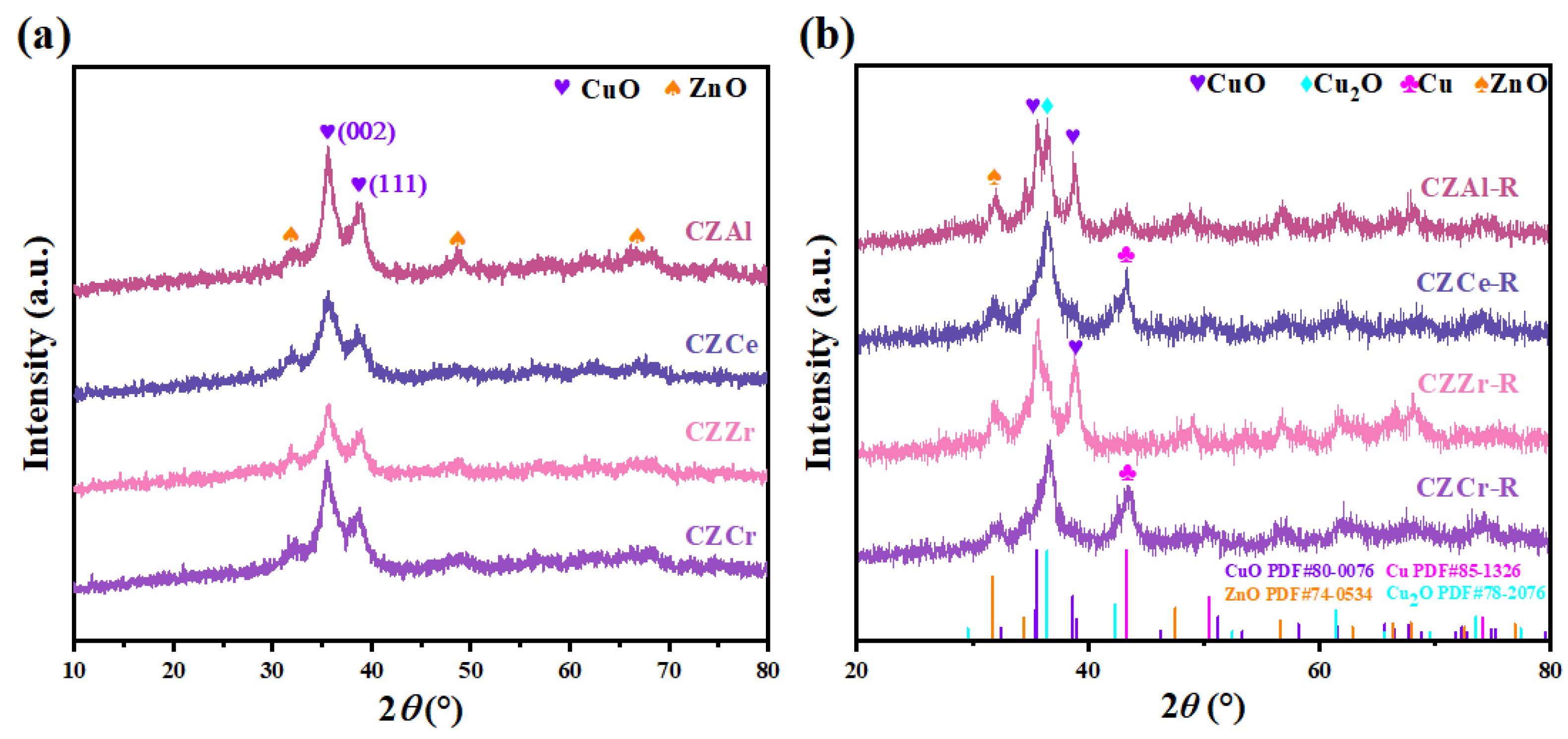
2.1. Crystal Structure Determined by XRD
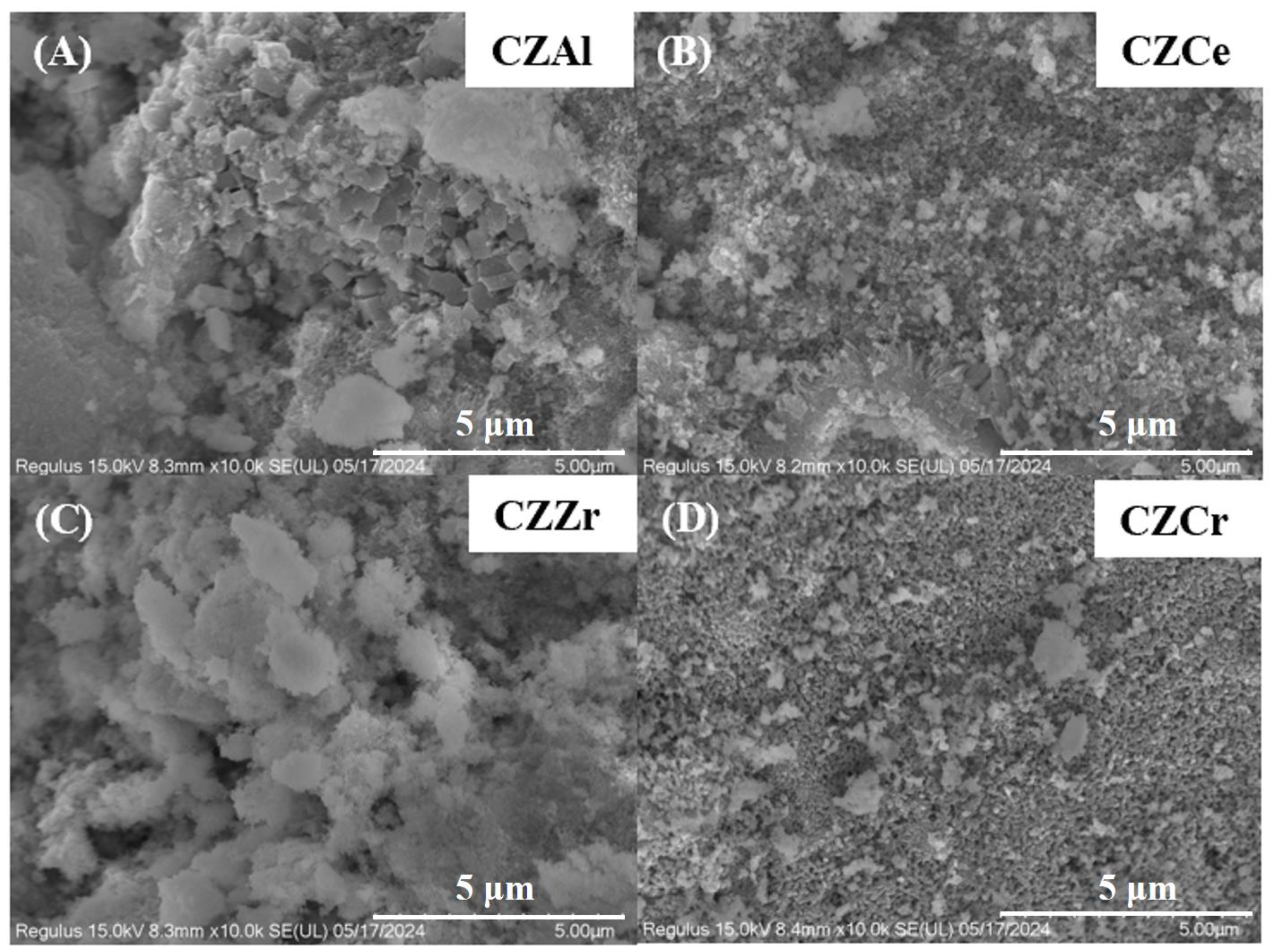
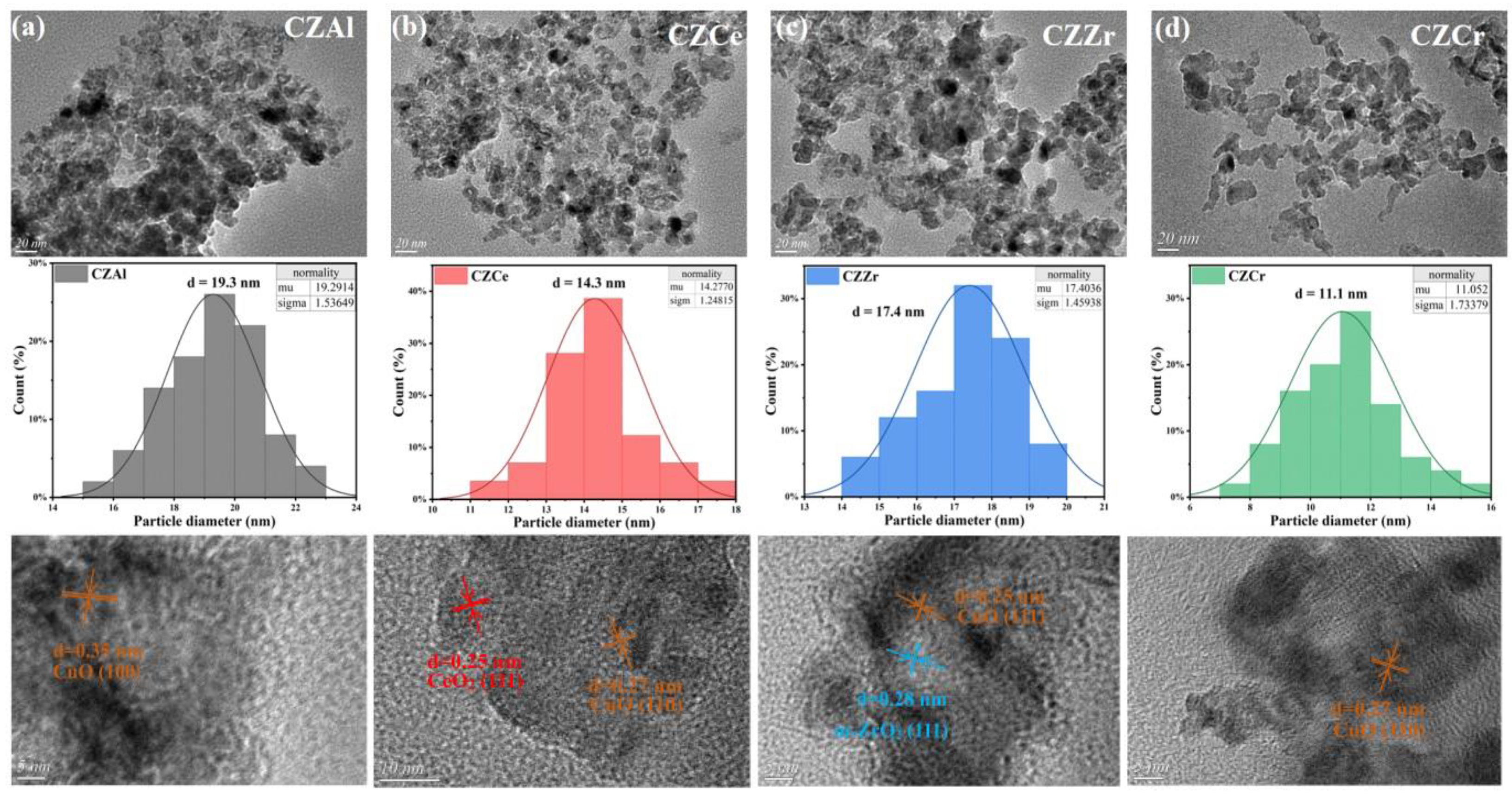
2.2. Catalyst Surface Morphology and Particle Size
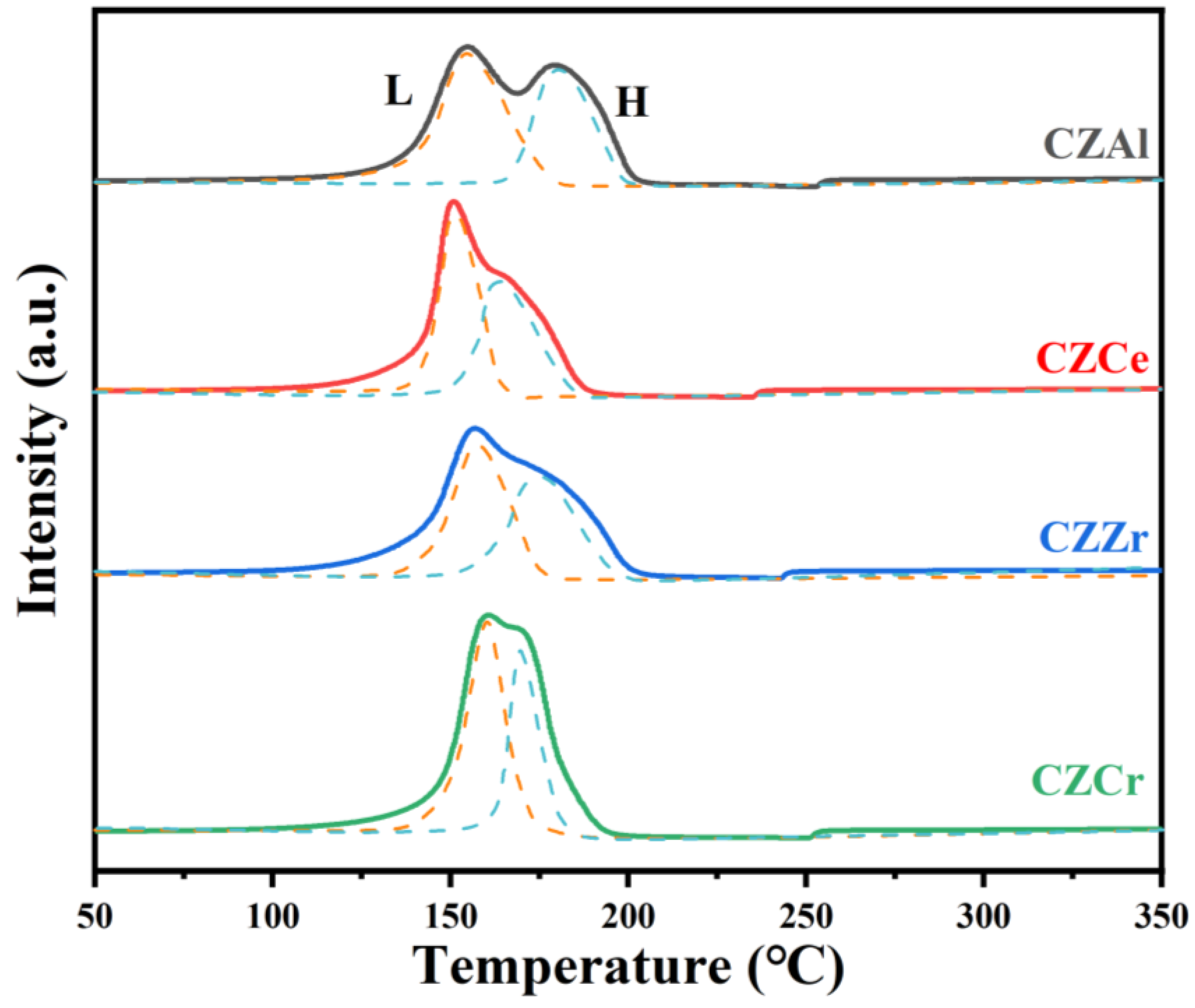
2.3. Catalyst Reduction Behavior by H2-TPR
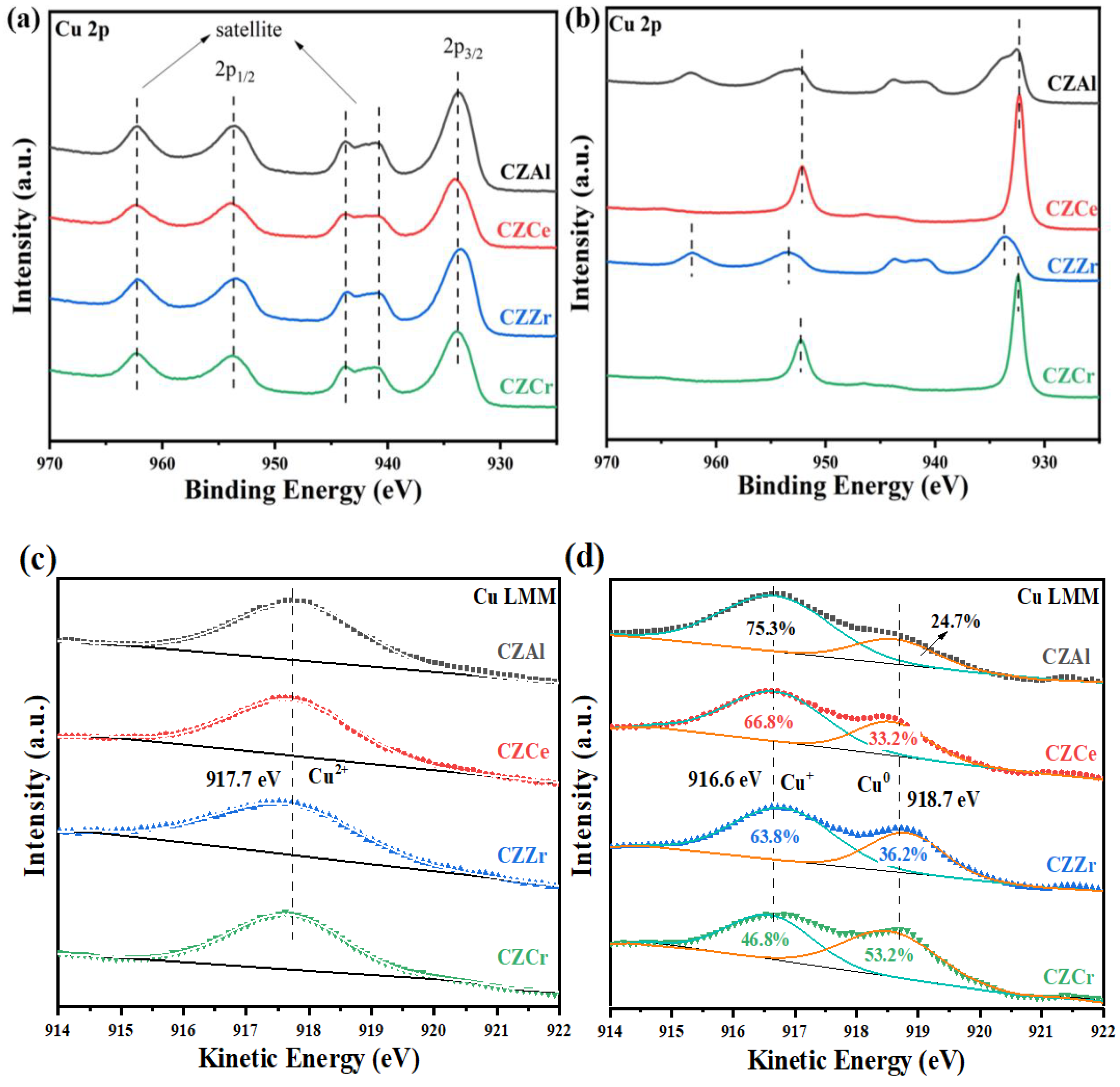
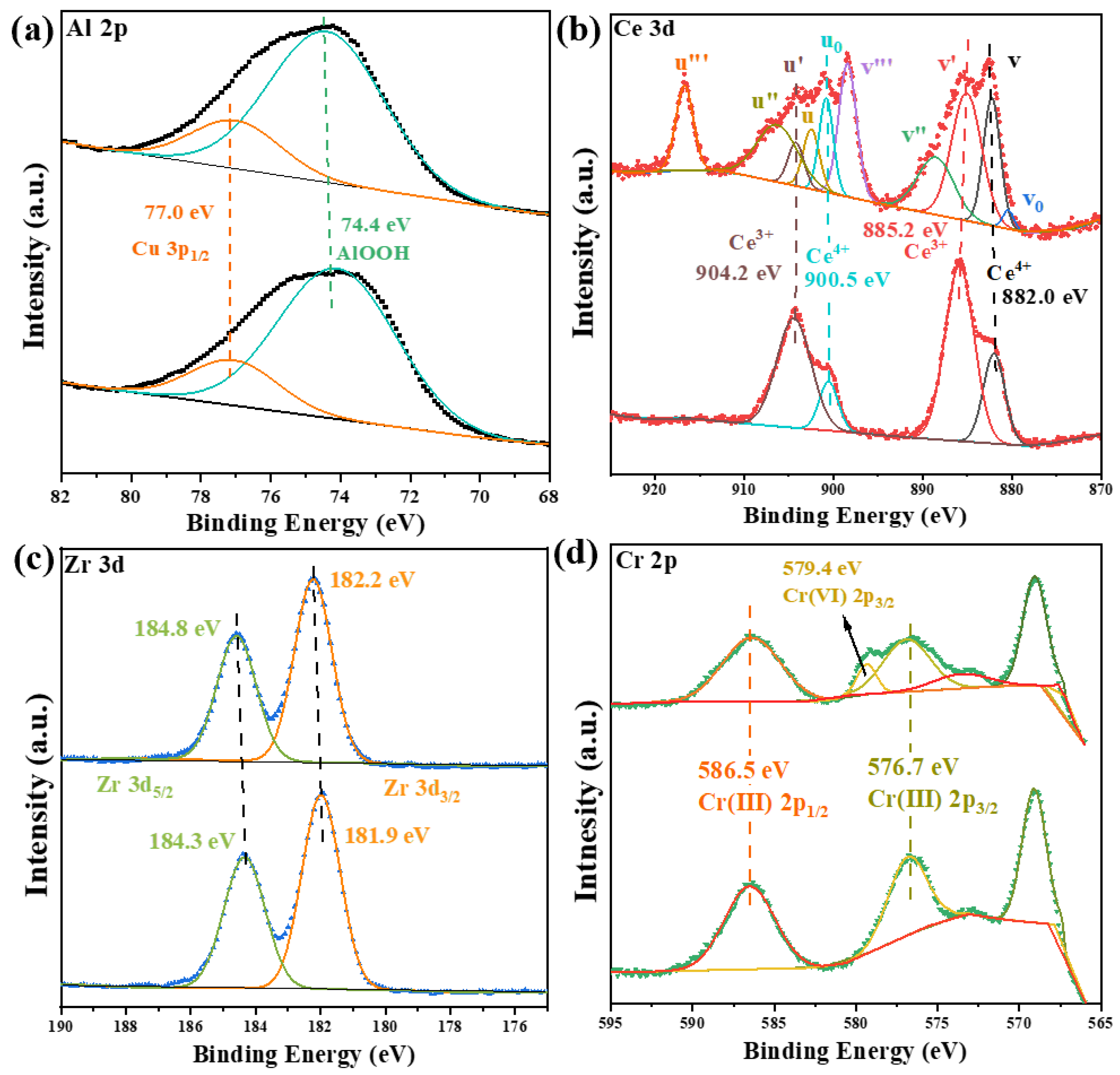
2.4. Surface Electronic State of Elements on the Catalysts
2.5. Desorption Behavior of Reactants on Catalyst Surfaces
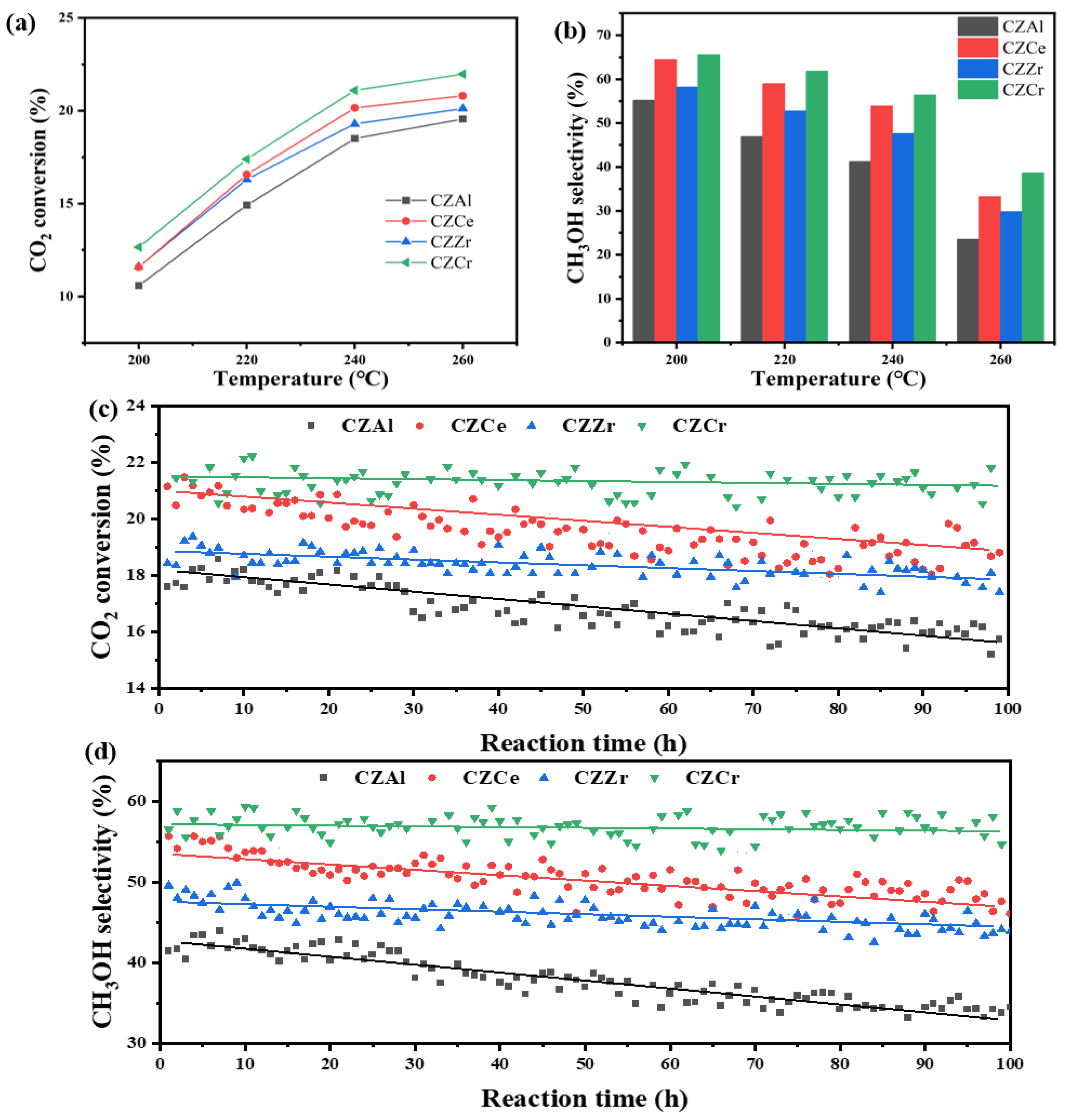
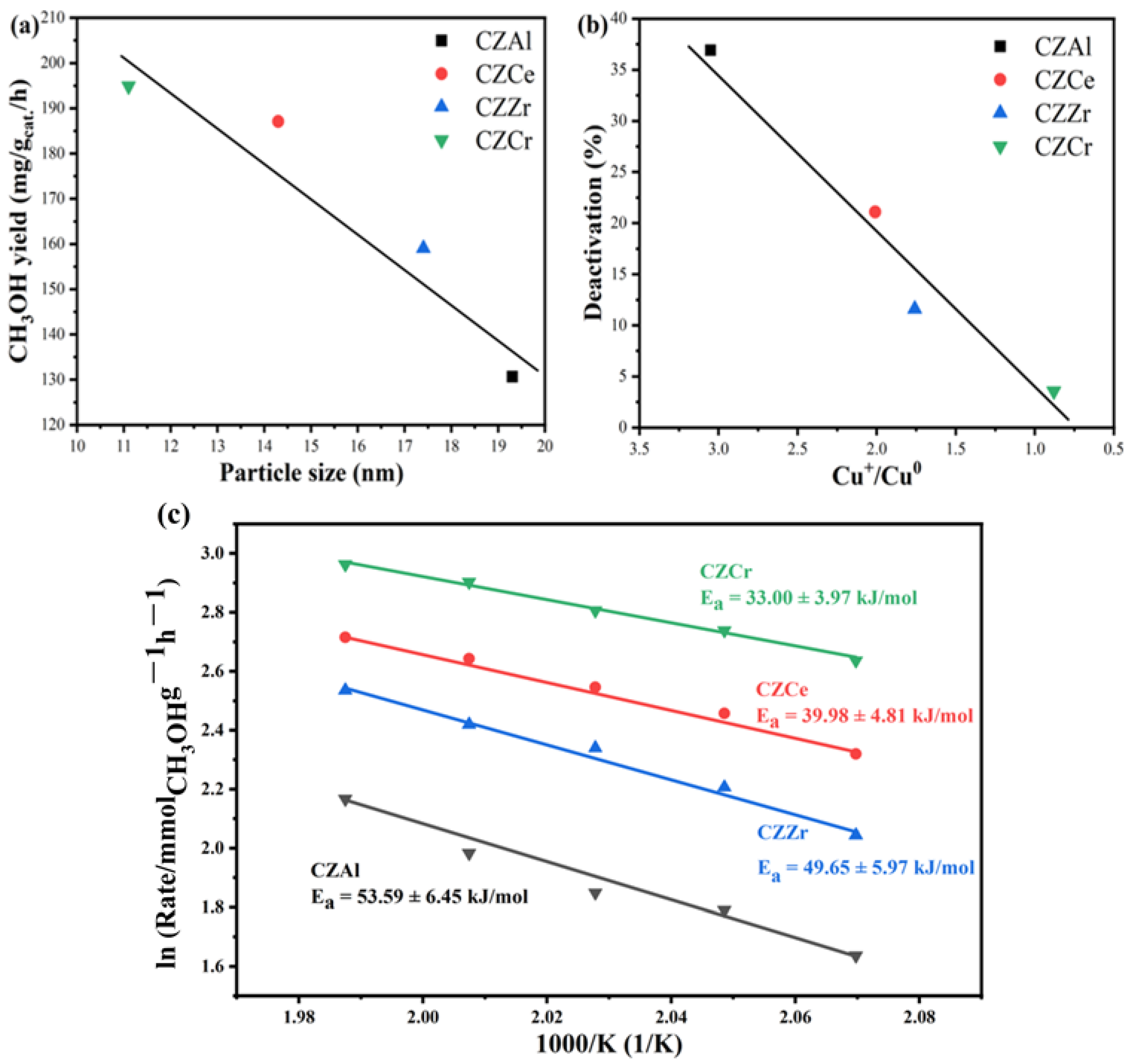
2.6. Evaluation Performance of Catalyst
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalyst Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Liu, F.; Geng, S.; Yao, M.; Ma, J.; Cao, J. Tuning the content of S vacancies in MoS2 by Cu doping for enhancing catalytic hydrogenation of CO2 to methanol. Mol. Catal. 2023, 547, 113288. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Xue, F.; Li, J.; Zhang, H.; Lu, S.; Su, X.; Cao, B.; Huo, W.; Fang, T. DFT investigation of CO2 hydrogenation to methanol over Ir-doped Cu surface. Mol. Catal. 2022, 528, 112460. [Google Scholar] [CrossRef]
- Kapiamba, K.F.; Otor, H.O.; Viamajala, S.; Alba-Rubio, A.C. Inverse oxide/metal catalysts for CO2 hydrogenation to methanol. Energy Fuels 2022, 36, 11691–11711. [Google Scholar] [CrossRef]
- Roy, S.; Mondal, D.K.; Chatterjee, S.; Chowdhury, A.; Khan, T.S.; Haider, M.A.; Mandal, S.; Chandra, D.; Hara, M.; Bhaumik, A. Selective CO2 reduction to methane catalyzed by mesoporous Ru-Fe3O4/CeOx-SiO2 in a fixed bed flow reactor. Mol. Catal. 2022, 528, 112486. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Varvoutis, G.; Binas, V.D.; Marnellos, G.E.; Konsolakis, M. Comparative assessment of first-row 3d transition metals (Ti-Zn) supported on CeO2 nanorods for CO2 hydrogenation. Catalysts 2024, 14, 611. [Google Scholar] [CrossRef]
- Murthy, P.S.; Liang, W.; Jiang, Y.; Huang, J. Cu-based nanocatalysts for CO2 hydrogenation to methanol. Energy Fuels 2021, 35, 8558–8584. [Google Scholar] [CrossRef]
