Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over a Bi-Promoted Pt/Al2O3 Catalyst
Abstract
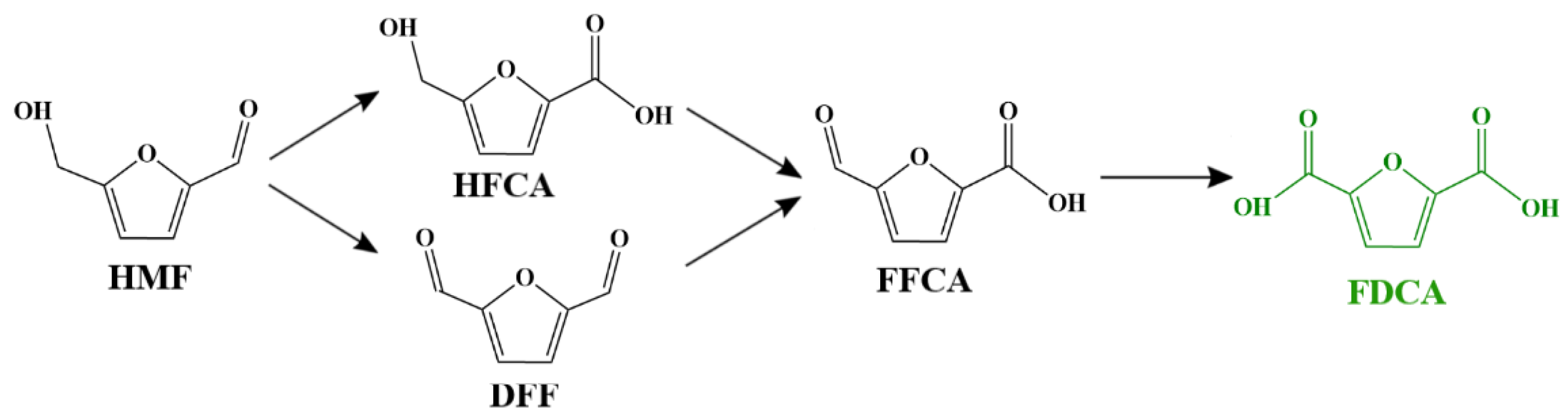
1. Introduction
2. Results and Discussion
2.1. Physiochemical Properties of 5Pt/Al2O3 and 5Pt–nBi/Al2O3 Catalysts
2.2. Catalytic Oxidation Performance of Pt/Al2O3
2.3. Catalytic Oxidation Performance of 5Pt–nBi/Al2O3
3. Experimental
3.1. Materials
3.2. Preparation of 5Pt/Al2O3 and 5Pt–nBi/Al2O3 Catalysts
3.3. Oxidation of HMF to FDCA
3.4. Characterization and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, W.; Bruining, H.C.; Heeres, H.J.; Yue, J. Insights into the Reaction Network and Kinetics of Xylose Conversion over Combined Lewis/Brønsted Acid Catalysts in a Flow Microreactor. Green Chem. 2023, 25, 5878–5898. [Google Scholar] [CrossRef]
- Li, X.L.; Zhu, R.; Xu, H.J. Recent Advances in Direct Synthesis of 2,5-Furandicarboxylic Acid from Carbohydrates. ACS Sustain. Chem. Eng. 2025, 13, 2223–2259. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Huang, W.; Hu, X.; Zhai, J.; Zhu, N.; Guo, K. Biorenewable Furan-Containing Polyamides. Mater. Today Sustain. 2020, 10, 100049. [Google Scholar] [CrossRef]
- Jiang, L.; Gonzalez-Diaz, A.; Ling-Chin, J.; Malik, A.; Roskilly, A.P.; Smallbone, A.J. Pef Plastic Synthesized from Industrial Carbon Dioxide and Biowaste. Nat. Sustain. 2020, 3, 761–767. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Yue, J.; Heeres, H.J.; Zhao, L.; Xi, Z. Experimental Study on the Synthesis of Biobased Poly(Ethylene-2,5-Furandicarboxylate) and Kinetic Modeling on the Esterification of 2,5-Furandicarboxylic Acid and Ethylene Glycol. ACS Sustain. Chem. Eng. 2025, 13, 7299–7317. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased Polyesters and Other Polymers from 2,5-Furandicarboxylic Acid: A Tribute to Furan Excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Zhong, X.; Yuan, P.; Wei, Y.; Liu, D.; Losic, D.; Li, M. Coupling Natural Halloysite Nanotubes and Bimetallic Pt–Au Alloy Nanoparticles for Highly Efficient and Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ACS Appl. Mater. Interfaces 2022, 14, 3949–3960. [Google Scholar] [CrossRef]
