Advances in In Situ Investigations of Heterogeneous Catalytic Ammonia Synthesis
Abstract
1. Introduction
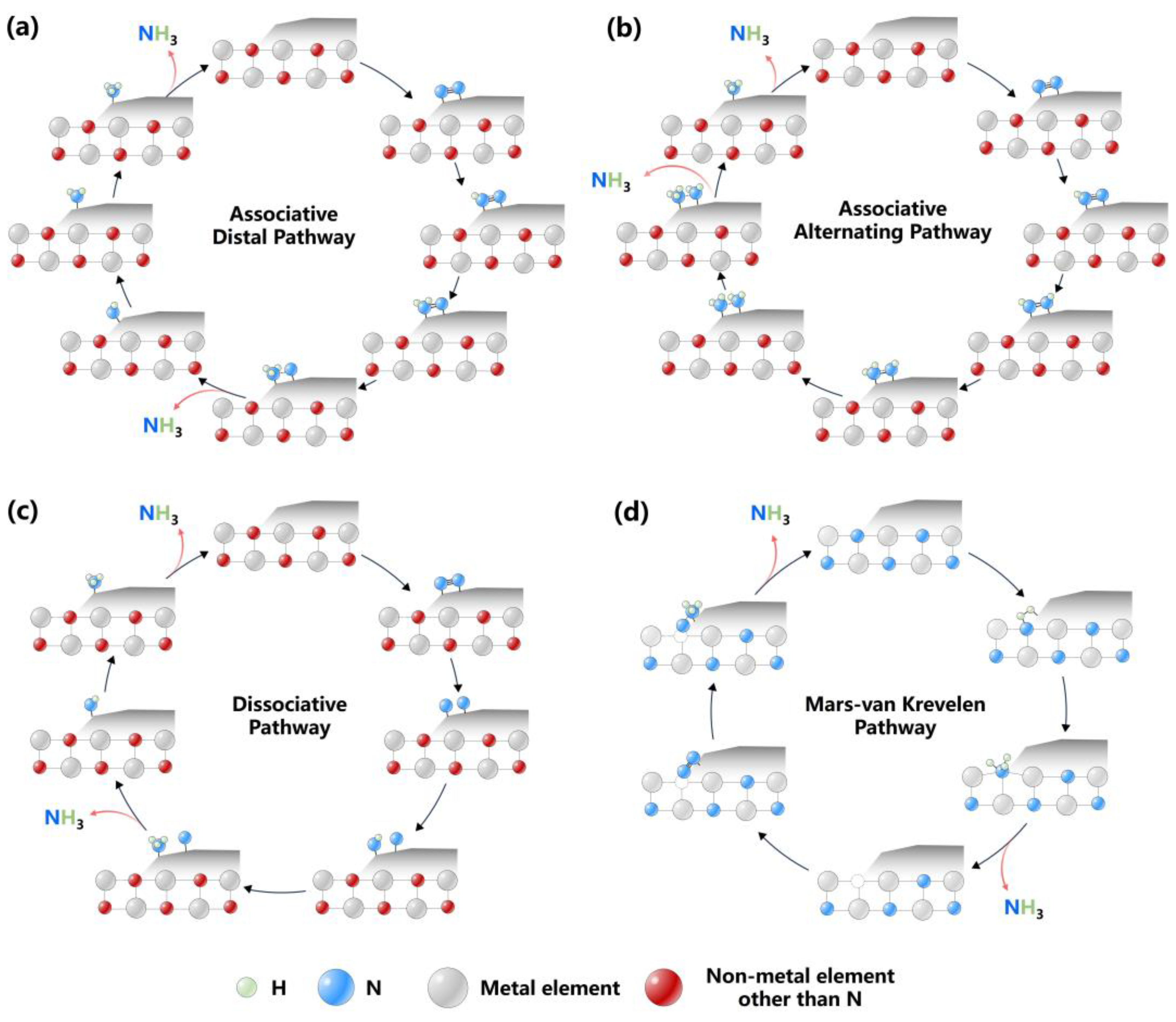
2. Reaction Mechanisms of Ammonia Synthesis
3. Recent Advances on In Situ Investigations
3.1. In Situ Diffuse Reflectance Fourier Transform Infrared Spectroscopy
3.1.1. Detection of Reaction Intermediates
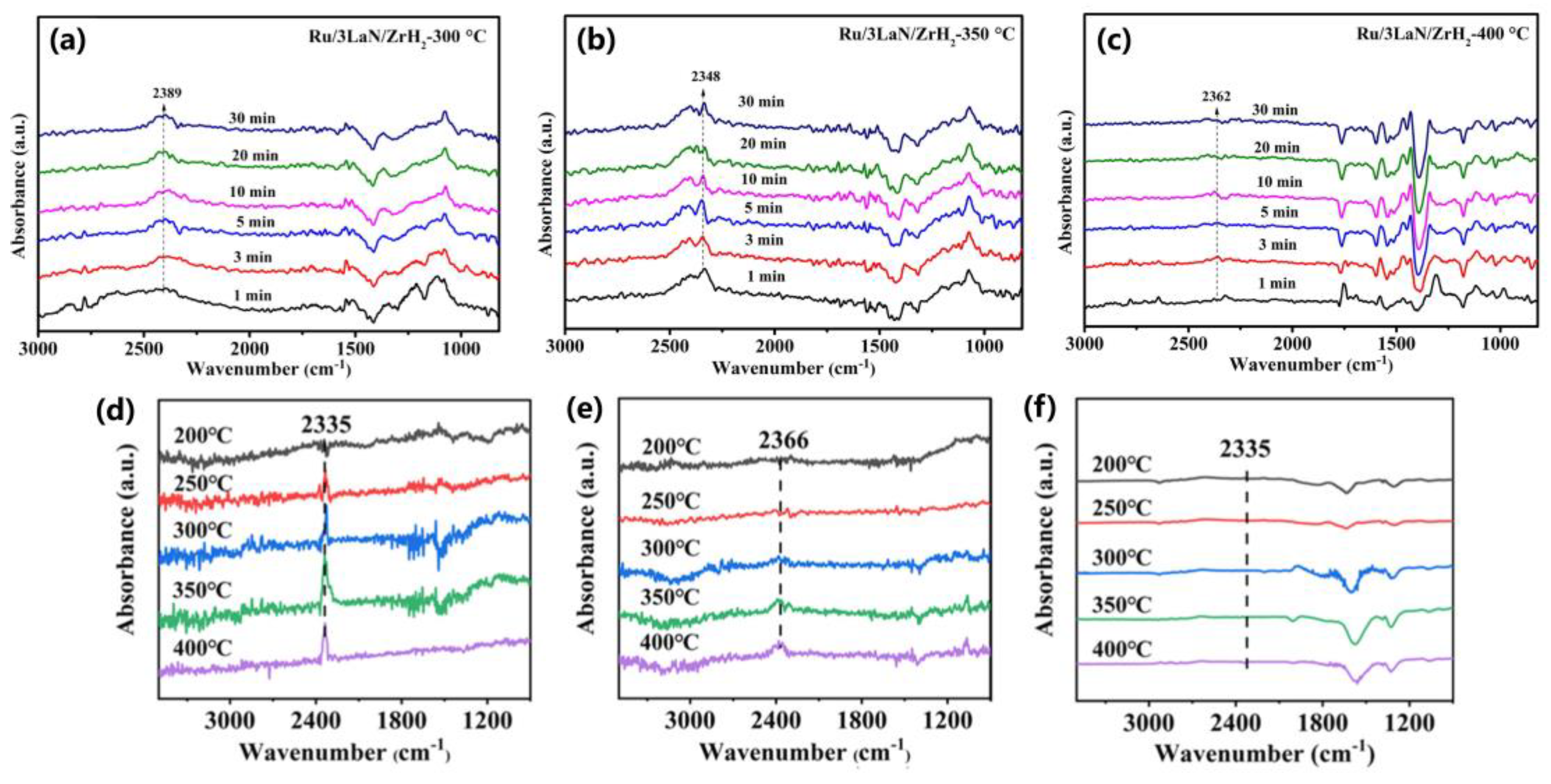
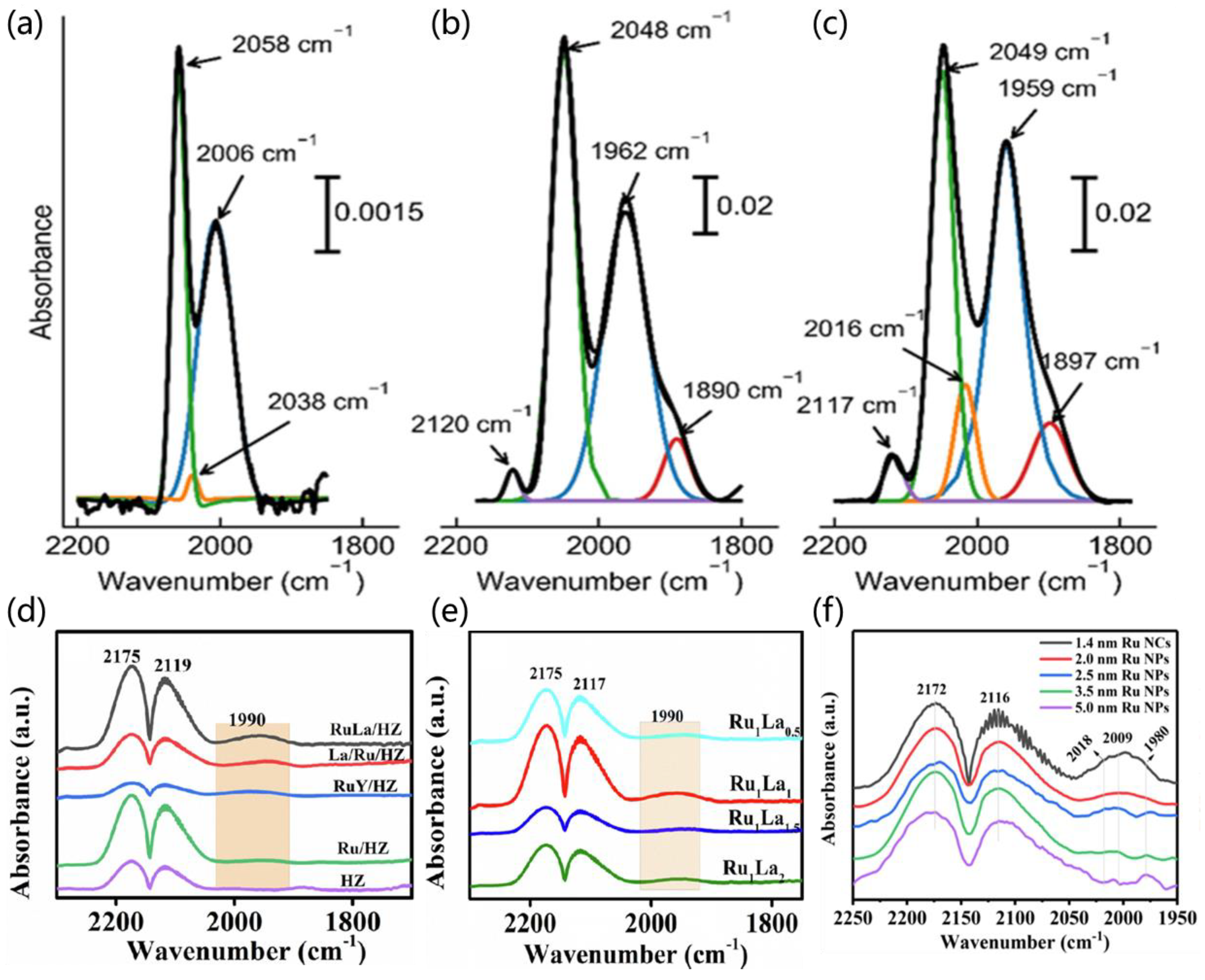
3.1.2. CO Molecular Probe Adsorption to Determine the Electronic Structures of Catalysts
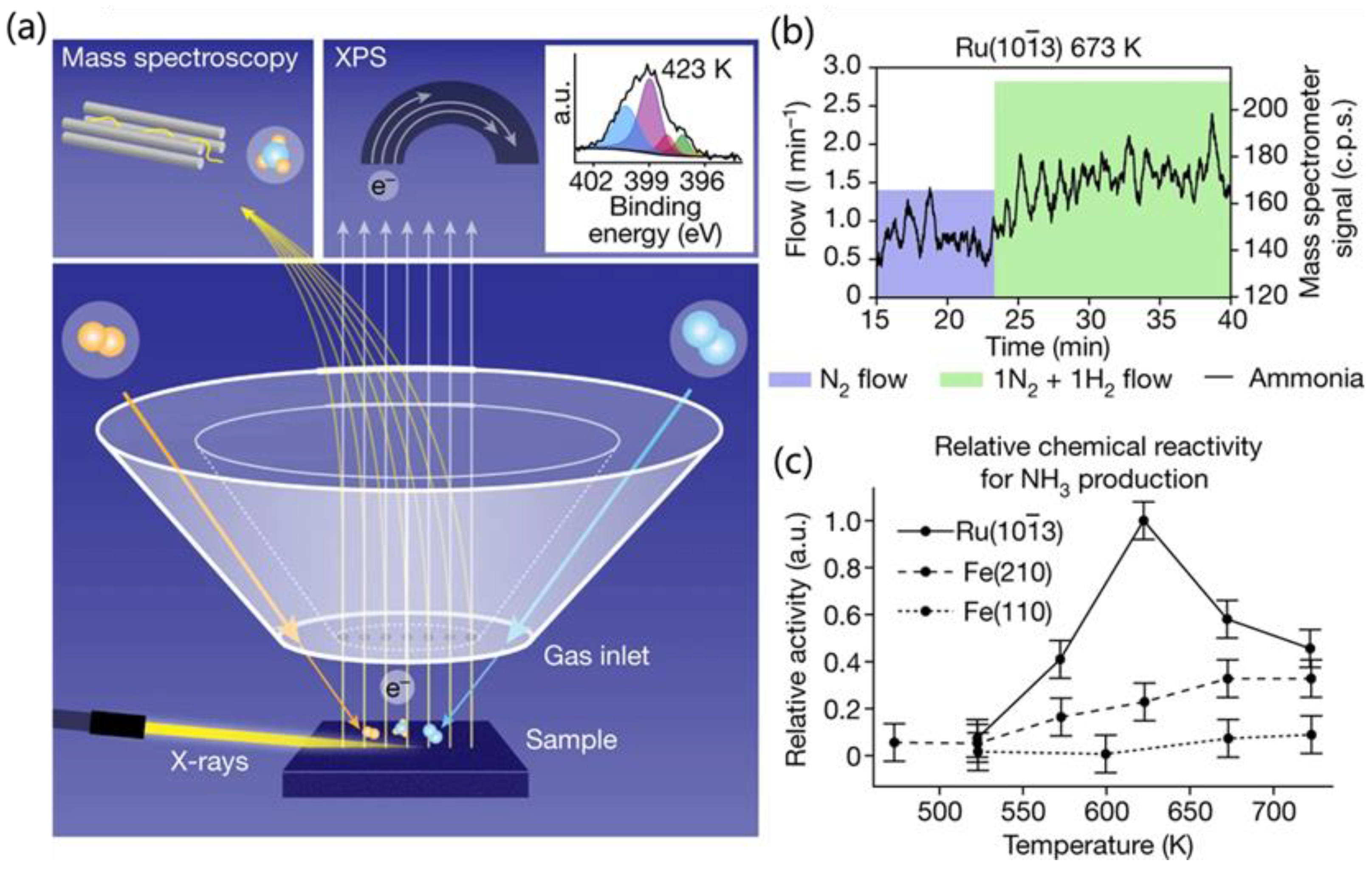
3.2. In Situ X-Ray Photoelectron Spectroscopy
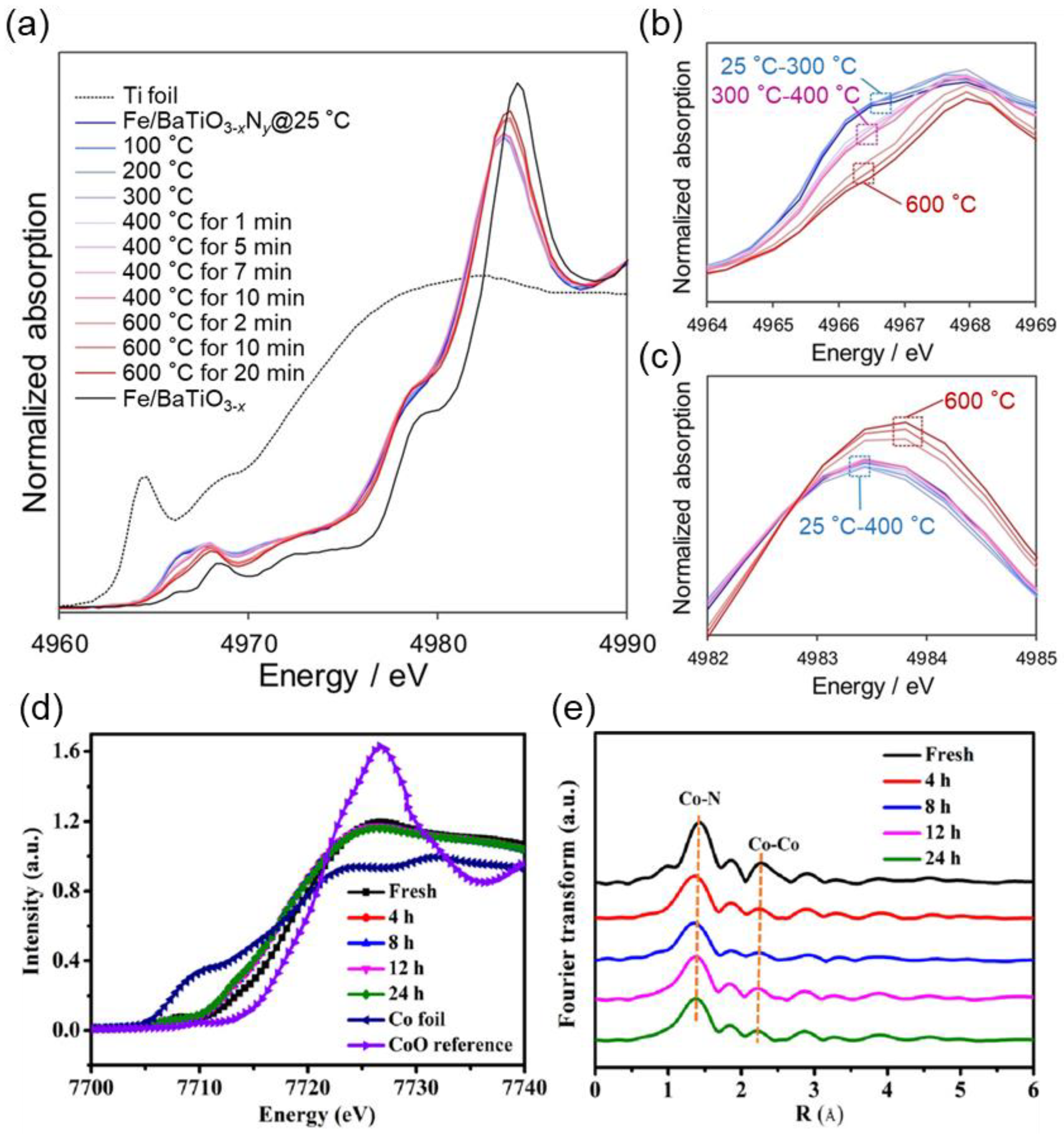
3.3. In Situ X-Ray Absorption Spectroscopy
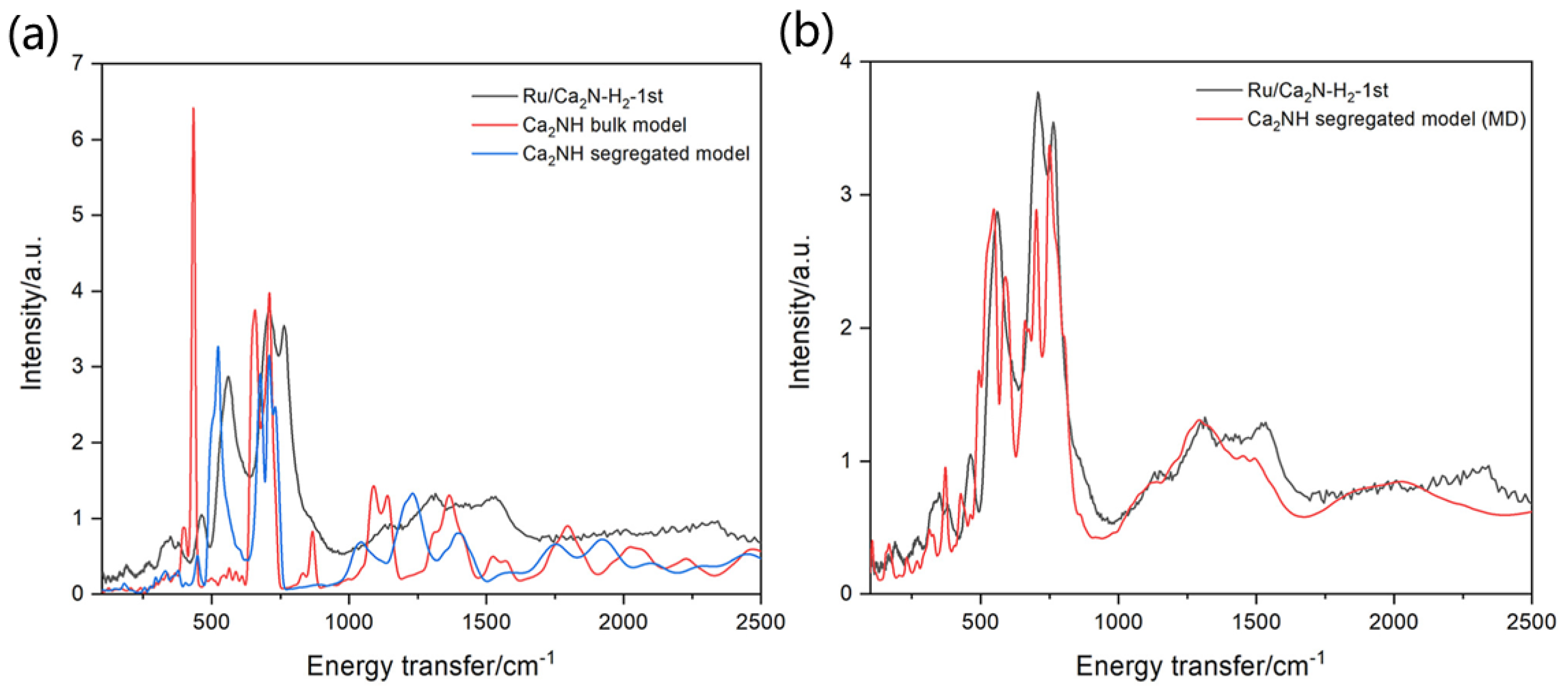
3.4. In Situ Neutron Diffraction
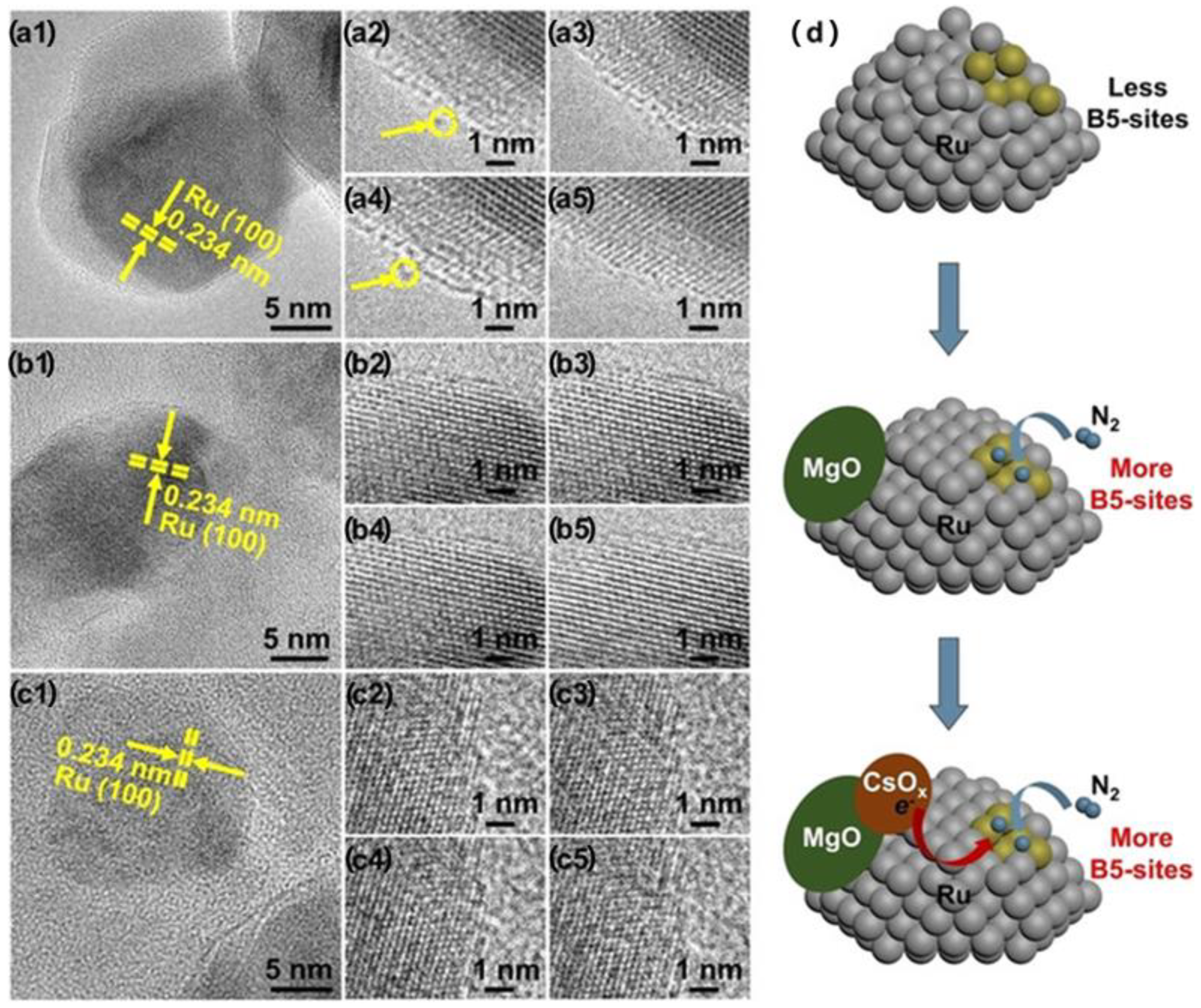
3.5. In Situ Environmental Transmission Electron Microscopy
4. Summary and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MvK | Mars van Krevelen |
| DRIFTS | Diffuse reflectance Fourier transform infrared spectroscopy |
| NP | Nanoparticle |
| NAP-XPS | Near-ambient pressure X-ray photoelectron spectroscopy |
| AP-XPS | Ambient pressure X-ray photoelectron spectroscopy |
| XAS | X-ray absorption spectroscopy |
| XAFS | X-ray absorption fine structure |
| XANES | X-ray absorption near-edge structure |
| EXAFS | Extended X-ray absorption fine structure |
| ACC | Atomic cluster |
| SAC | Single atom catalyst |
| XRD | X-ray diffraction |
| INS | Inelastic neutron scattering |
| ETEM | Environmental transmission electron microscopy |
| EELS | Electron energy loss spectroscopy |
References
- Fang, H.; Liu, D.; Luo, Y.; Zhou, Y.; Liang, S.; Wang, X.; Lin, B.; Jiang, L. Challenges and opportunities of Ru-based catalysts toward the synthesis and utilization of ammonia. ACS Catal. 2022, 12, 3938–3954. [Google Scholar] [CrossRef]
- Zhai, L.L.; Liu, S.Z.; Xiang, Z.H. Ammonia as a carbon-free hydrogen carrier for fuel cells: A perspective. Ind. Chem. Mater. 2023, 1, 332–342. [Google Scholar] [CrossRef]