- Kumar, A.; Daw, P.; Milstein, D. Homogeneous catalysis for sustainable energy: Hydrogen and methanol economies, fuels from biomass, and related topics. Chem. Rev. 2021, 122, 385–441. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Hui, X.; Zhang, C.; Cao, Y.; Xu, S.; He, P.; Li, H. Effective hydrogenation of carbonates to produce methanol over a ternary Cu/Zn/Al catalyst. RSC Adv. 2020, 10, 13083–13094. [Google Scholar] [CrossRef]
- Carvalho, D.F.; Almeida, G.C.; Monteiro, R.S.; Mota, C.J.A. Hydrogenation of CO2 to methanol and dimethyl ether over a bifunctional Cu·ZnO catalyst impregnated on modified γ-alumina. Energy Fuels 2020, 34, 7269–7274. [Google Scholar]
- Cheng, Z.; Wang, M.; Jiang, C.; Zhang, L.; He, X.; Sun, X.; Lan, G.; Qiu, Y.; Li, Y. Tuning lattice strain of copper particles in Cu/ZnO/Al2O3 catalysts for methanol steam reforming. Energy Fuels 2024, 38, 15611–15621. [Google Scholar] [CrossRef]
- Al Abdulghani, A.J.; Turizo-Pinilla, E.E.; Fabregas-Angulo, M.J.; Hagmann, R.H.; Ibrahim, F.; Jansen, J.H.; Agbi, T.O.; Bhat, S.; Sepúlveda-Pagán, M.; Kraimer, M.O.; et al. Realizing synergy between Cu, Ga, and Zr for selective CO2 hydrogenation to methanol. Appl. Catal. B 2024, 355, 124198. [Google Scholar] [CrossRef]
- Liang, B.; Ma, J.; Su, X.; Yang, C.; Duan, H.; Zhou, H.; Deng, S.; Li, L.; Huang, Y. Investigation on deactivation of Cu/ZnO/Al2O3 catalyst for CO2 hydrogenation to methanol. Ind. Eng. Chem. Res. 2019, 58, 9030–9037. [Google Scholar] [CrossRef]
- Santana, C.S.; Rasteiro, L.F.; Marcos, F.C.F.; Assaf, E.M.; Gomes, J.F.; Assaf, J.M. Influence of Al, Cr, Ga, or Zr as promoters on the performance of Cu/ZnO catalyst for CO2 hydrogenation to methanol. Mol. Catal. 2022, 528, 112512. [Google Scholar] [CrossRef]
- Chang, X.; Zi, X.; Li, J.; Liu, F.; Han, X.; Chen, J.; Hao, Z.; Zhang, H.; Zhang, Z.; Gao, P.; et al. An insight into synergistic metal-oxide interaction in CO2 hydrogenation to methanol over Cu/ZnO/ZrO2. Catalysts 2023, 13, 1337. [Google Scholar] [CrossRef]
- Singh, R.; Kundu, K.; Pant, K.K. CO2 hydrogenation to methanol over Cu-ZnO-CeO2 catalyst: Reaction structure-activity relationship, optimizing Ce and Zn ratio, and kinetic study. Chem. Eng. J. 2024, 479, 147783. [Google Scholar] [CrossRef]
