Development and Kinetic Study of Novel Denitrification Catalysts Based on C3H6 Reductant
Abstract
1. Introduction
2. Results and Discussion
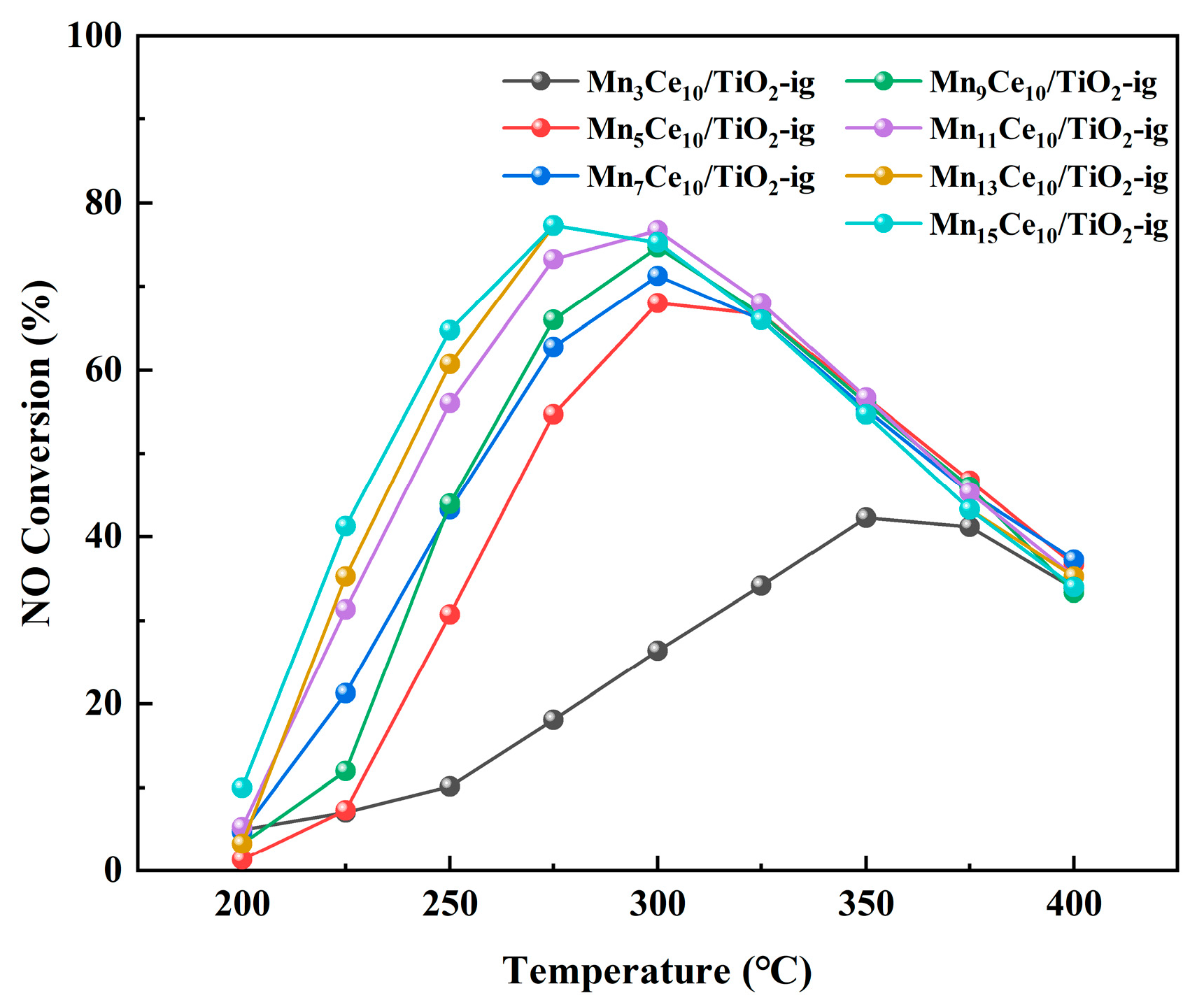
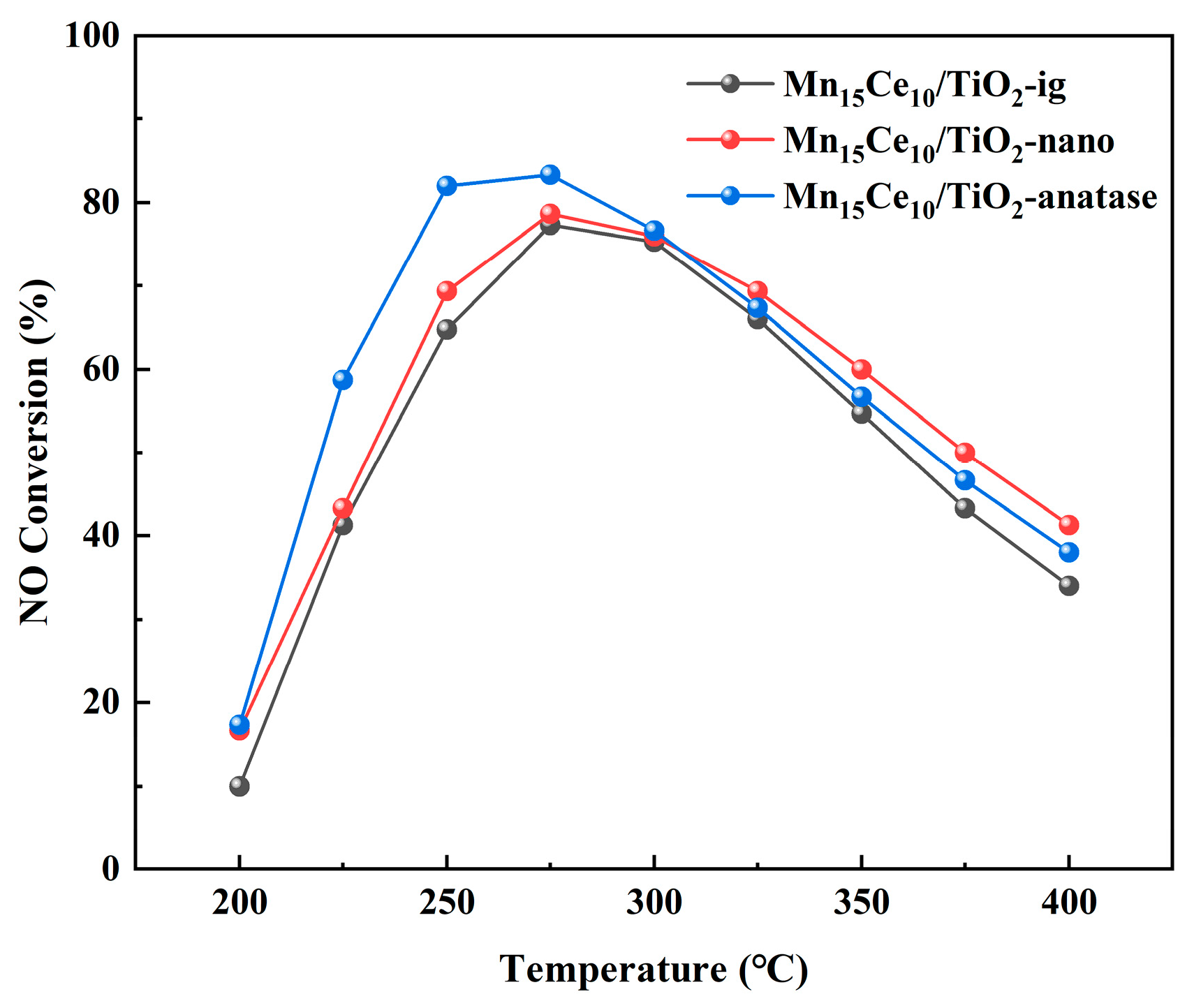
2.1. Catalytic Performance
2.2. Physical Property Analysis
2.2.1. Porosity Structure Analysis
2.2.2. Support Composition Analysis
2.3. Kinetic Study in C3H6-SCR
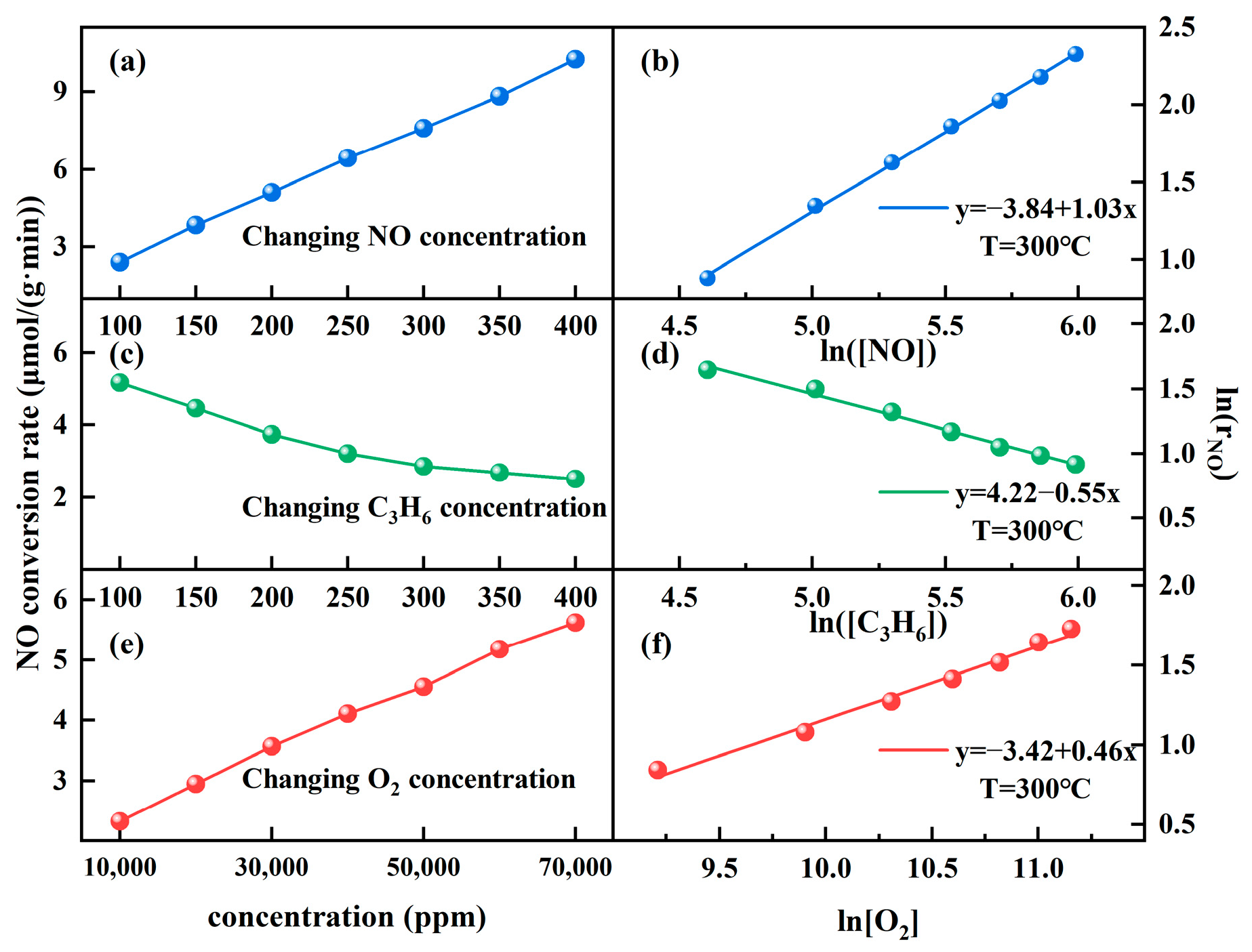
2.3.1. Reaction Order Determination
2.3.2. Determination of the Apparent Activation Energy and the Pre-Exponential Factor
3. Methods
3.1. Materials and Preparation
3.2. Catalytic Activity Test
3.3. Catalysts Characterization
3.4. Steady-State Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lakanen, L.; Grönman, K.; Väisänen, S.; Kasurinen, H.; Soininen, A.; Soukka, R. Applying the handprint approach to assess the air pollutant reduction potential of paraffinic renewable diesel fuel in the car fleet of the city of Helsinki. J. Clean. Prod. 2021, 290, 125786. [Google Scholar] [CrossRef]
- Park, Y.-K.; Kim, B.-S. Catalytic removal of nitrogen oxides (NO, NO2, N2O) from ammonia-fueled combustion exhaust: A review of applicable technologies. Chem. Eng. J. 2023, 461, 141958. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Tian, S.; Zhao, Y.; Long, L.; Yao, X. Mechanistic insights into the influence of preparation methods and Fe3+ doping on the low-temperature performance of MnCeOx catalyst for NH3-SCR reaction. Sep. Purif. Technol. 2024, 347, 127519. [Google Scholar] [CrossRef]