- Ye, D.; Tsang, S.C.E. Prospects and challenges of green ammonia synthesis. Nat. Synth. 2023, 2, 612–623. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Liu, Y.; Wei, Z.; Wang, K.; Shi, Z. A mini review on transition metal chalcogenides for electrocatalytic water splitting: Bridging material design and practical application. Energy Fuels 2023, 37, 2608–2630. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Bai, S.; Mu, J.; Liu, Y.; Zhang, S. Poly (ionic liquid)s-confined ultrafine Ru oxide with lattice strain induced by Fe doping as a durable catalyst for PEM water electrolysis. Appl. Catal. B-Environ. 2024, 365, 124959. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Humphreys, J.; Lan, R.; Tao, S. Development and recent progress on ammonia synthesis catalysts for Haber–Bosch process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Ravi, M.; Makepeace, J.W. Facilitating green ammonia manufacture under milder conditions: What do heterogeneous catalyst formulations have to offer? Chem. Sci. 2022, 13, 890–908. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021, 5, 2814–2839. [Google Scholar] [CrossRef]
- Chen, C.; Wu, K.; Ren, H.; Zhou, C.; Luo, Y.; Lin, L.; Au, C.; Jiang, L. Ru-based catalysts for ammonia decomposition: A mini-review. Energy Fuels 2021, 35, 11693–11706. [Google Scholar] [CrossRef]
- Jia, S.H.; Wu, L.M.; Xu, L.; Sun, X.F.; Han, B.X. Multicomponent catalyst design for CO2/N2/NOx electroreduction. Ind. Chem. Mater. 2023, 1, 93–105. [Google Scholar] [CrossRef]
- Wang, P.; Xie, H.; Guo, J.; Zhao, Z.; Kong, X.; Gao, W.; Chang, F.; He, T.; Wu, G.; Chen, M.; et al. The formation of surface lithium-iron ternary hydride and its function on catalytic ammonia synthesis at low temperatures. Angew. Chem. Int. Ed. 2017, 56, 8716–8720. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ruan, Y.K.; Iftikhar, R.; Khan, F.Z.; Li, W.X.; Hao, L.D.; Robertson, A.W.; Percoco, G.; Sun, Z.Y. Lithium-mediated electrochemical dinitrogen reduction reaction. Ind. Chem. Mater. 2023, 1, 563–581. [Google Scholar] [CrossRef]