- Zhan, H.; Shi, X.; Tang, B.; Wang, G.; Ma, B.; Liu, W. The performance of Cu/Zn/Zr catalysts of different Zr/(Cu+Zn) ratio for CO2 hydrogenation to methanol. Catal. Commun. 2021, 149, 106264. [Google Scholar] [CrossRef]
- Xiong, S.; Lian, Y.; Xie, H.; Liu, B. Hydrogenation of CO2 to methanol over Cu/ZnCr catalyst. Fuel 2019, 256, 115975. [Google Scholar] [CrossRef]
- Hengne, A.M.; Yuan, D.J.; Date, N.S.; Saih, Y.; Kamble, S.P.; Rode, C.V.; Huang, K.-W. Preparation and activity of copper-gallium nanocomposite catalysts for carbon dioxide hydrogenation to methanol. Ind. Eng. Chem. Res. 2019, 58, 21331–21340. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Yang, Y.; Chen, J.G.; Liu, P. Optimizing binding energies of key intermediates for CO2 hydrogenation to methanol over oxide-supported copper. J. Am. Chem. Soc. 2016, 138, 12440–12450. [Google Scholar] [CrossRef]
- Huang, C.J.; Zhang, S.N.; Wang, W.B.; Zhou, H.Z.; Shao, Z.L.; Xia, L.; Wang, H.; Sun, Y.H. Modulation of electronic metal-support interaction between Cu and ZnO by Er for effective low temperature CO2 hydrogenation to methanol. ACS Catal. 2024, 14, 1324–1335. [Google Scholar] [CrossRef]
- Phongamwong, T.; Chantaprasertporn, U.; Witoon, T.; Numpilai, T.; Poo-arporn, Y.; Limphirat, W.; Donphai, W.; Dittanet, P.; Chareonpanich, M.; Limtrakul, J. CO2 hydrogenation to methanol over CuO-ZnO-ZrO2-SiO2 catalysts: Effects of SiO2 contents. Chem. Eng. J. 2017, 316, 692–703. [Google Scholar] [CrossRef]
- Lei, T.; Dang, Q.; Wu, T.; Wu, Y.; Yu, J. The role of ZnO nanowires to improve methanol selectivity on Cu2O nanocubes during CO2 electroreduction. Appl. Catal. A 2023, 666, 119394. [Google Scholar] [CrossRef]
- Kordus, D.; Jelic, J.; Luna, M.L.; Divins, N.J.; Timoshenko, J.; Chee, S.W.; Rettenmaier, C.; Kröhnert, J.; Kühl, S.; Trunschke, A.; et al. Shape-dependent CO2 hydrogenation to methanol over Cu2O nanocubes supported on ZnO. J. Am. Chem. Soc. 2023, 145, 3016–3030. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Besenhard, M.O.; Hodzic, A.; Sergides, A.; Bogart, L.K.; Gavriilidis, A.; Thanh, N.T.K. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-Ray diffraction in solution. Nanoscale 2019, 11, 6620–6628. [Google Scholar] [CrossRef]