- Cong, H.; Yuan, H.; Tao, Z.; Bao, H.; Zhang, Z.; Jiang, Y.; Huang, D.; Liu, H.; Wang, T. Recent Advances in Catalytic Conversion of Biomass to 2,5-Furandicarboxylic Acid. Catalysts 2021, 11, 1113. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, Y.; Ban, H.; Zhang, Y.; Zheng, L.; Wang, L.; Li, X. Liquid-Phase Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Co/Mn/Br Catalyst. Ind. Eng. Chem. Res. 2020, 59, 17076–17084. [Google Scholar] [CrossRef]
- Zuo, X.B.; Venkitasubramanian, P.; Martin, K.J.; Subramaniam, B. Facile Production of 2,5-Furandicarboxylic Acid via Oxidation of Industrially Sourced Crude 5-Hydroxymethylfurfural. Chemsuschem 2022, 15, e202102050. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, L.; Li, B.; Bian, S.; Wang, J.; Zhang, H.; Zhao, C. Base Metal Catalyzed Oxidation of 5-Hydroxy-Methyl-Furfural to 2,5-Furan-Dicarboxylic Acid: A Review. Catal. Today 2023, 408, 64–72. [Google Scholar] [CrossRef]
- Li, M.; Hu, S.; Zhang, Y.; Qin, J.; Min, D.; Chen, C.; Zhang, P.; Jiang, L.; Zhang, J. Regulation of Oxygen Vacancy in Mn-Co Oxides to Enhance Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. Appl. Catal. B Environ. Energy 2025, 368, 125130. [Google Scholar] [CrossRef]
- Liu, Q.; Gong, Y.; Zeng, J.; Zhao, Y.; Lv, J.; Jiang, Z.; Hu, C.; Zhou, Y. Strategic Metal Doping in MnOx Catalysts Unlocks High-Yield 2,5-Furandicarboxylic Acid Production via Tailored Lattice Oxygen Activity and Oxygen Vacancies. ACS Sustain. Chem. Eng. 2025, 13, 2107–2119. [Google Scholar] [CrossRef]