- Guo, K.; Ji, J.; Song, W.; Sun, J.; Tang, C.; Dong, L. Conquering ammonium bisulfate poison over low-temperature NH3-SCR catalysts: A critical review. Appl. Catal. B Environ. 2021, 297, 120388. [Google Scholar] [CrossRef]
- Dai, G.; Liu, B.; Wang, R.; Wang, H.; Miao, Y.; Hou, S.; Lian, D.; Chen, M.; Li, C.; Zhang, Z.; et al. Pretreatment techniques in CO-SCR and NH3-SCR: Status, challenges, and perspectives. J. Catal. 2025, 442, 115925. [Google Scholar] [CrossRef]
- Ye, B.; Jeong, B.; Lee, M.J.; Kim, T.H.; Park, S.S.; Jung, J.; Lee, S.; Kim, H.D. Recent trends in vanadium-based SCR catalysts for NOx reduction in industrial applications: Stationary sources. Nano Converg. 2022, 9, 51. [Google Scholar] [CrossRef]
- Li, X.; Ren, S.; Jiang, Y.; Chen, Z.; Wang, L.; Liu, M.; Chen, T. In situ IR spectroscopy study of NO removal over CuCe catalyst for CO-SCR reaction at different temperature. Catal. Today 2023, 418, 114082. [Google Scholar] [CrossRef]
- Muhammad Farhan, S.; Pan, W.; Zhijian, C.; JianJun, Y. Innovative catalysts for the selective catalytic reduction of NOx with H2: A systematic review. Fuel 2024, 355, 129364. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, L.; Gao, S.; Li, X. New aspects on a low-medium temperature mechanism of H2-assisted C3H6-SCR over xAg-CeZr catalyst. Fuel 2021, 305, 121574. [Google Scholar] [CrossRef]