- Ban, H.; Li, C.; Asami, K.; Fujimoto, K. Influence of rare-earth elements (La, Nd and Pr) on the performance of Cu/Zn/Zr catalyst for CH3OH synthesis from CO2. Catal. Commun. 2014, 54, 50–54. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F.; Wang, X.; Lu, X.; Li, H.; Xu, G.; Su, F. CO methanation on ordered mesoporous Ni-Cr-Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Si, C.; Ban, H.; Chen, K.; Wang, X.; Cao, R.; Yi, Q.; Qin, Z.; Shi, L.; Li, Z.; Cai, W.; et al. Insight into the positive effect of Cu0/Cu+ ratio on the stability of Cu-ZnO-CeO2 catalyst for syngas hydrogenation. Appl. Catal. A 2020, 594, 117466. [Google Scholar] [CrossRef]
- Li, Z.; Men, Y.; Liu, S.; Wang, J.; Qin, K.; Tian, D.; Shi, T.; Zhang, L.; An, W. Boosting CO2 hydrogenation efficiency for methanol synthesis over Pd/In2O3/ZrO2 catalysts by crystalline phase effect. Appl. Surf. Sci. 2022, 603, 154420. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Lee, P.L. Reduction of CuO and Cu2O with H2: H embedding and kinetic effects in the formation of suboxides. J. Am. Chem. Soc. 2003, 125, 10684–10692. [Google Scholar] [CrossRef]
- Qi, T.Q.; Zhao, Y.M.; Chen, S.Y.; Li, W.Z.; Guo, X.W.; Zhang, Y.C.; Song, C.S. Bimetallic metal organic framework-templated synthesis of a Cu-ZnO/Al2O3 catalyst with superior methanol selectivity for CO2 hydrogenation. Mol. Catal. 2021, 514, 111870. [Google Scholar] [CrossRef]
- Vergara, T.; Gómez, D.; de Oliveira Campos, B.L.; Delgado, K.H.; Concepción, P.; Jiménez, R.; Karelovic, A. Combined role of Ce promotion and TiO2 support improves CO2 hydrogenation to methanol on Cu catalysts: Interplay between structure and kinetics. J. Catal. 2023, 426, 200–213. [Google Scholar] [CrossRef]
- Zhu, J.D.; Ciolca, D.; Liu, L.; Parastaev, A.; Kosinov, N.; Hensen, E.J.M. Flame synthesis of Cu/ZnO-CeO2 catalysts: Synergistic metal-support interactions promote CH3OH selectivity in CO2 Hydrogenation. ACS Catal. 2021, 11, 4880–4892. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Guo, Q.; Li, S.Z.; Hu, Y.K.; Yun, S.; Qian, Y.H. Effect of surface basicity over the supported Cu-ZnO catalysts on hydrogenation of CO2 to methanol. J. Catal. 2022, 407, 312–321. [Google Scholar] [CrossRef]
