1. Introduction
Biodiesel has emerged as a promising renewable energy source that is capable of replacing conventional diesel due to its favorable physicochemical properties and environmental benefits [
1]. Unlike petroleum-based diesel, biodiesel is biodegradable and renewable, and significantly reduces greenhouse gas emissions [
2]. Its compliance with international standards, such as EN 590 (EU) [
3] and ASTM D975 (US) [
3], highlights its suitability as a diesel substitute [
3]. Biodiesel offers a comparable density, viscosity, and flash point, ensuring compatibility with existing diesel engines. Additionally, its ultra-low sulfur content, high cetane number, and high oxygen content improve its combustion efficiency while minimizing harmful emissions. These advantages position biodiesel as a sustainable alternative to fossil fuels, aligning with global efforts to transition to cleaner energy sources. There has been a noticeable increase in the usage of thinner lubricant, even in the automotive sector. The new fuel economy standards promote lower viscosity over higher viscosity lubricants to increase the fluid friction and offset the fuel consumption of farmers [
4].
Table 1 compares the physicochemical properties of biodiesel with European and United States diesel standard specifications. The properties of biodiesel closely resemble those of conventional diesel, while ensuring advantages like a higher cetane number, better lubrication, and an ultralow sulfur content, leading to cleaner combustion and fewer emissions.
The growing global energy demand and the need for sustainable alternatives to fossil fuels have driven extensive research into renewable energy sources. Acetone–Butanol–Ethanol (ABE) has been identified as a promising oxygenated fuel blend that has an improved combustion efficiency and reduces emissions in diesel engines [
5,
6]. Among the types of ABEs, biodiesel has emerged as a promising candidate due to its environmental benefits and compatibility with existing diesel engines [
7]. Derived primarily from vegetable oils, animal fats, and waste cooking oils, biodiesel is a biodegradable, non-toxic, and renewable energy source that significantly reduces greenhouse gas emissions [
8] compared to petroleum-based diesel [
9,
10]. Its production involves transesterification, a chemical process where triglycerides react with alcohol in the presence of a catalyst to produce biodiesel (fatty acid methyl esters or FAMEs), and glycerol as a byproduct [
11,
12]. Despite its advantages, the commercialization of biodiesel faces significant challenges [
12], particularly related to its production efficiency, cost, and environmental impact [
13]. Central to addressing these challenges is the development and optimization of catalysts that can enhance the reaction efficiency while maintaining the sustainability of the process [
14].
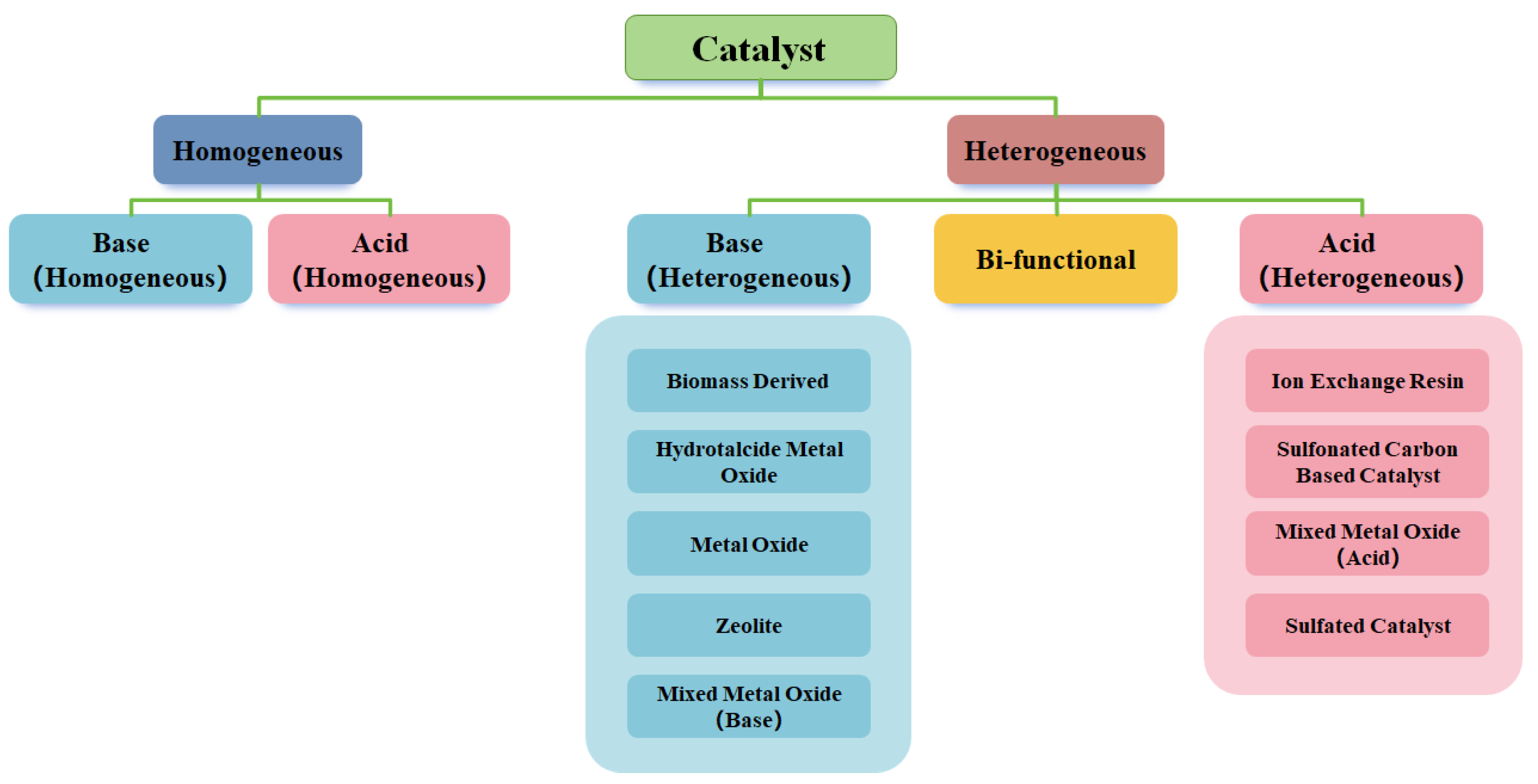
Figure 1 categorizes catalysts into homogeneous and heterogeneous systems. Traditional homogeneous catalysts, such as sodium hydroxide or sulfuric acid, have been widely used due to their high reaction rates [
15]. However, their drawbacks, including difficulties in their separation, their poor recyclability, and their generation of hazardous waste, have shifted the research focus towards heterogeneous catalysts [
16].
Solid transesterification catalysts offer significant advantages over traditional catalysts, making them a promising alternative for biodiesel production [
18]. These catalysts are highly stable under a wide range of thermal and chemical conditions, ensuring consistent performance and durability during extended operations. Their solid nature allows easy separation and recovery from the reaction mixture, enabling reuse in multiple cycles and reducing chemical waste. Unlike homogeneous catalysts, solid catalysts are resistant to impurities such as water and free fatty acids, which allows for the use of low-grade or waste feedstocks without compromising the biodiesel yield [
19]. Advanced designs, such as sulfonated zeolites, layered double hydroxides (LDHs), and metal–organic frameworks (MOFs), provide high selectivity and catalytic efficiency [
20], facilitating faster reactions and higher product purity. Furthermore, their environmental benefits include reduced reliance on corrosive substances and minimized waste generation, making them a greener choice for industrial applications [
21]. While the initial cost of solid catalysts may be higher, their reusability and efficiency lead to long-term cost savings. Additionally, their porous structures and large surface areas enhance mass transfer, enabling the effective processing of viscous systems [
22]. These features, combined with their versatility in processing diverse feedstocks and enabling other polyester-related reactions, highlight their transformative potential for sustainable biodiesel production and beyond.
The transesterification process requires specific conditions to achieve optimal yields, including elevated temperatures and pressures, precise alcohol-to-oil ratios, and prolonged reaction times [
12]. These stringent requirements increase operational costs and energy consumption, limiting the economic feasibility of biodiesel production. Moreover, side reactions, such as saponification and hydrolysis, can occur under suboptimal conditions, further complicating the process.
Acknowledging the pivotal role of catalysts in addressing these challenges, this review centers on the development and optimization of solid transesterification catalysts for biodiesel production. Solid catalysts represent a paradigm shift in biodiesel production, offering a sustainable and efficient alternative to traditional methods. Among the various types of heterogeneous catalysts, transesterification catalysts have gained attention due to their unique properties, including, high their catalytic efficiency. These catalysts exhibit excellent activity in transesterification reactions, enabling high conversion rates and biodiesel yields. The ability to modify the physical and chemical properties of transesterification catalysts allows for tailoring their performance to suit specific feedstocks and reaction conditions.
This review offers a comprehensive analysis of the catalytic properties and structural optimization of solid transesterification catalysts, emphasizing their influence on reaction efficiency. It explores the fundamental mechanisms underlying their activity, discusses strategies for their structural enhancement, and evaluates their performance in various reaction systems. By addressing these aspects, this review aims to establish a comprehensive understanding of the potential of transesterification catalysts in advancing biodiesel technology. Through a detailed discussion of the challenges in and opportunities related to biodiesel synthesis, this review underscores the importance of innovative catalyst designs in achieving sustainable energy solutions. By bridging the gap between laboratory research and industrial applications, solid transesterification catalysts hold the promise of transforming biodiesel production into a cost-effective and environmentally friendly process. The insights presented in this review are aimed to guide future research and development efforts, paving the way for the widespread adoption of biodiesel as a renewable energy source.
2. Literature Review
The transesterification of vegetable oils, animal fats, and recycled waste oils with alcohol in the presence of a catalyst is a well-established method for biodiesel production. This process produces methyl esters of long-chain fatty acids (biodiesel) and glycerol as a byproduct [
23]. The catalyst plays a pivotal role in providing active sites for the reactants, thereby reducing the overall activation energy required for the reaction. Various types of catalysts—homogeneous catalysts, heterogeneous catalysts, and biocatalysts—have been utilized in biodiesel synthesis.
The use of solid transesterification catalysts has revolutionized several chemical processes, particularly in the production of biodiesel and the recycling of polyesters [
24]. These catalysts stand out due to their unique combination of efficiency, stability, and environmental friendliness, which has made them a focal point of research. In recent years, various types of solid catalysts have been developed and investigated for their ability to drive ester-exchange reactions in polyesters, offering sustainable and practical alternatives to traditional homogeneous and enzymatic catalysts. Heterogeneous-based catalysts are unique because of their easy separation from the reaction mixture, enhanced reusability, and suitability for large-scale industrial applications [
25]. For example, titanium-based and zinc-based catalysts are recognized for their stability and tunable activity in both esterification and transesterification reactions. Antimony-based catalysts and heterogeneous acid catalysts, including sulfonated zeolites, provide strong acidic active sites that are effective for creating feedstocks with a high free fatty acid (FFA) content. Similarly, layered double hydroxides (LDHs) are versatile heterogeneous catalysts that exhibit a tunable basicity or acidity and high thermal stability, making them ideal for biodiesel production. The heterogeneity of these catalysts not only simplifies product purification but also aligns with the goals of sustainable and efficient biodiesel synthesis.