- Liu, Q.; Kashif, M.; Deng, W.; Zhao, B.; Heynderickx, P.M.; Su, Y. Exploring the role of Cu/Mn-BTC catalyst in the selective catalytic reduction of NOx by C3H6: Synthesis to in-situ DRIFTS study. Fuel 2024, 367, 131508. [Google Scholar] [CrossRef]
- Liu, Z.; An, Y.; Xu, G.; Yu, Y.; He, H. Insight into the Promotion Effect of Trace Pd Doping on the Catalytic Performance of Ag/Al2O3 for C3H6-SCR of NOx. Environ. Sci. Technol. 2023, 57, 14760–14767. [Google Scholar] [CrossRef]
- Hernández-Terán, M.E.; López Curiel, J.C.; Fuentes, G.A. Study of the Reversibility of the H2 Effect Over Ag/γ-Al2O3 Catalyst During Selective Catalytic Reduction (SCR) of NOx by Propane. Top. Catal. 2022, 65, 1505–1515. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, M.; Jiang, Z.; Shangguan, W. Performance and mechanism study for low-temperature SCR of NO with propylene in excess oxygen over Pt/TiO2 catalyst. J. Environ. Sci. 2010, 22, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Gunes, H.; Şanlı Yıldız, D.; Özener, B.; Hisar, G.; Rommel, S.; Aindow, M.; Bozbağ, S.E.; Erkey, C. Preparation of Pt/Al2O3 and PtPd/Al2O3 catalysts by supercritical deposition and their performance for oxidation of nitric oxide and propene. Catal. Today 2022, 388–389, 70–78. [Google Scholar] [CrossRef]
- Yang, L.; Yang, H.; Liu, Q.; Liu, F.; Chen, N. Study on the Reaction Performance of Ce- and Co-Modified Mn-Based Catalysts in C3H6-SCR. J. Phys. Chem. C 2023, 127, 15278–15289. [Google Scholar] [CrossRef]