- Lai, J.; Cheng, F.; Zhou, S.; Wen, S.; Guo, D.; Zhao, W.; Liu, X.; Yin, D. Base-Free Oxidation of 5-Hydroxymethylfurfural to 2, 5-Furan Dicarboxylic Acid over Nitrogen-Containing Polymers Supported Cu-Doped MnO2 Nanowires. Appl. Surf. Sci. 2021, 565, 150479. [Google Scholar] [CrossRef]
- Cheng, F.; Guo, D.; Lai, J.; Long, M.; Zhao, W.; Liu, X.; Yin, D. Efficient Base-Free Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Copper-Doped Manganese Oxide Nanorods with Tert-Butanol as Solvent. Front. Chem. Sci. Eng. 2021, 15, 960–968. [Google Scholar] [CrossRef]
- Bueno, A.; Viar, N.; Barredo, A.; Gandarias, I.; Requies, J.M. Integrated Process for 2,5-Furandicarboxylic Acid Production from Fructose via NaCl-Promoted Dehydration and Au/Ht-Catalyzed Oxidation. J. Ind. Eng. Chem. 2025, 147, 696–704. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, L.; Sun, Q.; Zong, B. High-Yield Synthesis of 2,5-Furandicarboxylic Acid from Fructose via a Two-Step Strategy without 5-Hydroxymethylfurfural Isolation Using Dioxane as a Bridging Solvent. Mol. Catal. 2024, 552, 113677. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, K.; He, Y.; Hou, Q.; Xie, C.; Lai, R.; Yu, G.; Xia, T.; Bai, X.; Xie, H.; et al. Engineering Crystal Plane of NiCo2O4 to Regulate Oxygen Vacancies and Acid Sites for Alkali-Free Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. Green Energy Environ. 2025, 10, 756–765. [Google Scholar] [CrossRef]
- Das, S.; Cibin, G.; Walton, R.I. Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural Catalyzed by an Iron-Grafted Metal-Organic Framework with a Sustainably Sourced Ligand. ACS Sustain. Chem. Eng. 2024, 12, 5575–5585. [Google Scholar] [CrossRef]
- Valentini, F.; Ferlin, F.; Vaccaro, L. Continuous Flow Tube-in-Tube Oxidation of Hmf to Fdca Using Heterogeneous Manganese Catalyst under Mild Conditions. Green Chem. 2025, 27, 12166–12175. [Google Scholar] [CrossRef]