2.1. Titanium-Based Catalysts
Titanium-based catalysts, particularly titanium dioxide (TiO
2), have demonstrated significant promise in polyester chemistry [
26]. TiO
2 is widely used in solid-state transesterification reactions, where it efficiently depolymerizes polyethylene terephthalate (PET) into its monomers, such as terephthalic acid and ethylene glycol [
27]. This property is crucial for recycling PET, one of the most commonly used polyesters in packaging and textiles. Additionally, TiO
2 exhibits remarkable thermal stability, allowing it to operate under high-temperature conditions without significant degradation [
28]. Its recyclability further enhances its appeal, as it can be recovered and reused in multiple reaction cycles. This property aligns with the growing emphasis on circular economic principles in polymer science. Research also indicates that TiO
2 can modify polyester structures, enabling the synthesis of novel materials with tailored properties, thereby expanding its utility in advanced applications.
2.2. Zinc-Based Catalysts
Zinc-based catalysts, such as zinc acetate (Zn(OAc)
2), are another widely studied class of solid catalysts used in polyesterification and transesterification reactions [
29]. Zn(OAc)
2 is particularly effective in facilitating ester-exchange reactions in PET synthesis, where it enables the reaction to proceed efficiently without requiring a homogeneous medium [
30]. This heterogeneous nature simplifies the catalyst recovery process, reducing waste and lowering operational costs. Moreover, zinc catalysts are known for their versatility, as they can be employed in various polymer systems and under different reaction conditions. Their ability to achieve high selectivity and conversion rates makes them a reliable choice for industrial-scale applications [
31].
2.3. Antimony-Based Catalysts
Antimony-based catalysts, such as antimony trioxide (Sb
2O
3), play a crucial role in polyester manufacturing, especially polyester manufacturing for PET production. Sb
2O
3 is highly effective in exchange reactions due to its ability to activate ester groups, ref. [
32] facilitating the breakdown and reformation of ester bonds. Its widespread use in the industrial production of PET highlights its importance as a robust and efficient catalyst. Despite concerns about the environmental impact of antimony compounds, advancements in catalyst design and waste management strategies are helping to address these challenges, ensuring their continued relevance in the field [
33].
2.4. Layered Double Hydroxides (LDHs)
Layered double hydroxides (LDHs), such as magnesium-aluminum (Mg-Al) LDHs, represent a highly tunable class of solid catalysts [
34]. The acidity and basicity of these materials can be adjusted by modifying their preparation conditions, making them suitable for a wide range of reactions. LDHs are particularly effective in transesterification and ester-exchange reactions in polyesters, where their layered structure facilitates the interaction between the reactants and active sites. Furthermore, their heterogeneous nature ensures easy separation from the reaction mixture, reducing the environmental impact and improving the overall process efficiency. The ability to tailor their properties also allows for the development of LDHs with enhanced catalytic activity, making them a valuable tool in both academic and industrial research [
35].
2.5. Heterogeneous Acid Catalysts
Heterogeneous acid catalysts, such as sulfonated zeolites (e.g., H-ZSM-5), are widely recognized for their efficiency in esterification and transesterification reactions [
36]. These catalysts are particularly useful for modifying or recycling polyesters, where their acidic active sites drive the reaction toward the desired products. Sulfonated zeolites are characterized by their high thermal stability, which allows them to function effectively under challenging reaction conditions [
19]. Additionally, their porous structure provides a large surface area, enhancing their mass transfer and catalytic efficiency. The environmental benefits of these catalysts are also noteworthy, as they reduce the need for corrosive substances and minimize waste generation, making them a sustainable choice for industrial applications.
Table 2 lists catalysts by kind, substance, and use. Active but corrosive homogeneous acids (e.g., H
2SO
4) fit the category of biodiesel but require neutralizing homogeneous bases (e.g., NaOH). While heterogeneous bases (e.g., CaO) are cheap, heterogeneous acids—such as zeolites—are reusable and stable. For applications involving polyester, solid catalysts (e.g., TiO
2, Mg-Al LDHs) provide recyclability and customizable characteristics. The reaction demands and sustainability of the material determine the catalyst choice.
3. Mechanistic Insights into Solid Ester-Exchange Catalysts
Solid ester-exchange catalysts are indispensable in achieving efficient and sustainable biodiesel production due to their high stability, reusability, and catalytic activity under diverse reaction conditions.
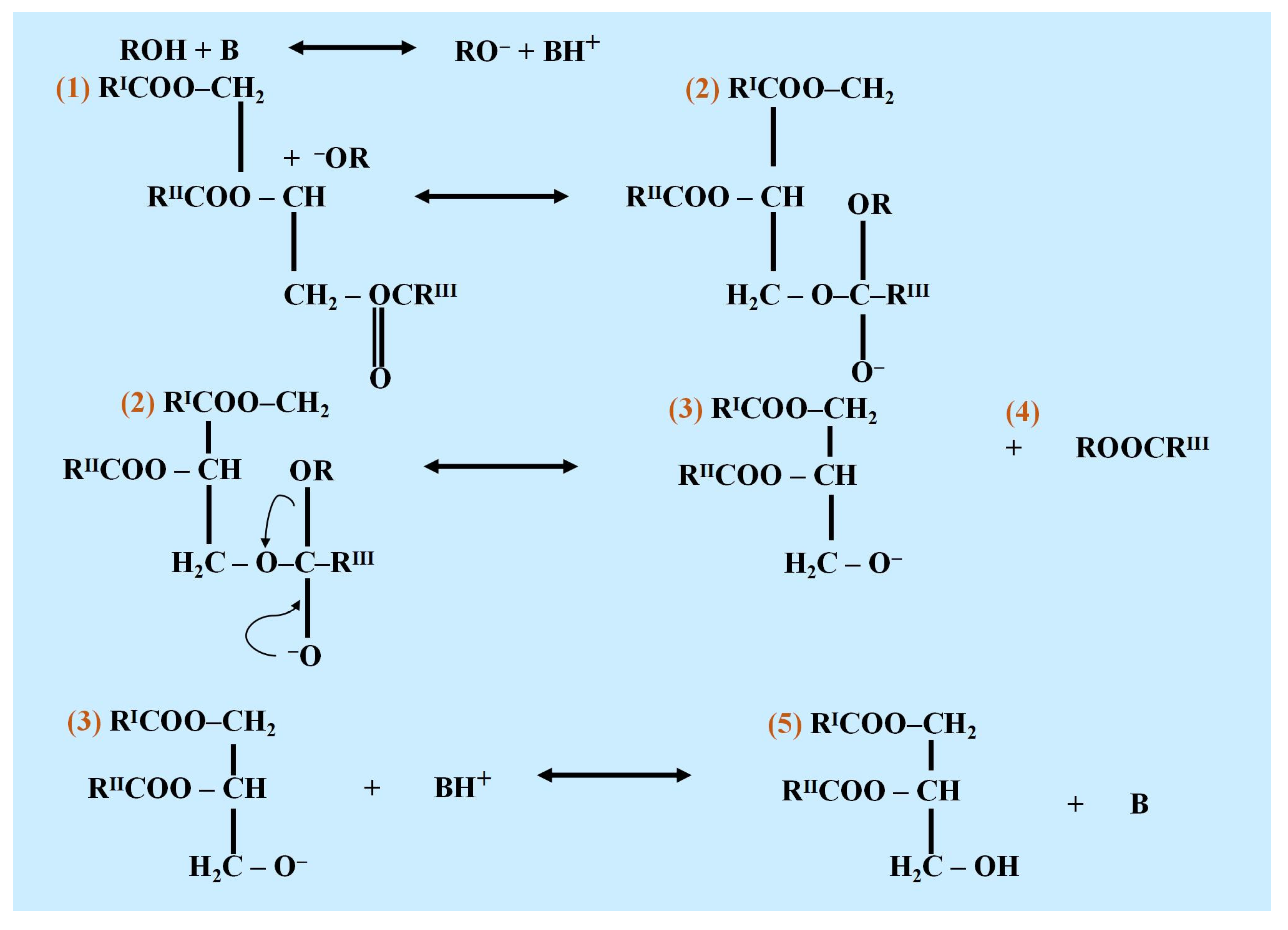
Figure 2 shows the process of base-catalyzed transesterification. A number of solid basic catalysts, including ZnO, CuO, zeolites, CaO, SrO, MgO, and BaO, and basic polymers and carbonates, such as MgCO
3, CaCO
3, BaCO
3, and SrCO
3, have garnered interest as heterogeneous transesterification catalysts.
Among these, titanium-based catalysts (e.g., TiO
2) have been widely studied for their superior thermal stability and ability to sustain catalytic activity in high-temperature environments [
44,
48]. This makes them particularly effective in industrial transesterification reactions. Mechanistically, titanium catalysts leverage their Lewis acid sites to promote ester bond cleavage and facilitate reactant adsorption on their active sites, ensuring high conversion efficiency [
49]. However, despite their promising attributes, titanium catalysts often exhibit moderate selectivity, necessitating precise surface modifications or doping strategies to enhance their performance and reduce the formation of byproducts [
50].
Zinc-based catalysts, particularly zinc acetate (Zn(OAc)
2), offer a compelling alternative due to their high selectivity and compatibility with feedstocks that contain impurities such as free fatty acids and water. Their ability to stabilize intermediate complexes during ester exchange reactions accelerates reaction kinetics and enhances product yields [
45]. Despite these advantages, zinc-based catalysts face limitations related to their thermal stability, which constrains their applicability in high-temperature or prolonged reaction conditions. Future research could focus on improving the thermal resilience of these catalysts through advanced preparation techniques and composite formulations.
Layered double hydroxides (LDHs), such as Mg-Al LDHs, represent a highly tunable class of solid catalysts that combine adjustable acidity/basicity with excellent thermal stability. Their layered structure facilitates the efficient diffusion of reactants to active sites, optimizing reaction pathways and ensuring high catalytic performance in both transesterification and polyester recycling applications [
47]. The structural tunability of LDHs enables their adaptation for specific feedstocks and reaction conditions. However, their complex synthesis methods, which often involve the precise control of precursor ratios and calcination parameters, remain a significant challenge. Moreover, the catalytic activity of LDHs depends heavily on the presence of uniform active sites, the optimal performance of which require meticulous preparation techniques.
