Promoting Effect of Copper Doping on LaMO3 (M = Mn, Fe, Co, Ni) Perovskite-Supported Gold Catalysts for Selective Gas-Phase Ethanol Oxidation
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalyst
2.2. Catalytic Performance
2.2.1. Support Effect
2.2.2. Isotope Effect
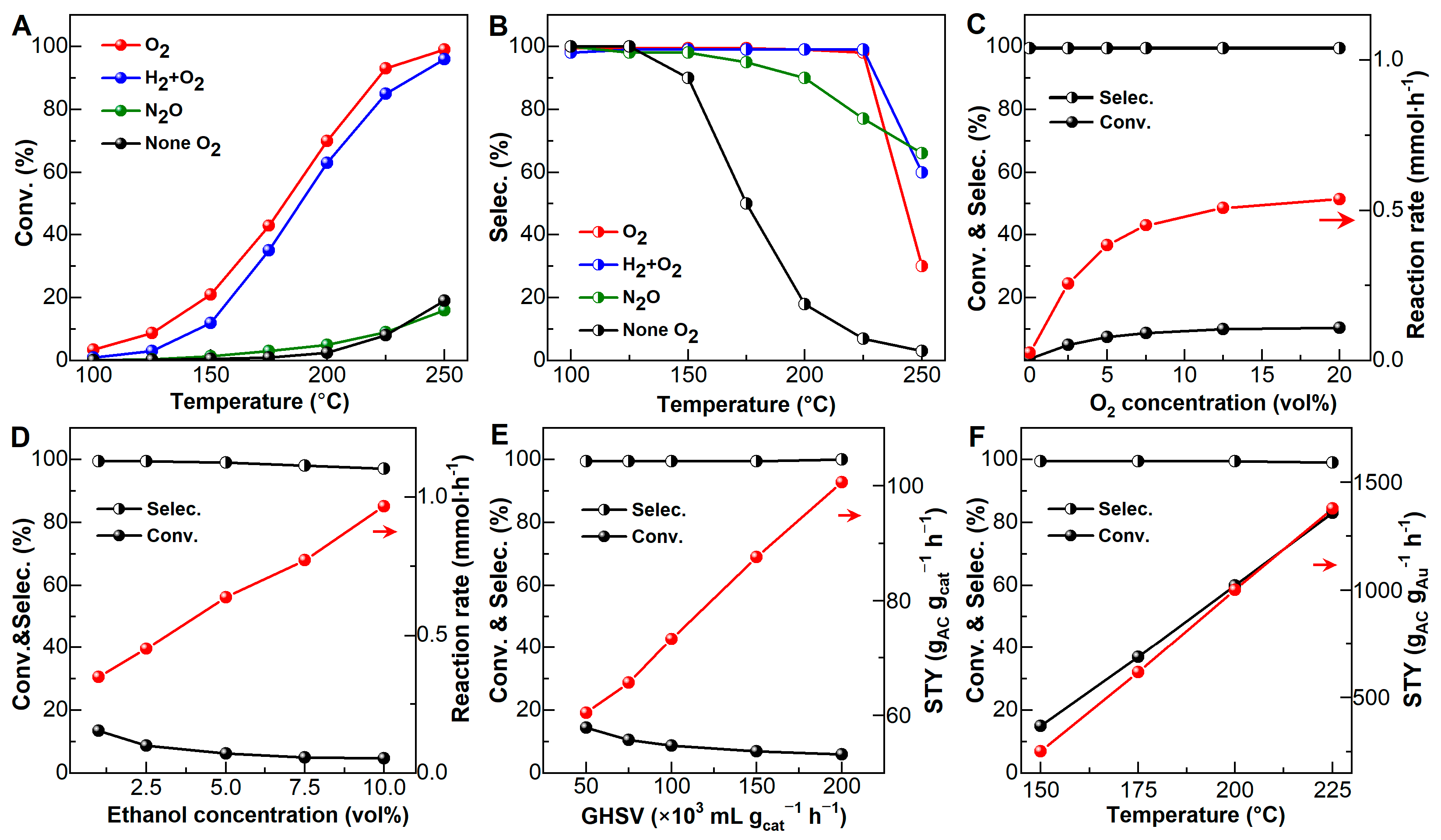
2.2.3. Influence of Reaction Conditions
3. Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eagan, N.M.; Kumbhalkar, M.D.; Buchanan, J.S.; Dumesic, J.A.; Huber, G.W. Chemistries and Processes for the Conversion of Ethanol into Middle-Distillate Fuels. Nat. Rev. Chem. 2019, 3, 223–249. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent Advances in Catalytic Conversion of Ethanol to Chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Dagle, R.A.; Winkelman, A.D.; Ramasamy, K.K.; Lebarbier Dagle, V.; Weber, R.S. Ethanol as a Renewable Building Block for Fuels and Chemicals. Ind. Eng. Chem. Res. 2020, 59, 4843–4853. [Google Scholar] [CrossRef]
- He, L.; Zhou, B.C.; Sun, D.H.; Li, W.C.; Lv, W.L.; Wang, J.; Liang, Y.Q.; Lu, A.H. Catalytic Conversion of Ethanol to Oxygen-Containing Value-Added Chemicals. ACS Catal. 2023, 13, 11291–11304. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Li, X.; Li, H.; Zheng, M.; Zhang, T. Advances in Catalytic Dehydrogenation of Ethanol to Acetaldehyde. Green Chem. 2021, 23, 7902–7916. [Google Scholar] [CrossRef]
- Liu, P.; Duan, J.; Ye, Q.; Mei, F.; Shu, Z.; Chen, H. Promoting Effect of Unreducible Metal Doping on OMS-2 Catalysts for Gas-Phase Selective Oxidation of Ethanol. J. Catal. 2018, 367, 115–125. [Google Scholar] [CrossRef]
- Malmusi, A.; Velasquez Ochoa, J.; Tabanelli, T.; Basile, F.; Lucarelli, C.; Agnoli, S.; Carraro, F.; Granozzi, G.; Cavani, F. Ethanol Aerobic and Anaerobic Oxidation with FeVO4 and V2O5 Catalysts. Appl. Catal. A Gen. 2019, 570, 139–147. [Google Scholar] [CrossRef]