- Suktong, K.; Phasi, J.; Longsomboon, P.; Chumkasorn, V.; Sane, A.; Wadaugsorn, K. Optimization of 2,5-Furandicarboxylic Acid (Fdca) Production in a Continuous Packed-Bed Reactor for Sustainable Pef Packaging Applications. ACS Omega 2025, 10, 42092–42101. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Khalid, A.J.; Narishetty, V.; Kumar, V.; Dutta, S.; Ahmad, E. Recent Advances in the Production of 2,5-Furandicarboxylic Acid from Biorenewable Resources. Mater. Sci. Energy Technol. 2023, 6, 502–521. [Google Scholar] [CrossRef]
- de Jong, E.; Visser, H.A.; Dias, A.S.; Harvey, C.; Gruter, G.-J.M. The Road to Bring Fdca and Pef to the Market. Polymers 2022, 14, 943. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward Biomass-Derived Renewable Plastics: Production of 2,5-Furandicarboxylic Acid from Fructose. Sci. Adv. 2018, 4, eaap9722. [Google Scholar] [CrossRef]
- Lv, G.; Liu, S.; Chen, X.; Chen, M.; Wu, Y.; Gao, Y.; Wang, S.; Tao, F.; Wang, J.; Niu, L. Thermodynamically Stable Synthesis of High Entropy Alloys and Efficiently Catalyzed Oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid under Base-Free Conditions. Green Chem. 2024, 26, 11316–11327. [Google Scholar] [CrossRef]
- Sahu, R.; Dhepe, P.L. Synthesis of 2,5-Furandicarboxylic Acid by the Aerobic Oxidation of 5-Hydroxymethyl Furfural over Supported Metal Catalysts. React. Kinet. Mech. Catal. 2014, 112, 173–187. [Google Scholar] [CrossRef]
- Ait Rass, H.; Essayem, N.; Besson, M. Selective Aqueous Phase Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Pt/C Catalysts: Influence of the Base and Effect of Bismuth Promotion. Green Chem. 2013, 15, 2240–2251. [Google Scholar] [CrossRef]
- Vinke, P.; van der Poel, W.; van Bekkum, H. On the oxygen tolerance of noble metal catalysts in liquid phase alcohol oxidations the influence of the support on catalyst deactivation. In Studies in Surface Science and Catalysis; Guisnet, M., Barrault, J., Bouchoule, C., Duprez, D., Pérot, G., Maurel, R., Montassier, C., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 59, pp. 385–394. [Google Scholar]