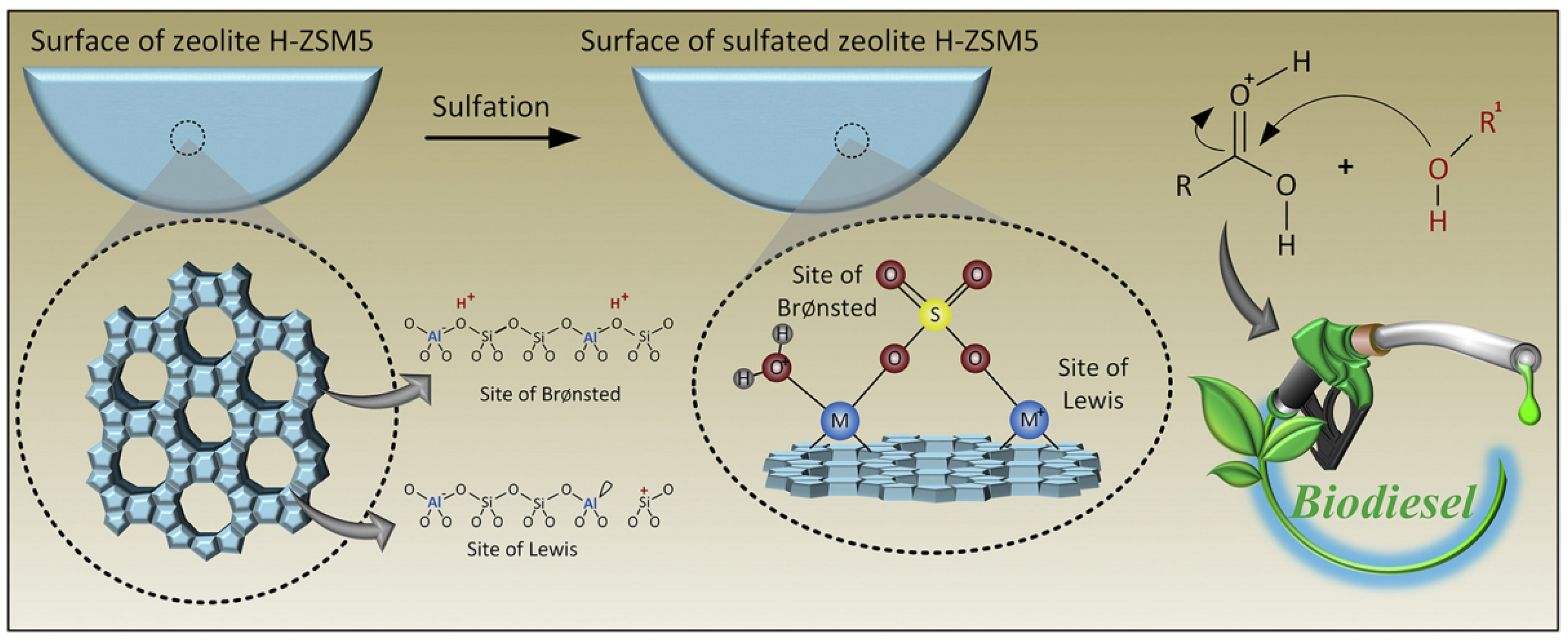
Heterogeneous acid catalysts, such as sulfonated zeolites (e.g., H-ZSM-5) [
36], are particularly effective in esterification reactions due to their strong acidic active sites. These catalysts operate by protonating ester groups, thereby enhancing their reactivity and facilitating bond cleavage.
Figure 3 shows the mechanism of esterification processes enabled by heterogeneous acid catalysts, such as sulfonated zeolites (e.g., H-ZSM-5). Reusable and stable at mild reaction conditions, these catalysts offer active acidic sites that protonate carbonyl groups, which facilitates nucleophilic assault by alcohols to produce esters. Their high thermal stability and reusability make them attractive for industrial applications. However, catalyst deactivation due to fouling or the accumulation of byproducts on the surface remains a challenge that needs to be addressed. Strategies such as the incorporation of protective layers or regeneration techniques can mitigate these issues, prolonging the catalyst’s operational lifespan.
4. Alkali-Doped Oxides
Alkali-doped oxides are among the most widely studied solid alkaline catalysts, owing to their high catalytic efficiency, stability, and versatility in a variety of chemical reactions [
52]. These catalysts are particularly important in processes like biodiesel synthesis, esterification, transesterification, and polyester depolymerization. These catalysts are formed by loading alkali metal salts such as potassium carbonate (K
2CO
3), sodium carbonate (Na
2CO
3), or lithium carbonate (Li
2CO
3) onto metal oxide carriers like magnesium oxide (MgO), calcium oxide (CaO), barium oxide (BaO), or iron oxide (Fe
3O
4) [
53]. The choice of alkali salt, carrier, and preparation method plays a critical role in determining the structural and catalytic properties of the resulting material.
4.1. Preparation and Structural Characteristics
The preparation of alkali-doped oxides typically involves methods such as impregnation, co-precipitation, or thermal calcination [
54]. These methods allow for precise control over the dispersion of alkali metal salts on the surface of metal oxides. For instance, wet impregnation ensures the uniform distribution of alkali species on the carrier, enhancing the availability of active sites. Co-precipitation techniques, on the other hand, result in strong interactions between the alkali metal salts and the metal oxide matrix, improving the catalyst’s thermal stability [
55].
The structural properties of the catalyst—such as its surface area, porosity, and basicity—are significantly influenced by the choice of carrier and alkali salt. MgO, for example, is known for its strong basic sites and thermal stability, which make it an excellent choice for high-temperature reactions [
56]. CaO is abundant, cost-effective, and offers moderate basicity, which is sufficient for many transesterification processes [
57]. Fe
3O
4, with its magnetic properties, facilitates catalyst recovery, enhancing its reusability [
58]. Barium oxide (BaO), though less commonly used, provides strong basic sites and high reactivity in specific applications [
59].
4.2. Different Types of Alkali-Doped Oxides
4.2.1. MgO-Based Catalysts
MgO doped with K
2CO
3 or Na
2CO
3 is among the most effective alkali-doped oxide catalysts. The alcohol doping introduces strong basic sites, enabling the catalyst to efficiently activate reactants in transesterification reactions. MgO-based catalysts also exhibit high thermal and chemical stability, making them suitable for industrial-scale biodiesel production [
54].
4.2.2. CaO-Based Catalysts
CaO doped with alkali salts is widely used due to its availability and low cost. These catalysts are highly active in processes such as biodiesel synthesis. Their strong basicity facilitates the activation of alcohols and esters, while their stability ensures consistent performance over multiple cycles [
55]. CaO-based catalysts often require calcination at high temperatures to remove moisture and enhance their reactivity.
4.2.3. Fe3O4-Based Catalysts
Fe
3O
4 doped with alkali metals combines catalytic efficiency with ease of recovery, thanks to its magnetic properties. These catalysts are particularly useful in batch processes, where the magnetic carrier allows for quick and effective separation of the catalyst from the reaction mixture. Fe
3O
4-based catalysts are often employed in esterification and transesterification reactions [
58].
4.2.4. BaO-Based Catalysts
BaO doped with K
2CO
3 or Na
2CO
3 provides strong basic sites and is particularly effective in high-temperature reactions. Although less common than MgO- or CaO-based systems, BaO-based catalysts have shown promise in specialized applications that require high reactivity [
53].
4.3. Catalytic Mechanism
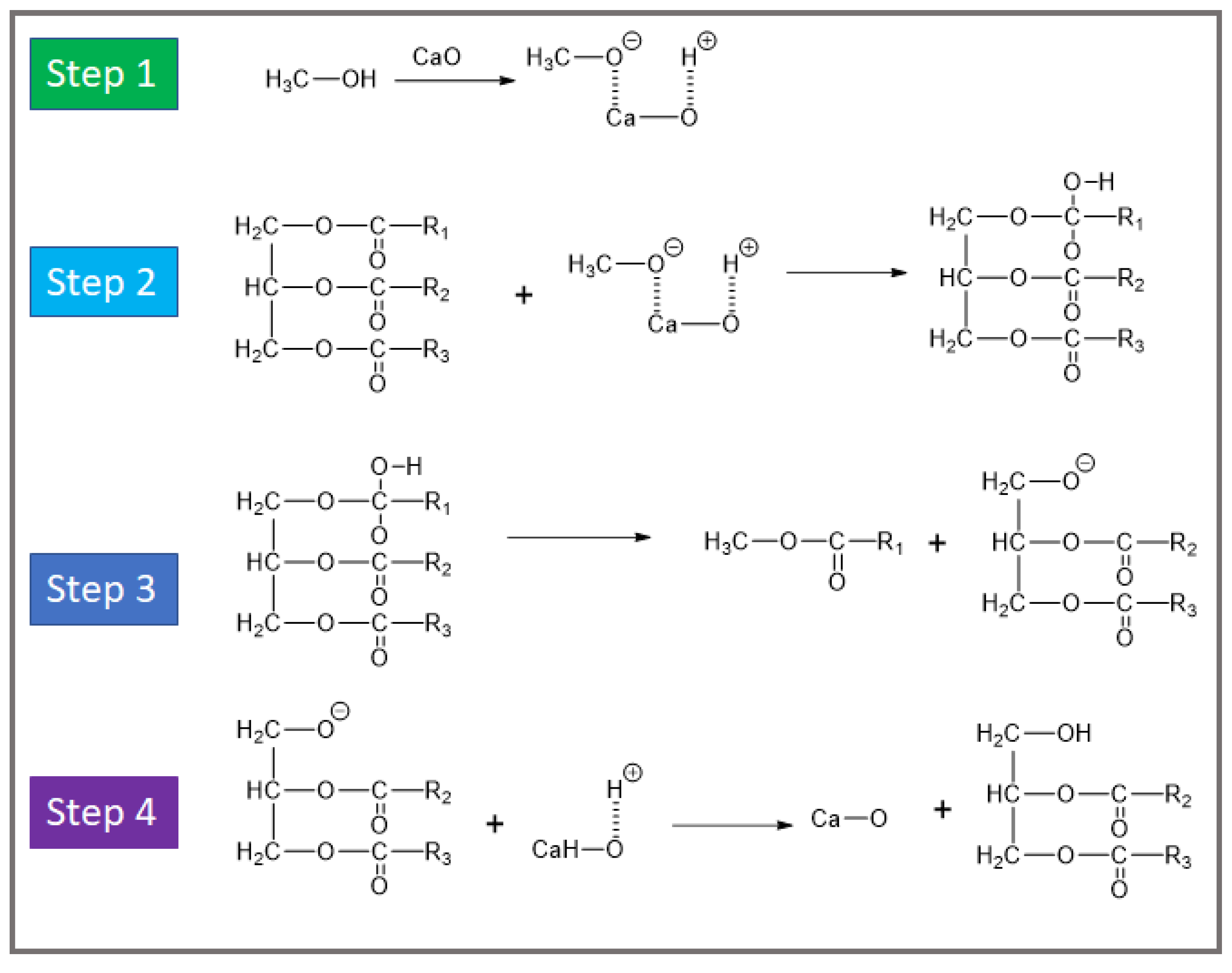
The mechanism of alkali-doped oxides typically involves the activation of reactants at the basic sites provided by the catalyst. These basic sites, created by the interaction of alkali metal salts with the oxide surface, facilitate the deprotonation of alcohols in transesterification reactions or the activation of ester bonds in ester-exchange processes. For example, in biodiesel synthesis, the catalyst promotes the reaction between triglycerides and methanol by generating methoxide ions, which attack the carbonyl carbon of the triglyceride, leading to the formation of methyl esters (biodiesel) and glycerol. The strength and density of the basic sites play a crucial role in determining the reaction rate and yield. Diagrams illustrating this mechanism can provide a clearer understanding of the interaction between the reactants and the active sites on the catalyst surface [
60]. The mechanism of Cao-based heterogeneous catalyst creation is shown in
Figure 4.