- Li, S.Z.; Guo, L.M.; Ishihara, T. Hydrogenation of CO2 to methanol over Cu/AlCeO catalyst. Catal. Today 2020, 339, 352–361. [Google Scholar] [CrossRef]
- Shi, P.; Han, J.; Yan, Z.; Luo, P.; Wang, J.; Ban, H.; Zhang, X.; Li, C. Insight into the correlation between Cu species and methanol selectivity from CO2 hydrogenation over Cu-based catalyst. Mol. Catal. 2024, 563, 114278. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Wang, Y.; Shan, B.; Zhang, J.; Wang, S.; Ma, X. Efficient tuning of surface copper species of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. Chem. Eng. J. 2017, 313, 759–768. [Google Scholar] [CrossRef]
- Yilmaz, M.; Handoko, A.D.; Parkin, I.P.; Sankar, G. Probing the electronic and geometric structures of photoactive electrodeposited Cu2O films by X-ray absorption spectroscopy. J. Catal. 2020, 389, 483–491. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Zhang, L.; Qing, S.J.; Zhang, C.S.; Liu, Y.J.; Gao, Z.X. Cu-Zn-Al spinel catalyst for hydrogen production from methanol steam reforming. J. Fuel Chem. Technol. 2022, 50, 494–502. [Google Scholar]
- Liu, J.; Ren, H.; Fan, J.C.; Hang, W. Effects of heat treatment methods on the structure of Cu-Zn-Al catalyst prepared by liquid phase method and the catalytic performance of CO hydrogenation. J. Mol. Catal. 2019, 33, 166–173. [Google Scholar]
- Gong, N.; Zhang, T.; Tan, M.; Wang, L.; Yang, J.; Tan, L.; Yang, G.; Wu, P.; Wu, Y.; Tan, Y. Realizing and revealing complex isobutyl alcohol production over a simple Cu-ZrO2 catalyst. ACS Catal. 2023, 13, 3563–3574. [Google Scholar] [CrossRef]
- Marcos, F.C.F.; Alvim, R.S.; Lin, L.; Betancourt, L.E.; Petrolini, D.D.; Senanayake, S.D.; Alves, R.M.B.; Assaf, J.M.; Rodriguez, J.A.; Giudici, R.; et al. The role of copper crystallization and segregation toward enhanced methanol synthesis via CO2 hydrogenation over CuZrO2 catalysts: A combined experimental and computational study. Chem. Eng. J. 2023, 452, 139519. [Google Scholar] [CrossRef]