- Wang, P.; Duan, J.; Wang, J.; Mei, F.; Liu, P. Elucidating Structure-Performance Correlations in Gas-Phase Selective Ethanol Oxidation and CO Oxidation over Metal-Doped γ-MnO2. Chin. J. Catal. 2020, 41, 1298–1310. [Google Scholar] [CrossRef]
- Oefner, N.; Heck, F.; Dürl, M.; Schumacher, L.; Khatoon Siddiqui, H.; Kramm, U.I.; Hess, C.; Möller, A.; Albert, B.; Etzold, B.J.M. Activity, Selectivity and Initial Degradation of Iron Molybdate in the Oxidative Dehydrogenation of Ethanol. ChemCatChem 2022, 14, e202101219. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Feng, Z.; Liu, H.; Su, D. Multi-Walled Carbon Nanotubes as a Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde. ChemSusChem 2016, 9, 1820–1826. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Zhang, Y.; Diao, J.; Zhang, J.; Liu, H.; Su, D. Nitrogen-Doped Carbon Nanotubes as Bifunctional Catalysts with Enhanced Catalytic Performance for Selective Oxidation of Ethanol. Carbon 2017, 111, 519–528. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, X.; Yang, S.; Li, T.; Hensen, E.J.M. On the Metal-Support Synergy for Selective Gas-Phase Ethanol Oxidation over MgCuCr2O4 Supported Metal Nanoparticle Catalysts. J. Catal. 2015, 331, 138–146. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhu, L.; Wu, Q.; Chen, C.; Wang, X.; Ji, Y.; Meng, X.; Xiao, F. Solvent-Free Synthesis of Zeolite Crystals Encapsulating Gold-Palladium Nanoparticles for the Selective Oxidation of Bioethanol. ChemSusChem 2015, 8, 2867–2871. [Google Scholar] [CrossRef]