4.4. Performance Metrics and Comparison
The performance of alkali-doped oxides is evaluated based on metrics such as their catalytic activity, selectivity, stability, and reusability. For instance, MgO-based catalysts doped with K
2CO
3 have shown high conversion rates (up to 95%) in biodiesel synthesis, as well as excellent reusability over multiple cycles. CaO-based catalysts, while slightly less efficient, offer the advantage of a lower cost and widespread availability. Fe
3O
4-based catalysts, on the other hand, excel in processes where catalyst recovery is a priority, achieving high separation efficiency due to their magnetic properties [
54].
5. Literature Analysis
A detailed analysis of the existing studies reveals that alkali-doped oxides have been extensively studied for their role in biodiesel production, with a focus on optimizing their preparation methods and improving their performance. Research has demonstrated that doping MgO with K
2CO
3 results in a catalyst with enhanced basicity and stability, which is ideal for high-temperature reactions [
61]. Similarly, studies on CaO doped with Na
2CO
3 highlight its cost-effectiveness and efficiency in transesterification processes. Fe
3O
4-based catalysts have been investigated for their unique magnetic properties which simplify catalyst recovery and improve the efficiency of the process.
6. Challenges and Future Directions
A significant barrier to the industrial application of solid transesterification catalysts is their gradual deactivation, which lowers their catalytic efficacy over a number of reaction cycles. The three primary reasons for the occurrence of deactivation are thermal sintering, surface contamination (pollutant adsorption), and metal leaching, which reduce the catalyst lifetime; therefore, stabilization and regeneration strategies must be devised to maintain the performance of catalysts in the production of biodiesel [
62].
Metal leaching is one of the most significant deactivation mechanisms, especially in liquid-phase transesterification reactions where transition metals (like ZnO and TiO
2) and alkaline earth metals (like CaO and MgO) gradually dissolve into the reaction media. Leaching is accelerated by high reaction temperatures, prolonged exposure to polar solvents such as methanol and ethanol, and acidic byproducts that break down metal–oxygen bonds [
63]. This loss of active metal species reduces the catalytic activity and contaminates the biodiesel output, necessitating additional purification procedures. Numerous methods have been developed to counteract leaching. Barriers made of protective coatings, like SiO
2 or carbon layers, prevent active sites from coming into direct contact with the reaction fluid. The catalyst structure is strengthened and metal solubility is decreased by doping with stabilizing elements such as La
1⁺, Ce
3⁺, and Al
2O
3 [
64]. Core-shell architectures also provide long-term durability, as they contain active metal species within a stable framework. For instance, TiO
2/SiO
2 composites have demonstrated exceptional resistance to dissolution, maintaining over 95% catalytic activity after eight reaction cycles; MgO–zeolite composites significantly reduce Mg
2⁺ leaching while maintaining their catalytic efficiency over multiple cycles [
65].
Another common reason for deactivation is surface contamination, which occurs when the surfaces of catalysts are covered in organic residues, chemical byproducts, and impurities that obstruct active sites. The most problematic contaminants from biodiesel feedstocks include glycerol, free fatty acids (FFAs), and heavy metal pollution. These chemicals’ strong adhesiveness to the surfaces of catalysts reduces the catalytic efficiency by preventing reactants from reaching active regions [
66]. Additionally, coke formation, in which carbonaceous deposits accumulate due to incomplete reactions, deactivates catalysts by obstructing their pores and decreasing their surface area. These effects can be countered by periodic oxidative regeneration, in which carbon residues are burned off by heating the catalyst in an oxygen-rich environment. For example, calcination at 500–600 °C effectively removes coke deposits from sulfonated zeolites (H-ZSM-5), thereby maintaining their function [
67]. Another strategy is to use hydrophobic surface changes, like siloxane layers or fluorinated coatings (-CF
3), which reject polar contaminants and prevent the accumulation of glycerol or FFAs. Some catalysts, including those based on TiO
2, also exhibit photo-induced self-cleaning properties, in which organic impurities are broken down by UV radiation, which helps to maintain their long-term efficacy.
A third major issue is thermal sintering, where catalyst particles aggregate at high temperatures, decreasing surface the area and active site availability [
68]. This phenomenon is particularly problematic for nanostructured catalysts, such as ZnO and TiO
2, whose high surface energy makes them vulnerable to particle growth and coalescence. To avoid sintering, researchers have focused on including thermal-resistant supports, such as SiO
2 and AlO
3, which provide structural stability and inhibit particle fusion [
69]. Additionally, controlled calcination methods that gradually heat the catalyst under optimal conditions aid in maintaining particle dispersion and reducing agglomeration.
Resolving specific deactivation mechanisms is essential to increasing catalyst stability and industrial viability. When combined, protection coatings, structural doping, and enhanced regeneration methods significantly extend the life of catalysts and reduce operating expenses. The primary focus of future research should be on creating stronger catalyst topologies with increased resistance to leaching, reduced surface contamination, and enhanced thermal stability. By improving these properties, solid transesterification catalysts can be further enhanced to enable the production of affordable, industrial-scale biodiesel that complies with sustainability standards. The stability of catalysts is essential to their use in industry. A highly effective catalyst must maintain 90% or more of its initial activity over 10 or more reaction cycles in order to be both efficient and lucrative. Future research should concentrate on developing regenerable catalysts with high conversion efficiencies, low deactivation rates, and great stability. Current material engineering approaches, bimetallic or hybrid catalyst systems that balance durability with activity, and even porous nanostructures that enhance the diffusion properties of catalysts can all be used to accomplish this goal. AI-driven catalyst design may speed up the creation of new materials with improved stability and resistance to deterioration.
7. Comparison and Applications
The advancements in solid transesterification catalysts highlight their growing importance and versatility compared to traditional catalyst systems. Unlike homogeneous catalysts, which are often associated with challenges such as corrosion, difficulty in their recovery, and environmental concerns due to chemical waste, solid catalysts provide significant advantages in terms of stability, reusability, and resistance to impurities in the feedstock [
70]. These characteristics make them an ideal choice for industrial applications, particularly in biodiesel production, where they have been instrumental in enabling the use of low-grade or waste feedstocks. By facilitating the efficient conversion of triglycerides into biodiesel with minimal byproduct formation, solid catalysts not only enhance the process’s sustainability but also reduce operational costs by simplifying product purification and catalyst recovery. Another key advantage of solid transesterification catalysts lies in their tunable properties. Through innovations in catalyst design, such as nanostructuring, doping with metal or non-metal elements, and functionalization with acidic or basic groups, researchers have significantly improved the catalytic activity, selectivity, and thermal stability of catalysts. These modifications allow for precise control over reaction conditions, enabling catalysts to perform efficiently even under mild conditions. Moreover, the incorporation of hybrid systems, such as MgO–zeolite composites or TiO
2/SiO
2 materials, has expanded the scope of the application of catalysts. These hybrid catalysts combine the benefits of multiple materials, balancing acidity and basicity while maintaining high structural integrity, which is critical for long-term industrial use.
Beyond biodiesel production, solid transesterification catalysts are proving to be highly versatile in other areas of polymer science. Their ability to facilitate ester-exchange reactions with exceptional selectivity and yields makes them valuable for processes like polyester recycling, where they aid in breaking down and reprocessing post-consumer plastics. This capability supports circular economy initiatives by converting waste plastics into raw materials for new polymer production. Additionally, the use of these catalysts is being explored in advanced material synthesis, such as the development of biodegradable polymers and functionalized polyesters for biomedical applications. For instance, bio-based solid catalysts, such as chitosan derivatives, offer a renewable and eco-friendly alternative for producing high-performance polymers while reducing the environmental footprint of the process [
71,
72].
8. Structural Optimization of Solid Transesterification Catalysts
Structural optimization is crucial for enhancing the catalytic performance of solid transesterification catalysts. This involves tailoring the surface area, porosity, acidity, basicity, particle size, and morphology of the catalyst, and doping it with active elements. As shown in
Table 3, optimizing the surface area and porosity of the catalyst enhances the reactant diffusion by increasing the accessibility to active sites, reducing mass transfer limitations, and improving the overall catalytic efficiency. Catalysts with mesoporous structures exhibit superior performance in transesterification reactions, leading to higher biodiesel yields and improved reusability. These modifications improve the reaction efficiency, selectivity, and catalyst stability, enabling applications in diverse chemical processes. The following table summarizes key optimization strategies and their corresponding benefits:
This structured approach allows for the systematic improvement of catalyst performance, paving the way for efficient and eco-friendly chemical processes.
8.1. Influence of Structural Properties on Catalytic Activity
The catalytic activity of solid transesterification catalysts is greatly influenced by their structural properties [
79]. These properties determine how efficiently the catalysts interact with reactants, how effectively they facilitate chemical transformations, and how well they maintain their performance over time. The key structural properties that influence catalytic activity include the surface area, pore structure, active site distribution, acidity and basicity, particle size, and morphology of the catalyst, as well as the presence of dopants or functional groups [
80]. By modifying these structural characteristics, researchers can optimize catalysts for specific applications, enhancing their performance in various chemical processes, including esterification, transesterification, and polyester depolymerization.
8.2. Surface Area and Porosity
The surface area of the catalyst is a critical determinant of its catalytic activity because it directly correlates with the number of available active sites where chemical reactions can occur [
81]. A larger surface area means more contact between the catalyst and the reactants, which can enhance the reaction rate. Materials with high surface areas, such as mesoporous catalysts or nanostructured solids, are particularly effective because they allow for the more efficient diffusion of reactants into the pores, ensuring that reactions occur throughout the entire catalyst volume. For example, mesoporous TiO
2 and ZnO have been shown to offer improved catalytic activity in transesterification reactions due to their high surface area and well-distributed pores. These properties minimize mass transfer limitations and provide a greater number of active sites for ester exchange reactions.