- Li, N.; Zhang, T.; Wu, Z.; Li, J.; Wang, W.; Zhu, J.; Yao, S.; Gao, E. Rationally tailored redox ability of Sn/γ-Al2O3 with Ag for enhancing the selective catalytic reduction of NOx with propene. RSC Adv. 2023, 13, 1738–1750. [Google Scholar] [CrossRef]
- La Greca, E.; Kharlamova, T.S.; Grabchenko, M.V.; Consentino, L.; Savenko, D.Y.; Pantaleo, G.; Kibis, L.S.; Stonkus, O.A.; Vodyankina, O.V.; Liotta, L.F. Ag Catalysts Supported on CeO2, MnO2 and CeMnOx Mixed Oxides for Selective Catalytic Reduction of NO by C3H6. Nanomaterials 2023, 13, 873. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Yuan, S.; Zhu, X.; Sun, C.; Liu, H.; Chen, D.-Z.; Huang, F.; Dong, L. d-π Orbital Interaction Promoting NOx Selective Reduction on the Mn-Doped α-Fe2O3(001) Catalyst. Environ. Sci. Technol. 2025, 59, 4036–4046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhong, Q.; Wang, Y. Effect of rutile phase on V2O5 supported over TiO2 mixed phase for the selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2014, 314, 112–118. [Google Scholar] [CrossRef]
- Tang, T.; Du, X.; Chen, Y.; Xue, J.; Chen, K.; Li, X. Study on the mechanism of selective catalytic reduction with propylene (C3H6-SCR) on the MnOx-based catalysts by a doping experiment and DFT+U calculation. J. Environ. Chem. Eng. 2023, 11, 109831. [Google Scholar] [CrossRef]