- Silbaugh, T.L.; Devlaminck, P.; Sofranko, J.A.; Barteau, M.A. Selective Oxidation of Ethanol over Ag, Cu and Au Nanoparticles Supported on Li2O/γ-Al2O3. J. Catal. 2018, 364, 40–47. [Google Scholar] [CrossRef]
- Wang, J.; Luo, H.; Liu, P. Highly Dispersed Gold Nanoparticles on Metal-Doped α-MnO2 Catalysts for Aerobic Selective Oxidation of Ethanol. Catal. Commun. 2020, 142, 106030. [Google Scholar] [CrossRef]
- Wang, P.; Luo, H.; Wang, J.; Han, B.; Mei, F.; Liu, P. Synergistic Effect between Gold Nanoparticles and Fe-doped γ-MnO2 toward Enhanced Aerobic Selective Oxidation of Ethanol. Catal. Sci. Technol. 2020, 10, 4332–4339. [Google Scholar] [CrossRef]
- Song, W.; Liu, P.; Hensen, E.J.M. A Mechanism of Gas-Phase Alcohol Oxidation at the Interface of Au Nanoparticles and a MgCuCr2O4 Spinel Support. Catal. Sci. Technol. 2014, 4, 2997–3003. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Meng, X.; Xiao, F.S. New Strategies for the Preparation of Sinter-Resistant Metal-Nanoparticle-Based Catalysts. Adv. Mater. 2019, 31, 1901905. [Google Scholar] [CrossRef]
- Takei, T.; Iguchi, N.; Haruta, M. Synthesis of Acetaldehyde, Acetic Acid, and Others by the Dehydrogenation and Oxidation of Ethanol. Catal. Surv. Asia 2011, 15, 80–88. [Google Scholar] [CrossRef]
- Takei, T.; Iguchi, N.; Haruta, M. Support Effect in the Gas Phase Oxidation of Ethanol over Nanoparticulate Gold Catalysts. New J. Chem. 2011, 35, 2227–2233. [Google Scholar] [CrossRef]
- Liu, P.; Hensen, E.J.M. Highly Efficient and Robust Au/MgCuCr2O4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef]
- Liu, P.; Li, T.; Chen, H.; Hensen, E.J.M. Optimization of Au0-Cu+ Synergy in Au/MgCuCr2O4 Catalysts for Aerobic Oxidation of Ethanol to Acetaldehyde. J. Catal. 2017, 347, 45–56. [Google Scholar] [CrossRef]