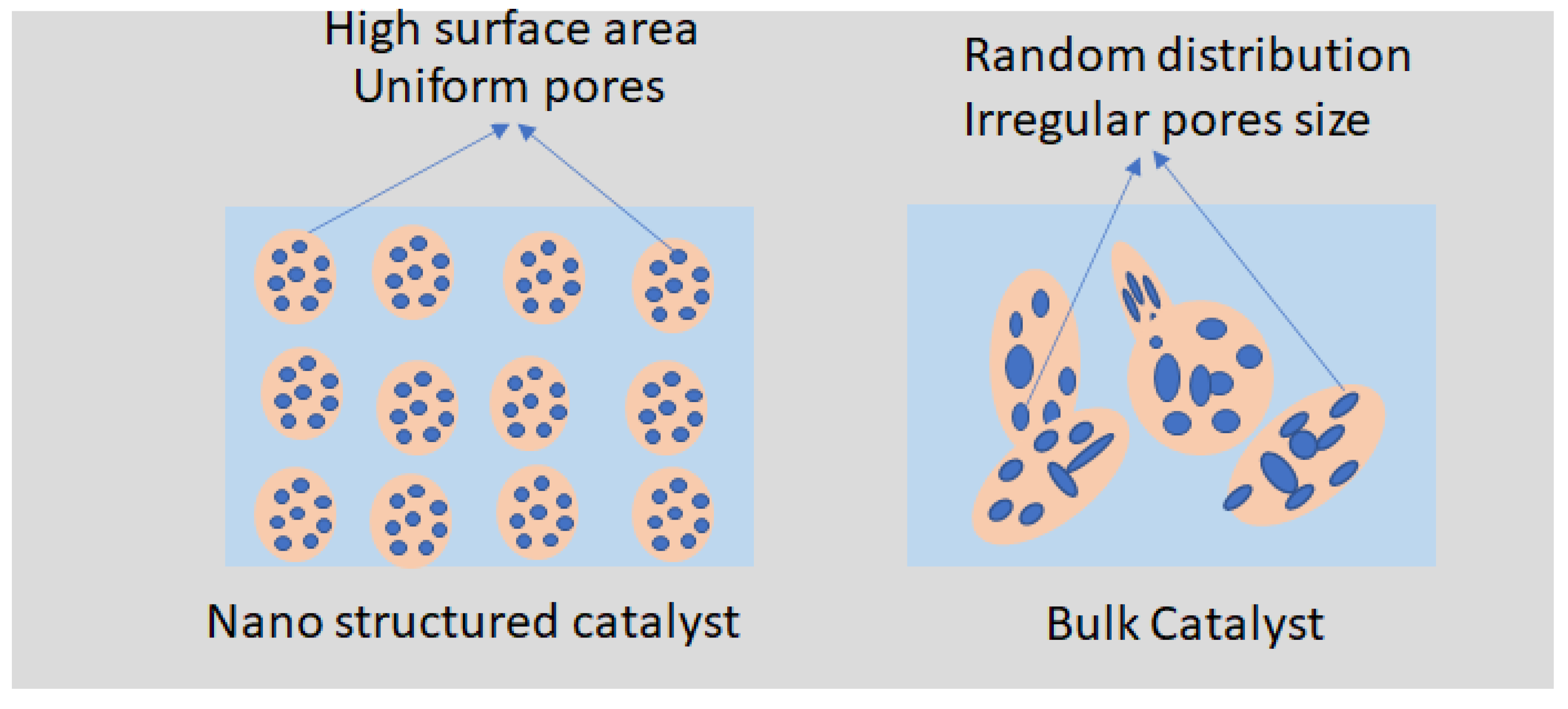
Figure 5 illustrates the difference in pore distribution between nanostructured and bulk catalysts. Nanostructured catalysts exhibit a high surface area with small, uniform pores, allowing better reactant diffusion and increased accessibility to active sites, which enhances the catalytic efficiency. In contrast, bulk catalysts have fewer, larger, and more irregular pores, resulting in a lower surface area and limited reactant accessibility, which can hinder the catalytic performance. This comparison highlights the importance of optimizing the porosity and surface area to improve the effectiveness and reusability of solid transesterification catalysts.
Figure 5 compares the pore distributions of nanostructured and bulk catalysts, highlighting the superior surface area and more uniform porosity of nanostructured materials, which in turn enhance the reactant diffusion and catalytic efficiency.
Similarly, the porosity of a catalyst determines the accessibility of its active sites to reactants [
82,
83]. Materials with well-defined pore structures, such as zeolites and layered double hydroxides (LDHs), exhibit improved catalytic performance due to the efficient transport of reactants and products through their pores. Zeolites, particularly sulfonated varieties like H-ZSM-5, have a regular pore structure that facilitates selective access to active sites, enhancing their efficiency in transesterification and esterification reactions. The optimal pore size also ensures that larger reactant molecules can enter the pores and interact with active sites, leading to more efficient catalytic processes [
84].
8.3. Acidity and Basicity
The acidity and basicity of a catalyst play a crucial role in determining its catalytic activity, especially in ester-exchange reactions. Catalysts with strong acidic sites are effective in esterification reactions, where they activate alcohols to promote ester bond formation [
84]. In contrast, catalysts with strong basic sites are more suitable for transesterification, where they facilitate the exchange of ester groups. Modifying the acidity and basicity of catalysts can significantly improve their selectivity and efficiency [
85]. For example, sulfonated zeolites, which possess strong acidic sites (–SO
3H), are widely used in esterification reactions due to their ability to protonate ester groups, making them more reactive. On the other hand, basic catalysts like MgO, especially when doped with alkali metals such as K
2O, exhibit improved basicity, facilitating the deprotonation of alcohols during transesterification. The balance between acidity and basicity can be fine-tuned to optimize the catalyst for specific reactions, thereby its improving catalytic activity and selectivity.
8.4. Particle Size and Morphology
The size and morphology of catalyst particles also influence the catalytic activity. Smaller particles generally offer a higher surface-to-volume ratio, providing more active sites for reactions [
86]. Nanoparticles or nanoscale catalysts, such as those of TiO
2 and ZnO, are particularly efficient because they expose more surface area and thus improve reactant interaction. Moreover, controlling the morphology of catalysts—such as producing rod-like or sheet-like structures—can further enhance the catalytic activity by exposing specific crystal facets that possess higher intrinsic reactivity [
81,
87].
In addition, the morphology of catalysts affects their diffusion properties. For instance, spherical particles provide more uniform diffusion paths, while elongated particles can offer an increased surface area per unit volume, enhancing reaction rates. Tailoring the particle size and morphology of catalysts through synthetic methods such as sol-gel processes or hydrothermal treatments can lead to catalysts with optimized catalytic properties.
8.5. Doping and Functionalization
Doping solid catalysts with transition metals or non-metallic elements is an effective strategy for improving their catalytic activity. Doping can modify the electronic structure of the catalyst, enhancing its ability to interact with reactants [
88]. For example, doping TiO
2 with metals like vanadium or tungsten can enhance its electronic properties, making it more effective in polyester depolymerization reactions. Similarly, introducing heteroatoms like nitrogen into the framework of LDHs or MgO can create new active sites, improving the catalytic efficiency and selectivity. Functionalizing the surface of catalysts with specific groups also plays a significant role in enhancing catalytic activity. For instance, adding sulfonic acid (-SO
3H) or carboxylic acid (-COOH) groups to the surface of zeolites can increase their proton donation capacity, improving their effectiveness in esterification reactions. Surface functionalization can also enhance the interaction between catalysts and reactants, improving the reaction rates and selectivity of the catalyst.
Table 4 compares nanostructured, acidity/basicity-tuned, and hybrid catalysts based on their efficiency, stability, and reusability. Nanostructured catalysts offer a high surface area but may aggregate. Acidity/basicity tuning improves catalyst selectivity but requires careful doping. Hybrid catalysts provide dual functionality and enhanced stability but have higher synthesis costs.
9. Performance Evaluation of Solid Transesterification Catalysts
The performance of solid transesterification catalysts is critical in determining their applicability and efficiency in various catalytic processes, such as esterification, transesterification, and polyester depolymerization. Evaluating the performance of these catalysts involves assessing their catalytic activity, selectivity, stability, and reusability under different reaction conditions [
89,
90]. These performance metrics provide essential insights into the suitability of a catalyst for industrial-scale applications, helping researchers identify the most efficient and cost-effective catalysts. This section focuses on the key parameters used to evaluate the performance of solid transesterification catalysts and the various techniques employed to assess their effectiveness.
9.1. Catalytic Activity
The catalytic activity is one of the primary indicators of a catalyst’s performance. It refers to the catalyst’s ability to accelerate the reaction rate and convert reactants into products within a specific time frame. For transesterification reactions, the activity is often determined by measuring the conversion of reactants, such as polyesters (e.g., PET), alcohols, or esters, into desired products [
91]. A high catalytic activity is essential to obtaining efficient reaction kinetics, which in turn reduces the reaction time and energy consumption [
80]. For example, nanostructured titanium dioxide (TiO
2) catalysts exhibit high biodiesel yields, achieving 96–98% conversion efficiency over 5–7 cycles (
Table 5), making them highly effective for transesterification reactions. The activity can be influenced by several factors, including the surface area, porosity, acidity, basicity, and particle size of the catalyst, as these properties directly impact the availability of active sites and the ease of reactant diffusion. A common method for evaluating catalytic activity is using conversion rates or the turnover frequency (TOF) [
92]. The TOF is calculated as the number of moles of product formed per moles of active sites per unit time. In transesterification reactions, catalysts with higher TOF values are considered more efficient as they can process reactants faster and at lower temperatures. Additionally, temperature-dependent studies are often conducted to determine the optimal operating temperature at which the catalyst exhibits the highest activity. MgO–zeolite composites exhibit excellent catalytic performance, achieving a 93–96% biodiesel yield over 7–10 reaction cycles, demonstrating high stability and reusability. In contrast, bio-based chitosan catalysts provide an 89–93% biodiesel yield but with lower reusability due to structural degradation over multiple cycles (
Table 5). These variations highlight the trade-off between catalyst efficiency and long-term usability, influencing their suitability for industrial applications.
9.2. Selectivity
Selectivity refers to the catalyst’s ability to favor the formation of specific products while minimizing the production of undesired byproducts [
94]. In transesterification reactions, high selectivity is important to ensure that the desired ester or polymer structure is produced without the formation of unwanted side products, such as oligomers, byproducts from degradation, or unreacted intermediates [
80]. The selectivity of a catalyst depends on the nature of its active sites, the presence of functional groups, and the reaction conditions. To evaluate selectivity, researchers often conduct product analysis using techniques such as gas chromatography (GC), high-performance liquid chromatography (HPLC), and mass spectrometry (MS). These methods enable the identification and quantification of individual products and byproducts, providing insights into the catalyst’s ability to direct the reaction towards the desired product. Catalysts with high selectivity are crucial for industrial applications where high-purity products are required, such as in the production of high-quality biodiesel, polyesters, and fine chemicals [
95].