- Chen, J.; Fu, W.; Cai, C.; Ning, S.; Kashif, M.; Deng, W.; Zhao, B.; Su, Y. Activity of CuCoCe layered double hydroxides catalysts and mechanism for C3H6-SCR. Fuel 2024, 369, 131789. [Google Scholar] [CrossRef]
- Feng, B.; Zhao, T.; Du, J.; Hu, J.; Shi, Y.; Zhao, J.; Chen, J. Reaction and deactivation mechanisms of a CeIn/HBEA catalyst with dual active sites for selective catalytic reduction of NOx by CH4. Appl. Catal. B Environ. Energy 2024, 358, 124343. [Google Scholar] [CrossRef]
- Kashif, M.; Khan, M.N.; Su, Y.; Heynderickx, P.M. Most efficient mesoporous Mn/Ga-PCH catalyst for low-temperature selective catalytic reduction of NO with C3H6. Vacuum 2022, 198, 110879. [Google Scholar] [CrossRef]
- Chen, X.; Gao, P.; Huang, L.; Hu, Y.; Wang, J.; Liu, Z.; Shen, Y. Ultra-low temperature selective catalytic reduction of NOx into N2 by micron spherical CeMnOx in high-humidity atmospheres containing SO2. Appl. Catal. B Environ. Energy 2025, 360, 124552. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Xing, L.; Liang, Y.; Li, H.; Liu, M. Effect of Cu loading on the performance and kinetics of Cu/SAPO-34 catalysts for selective catalytic reduction with NH3. Environ. Sci. Pollut. Res. 2023, 30, 64682–64699. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- Qin, C.; Ruan, S.; Xu, K.; He, C.; Shi, Y.; Feng, B.; Zhang, L. Theoretical study on the reaction kinetics of CO oxidation by nitrogen-doped graphene catalysts with different ligand structures. Mol. Catal. 2023, 541, 113103. [Google Scholar] [CrossRef]