- Du, X.; Fu, N.; Zhang, S.; Chen, C.; Wang, D.; Li, Y. Au/CuSiO3 Nanotubes: High-Performance Robust Catalysts for Selective Oxidation of Ethanol to Acetaldehyde. Nano Res. 2016, 9, 2681–2686. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Chen, S.; Su, Y.; Deng, P.; Qi, R.; Zhu, J.; Chen, J.; Wang, Z.; Zhou, L.; Guo, X.; Xia, B.Y. Highly Selective Carbon Dioxide Electroreduction on StructureEvolved Copper Perovskite Oxide toward Methane Production. ACS Catal. 2020, 10, 4640–4646. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, X.; Wu, C.; Wen, Y.; Xue, Y.; Chen, R.; Zhang, Z.; Shan, B.; Yin, H.; Wang, W.G. NO Oxidation Catalysis on Copper Doped Hexagonal Phase LaCoO3: A Combined Experimental and Theoretical Study. Phys. Chem. Chem. Phys. 2014, 16, 5106–5112. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, J.; Ding, J.; Tan, K.B.; Zhan, G.; Huang, J.; Li, Q. Activation of Molecular Oxygen over Mn-Doped La2CuO4 Perovskite for Direct Epoxidation of Propylene. Catal. Sci. Technol. 2022, 12, 2426–2437. [Google Scholar] [CrossRef]
- Duma, Z.G.; Swartbooi, A.; Musyoka, N.M. Thermocatalytic Decomposition of Methane to Low-Carbon Hydrogen using LaNi1-xCuxO3 Perovskite Catalysts. Appl. Catal. A Gen. 2024, 677, 119703. [Google Scholar] [CrossRef]
- Arrii, S.; Morfin, F.; Renouprez, A.J.; Rousset, J.L. Oxidation of CO on Gold Supported Catalysts Prepared by Laser Vaporization: Direct Evidence of Support Contribution. J. Am. Chem. Soc. 2004, 126, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Degirmenci, V.; Hensen, E.J.M. Unraveling the Synergy Between Gold Nanoparticles and Chromium-Hydrotalcites in Aerobic Oxidation of Alcohols. J. Catal. 2014, 313, 80–91. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y.; Zhu, K.; Zhang, H. Understanding oxygen-deficient La2CuO4-δ perovskite activated peroxymonosulfate for bisphenol A degradation: The role of localized electron within oxygen vacancy. Appl. Catal. B Environ. 2021, 284, 119732. [Google Scholar] [CrossRef]