- Zhang, F.; Gutiérrez, R.A.; Lustemberg, P.G.; Liu, Z.; Rui, N.; Wu, T.; Ramírez, P.J.; Xu, W.; Idriss, H.; Ganduglia-Pirovano, M.V.; et al. Metal-support interactions and C1 chemistry: Transforming Pt-CeO2 into a highly active and stable catalyst for the conversion of carbon dioxide and methane. ACS Catal. 2021, 11, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, S.; Wu, P.; Guo, L. Nitrogen-doped CeOx nanoparticles modified graphitic carbon nitride for enhanced photocatalytic hydrogen production. Green Chem. 2015, 17, 509–517. [Google Scholar] [CrossRef]
- Xiao, Y.; Tan, S.; Wang, D.; Wu, J.; Jia, T.; Liu, Q.; Qi, Y.; Qi, X.; He, P.; Zhou, M. CeO2/BiOIO3 heterojunction with oxygen vacancies and Ce4+/Ce3+ redox centers synergistically enhanced photocatalytic removal heavy metal. Appl. Surf. Sci. 2020, 530, 147116. [Google Scholar] [CrossRef]
- Lin, S.D.; Hsiao, T.C.; Chen, L.-C. The steady state methanol decomposition reaction over Cu/Zn and Cu/Cr catalysts: Pretreatment, operando EXAFS, and activity study. Appl. Catal. A 2009, 360, 226–231. [Google Scholar] [CrossRef]
- Gholami, S.; Alavi, S.M.; Rezaei, M. CO2 methanation over nanocrystalline Ni catalysts supported on mechanochemically synthesized Cr2O3-M (M = Fe, Co, La, and Mn) carriers. Int. J. Hydrogen Energy 2021, 46, 35571–35584. [Google Scholar] [CrossRef]
- Zuo, J.; Na, W.; Zhang, P.; Yang, X.; Wen, J.; Zheng, M.; Wang, H. Enhanced activity of CexZr1-xO2 solid solutions supported Cu-based catalysts for hydrogenation of CO2 to methanol. Mol. Catal. 2022, 526, 112357. [Google Scholar] [CrossRef]
- Proaño, L.; Jones, C.W. CO2 hydrogenation to methanol over ceria-zirconia NiGa alloy catalysts. Appl. Catal. A 2024, 669, 119485. [Google Scholar] [CrossRef]
- Bronsato, B.J.; Souza, E.F.; Gonzalez, G.G.; Chagas, L.H.; Zonetti, P.C.; Mendoza, C.D.; Huaman, N.R.; de Avillez, R.R.; Appel, L.G. What role does Al3+ play in the methanol synthesis from CO2 hydrogenation using Cu/ZnO/Al catalysts. Fuel 2024, 375, 132533. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Z.; Guo, X.; Yan, Z.; Ban, H.; Wang, P.; Yao, R.; Li, L.; Li, C. Modulating electronic interaction over Zr-ZnO catalysts to enhance CO2 hydrogenation to methanol. ACS Catal. 2023, 14, 508–521. [Google Scholar] [CrossRef]