- Shipilin, M.; Degerman, D.; Lömker, P.; Goodwin, C.M.; Rodrigues, G.L.S.; Wagstaffe, M.; Gladh, J.; Wang, H.Y.; Stierle, A.; Schlueter, C.; et al. In-situ surface-sensitive investigation of multiple carbon phases on Fe (110) in the Fischer–Tropsch synthesis. ACS Catal. 2022, 12, 7609–7621. [Google Scholar] [CrossRef]
- Schlögl, R. Ammonia Iron: An Epistemic Challenge with Practical Consequences. J. Phys. Chem. C 2024, 46, 19601–19620. [Google Scholar] [CrossRef]
- Liu, H. Ammonia synthesis catalyst 100 years: Practice, enlightenment and challenge. Chin. J. Catal. 2014, 35, 1619–1640. [Google Scholar] [CrossRef]
- Rohr, B.A.; Singh, A.R.; Nørskov, J.K. A theoretical explanation of the effect of oxygen poisoning on industrial Haber-Bosch catalysts. J. Catal. 2019, 372, 33–38. [Google Scholar] [CrossRef]
- Pappas, I.; Chirik, P.J. Ammonia synthesis by hydrogenolysis of titanium–nitrogen bonds using proton coupled electron transfer. J. Am. Chem. Soc. 2015, 137, 3498–3501. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; An, Q.; Fortunelli, A.; Nielsen, R.J.; Goddard, W.A., 3rd. Reaction mechanism and kinetics for ammonia synthesis on the Fe (111) surface. J. Am. Chem. Soc. 2018, 140, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Ertl, G. Elementary Steps in Heterogeneous Catalysis. Angew. Chem. Int. Ed. 1990, 29, 1219–1227. [Google Scholar] [CrossRef]
- Medford, A.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Y.F.; Zhang, T.; Cai, H.; Zhou, Y.; Lin, B.; Lin, X.; Zheng, Y.; Zheng, L.; Wang, X.; et al. Size sensitivity of supported Ru catalysts for ammonia synthesis: From nanoparticles to subnanometric clusters and atomic clusters. Chem 2022, 8, 749–768. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Chen, P. Recent progress towards mild-condition ammonia synthesis. J. Energy Chem. 2019, 36, 25–36. [Google Scholar] [CrossRef]