9.3. Stability and Lifetime
The stability and reusability of solid transesterification catalysts are critical to their viability for large-scale biodiesel production. The most crucial factors that determine a catalyst’s lifetime are its resistance to poisoning or deactivation processes, chemical stability, and heat resistance. A powerful catalyst should withstand numerous cycles without seeing a noticeable decline in effectiveness in order to ensure both financial and environmental benefits [
96].
For a catalyst to be considered practical for industrial usage, it must maintain at least 90% of its initial catalytic activity over 10 reaction cycles [
97]. Additionally, in order to maintain catalytic performance without contaminating the final product, the metal leaching should be kept below 5 ppm for every cycle. Resistance to coke formation is also crucial since excessive carbon deposition can lead to catalyst fouling and the requirement for regular regeneration, which raises operating expenses.
According to recent studies, several solid catalysts exhibit exceptional long-term stability. For instance, MgO–zeolite composites, which are highly suitable for biodiesel production, sustain 93–96% conversion efficiency through 7–10 cycles. Likewise, nanostructured TiO2 catalysts maintain 96–98% activity through 5–7 cycles, but require additional modifications to reduce their metal leaching. Despite the high selectivity and catalytic efficiency demonstrated by sulfonated zeolites (H-ZSM-5), their acidic nature renders them vulnerable to deactivation as a result of surface fouling; hence, periodic regeneration is required to sustain performance.
Long-term catalyst stability can be increased using a variety of strategies. Regarding doping techniques, stabilizing compounds and metal oxides are used to enhance metal retention and prevent leaching. Surface coatings, such as silica layers or organic functionalization, also serve as protective layers, reducing coke formation and extending the catalyst life. Another tactic is the creation of regeneration-friendly catalysts, which can be easily restored with minimal function loss through mild chemical reactivation or straightforward heat treatments [
98].
Going forward, catalyst research should focus on optimizing scalable, cost-effective substitutes that maintain a high reaction efficiency while enhancing the durability of the resulting products. Hybrid catalyst systems, which blend basic and acidic qualities, can contribute to increased stability by decreasing the number of breakdown routes. Furthermore, by revealing more about catalyst activity under real-time reaction conditions, advanced characterization techniques like operando X-ray diffraction and in situ spectroscopy aid in the optimization of targeted material design.
9.4. Reusability
Reusability is an important characteristic of solid catalysts, as it directly influences the economic viability of catalytic processes [
99]. A catalyst’s ability to maintain its activity and selectivity over multiple reaction cycles can reduce operational costs and improve sustainability by minimizing the need for frequent catalyst replacement. The reusability of a catalyst is typically assessed by conducting repeated reaction cycles, where the catalyst is recovered, cleaned, and reused [
100]. During each cycle, the catalyst is evaluated for changes in activity, selectivity, and structural integrity. The number of cycles a catalyst can undergo before exhibiting a significant loss of activity or selectivity is a key indicator of its reusability [
101]. In many cases, solid transesterification catalysts, particularly those that are well-structured and resistant to degradation, can maintain their performance over dozens or even hundreds of cycles. Reusability studies often involve using the catalyst in a batch or continuous-flow reactor, followed by regeneration treatments (e.g., calcination, washing, or chemical treatment) to restore its activity.
10. Common Methods of Biodiesel Yield Testing
Biodiesel yield testing is a critical step in evaluating the efficiency of catalysts used in transesterification reactions. Accurate and reliable methods are required to quantify the conversion of triglycerides into fatty acid methyl esters (FAMEs) while identifying the byproducts. Several analytical and chemical methods have been established for this purpose, each with its strengths and limitations. These methods provide insights into catalytic activity, selectivity, stability, and reusability.
10.1. Gas Chromatography (GC)
Gas chromatography (GC) is the gold standard for biodiesel yield testing due to its precision in separating and quantifying FAMEs [
93]. In GC, a sample is vaporized and passed through a capillary column, where individual components are separated based on their volatility. A flame ionization detector (FID) then detects the separated components, providing quantitative data on the concentration of FAMEs. GC is particularly valuable for ensuring compliance with biodiesel standards such as ASTM D6751 and EN 14214. This method also detects impurities, such as residual mono-, di-, and tri-glycerides, which can affect the quality of biodiesel. GC offers unmatched sensitivity and accuracy, making it indispensable for biodiesel analysis. However, it requires extensive sample preparation and calibration using FAME standards. Additionally, the high cost of the necessary equipment and maintenance makes GC more suitable for advanced laboratories rather than routine field testing.
10.2. High-Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography (HPLC) is another widely used method for biodiesel analysis, especially for determining the concentrations of triglycerides, diglycerides, monoglycerides, and FAMEs in biodiesel. In HPLC, a liquid sample is pumped through a stationary phase, separating components based on their interactions with the column material. Reversed-phase HPLC, which uses nonpolar stationary phases, is particularly effective for analyzing biodiesel. Methanol or acetonitrile is often used as the mobile phase for improved separation efficiency [
102]. HPLC provides detailed information on unreacted glycerides, making it an excellent complement to GC in assessing catalyst performance. While HPLC is highly versatile, its limitations include a lower sensitivity for FAMEs compared to GC and a dependence on proper calibration to achieve reliable results.
10.3. Mass Spectrometry (MS)
Mass spectrometry (MS), often coupled with GC or HPLC, offers unparalleled accuracy in identifying the molecular composition of biodiesel. MS works by ionizing molecules and analyzing their mass-to-charge ratios. This technique is particularly useful for detecting trace impurities, such as sulfur or nitrogen-containing compounds, which can impact biodiesel quality and engine performance [
103]. The combination of GC with MS or HPLC with MS is highly effective for biodiesel analysis, as it combines the separation capabilities of chromatography with the molecular specificity of mass spectrometry. However, the high cost of MS instruments and the expertise required to interpret the results limit its use to specialized research facilities.
10.4. Glycerol Titration
Glycerol titration is a traditional chemical method for measuring the free and total glycerol contents in biodiesel. This method involves the titration of glycerol with reagents like sodium periodate, which oxidizes glycerol to form measurable products. The amount of titrant used is proportional to the glycerol concentration, providing a simple way to estimate the reaction’s completeness [
104]. While glycerol titration is cost-effective and straightforward, it lacks the sensitivity of chromatographic techniques. It is best suited for preliminary analyses or as a supplementary method in biodiesel production facilities.
10.5. Glycerol Copper Colorimetry
Glycerol copper colorimetry is a spectrophotometric technique that measures the glycerol content in biodiesel by forming a colored complex with copper ions. The intensity of the color, measured at a specific wavelength, is directly related to the glycerol concentration. This method is rapid and suitable for routine testing, especially in industrial settings where quick results are needed [
104]. However, this technique is less accurate in detecting low glycerol concentrations and is often used in conjunction with more precise methods like GC or HPLC for comprehensive analysis.
Table 5 provides a comparative analysis of different biodiesel yield testing methods, highlighting their sensitivity, cost, and most suitable applications.
11. Characterization Techniques for Performance Evaluation
The performance of solid transesterification catalysts is determined by their physical, chemical, and structural properties. These characteristics are closely linked to their catalytic activity, selectivity, stability, and reusability. A range of advanced characterization techniques is employed to evaluate these properties and understand how they influence catalytic efficiency in biodiesel synthesis.
11.1. X-Ray Diffraction (XRD)
X-ray diffraction is an essential technique used to analyze the crystallinity and phase composition of catalysts. By determining the arrangement of atoms and identifying crystalline phases, XRD provides insights into structural changes that may occur during catalyst preparation or reaction processes. For example, the presence of anatase TiO2 or well-formed layered double hydroxides (LDHs) indicates active phases responsible for catalysis. This method also identifies the formation of mixed or amorphous phases, which can either enhance or diminish the catalytic performance, depending on their interaction with the reactants.
11.2. Scanning Electron Microscopy (SEM)
Scanning electron microscopy and transmission electron microscopy (TEM) are critical for examining the morphology, surface structure, and particle size of catalysts. SEM generates high-resolution images of catalyst surfaces, revealing their porosity, cracks, or roughness, which influence mass transfer during reactions. TEM, on the other hand, provides nanoscale images of the internal structure of the catalyst, showing particle distributions, layered morphologies in LDHs, or the dispersion of doped metals. These microscopy techniques are indispensable for visualizing the effects of treatments like calcination or functionalization on the catalyst structure and ensuring uniformity in its particle morphology.
11.3. Fourier-Transform Infrared (FTIR)
Fourier-tranform infrared spectroscopy identifies functional groups and examines the surface chemistry of catalysts. It detects active groups, such as –SO3H in sulfonated zeolites or –OH in metal oxides, which are critical for driving esterification and transesterification reactions. FTIR spectroscopy can also reveal chemical interactions between reactants and the catalyst surface, providing valuable information about reaction mechanisms and the functional modifications achieved through doping or hybridization.
11.4. Nuclear Magnetic Resonance (NMR)
Nuclear magnetic resonance spectroscopy offers molecular-level insights into the chemical environment of functional groups on catalysts. This technique is particularly valuable for identifying acidic or basic groups that facilitate the conversion of triglycerides into biodiesel. For instance, the presence of active hydrogen in hydroxyl or sulfonic groups can be detected, helping researchers understand how these groups interact with alcohols or esters during catalytic reactions. NMR is a powerful tool for correlating the chemical structure of catalysts with their performance in biodiesel synthesis.
11.5. BET Surface Area Analysis
BET surface area analysis, based on the Brunauer–Emmett–Teller method, is widely used to measure the surface area, pore size, and pore volume of catalysts. These properties are crucial for maximizing reactant–catalyst interactions. Mesoporous materials like SBA-15 or hybrid TiO2–LDH systems often show enhanced performance due to their high surface area and optimal pore structure, which facilitate reactant diffusion and the access of the reactant to active sites. BET analysis also helps evaluate the impact of treatments such as calcination or doping on the structural properties of catalysts.
11.6. X-Ray Photoelectron Spectroscopy (XPS)
Analysis of the surface chemical state of solid transesterification catalysts can be easily accomplished with X-ray photoelectron spectroscopy (XPS). XPS offers a comprehensive understanding of the elemental composition, oxidation states, and electronic interactions of catalysts, thus elucidating the nature of active sites that are accountable for catalytic activity. Examining catalysts with doped or functionalized surfaces using this method is very helpful in verifying the presence of components that affect catalyst performance, such as sulfur, nitrogen, or alkali metals. XPS’s great surface sensitivity lets one precisely characterize chemical changes that improve catalytic efficiency.
11.7. Atomic Force Microscopy (AFM)
The surface shape and roughness of catalysts, at the nanoscale, are investigated using atomic force microscopy (AFM). Unlike SEM or TEM, which offer structural images, AFM measures the surface texture with atomic-level accuracy by means of topographical fluctuations. This method clarifies the way that the active sites, surface porosity, and roughness affect reactant diffusion. By providing quantitative roughness measurements which are vital in optimizing catalyst design for enhanced biodiesel synthesis, AFM data enhance other data imaging methods.