- Ay, S.; Ozdemir, M.; Melikoglu, M. Effects of magnesium and chromium addition on stability, activity and structure of copper-based methanol synthesis catalysts. Int. J. Hydrogen Energy 2021, 46, 12857–12873. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.S.; Lv, Y.; Liu, P.L.; Hao, F.; Xiong, W.; Luo, H. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: Effects of ZnO morphology and oxygen vacancy. Fuel 2022, 314, 123035. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, C.L.; Chen, L.M.; Zhang, Y.J.; Fu, M.L.; Wu, J.L.; Ye, D.Q. Active site structure study of Cu/Plate ZnO model catalysts for CO2 hydrogenation to methanol under the real reaction conditions. J. CO2 Util. 2020, 37, 55–64. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.S.; Xiang, L.; Komarneni, S. Defect-rich ZnO nanosheets of high surface area as an efficient visible-light photocatalyst. Appl. Catal. B Environ. 2016, 192, 8–16. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.F.; Meng, D.M.; Guo, Y.; Guo, Y.L.; Lu, G.Z. Effect of ceria crystal plane on the physicochemical and catalytic properties of Pd/ceria for CO and propane oxidation. ACS Catal. 2016, 6, 2265–2279. [Google Scholar] [CrossRef]
- Luo, P.C.; Shi, P.X.; Yan, Z.Q.; Han, J.H.; Wang, J.J.; Li, Y.C.; Ban, H.Y.; Cai, W.J.; Li, C.M. Ternary synergistic interaction of Cu-ZnO-ZrO2 promoting CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2025, 689, 120006. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.B.; Liu, X.H. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Singh, R.; Pandey, V.; Pant, K.K. Promotional role of oxygen vacancy defects and Cu-Ce interfacial sites on the activity of Cu/CeO2 catalyst for CO2 hydrogenation to methanol. ChemCatChem 2022, 14, e202201053. [Google Scholar] [CrossRef]
- Qin, H.; Ye, Y.K.; Lin, G.L.; Zhang, J.Y.; Jia, W.Q.; Xia, W.; Jiao, L.F. Regulating the electrochemical microenvironment of Ni(OH)2 by Cr doping for highly efficient methanol electrooxidation. ACS Catal. 2024, 14, 16234–16244. [Google Scholar] [CrossRef]


| Catalyst | Element | Mass Percentage Content (wt%) |
|---|---|---|
| CZAl | Cu | 60.761 |