- Guo, J.; Che, P. Interplay of alkali, transition metals, nitrogen, and hydrogen in ammonia synthesis and decomposition reactions. Acc. Chem. Res. 2021, 54, 2434–2444. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Gaigneaux, E.M. Recent advances in heterogeneous catalysis for ammonia synthesis. ChemCatChem 2020, 12, 5838–5857. [Google Scholar] [CrossRef]
- Van Der Ham, C.J.M.; Koper, M.T.M.; Hetterscheid, D.G.H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.N.; Park, S.W.; Lu, Y.; Li, J.; Wu, J.; Sasase, M.; Kitano, M.; Hosono, H. Dissociative and associative concerted mechanism for ammonia synthesis over Co-based catalyst. J. Am. Chem. Soc. 2021, 143, 12857–12866. [Google Scholar] [CrossRef]
- Sun, K.; Zou, X.; Sun, X.; Pang, W.; Hao, X.; Xu, Y.; Su, H.Y. Structure and reaction condition dependent mechanism for ammonia synthesis on Ru-based catalyst. Appl. Surf. Sci. 2023, 613, 156060. [Google Scholar] [CrossRef]
- Kuganathan, N.; Hosono, H.; Shluger, A.L.; Sushko, P.V. Enhanced N2 dissociation on Ru-loaded inorganic electride. J. Am. Chem. Soc. 2014, 136, 2216–2219. [Google Scholar] [CrossRef]
- Ertl, G. Reactions at surfaces: From atoms to complexity (Nobel Lecture). Angew. Chem. Int. Ed. 2008, 47, 3524–3535. [Google Scholar] [CrossRef] [PubMed]
- Daisley, A.; Hargreaves, J.S.J. Metal nitrides, the Mars-van Krevelen mechanism and heterogeneously catalysed ammonia synthesis. Catal. Today 2023, 423, 113874. [Google Scholar] [CrossRef]
- Cai, J.; Wang, C.; Liu, Y.; Ni, J.; Lin, B.; Wang, X.; Lin, J.; Jiang, L. Effect of pore-size distribution on Ru/ZSM-5 catalyst for enhanced N2 activation to ammonia via dissociative mechanism. J. Rare Earths 2020, 38, 873–882. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Cai, J.; Cai, H.; Ni, J.; Lin, B.; Lin, J.; Wang, X.; Zheng, L.; Au, C.T.; et al. Operando spectroscopic and isotopic-label-directed observation of LaN-promoted Ru/ZrH2 catalyst for ammonia synthesis via associative and chemical looping route. J. Catal. 2020, 389, 218–228. [Google Scholar] [CrossRef]