- Zhang, Y.; Liu, Y.; Xia, Q.; Chen, Y.; Kong, L.; Yan, X.; Guan, W.; Pan, J. Ambient Temperature Catalyzed Air-Oxidation of 5-Hydroxymethylfurfural via Ternary Metal and Oxygen Vacancies. Green Energy Environ. 2025, 10, 1568–1582. [Google Scholar] [CrossRef]
- Molinaro, J.M.; Swartzentruber, J.; Ledger, V.W.; Fredericks, Z.T.; Alonso, D.M.; Wettstein, S.G. Recent Developments in Solvent and Catalyst Selection for 5-Hydroxymethylfurfural Oxidation to 2,5-Furandicarboxylic Acid. EES Catal. 2025, 3, 595–620. [Google Scholar] [CrossRef]
- Mallat, T.; Bodnar, Z.; Hug, P.; Baiker, A. Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde with Air over Bi-Pt/Alumina Catalysts. J. Catal. 1995, 153, 131–143. [Google Scholar] [CrossRef]
- Rass, H.A.; Essayem, N.; Besson, M. Selective Aerobic Oxidation of 5-HMF into 2,5-Furandicarboxylic Acid with Pt Catalysts Supported on TiO2- and ZrO2-Based Supports. Chemsuschem 2015, 8, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Maizkurrena, P.; Requies, J.; Iriondo, A.; Macías-Villasevil, M. Characterization and Performance of Carbon Supported Platinum-Bismuth Bimetallic Catalysts Tested in 5-Hydroxymethylfurfural Aerobic Oxidation to 2,5-Furandicarboxylic Acid. Biomass Bioenergy 2025, 192, 107505. [Google Scholar] [CrossRef]
- Díaz-Maizkurrena, P.; Requies, J.; Iriondo, A.; Macías-Villasevil, M. Addressing the Influence of Alkaline Compounds in 5-Hydroxymethylfurfu-Ral Oxidation and Separation Towards 2,5-Furandicarboxylic Acid. Catal. Today 2025, 456, 115338. [Google Scholar] [CrossRef]
- Miao, Z.; Wu, T.; Li, J.; Yi, T.; Zhang, Y.; Yang, X. Aerobic Oxidation of 5-Hydroxymethylfurfural (HMF) Effectively Catalyzed by a Ce0.8Bi0.2O2−δ Supported Pt Catalyst at Room Temperature. RSC Adv. 2015, 5, 19823–19829. [Google Scholar] [CrossRef]