- Sun, M.; Li, W.; Zhang, B.; Cheng, G.; Lan, B.; Ye, F.; Zheng, Y.; Cheng, X.; Yu, L. Enhanced Catalytic Performance by Oxygen Vacancy and Active Interface Originated from Facile Reduction of OMS-2. Chem. Eng. J. 2018, 331, 626–635. [Google Scholar] [CrossRef]
- Yin, X.; Shen, L.; Wang, S.; Wang, B.; Shen, C. Double adjustment of Co and Sr in LaMnO3+δ perovskite oxygen carriers for chemical looping steam methane reforming. Appl. Catal. B Environ. 2022, 301, 120816. [Google Scholar] [CrossRef]
- Hu, W.; Li, D.; Yang, Y.; Li, T.; Chen, H.; Liu, P. Copper Ferrite Supported Gold Nanoparticles as Efficient and Recyclable Catalyst for Liquid-Phase Ethanol Oxidation. J. Catal. 2018, 357, 108–117. [Google Scholar] [CrossRef]
- Chu, K.; Zong, W.; Xue, G.; Guo, H.; Qin, J.; Zhu, H.; Zhang, N.; Tian, Z.; Dong, H.; Miao, Y.E.; et al. Cation Substitution Strategy for Developing Perovskite Oxide with Rich Oxygen Vacancy-Mediated Charge Redistribution Enables Highly Efficient Nitrate Electroreduction to Ammonia. J. Am. Chem. Soc. 2023, 145, 21387–21396. [Google Scholar] [CrossRef]
- Deka, D.J.; Gunduz, S.; Fitzgerald, T.; Miller, J.T.; Co, A.C.; Ozkan, U.S. Production of syngas with controllable H2/CO ratio by high temperature co-electrolysis of CO2 and H2O over Ni and Co-doped lanthanum strontium ferrite perovskite cathodes. Appl. Catal. B Environ. 2019, 248, 487–503. [Google Scholar] [CrossRef]