- Zhou, Y.; Sai, Q.; Tan, Z.; Wang, C.; Wang, X.; Lin, B.; Ni, J.; Lin, J.; Jiang, L. Highly efficient subnanometer Ru-based catalyst for ammonia synthesis via an associative mechanism. Chin. J. Chem. Eng. 2022, 43, 177–184. [Google Scholar] [CrossRef]
- Peng, X.; Chen, X.; Zhou, Y.; Sun, F.; Zhang, T.; Zheng, L.; Jiang, L.; Wang, X. Size-dependent activity of supported Ru catalysts for ammonia synthesis at mild conditions. J. Catal. 2022, 408, 98–108. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, X.; Zhang, T.; Cai, H.; Lin, B.; Zheng, L.; Wang, X.; Jiang, L. Essential Role of Ru–anion Interaction in Ru-based ammonia synthesis catalysts. ACS Catal. 2022, 12, 7633–7642. [Google Scholar] [CrossRef]
- Aslan, M.Y.; Mete, E.; Uner, D. In search of the bottlenecks of ammonia synthesis over Ru/Vulcan under ambient conditions. Faraday Discuss. 2023, 243, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, H.; Li, J.; Gao, Y.; Li, L.; Zhang, M.; Peng, X.; Zhou, Y.; Ni, J.; Lin, B.; et al. Tuning clusters-metal oxide promoters electronic interaction of Ru-based catalyst for ammonia synthesis under mild conditions. J. Catal. 2024, 60, 209–218. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; Wang, J.; Peng, X.; Deng, J.; Wang, S.; Song, Y.; Zhou, Y.; Ni, J.; Wang, X.; et al. Ru alloying with La or Y for ammonia synthesis via integrated dissociative and associative mechanism with superior operational stability. Chem. Eng. Sci. 2022, 252, 117255. [Google Scholar] [CrossRef]
- Qiu, J.Z.; Hu, J.; Lan, J.; Wang, L.-F.; Fu, G.; Xiao, R.; Ge, B.; Jiang, J. Pure siliceous zeolite-supported Ru single-atom active sites for ammonia synthesis. Chem. Mater. 2019, 31, 9413–9421. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, C.Q.; Tan, Z.; Cai, H.; Wang, X.; Li, J.; Zheng, L.; Au, C.T.; Li, J.; Jiang, L. Integrating dissociative and associative routes for efficient ammonia synthesis over a TiCN-promoted Ru-based catalyst. ACS Catal. 2022, 12, 2651–2660. [Google Scholar] [CrossRef]