| Zn | 29.126 | |
| Al | 9.732 | |
| CZCe | Cu | 60.169 |
| Zn | 29.267 | |
| Ce | 9.8 | |
| CZZr | Cu | 59.867 |
| Zn | 28.475 | |
| Zr | 11.137 | |
| CZCr | Cu | 61.430 |
| Zn | 28.194 | |
| Cr | 10.078 |
| Sample | DCu 1 (%) | SCu 1 (m2/g) | dCu 1/nm | dCu 2/nm | dCu 3/nm |
|---|---|---|---|---|---|
| CZAl | 6.31 | 42.69 | 16.52 | 17 ± 0.2 | 19.3 ± 0.4 |
| CZCe | 7.52 | 50.87 | 13.86 | 14 ± 0.4 | 14.3 ± 0.5 |
| CZZr | 6.08 | 41.15 | 17.13 | 16 ± 0.2 | 17.4 ± 0.3 |
| CZCr | 9.78 | 66.17 | 10.65 | 11 ± 0.3 | 11.1 ± 0.3 |
| Sample | Peak L | Peak H | ||
|---|---|---|---|---|
| T/°C | Concentration/% | T/°C | Concentration/% | |
| CZAl | 154.3 | 51.8 | 180.8 | 48.2 |
| CZCe | 150.9 | 57.5 | 164.3 | 42.5 |
| CZZr | 156.8 | 56.6 | 175.1 | 43.4 |
| CZCr | 160.0 | 57.9 | 169.6 | 42.1 |
| Sample | Cu+ (%) | Cu0 (%) | Cu+/Cu0 |
|---|---|---|---|
| CZAl | 75.3 | 24.7 | 3.05 |
| CZCe | 66.8 | 33.2 | 2.01 |
| CZZr | 63.8 | 36.2 | 1.76 |
| CZCr | 46.8 | 53.2 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shi, P.; Han, J.; Tian, Y.; Yan, Z.; Luo, P.; Ban, H.; Li, Y.; Cai, W.; Lin, G.; et al. Investigation of Ternary CuZnM (M = Cr, Ce, Zr, Al) Catalysts in CO2 Hydrogenation for Methanol Synthesis. Catalysts 2025, 15, 250. https://doi.org/10.3390/catal15030250
Wang J, Shi P, Han J, Tian Y, Yan Z, Luo P, Ban H, Li Y, Cai W, Lin G, et al. Investigation of Ternary CuZnM (M = Cr, Ce, Zr, Al) Catalysts in CO2 Hydrogenation for Methanol Synthesis. Catalysts. 2025; 15(3):250. https://doi.org/10.3390/catal15030250
Chicago/Turabian StyleWang, Jingjing, Peixiang Shi, Jiahao Han, Yuhao Tian, Zhiqiang Yan, Pengcheng Luo, Hongyan Ban, Yanchun Li, Weijie Cai, Guanjing Lin, and et al. 2025. "Investigation of Ternary CuZnM (M = Cr, Ce, Zr, Al) Catalysts in CO2 Hydrogenation for Methanol Synthesis" Catalysts 15, no. 3: 250. https://doi.org/10.3390/catal15030250
APA StyleWang, J., Shi, P., Han, J., Tian, Y., Yan, Z., Luo, P., Ban, H., Li, Y., Cai, W., Lin, G., Zhai, Z., & Li, C. (2025). Investigation of Ternary CuZnM (M = Cr, Ce, Zr, Al) Catalysts in CO2 Hydrogenation for Methanol Synthesis. Catalysts, 15(3), 250. https://doi.org/10.3390/catal15030250