- Hou, Y.C.; Ding, M.W.; Liu, S.K.; Wu, S.K.; Lin, Y.C. Ni-Substituted LaMnO3 Perovskites for Ethanol Oxidation. RSC Adv. 2014, 4, 5329–5338. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Fang, Y.; Hoang, S.; Li, L.; Yang, W.; Liang, Z.; Wu, J.; Hu, J.; Xiao, W.; et al. Oxygen Vacancy Promoted O2 Activation over Perovskite Oxide for Low-Temperature CO Oxidation. ACS Catal. 2019, 9, 9751–9763. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Relationship between Catalytic Deactivation and Physicochemical Properties of LaMnO3 Perovskite Catalyst during Catalytic Oxidation of Vinyl Chloride. Appl. Catal. B Environ. 2016, 186, 173–183. [Google Scholar] [CrossRef]
- Qin, Y.; Shen, F.; Zhu, T.; Hong, W.; Liu, X. Catalytic Oxidation of Ethyl Acetate over LaBO3 (B = Co, Mn, Ni, Fe) Perovskites Supported Silver Catalysts. RSC Adv. 2018, 8, 33425–33431. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Zhang, W.; Ferronato, C.; Valverde, J.L.; Giroir-Fendler, A. Boosting Propene Oxidation Activity over LaFeO3 Perovskite Catalysts by Cobalt Substitution. Appl. Catal. A Gen. 2022, 643, 118779. [Google Scholar] [CrossRef]
- Li, X.; Dai, H.; Deng, J.; Liu, Y.; Xie, S.; Zhao, Z.; Wang, Y.; Guo, G.; Arandiyan, H. Au/3DOM LaCoO3: High-Performance Catalysts for the Oxidation of Carbon Monoxide and Toluene. Chem. Eng. J. 2013, 228, 965–975. [Google Scholar] [CrossRef]
- Liu, P.; Guan, Y.; van Santen, R.A.; Li, C.; Hensen, E.J.M. Aerobic Oxidation of Alcohols over Hydrotalcite-Supported Gold Nanoparticles: The Promotional Effect of Transition Metal Cations. Chem. Commun. 2011, 47, 11540–11542. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, C.; Hensen, E.J.M. Efficient Tandem Synthesis of Methyl Esters and Imines by Using Versatile Hydrotalcite-Supported Gold Nanoparticles. Chem. Eur. J. 2012, 18, 12122–12129. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Zhang, L.; Gao, B.; Wang, Y.; Li, X.; Xie, S.; Guo, G. PMMA-Templating Generation and High Catalytic Performance of Chain-like Ordered Macroporous LaMnO3 Supported Gold Nanocatalysts for the Oxidation of Carbon Monoxide and Toluene. Appl. Catal. B Environ. 2013, 140–141, 317–326. [Google Scholar] [CrossRef]
- Mielby, J.; Abildstrøm, J.O.; Wang, F.; Kasama, T.; Weidenthaler, C.; Kegnæs, S. Oxidation of Bioethanol Using Zeolite-Encapsulated Gold Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 12513–12516. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.T.; Koltunov, K.Y. Gas-phase Oxidation of Alcohols with O2 and N2O Catalyzed by Au/TiO2: A Comparative Study. Catal. Lett. 2015, 145, 583–588. [Google Scholar] [CrossRef]
- Song, W.; Perez Ferrandez, D.M.; van Haandel, L.; Liu, P.; Alexander Nijhuis, T.; Hensen, E.J.M. Selective Propylene Oxidation to Acrolein by Gold Dispersed on MgCuCr2O4 Spinel. ACS Catal. 2015, 5, 1100–1111. [Google Scholar] [CrossRef]
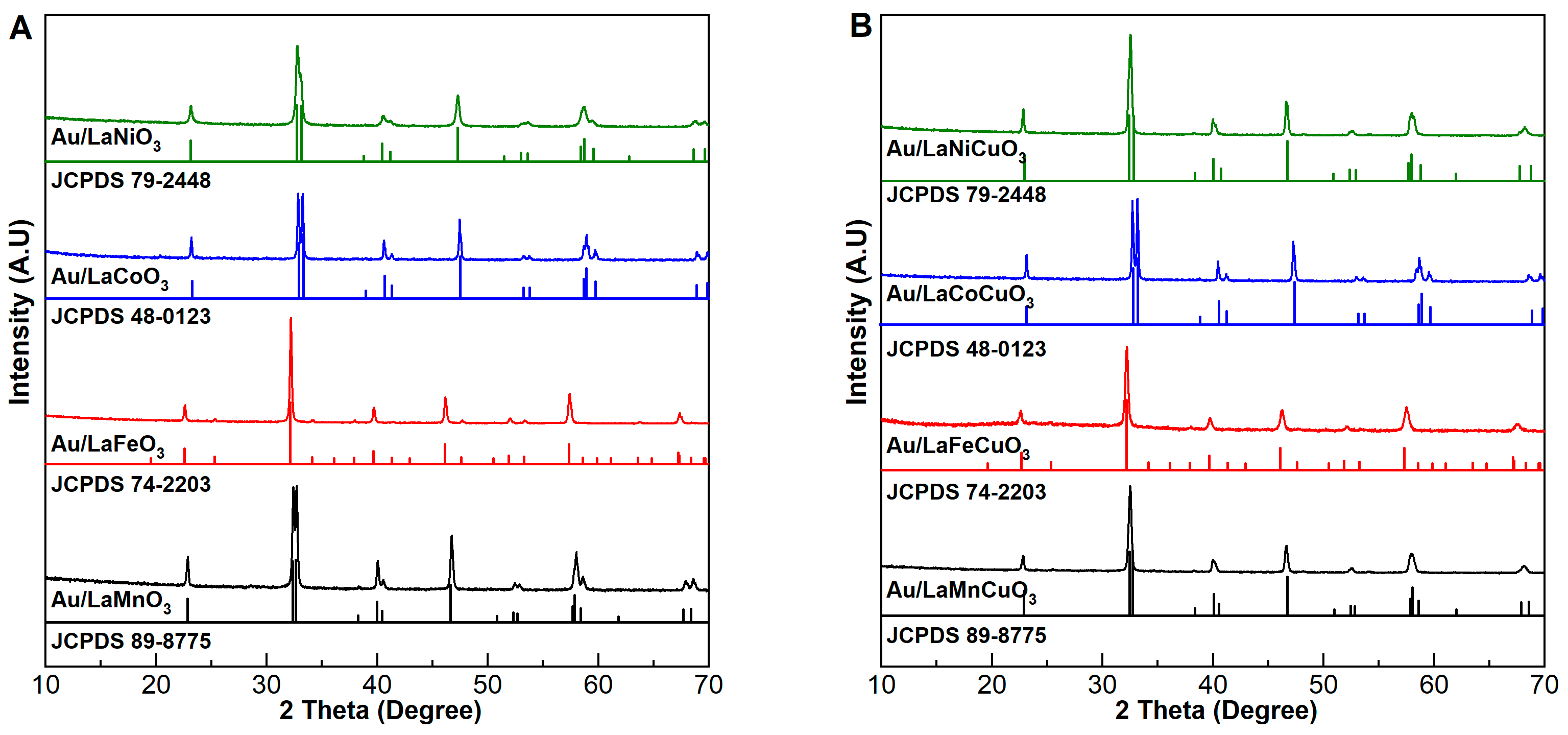

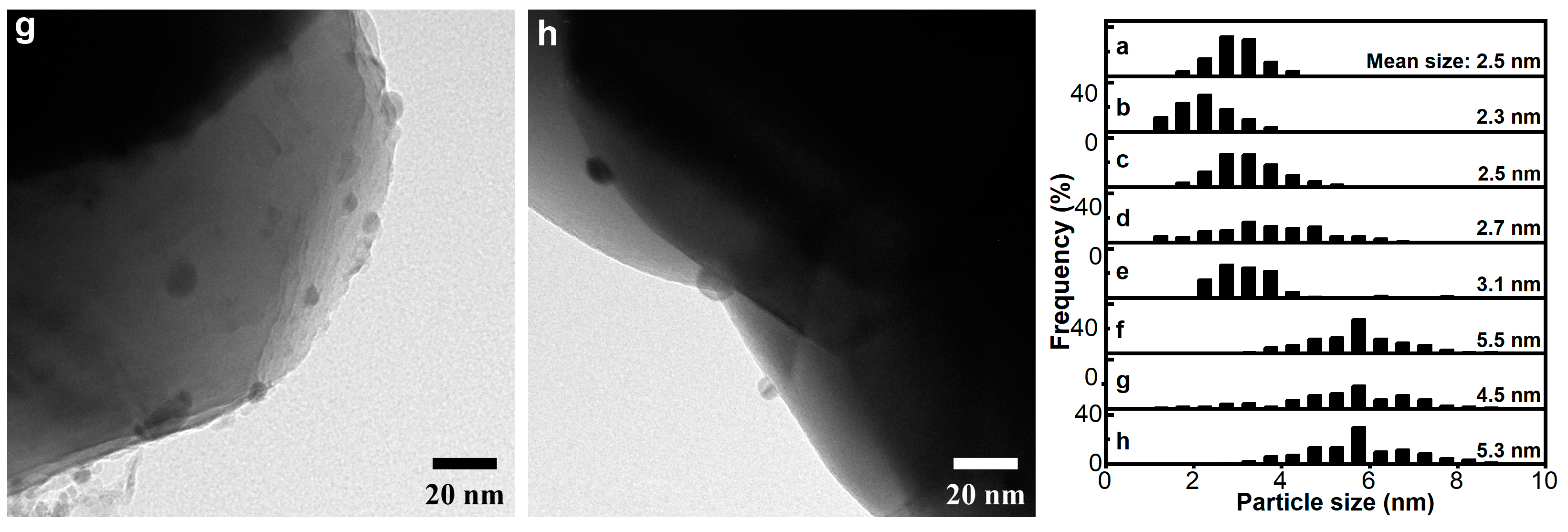
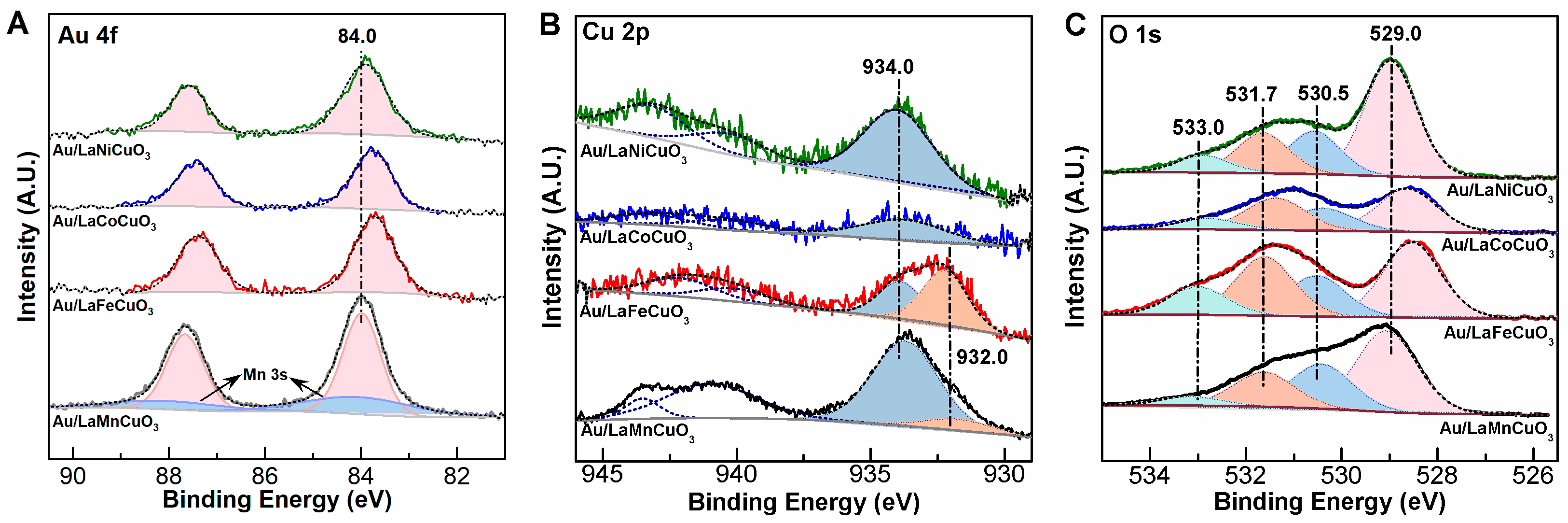


| Catalyst | dAu a (nm) | [Au] b (wt%) | SBET (m2/g) | H2 Uptake c (mmol/g) | Acidity d (μmol/g) | Basicity e (μmol/g) |
|---|---|---|---|---|---|---|
| Au/LaMnO3 | 2.5 | 0.95 | 5.3 | 3.3 (3.3) | 78.9 | 149.7 |
| Au/LaFeO3 | 2.3 | 0.98 | 4.8 | 0.2 (0.1) | 73.5 | 145.3 |
| Au/LaCoO3 | 2.5 | 0.97 | 3.8 | 5.8 (5.8) | 67 | 116.1 |
| Au/LaNiO3 | 2.7 | 0.93 | 6.5 | 5.9 (5.8) | 72.9 | 123.5 |
| Au/LaMnCuO3 | 3.1 | 0.45 | 2.2 | 3.6 (3.5) | 14.7 | 50.1 |
| Au/LaFeCuO3 | 5.5 | 0.47 | 4.2 | 1.2 (0.9) | 65.2 | 107.7 |
| Au/LaCoCuO3 | 4.5 | 0.43 | 2 | 6.3 (6.0) | 33.5 | 81.2 |