- Tan, S.; Yao, Z.; Huang, H.; Liu, F.; Liu, Z.; Wang, X. Mn-Ce Oxide Nanoparticles Supported on Nitrogen-Doped Graphene for Low-Temperature Catalytic Reduction of NOx: De-Nitration Characteristics and Kinetics. Crystals 2023, 13, 313. [Google Scholar] [CrossRef]
- Yu, B.; Liu, X.; Wu, S.; Yang, H.; Zhou, S.; Yang, L.; Liu, F. Study on Novel SCR Catalysts for Denitration of High Concentrated Nitrogen Oxides and Their Reaction Mechanisms. Catalysts 2024, 14, 406. [Google Scholar] [CrossRef]
- Obalová, L.; Klegova, A.; Matějová, L.; Pacultová, K.; Fridrichová, D. Must the Best Laboratory Prepared Catalyst Also Be the Best in an Operational Application? Catalysts 2019, 9, 160. [Google Scholar] [CrossRef]
- Mbatha, S.; Thomas, S.; Parkhomenko, K.; Roger, A.-C.; Louis, B.; Cui, X.; Everson, R.; Langmi, H.; Musyoka, N.; Ren, J. Development of an Improved Kinetic Model for CO2 Hydrogenation to Methanol. Catalysts 2023, 13, 1349. [Google Scholar] [CrossRef]
- Li, Z.; Shen, L.-T.; Huang, W.; Xie, K.-C. Kinetics of selective catalytic reduction of NO by NH3 on Fe-Mo/ZSM-5 catalyst. J. Environ. Sci. 2007, 19, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Long, R.Q.; Yang, R.T. Kinetics of selective catalytic reduction of NO with NH3 on Fe-ZSM-5 catalyst. Appl. Catal. A Gen. 2002, 235, 241–251. [Google Scholar] [CrossRef]

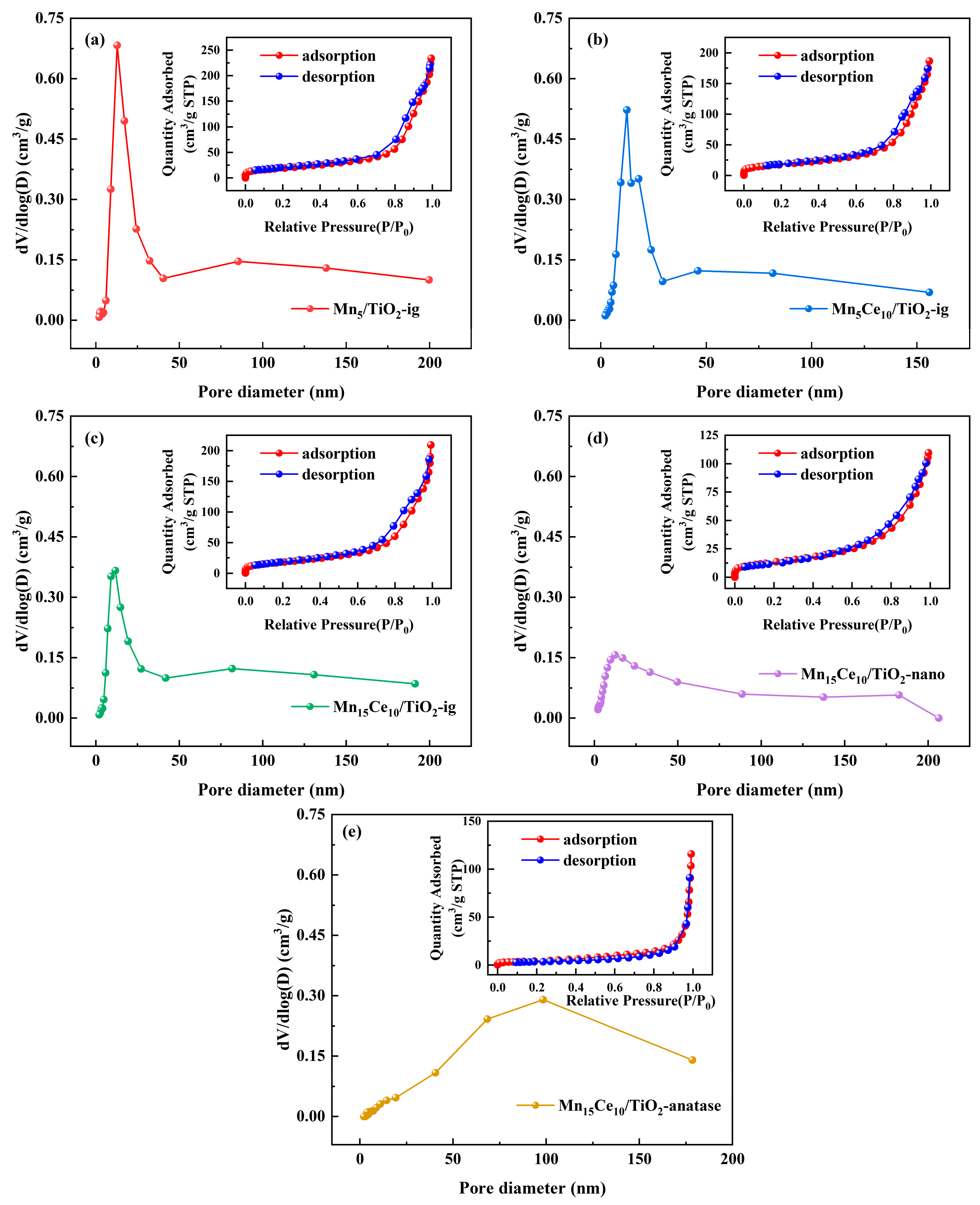




| Catalysts | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Mn5/TiO2-ig | 68.57 | 0.36 | 15.93 |