- Drummond, S.M.; Naglic, J.; Onsree, T.; Balijepalli, S.K.; Allegro, A.; Orraca Albino, S.N.; O’Connell, K.M.; Lauterbach, J. Promoted Ru/PrOx catalysts for mild ammonia synthesis. Catalysts 2024, 14, 572. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Chen, W.; Liu, G.; Zheng, A.; Zheng, L.; Ni, J.; Au, C.T.; Jiang, L. Insight into dynamic and steady-state active sites for nitrogen activation to ammonia by cobalt-based catalyst. Nat. Commun. 2020, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.; Fellowes, J.; Fang, H.; Large, A.; Wu, S.; Held, G.; Raine, E.; Ho, P.L.; Tang, C.; Tsang, S.C.E. Importance of hydrogen migration in catalytic ammonia synthesis over yttrium-doped barium zirconate-supported ruthenium nanoparticles: Visualization of proton trap sites. J. Phys. Chem. C 2021, 125, 23058–23070. [Google Scholar] [CrossRef]
- Goodwin, C.M.; Lomker, P.; Degerman, D.; Davies, B.; Shipilin, M.; Garcia-Martinez, F.; Koroidov, S.; Katja Mathiesen, J.; Rameshan, R.; Rodrigues, G.L.S.; et al. Operando probing of the surface chemistry during the Haber-Bosch process. Nature 2024, 625, 282–286. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, L.; Wang, C.; Sun, F.; Zheng, L.; Qi, H.; Wang, B.; Wang, X.; Au, C.T.; Wang, J.; et al. Precious-metal-free Mo-MXene catalyst enabling facile ammonia synthesis via dual sites bridged by H-spillover. J. Am. Chem. Soc. 2024, 146, 23054–23066. [Google Scholar] [CrossRef]
- Gondo, A.; Manabe, R.; Sakai, R.; Murakami, K.; Yabe, T.; Ogo, S.; Ikeda, M.; Tsuneki, H.; Sekine, Y. Ammonia synthesis over Co catalyst in an electric field. Catal. Lett. 2018, 148, 1929–1938. [Google Scholar] [CrossRef]