- Lilga, M.A.; Hallen, R.T.; Gray, M. Production of Oxidized Derivatives of 5-Hydroxymethylfurfural (HMF). Top. Catal. 2010, 53, 1264–1269. [Google Scholar] [CrossRef]
- Maphoru, M.V.; Heveling, J.; Kesavan Pillai, S. Impact of the Promoter on the Performance of Carbon-Supported Pt-Bi and Pt-Sb Catalysts for the Oxidative Coupling of 2-Methyl-1-Naphthol. React. Kinet. Mech. Catal. 2021, 134, 95–107. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Varma, A. Low-Temperature Selective Oxidation of Methanol over Pt-Bi Bimetallic Catalysts. J. Catal. 2018, 363, 144–153. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Tian, H.; Wei, H.; Wang, Y.; Chen, X.; Fang, X. Ni-Pt Bimetallic Adsorbent for Deep Desulphurization of Coked Benzene. Adsorption 2025, 31, 66. [Google Scholar] [CrossRef]
- Wu, B.; Chen, B.; Zhu, X.; Yu, L.; Shi, C. Lower Loading of Pt on Hydrophobic TS-1 Zeolite: A High-Efficiency Catalyst for Benzene Oxidation at Low Temperature. Catal. Today 2020, 355, 512–517. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Hsieh, Y.-C.; Tsai, H.-C.; Lu, I.T.; Wu, Y.-H.; Yu, T.H.; Lee, J.-F.; Merinov, B.V.; Goddard, W.A.; Wu, P.-W. Dealloyed Pt2os Nanoparticles for Enhanced Oxygen Reduction Reaction in Acidic Electrolytes. Appl. Catal. B Environ. 2014, 150-151, 636–646. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Li, H.; Liu, H. Controllable Synthesis of Metallic Bi from Commercial Bi2o3 via One-Pot Solvothermal Reduction Method. Chin. J. Chem. Eng. 2017, 25, 1202–1206. [Google Scholar] [CrossRef]
- Wang, X.; Huang, C.; Wu, B.; Yang, W.; Zhang, Y.; Xu, Y.; Jiao, Y.; Zhong, L.; Wang, J. A Pt Catalyst with Enhanced Three-Way Catalytic Activity Supported on Ceo2 Modified with Al2O3 for Natural Gas Vehicles. Ind. Eng. Chem. Res. 2025, 64, 5854–5863. [Google Scholar] [CrossRef]
- de Miguel, S.R.; Scelza, O.A.; Román-Martínez, M.C.; Salinas-Martínez de Lecea, C.; Cazorla-Amorós, D.; Linares-Solano, A. States of Pt in Pt/C Catalyst Precursors after Impregnation, Drying and Reduction Steps. Appl. Catal. A Gen. 1998, 170, 93–103. [Google Scholar] [CrossRef]