A vital addition to the existing evaluation methods is CO2-TPD (Temperature-Programmed Desorption) and NH3-TPD, which are used to assess the basic and acidic properties of catalysts. In CO2-TPD, carbon dioxide is adsorbed onto basic sites, and its desorption is monitored as the temperature rises. Low-temperature desorption indicates weak basicity, which is commonly associated with surface hydroxyl groups, while medium- to high-temperature desorption (200–500 °C) reflects the presence of moderate to strong basic sites, such as those formed through alkali metal doping. These strong basic sites are crucial for alcohol activation and deprotonation during transesterification.
Similarly, NH3-TPD involves the adsorption of ammonia onto acidic sites, with the desorption temperatures reflecting the strength of these sites. Weak acidic sites desorb ammonia at low temperatures (100–200 °C), while stronger sites release it at higher temperatures (200–400 °C or beyond). Catalysts like sulfonated zeolites exhibit pronounced high-temperature NH3-TPD peaks, indicating the presence of strong acidic sites that are essential for esterification reactions to take place. Comparing TPD profiles across different catalysts, such as MgO, TiO2–LDH composites, and sulfonated resins, provides valuable insights into their suitability for specific biodiesel feedstocks or reaction conditions. Together, these techniques form a comprehensive toolkit for characterizing catalysts and optimizing their design for biodiesel production. XRD and microscopy provide structural and morphological insights, while FTIR and NMR focus on the surface chemistry and functional groups. BET analysis highlights the importance of the surface area and porosity, while TPD methods quantify the strength and distribution of active sites. By integrating these methods, researchers can design catalysts with enhanced performance and which are tailored to meet specific biodiesel production challenges.
12. Heterogeneous Catalysts with Tailored Acidity/Basicity
Another significant development is the synthesis of heterogeneous catalysts with a finely tuned acidity or basicity [
25]. The acidity or basicity of a catalyst plays a crucial role in its activity and selectivity, especially in ester exchange reactions. Researchers have made strides in designing catalysts with an adjustable acidity or basicity, such as layered double hydroxides (LDHs) and metal-doped catalysts, which can be optimized to suit specific reaction conditions. These catalysts offer the advantage of enhancing the selectivity of reactions by selectively activating ester bonds or modifying polyester structures without causing unwanted side reactions.
12.1. Hybrid and Composite Catalysts
Hybrid catalysts that combine solid acids, bases, and other materials have shown promising results in transesterification reactions. The combination of different catalytic components in a single catalyst system allows synergistic effects between the individual components, improving the overall catalytic performance [
105]. For example, the combination of metal oxides with zeolites or LDHs has been explored to enhance both the activity and selectivity of transesterification catalysts. These hybrid catalysts can be fine-tuned to achieve optimal catalytic performance and improve the stability and reusability of catalyst systems.
12.2. Green Catalysis and Environmentally Friendly Processes
The focus on green chemistry and environmentally friendly catalytic processes has driven significant progress in the development of solid transesterification catalysts [
106]. Researchers have increasingly turned to non-toxic, sustainable materials such as bio-based catalysts, recyclable catalysts, and catalysts derived from renewable resources. These catalysts reduce the environmental impact of transesterification reactions and contribute to the sustainability of industrial processes. The development of non-toxic solid acid catalysts and environmentally benign reaction conditions has garnered attention for its potential to reduce the carbon footprint of industrial-scale reactions.
12.3. Catalysts for Polyester Recycling
One of the most exciting areas of research is the use of solid transesterification catalysts for the recycling of polyesters, particularly polyethylene terephthalate (PET) [
24]. PET is one of the most widely used plastics but is notoriously difficult to recycle due to its strong ester bonds. Recent advancements have focused on the design of solid catalysts capable of efficiently depolymerizing PET into its monomers, such as terephthalic acid (TPA) and ethylene glycol (EG). These catalysts enable the circular economy by allowing for the reprocessing of PET waste into valuable raw materials, reducing the need for virgin materials and minimizing environmental pollution.
12.4. Catalyst Design for Industrial-Scale Applications
While significant progress has been made in laboratory-scale catalyst development, the translation of these catalysts to industrial-scale applications remains a challenge [
107]. Future research should focus on optimizing catalysts for large-scale processes, including improving their stability under industrial conditions, enhancing their resistance to deactivation, and scaling up synthesis methods. There is also a need for catalysts that can operate under mild conditions (e.g., lower temperatures and pressures) to reduce energy consumption and improve cost-effectiveness.
12.5. Integration of Artificial Intelligence and Machine Learning
The creation of numerical simulations has yielded a wealth of valuable data that have furthered catalyst development. Due to the widespread use of data science techniques in many fields, particularly machine learning (ML), many individuals are interested in using big data to find new catalysts [
108]. Finding new catalysts with good performance is challenging since a number of factors, including the support [
109], composition, and particle size of the catalyst affect a chemical reaction’s success [
110]. The process of selecting the best classical catalyst candidate was costly, time-consuming, and primarily dependent on empirical data. However, machine learning (ML) provides a fresh approach to discovering catalysts and aids in selecting those that are effective. Additionally, less time is spent on research. ML has the potential to produce accurate prediction results because it is based on enough pertinent data and many elements that are taken into account collectively by the model to find the best feasible outcomes [
109]. The integration of artificial intelligence (AI) and machine learning (ML) into catalyst design is a promising direction for future research [
111]. AI and ML algorithms can be used to predict the properties and performance of new catalysts, as well as to optimize reaction conditions and catalyst regeneration processes. These technologies can accelerate the discovery of new catalyst materials, improve the efficiency of catalytic processes, and provide insights into reaction mechanisms that are difficult to obtain through traditional methods. In particular, AI could assist in the development of hybrid or composite catalysts by simulating the effects of different materials and structures on catalytic performance.
12.6. Enhanced Catalyst Characterization Techniques
As catalyst design becomes more complex, advanced characterization techniques will be crucial for understanding the structure–property relationships that govern catalytic performance [
112]. Techniques such as in situ X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and advanced microscopy methods (e.g., atomic force microscopy) will be increasingly important in monitoring the behavior of catalysts under different reaction conditions. These methods will provide valuable information on the evolution of the catalyst’s surface structure, the interaction between the reactants and active sites, and the potential degradation mechanisms.
13. Catalysts for Sustainable Chemical Production
The demand for sustainable chemical processes has fueled the development of advanced solid transesterification catalysts that contribute to the production of biofuels, biodegradable plastics, and green chemicals [
113]. These catalysts play a pivotal role in enabling eco-friendly manufacturing by facilitating efficient, selective, and low-impact reactions. The focus on sustainability underscores the importance of designing catalysts that not only deliver high performance but that also align with principles such as waste reduction, energy efficiency, and reliance on renewable resources.
One of the most significant areas of application is in the production of biofuels, such as biodiesel, where solid transesterification catalysts have demonstrated their ability to enhance the efficiency of reactions [
114]. By enabling transesterification and esterification reactions with high selectivity and minimal byproduct formation, these catalysts reduce the energy requirements and environmental impact associated with conventional processes. For instance, catalysts based on MgO, CaO, and TiO
2 have shown remarkable efficiency in converting waste oils and low-grade feedstocks into biodiesel under mild reaction conditions. Incorporating renewable feedstocks such as used cooking oil or algae-derived triglycerides further enhances the sustainability of these processes, reducing reliance on fossil resources [
114].
Another critical area of research involves the production of biodegradable plastics from bio-based monomers. Solid transesterification catalysts facilitate the synthesis of polyesters like polylactic acid (PLA) and polyhydroxyalkanoates (PHA) from renewable monomers such as lactic acid and hydroxyalkanoic acids. These bioplastics offer a viable alternative to conventional plastics by addressing concerns over long-term environmental degradation and microplastic pollution. Recent advancements in catalyst design have focused on achieving high polymerization rates and high molecular weights under green reaction conditions, such as solvent-free systems or reactions conducted at ambient temperatures [
113].
The production of green chemicals, including solvents, polymers, and intermediates derived from biomass, is another area where solid catalysts have shown significant promise. For example, the catalytic conversion of glycerol, a biodiesel byproduct, into value-added chemicals such as acrolein, 1,2-propanediol, and epichlorohydrin has been successfully achieved using solid acid and base catalysts. The integration of renewable feedstocks into these processes not only reduces their carbon footprint but also promotes the circular economy by converting waste streams into valuable resources.
In line with sustainability goals, there is a growing emphasis on bio-based catalysts and precursors. Researchers are exploring the use of bio-derived materials, such as chitosan, cellulose, and lignin, as supports or precursors for solid catalysts. These materials are abundant, renewable, and biodegradable, making them ideal for applications where the environmental impact is a concern. Bio-based supports can be functionalized with acidic or basic groups to create catalysts that rival traditional systems in performance while significantly reducing the environmental harm [
84,
115].
Future advancements in the field will likely involve the development of hybrid catalysts that combine the benefits of multiple materials to achieve superior performance. For instance, combining metal oxides like ZnO and MgO with bio-based supports can result in catalysts that exhibit high activity, stability, and compatibility with renewable feedstocks. Moreover, the integration of nanotechnology into catalyst design is expected to further enhance their performance by increasing the number of active sites and improving reactant accessibility.
14. Optimization of Catalyst Recycling and Regeneration
The recyclability and regeneration of solid catalysts are critical to their long-term industrial viability and cost-effectiveness. Effective regeneration methods enable the reuse of catalysts over multiple cycles with a minimal loss of activity, reducing waste and ensuring sustainability [
116]. Various regeneration strategies, such as thermal treatments, solvent washing, and chemical regeneration, are being optimized to restore the activity of catalysts while maintaining their structural integrity. Advanced solid catalysts, particularly those used in biodiesel production and polyester recycling, are now designed to withstand multiple reaction cycles without degradation.
Table 6, below, is a table summarizing the biodiesel yield and catalyst reusability of various improved catalyst systems.
14.1. Nanostructured TiO2 Catalysts
Nanostructured TiO2 catalysts are primarily synthesized using sol-gel or hydrothermal methods, which provide precise control over the particle size and surface properties. Enhancements in catalyst activity and stability are achieved by incorporating dopants such as metals or non-metals. The optimization process involves controlling the calcination temperatures and using template agents to improve the surface area and porosity, leading to an increased availability of active sites. These catalysts are known for their high thermal stability and minimal deactivation over multiple reaction cycles, with enhanced activity due to their high dispersion of active sites and improved mass transfer properties.