- Kikugawa, M.; Goto, Y.; Kobayashi, K.; Nanba, T.; Matsumoto, H.; Imagawa, H. Efficient ammonia synthesis over Ru/CeO2-PrOx catalysts with controlled Ru dispersion by Ru-Pr interaction. J. Catal. 2022, 413, 934–942. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ikejima, K.; Ogasawara, K.; Kitano, M.; Hosono, H. Ammonia synthesis over Fe-supported catalysts mediated by face-sharing nitrogen sites in BaTiO(3−x)N(y) oxynitride. ChemSusChem 2023, 16, e202300551. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cai, H.; Zhou, Y.; Ni, J.; Wang, X.; Lin, B.; Lin, J.; Zheng, L.; Au, C.T.; Jiang, L. Studies of a highly active cobalt atomic cluster catalyst for ammonia synthesis. ACS Sustain. Chem. Eng. 2022, 10, 1951–1960. [Google Scholar] [CrossRef]
- Herzog, B.; Herein, D.; Schlögl, R. In situ X-ray powder diffraction analysis of the microstructure of activated iron catalysts for ammonia synthesis. Appl. Catal. A 1996, 141, 71–104. [Google Scholar] [CrossRef]
- Laassiri, S.; Zeinalipour-Yazdi, C.D.; Bion, N.; Catlow, C.R.A.; Hargreaves, J.S.J. Combination of theoretical and in situ experimental investigations of the role of lithium dopant in manganese nitride: A two-stage reagent for ammonia synthesis. Faraday Discuss. 2021, 229, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Cheng, Y.; Daemen, L.; Novak, E.; Ramirez-Cuesta, A.J.; Wu, Z. On the structural transformation of Ni/BaH2 during a N2-H2 chemical looping process for ammonia synthesis: A joint in situ inelastic neutron scattering and first-principles simulation study. Top. Catal. 2021, 64, 685–692. [Google Scholar] [CrossRef]
- Kammert, J.; Moon, J.; Cheng, Y.; Daemen, L.; Irle, S.; Fung, V.; Liu, J.; Page, K.; Ma, X.; Phaneuf, V.; et al. Nature of reactive hydrogen for ammonia synthesis over a Ru/C12A7 electride catalyst. J. Am. Chem. Soc. 2020, 142, 7655–7667. [Google Scholar] [CrossRef]
- Yu, X.; Moon, J.; Cheng, Y.; Daemen, L.; Liu, J.; Kim, S.W.; Kumar, A.; Chi, M.; Fung, V.; Ramirez-Cuesta, A.J.; et al. In situ neutron scattering study of the structure dynamics of the Ru/Ca2N:e− catalyst in ammonia synthesis. Chem. Mater. 2023, 35, 2456–2462. [Google Scholar] [CrossRef]