- Baer, Y.; Myers, H.P. An XPS Study of the Valence Bands in Solid and Liquid Bismuth. Solid State Commun. 1977, 21, 833–835. [Google Scholar] [CrossRef]
- Dias, L.P.; Correia, F.C.; Ribeiro, J.M.; Tavares, C.J. Photocatalytic Bi2o3/Tio2:N Thin Films with Enhanced Surface Area and Visible Light Activity. Coatings 2020, 10, 445. [Google Scholar] [CrossRef]
- Yan, D.; Wang, G.; Gao, K.; Lu, X.; Xin, J.; Zhang, S. One-Pot Synthesis of 2,5-Furandicarboxylic Acid from Fructose in Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 1851–1858. [Google Scholar] [CrossRef]
- Danielli da Fonseca Ferreira, A.; Dorneles de Mello, M.; da Silva, M.A.P. Catalytic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Ru/Al2O3 in a Trickle-Bed Reactor. Ind. Eng. Chem. Res. 2019, 58, 128–137. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the Mechanism of Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Supported Pt and Au Catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Kerdi, F.; Ait Rass, H.; Pinel, C.; Besson, M.; Peru, G.; Leger, B.; Rio, S.; Monflier, E.; Ponchel, A. Evaluation of Surface Properties and Pore Structure of Carbon on the Activity of Supported Ru Catalysts in the Aqueous-Phase Aerobic Oxidation of HMF to FDCA. Appl. Catal. A Gen. 2015, 506, 206–219. [Google Scholar] [CrossRef]
- Siankevich, S.; Savoglidis, G.; Fei, Z.; Laurenczy, G.; Alexander, D.T.L.; Yan, N.; Dyson, P.J. A Novel Platinum Nanocatalyst for the Oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid under Mild Conditions. J. Catal. 2014, 315, 67–74. [Google Scholar] [CrossRef]
- Miao, Z.Z.; Zhang, Y.B.; Pan, X.Q.; Wu, T.X.; Zhang, B.; Li, J.W.; Yi, T.; Zhang, Z.D.; Yang, X.G. Superior Catalytic Performance of Ce1-xBixO2-δ Solid Solution and Au/Ce1-xBixO2-δ for 5-Hydroxymethylfurfural Conversion in Alkaline Aqueous Solution. Catal. Sci. Technol. 2015, 5, 1314–1322. [Google Scholar] [CrossRef]