| Au/LaNiCuO3 | 5.3 | 0.48 | 4.6 | 6.1 (6.0) | 17.8 | 41.3 |
| Catalyst | Conv. (%) a | Selec. (%) a | Yield (%) a | STY (h−1) b | Ea (kJ/mol) c |
|---|---|---|---|---|---|
| Au/LaMnO3 | 97 | 5 | 5 | 19 | 66 |
| Au/LaFeO3 | 15 | 95 | 14 | 55 | 76 |
| Au/LaCoO3 | 32 | 90 | 29 | 112 | 68 |
| Au/LaNiO3 | 45 | 85 | 38 | 155 | 65 |
| Au/LaMnCuO3 | 93 | 98 | 91 | 764 | 47 |
| Au/LaFeCuO3 | 39 | 97 | 38 | 304 | 70 |
| Au/LaCoCuO3 | 62 | 97 | 60 | 528 | 65 |
| Au/LaNiCuO3 | 66 | 96 | 63 | 498 | 58 |
| Reactant | Conv. | Selec. | Activity | KIE (kH/kD) |
|---|---|---|---|---|
| (%) | (%) | (mmol gcat−1 h−1) | ||
| C2H5OH | 8.8 | 99.5 | 7.5 | -- |
| C2H5OD | 6.0 | 99.0 | 5.1 | 1.5 |
| C2D5OD | 3.2 | 99.0 | 2.7 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, L.; Wang, J.; Liu, P. Promoting Effect of Copper Doping on LaMO3 (M = Mn, Fe, Co, Ni) Perovskite-Supported Gold Catalysts for Selective Gas-Phase Ethanol Oxidation. Catalysts 2025, 15, 176. https://doi.org/10.3390/catal15020176
Yue L, Wang J, Liu P. Promoting Effect of Copper Doping on LaMO3 (M = Mn, Fe, Co, Ni) Perovskite-Supported Gold Catalysts for Selective Gas-Phase Ethanol Oxidation. Catalysts. 2025; 15(2):176. https://doi.org/10.3390/catal15020176
Chicago/Turabian StyleYue, Lijun, Jie Wang, and Peng Liu. 2025. "Promoting Effect of Copper Doping on LaMO3 (M = Mn, Fe, Co, Ni) Perovskite-Supported Gold Catalysts for Selective Gas-Phase Ethanol Oxidation" Catalysts 15, no. 2: 176. https://doi.org/10.3390/catal15020176
APA StyleYue, L., Wang, J., & Liu, P. (2025). Promoting Effect of Copper Doping on LaMO3 (M = Mn, Fe, Co, Ni) Perovskite-Supported Gold Catalysts for Selective Gas-Phase Ethanol Oxidation. Catalysts, 15(2), 176. https://doi.org/10.3390/catal15020176