- Hansen, T.W.; Wagner, J.B.; Hansen, P.L.; Dahl, S.; Topsøe, H.; Jacobsen, C.J.H. Atomic-resolution in situ transmission electron microscopy of a promoter of a heterogeneous catalyst. Science 2001, 294, 1508–1510. [Google Scholar] [CrossRef]
- Ward, M.R.; Mitchell, R.W.; Boyes, E.D.; Gai, P.L. Visualization of atomic scale reaction dynamics of supported nanocatalysts during oxidation and ammonia synthesis using in-situ environmental (scanning) transmission electron microscopy. J. Energy Chem. 2021, 57, 281–290. [Google Scholar] [CrossRef]
- Ding, J.; Wang, L.; Wu, P.; Li, A.; Li, W.; Stampfl, C.; Liao, X.; Haynes, B.S.; Han, X.; Huang, J. Confined Ru nanocatalysts on surface to enhance ammonia synthesis: An in situ ETEM study. ChemCatChem 2020, 13, 534–538. [Google Scholar] [CrossRef]
- Boniface, M.; Plodinec, M.; Schlögl, R.; Lunkenbein, T. Quo Vadis Micro-electro-mechanical systems for the study of heterogeneous catalysts inside the electron microscope? Top. Catal. 2020, 63, 1623–1643. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Cheng, X.; Shang, S.; Pan, R.; Qi, M.; Sang, Q.; Xie, Z.; Zhang, H.; Wang, K.; Liu, Y. Advances in In Situ Investigations of Heterogeneous Catalytic Ammonia Synthesis. Catalysts 2025, 15, 160. https://doi.org/10.3390/catal15020160
Su W, Cheng X, Shang S, Pan R, Qi M, Sang Q, Xie Z, Zhang H, Wang K, Liu Y. Advances in In Situ Investigations of Heterogeneous Catalytic Ammonia Synthesis. Catalysts. 2025; 15(2):160. https://doi.org/10.3390/catal15020160
Chicago/Turabian StyleSu, Weiyi, Xi Cheng, Suokun Shang, Runze Pan, Miao Qi, Qinqin Sang, Zhen Xie, Honghua Zhang, Ke Wang, and Yanrong Liu. 2025. "Advances in In Situ Investigations of Heterogeneous Catalytic Ammonia Synthesis" Catalysts 15, no. 2: 160. https://doi.org/10.3390/catal15020160
APA StyleSu, W., Cheng, X., Shang, S., Pan, R., Qi, M., Sang, Q., Xie, Z., Zhang, H., Wang, K., & Liu, Y. (2025). Advances in In Situ Investigations of Heterogeneous Catalytic Ammonia Synthesis. Catalysts, 15(2), 160. https://doi.org/10.3390/catal15020160