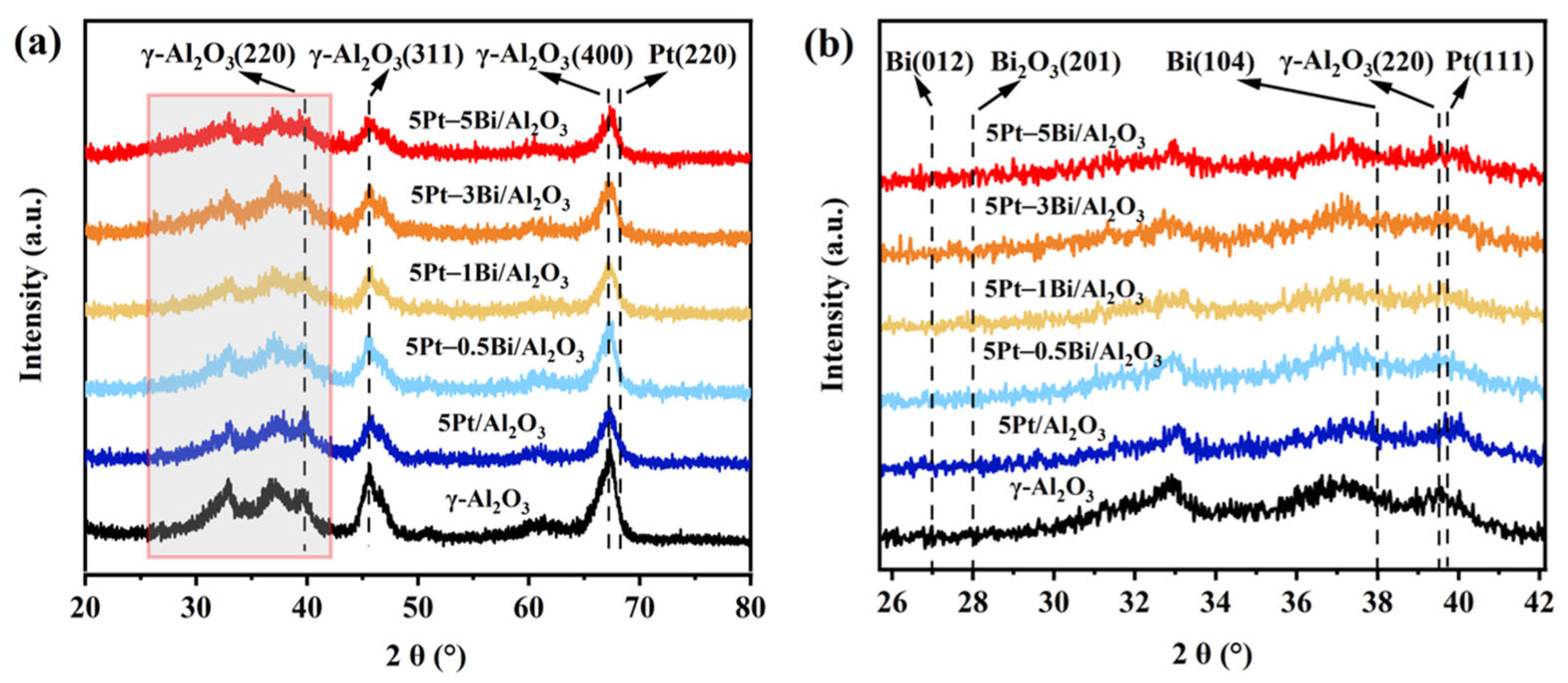

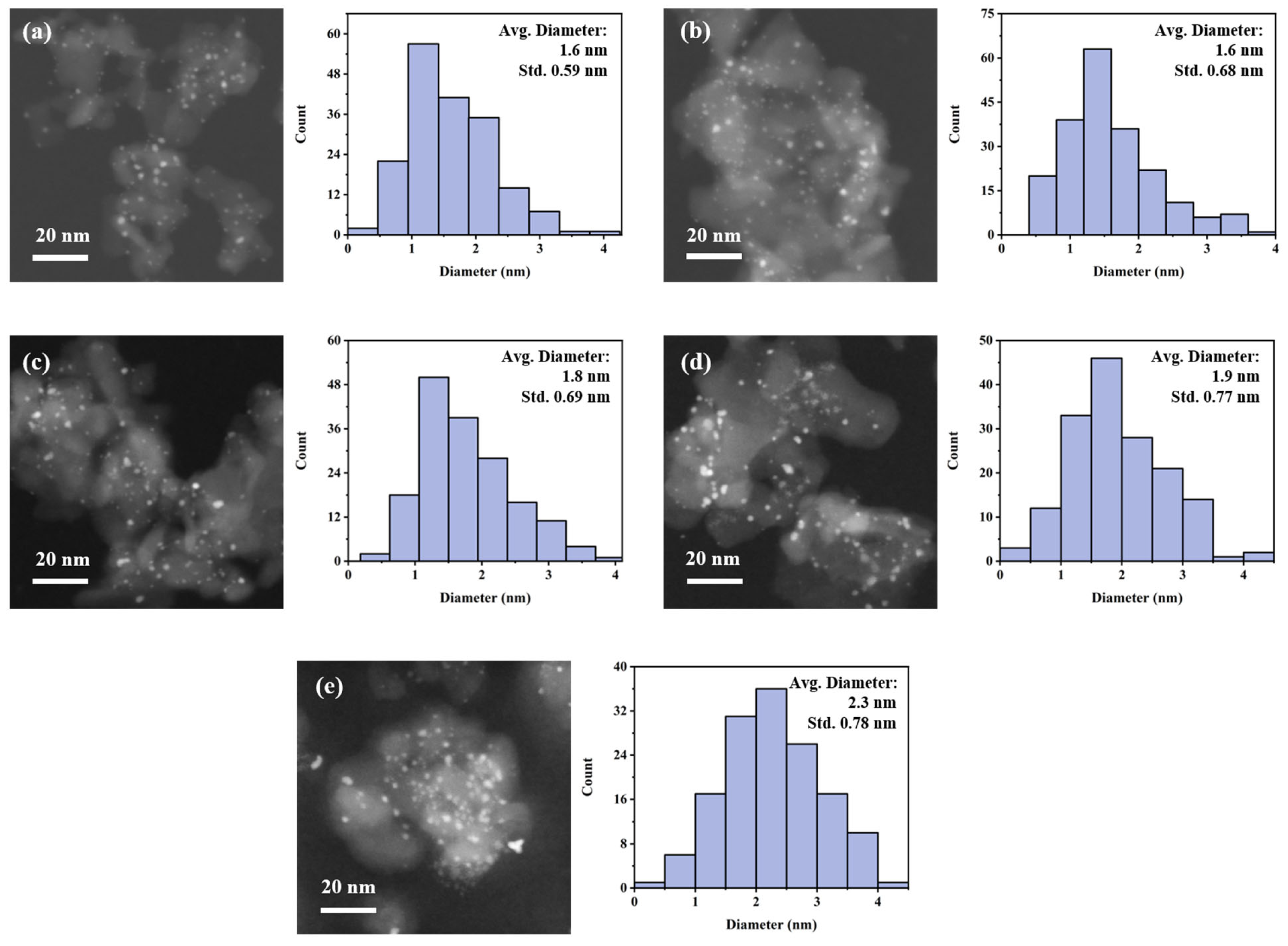

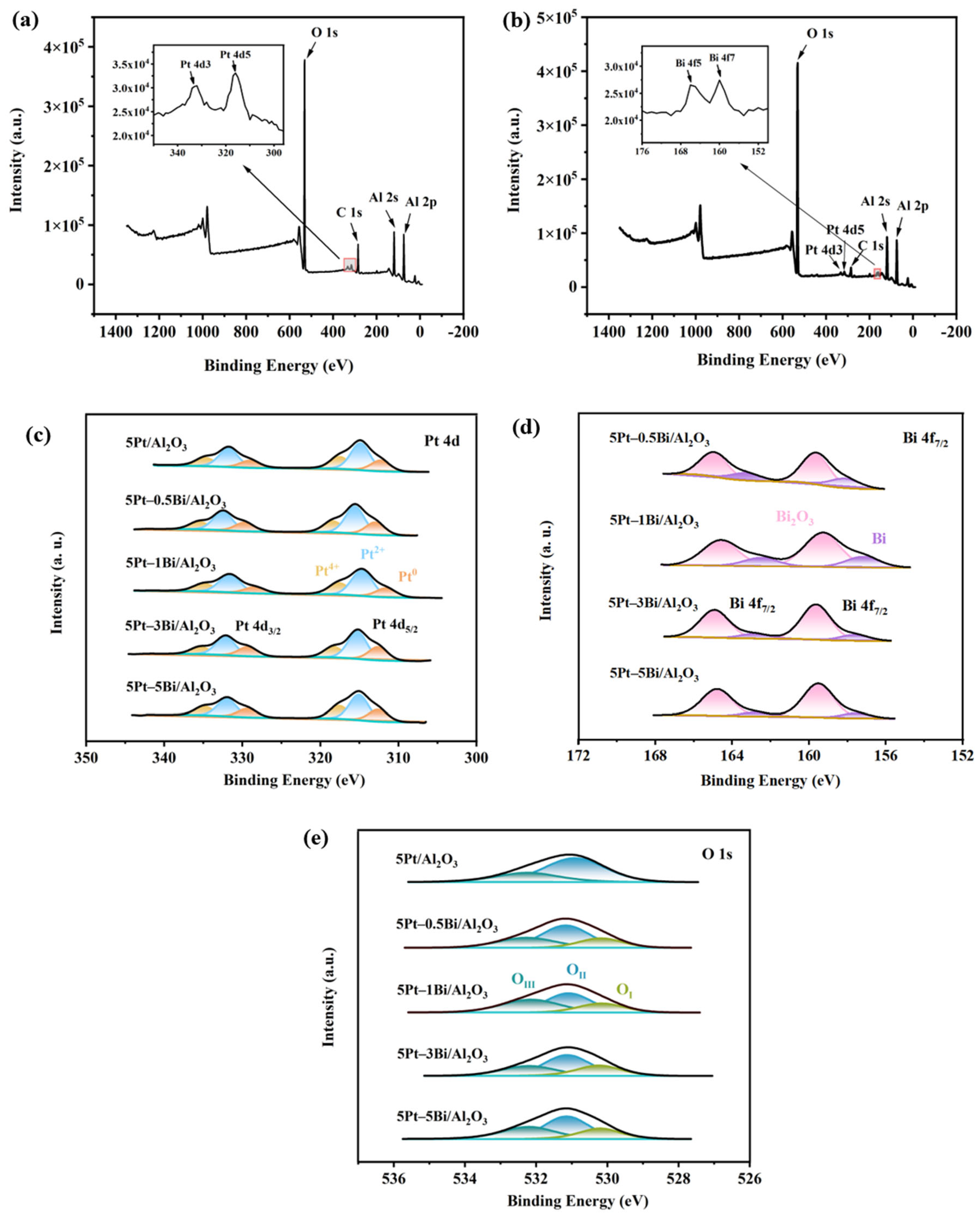




| Samples | d a (nm) | Total Content (wt%) b | DPt c | Pt0/ (Pt2+ + Pt4+) d | Bi/ B2O3 d | SBET e (m2/g) | D e (nm) | V e (cm3/g) | |
|---|---|---|---|---|---|---|---|---|---|
| Pt | Bi | ||||||||
| 5Pt/Al2O3 | 1.6 | 5.4 | - | 48.1% | 30.6% | - | 176.7 | 16.0 | 0.7 |
| 5Pt–0.5Bi/Al2O3 | 1.6 | 4.8 | 0.6 | 44.2% | 32.9% | 34.5% | 146.7 | 14.2 | 0.6 |
| 5Pt–1Bi/Al2O3 | 1.8 | 4.7 | 1.2 | 31.9% | 33.0% | 31.5% | n.d. f | n.d. f | n.d. f |
| 5Pt–3Bi/Al2O3 | 1.9 | 5.9 | 5.7 | 3.8% | 35.4% | 17.1% | 153.9 | 11.6 | 0.5 |
| 5Pt–5Bi/Al2O3 | 2.3 | 5.7 | 7.2 | 0.6% | 17.6% | 14.6% | 135.3 | 12.1 | 0.4 |
| 5Pt–1Bi/Al2O3 g | 3.3 | 4.0 | 1.2 | n.d. f | 44.0% | 54.8% | n.d. f | n.d. f | n.d. f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Qiu, W.; Ayaz, S.; Long, J.; Guo, W.; Zhao, L.; Xi, Z. Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over a Bi-Promoted Pt/Al2O3 Catalyst. Catalysts 2025, 15, 1088. https://doi.org/10.3390/catal15111088
Du J, Qiu W, Ayaz S, Long J, Guo W, Zhao L, Xi Z. Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over a Bi-Promoted Pt/Al2O3 Catalyst. Catalysts. 2025; 15(11):1088. https://doi.org/10.3390/catal15111088
Chicago/Turabian StyleDu, Juan, Wanting Qiu, Sunbal Ayaz, Jian Long, Wenze Guo, Ling Zhao, and Zhenhao Xi. 2025. "Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over a Bi-Promoted Pt/Al2O3 Catalyst" Catalysts 15, no. 11: 1088. https://doi.org/10.3390/catal15111088
APA StyleDu, J., Qiu, W., Ayaz, S., Long, J., Guo, W., Zhao, L., & Xi, Z. (2025). Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over a Bi-Promoted Pt/Al2O3 Catalyst. Catalysts, 15(11), 1088. https://doi.org/10.3390/catal15111088