14.2. Layered Double Hydroxides (LDHs)
LDHs are synthesized using co-precipitation techniques, followed by calcination to produce mixed metal oxides. Careful control of the molar ratio of metal precursors and the reaction medium’s pH ensures the desired acidity or basicity. Functionalization with organic acids or doping with transition metals improves the selectivity and stability of the process. Additionally, tuning metal compositions, such as Mg-Al or Zn-Cu systems, allows LDHs to target specific feedstocks. The layered structure of LDHs provides exceptional thermal stability and reusability, as well as a highly tunable acidity and basicity, making them versatile catalysts.
14.3. ZnO Nanoparticles
ZnO nanoparticles are typically synthesized through precipitation or hydrothermal methods, often with surfactants to control their particle size and morphology. Surface modification with alkali metals or sulfur-containing groups enhances their basicity and reactivity. Calcination under controlled conditions further improves their crystallinity and the exposure of their active sites. ZnO catalysts exhibit high reusability and minimal leaching, making them highly suitable for the transesterification of low-grade feedstocks.
14.4. MgO–Zeolite Composites
MgO–zeolite composites are prepared by impregnating MgO into the porous framework of zeolites, followed by calcination. This hybrid structure combines the basicity of MgO with the acidity and structural stability of zeolites. Optimization of these composites involves adjusting the MgO loading and calcination temperature to achieve an optimal balance of acidity and basicity, which is essential for processing feedstocks containing both triglycerides and free fatty acids (FFAs). The synergistic effects of the hybrid structure result in enhanced performance, high biodiesel yields, and prolonged catalyst stability over multiple cycles.
14.5. Sulfonated Zeolites (H-ZSM-5)
Sulfonated zeolites are produced by treating zeolites with concentrated sulfuric acid or sulfonic acid groups, introducing strongly acidic sites while maintaining the crystalline structure. The strength and density of acidic sites are tailored through controlled sulfonation and calcination processes. Adjustments to the pore size and structure enhance the accessibility of the reactant. These catalysts are particularly effective for esterification, especially with high-FFA feedstocks, due to their strongly acidic sites, which ensure excellent selectivity and conversion efficiency.
14.6. TiO2/SiO2 Composites
TiO2/SiO2 composites are synthesized using sol-gel methods, followed by calcination to create a mesoporous structure. The incorporation of SiO2 improves their thermal stability and increases the dispersion of active TiO2 sites. By optimizing the Ti–Si ratio, the acidic and basic properties can be balanced, allowing for versatile catalytic applications. These composites are effective for both transesterification and esterification reactions, making them ideal for biodiesel production from a wide range of feedstocks.
14.7. Bio-Based Catalysts (e.g., Chitosan)
Bio-based catalysts are prepared by functionalizing renewable materials such as chitosan with acidic or basic groups. Sulfonation introduces acidic sites, while impregnation with alkali metals generates basic sites. Crosslinking and thermal treatments are used to enhance the catalyst’s mechanical stability and reusability. Additionally, the functional group density is optimized to maximize the catalytic activity. These renewable, biodegradable, and cost-effective catalysts are particularly attractive for green chemistry applications, including the synthesis of biodegradable fuels.
15. Advancements and Future Directions
The field of solid transesterification catalysts has witnessed remarkable progress in recent years, driven by the demand for sustainable, efficient, and cost-effective catalytic processes. These advancements address the challenges of conventional catalytic systems, such as their limited reusability, poor feedstock compatibility, and high energy requirements. By enhancing the activity, selectivity, stability, and reusability of catalysts, researchers have paved the way for diverse industrial applications, including biodiesel production, polyester recycling, and fine chemical synthesis. The integration of innovative design strategies, advanced materials, and nanotechnology has played a pivotal role in shaping this progress.
One of the most transformative developments is the introduction of nanostructured catalysts. Nanoparticles, owing to their high surface area and tunable surface properties, provide a significantly larger number of active sites compared to bulk materials [
123]. This increase in surface area enhances the adsorption of reactants and their subsequent conversion, resulting in improved catalytic activity and selectivity. Furthermore, the small size of nanoparticles facilitates the better diffusion of reactants to the active sites, minimizing mass transfer limitations and accelerating the reaction kinetics. Recent studies have shown that nanoparticle-based catalysts, such as those derived from TiO
2, ZnO, and MgO, outperform their bulk counterparts in esterification and transesterification reactions [
124]. These catalysts exhibit superior thermal stability, higher conversion rates, and reduced byproduct formation, making them ideal for biodiesel production.
The tunability of nanostructured catalysts extends to their acidity and basicity, which can be precisely engineered through doping, functionalization, or the incorporation of hybrid systems. For example, doping MgO nanoparticles with alkali metals like K
2CO
3 enhances their basicity, as evidenced by pronounced high-temperature desorption peaks observed in CO
2-TPD analyses.101 Similarly, sulfonating TiO
2 nanoparticles increases their acidic site density, as confirmed by NH
3-TPD profiles, enabling their application in esterification reactions with high-free fatty acid (FFA) feedstocks. These modifications not only improve the catalytic performance but also broaden the range of compatible feedstocks, which includes low-grade or waste oils [
125].
Another significant advancement involves the development of hybrid catalysts that combine the properties of different materials to achieve synergistic effects. For instance, TiO
2–zeolite composites exhibit both acidic and basic sites, enabling dual functionality for transesterification and esterification reactions [
126]. These hybrid catalysts leverage the porosity and structural stability of zeolites with the high reactivity and surface area of TiO
2 nanoparticles, resulting in exceptional performance in biodiesel production. BET surface area analyses of such systems reveal their mesoporous nature, which ensures efficient reactant diffusion and prevents catalyst fouling.
Beyond nanostructuring and hybrid systems, researchers have focused on structural optimization to enhance the stability and reusability of catalysts. Techniques such as controlled calcination, hydrothermal synthesis, and functional group grafting have been employed to strengthen the catalyst framework and protect active sites from deactivation. For example, MgO–LDH composites prepared through hydrothermal synthesis exhibit robust structural integrity, allowing them to maintain high activity over multiple reaction cycles. SEM and TEM imaging of these catalysts reveals well-dispersed active sites and a uniform particle morphology, which contribute to their longevity [
127,
128].
The integration of characterization techniques, such as XRD, FTIR, and TPD, has been instrumental in guiding these advancements. XRD analysis helps to identify crystalline phases that contribute to catalytic activity, while FTIR spectroscopy confirms the successful incorporation of functional groups like –SO
3H or –COOH. TPD techniques provide quantitative data on the strength and distribution of acidic and basic sites, offering valuable insights into how structural modifications impact catalytic behavior. These techniques have also highlighted the importance of balancing acidity and basicity in catalysts to optimize their performance in specific reactions. Looking ahead, future directions in catalyst development include sustainability and scalability. The development of AI-driven catalyst design and hybrid catalytic systems to improve feedstock flexibility and the general process efficiency should take center stage in future biomass transformation efforts. By allowing the quick screening of catalyst formulations, reaction outcome prediction, and process parameter optimization, artificial intelligence and machine learning can help to revolutionize catalyst development. This method can greatly hasten the search for affordable and high-performance catalysts, particularly those using earth-abundant materials, thus lowering dependency on costly noble metals [
129]. Furthermore, the investigation of hybrid catalytic systems, which combine the advantages of homogeneous and heterogeneous catalysts, is providing interesting paths for enhancing reaction selectivity, stability, and scalability. These systems can be customized to manage the structural complexity and unpredictability of various biomass feedstocks, such as lignocellulosic, algal, and waste biomass, thereby guaranteeing more effective and sustainable conversion methods [
130]. Using these cutting-edge technologies can help scientists solve important problems in catalyst deactivation, economic viability, and environmental sustainability, thus promoting the shift to a circular bioeconomy and lowering world reliance on fossil fuels. The use of bio-based materials, such as chitosan or lignocellulosic derivatives, as catalyst precursors represents a promising approach to reducing the environmental impact of catalyst production. Additionally, the integration of machine learning and artificial intelligence in catalyst design is expected to accelerate the discovery of novel materials and optimize synthesis parameters. AI-driven models can predict catalyst performance based on structural and chemical data, enabling the rapid screening of potential candidates and the identification of optimal operating conditions.
Additional developments in the application of density functional theory (DFT), such as computational models and in situ operando characterization techniques, will enhance our knowledge of heterogeneous-based transesterification catalysts, which in turn will encourage and drive further research into these catalysts. Much effort remains to be made to comprehend the kinetics and reaction processes for of catalyst activity, even though a number of research studies on the synthesis of biodiesel using transesterification over green catalysts have been published in the last 10 years. Furthermore, it is currently imperative to use architectural design techniques to obtain new functional nanostructured catalysts (such as porous nanocarbons, metal–organic frameworks, etc.) in order to increase the activity of heterogeneous catalysts. As transesterification is widely recognized as the most effective biodiesel processing technique, new catalytic trans/esterification technologies that can combine the esterification and transesterification processes are crucial to closing the price gap between biodiesel and production costs. Furthermore, the development of heterogeneous catalysis systems to encourage the value-adding of biodiesel by-products is still essential to the industry’s sustainability. Additionally, in order to advance biodiesel production, a great deal of cooperation between various professionals, such as materials chemists, chemical engineers, and microbiologists, is required. This includes designing and developing an efficient catalyst that is suitable at low temperatures and that is also easily regenerated and stable.
16. Conclusions
Thanks to advancements in solid transesterification catalysts that solve major problems such catalyst deactivation, stability, and reusability, biodiesel synthesis is now much more efficient. Notable advancements in performance have been made in achieving catalysts that are acidity/basicity-tuned, nanostructured, and in hybrid catalytic systems. Nanostructured TiO2 and ZnO catalysts have generated 96–98% biodiesel over 5–7 cycles, whereas MgO–zeolite hybrids have proven their stability and commercial viability by sustaining 93–96% efficiency over 7–10 cycles.
These developments are helpful, but they are not enough to satisfy industrial needs. For the catalysts of the future to be financially feasible, they need to keep a biodiesel output of at least 95% for 10–15 cycles. For long-term use, it is particularly necessary to minimize coke production (<10% surface coating after several cycles) and keep metal leaching rates low (<5 ppm per cycle). The stability of the active sites and the prevention of deactivation are achieved through surface changes, such as protective coatings (SiO2, carbon layers) and hybrid structures (TiO2/SiO2, MgO–zeolite composites). To further enhance reactant transport and catalytic activity, mesoporous catalysts can have their pore structure and surface area optimized to be greater than 200 m2/g.
In order to accomplish these goals and make feedstocks more compatible, researchers should work on developing multifunctional catalysts that can undergo both esterification and transesterification. By incorporating AI and ML into catalyst design, new formulations can be rapidly discovered that improve the stability, selectivity, and reusability of catalysts. For biodiesel production to be both sustainable and economical, it is crucial to develop green catalysts from bio-based materials. Solid transesterification catalysts will play a key role in the next generation of eco-friendly, cost-effective biodiesel systems when these technologies are combined.