| Mn5Ce10/TiO2-ig | 63.03 | 0.29 | 14.06 |
| Mn15Ce10/TiO2-ig | 56.9 | 0.28 | 14.2 |
| Mn15Ce10/TiO2-nano | 48.66 | 0.17 | 10.76 |
| Mn15Ce10/TiO2-anatase | 16.84 | 0.18 | 42.61 |
| Components | Content (%) |
|---|---|
| TiO2 | 98.237 |
| SO3 | 1.312 |
| P2O5 | 0.164 |
| Cl | 0.080 |
| CaO | 0.070 |
| Nb2O5 | 0.060 |
| SiO2 | 0.058 |
| ZrO2 | 0.010 |
| SrO | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Ning, J.; Liu, X.; Liu, X.; Xie, S.; Liu, J.; Pu, X.; Yu, B.; Yang, L.; Liu, F. Development and Kinetic Study of Novel Denitrification Catalysts Based on C3H6 Reductant. Catalysts 2025, 15, 1087. https://doi.org/10.3390/catal15111087
Tang Z, Ning J, Liu X, Liu X, Xie S, Liu J, Pu X, Yu B, Yang L, Liu F. Development and Kinetic Study of Novel Denitrification Catalysts Based on C3H6 Reductant. Catalysts. 2025; 15(11):1087. https://doi.org/10.3390/catal15111087
Chicago/Turabian StyleTang, Zhonghua, Jingshu Ning, Xingyu Liu, Xingyu Liu, Shugang Xie, Junqiang Liu, Xin Pu, Bo Yu, Li Yang, and Fang Liu. 2025. "Development and Kinetic Study of Novel Denitrification Catalysts Based on C3H6 Reductant" Catalysts 15, no. 11: 1087. https://doi.org/10.3390/catal15111087
APA StyleTang, Z., Ning, J., Liu, X., Liu, X., Xie, S., Liu, J., Pu, X., Yu, B., Yang, L., & Liu, F. (2025). Development and Kinetic Study of Novel Denitrification Catalysts Based on C3H6 Reductant. Catalysts, 15(11), 1087. https://doi.org/10.3390/catal15111087





