Ru-Modified α-MnO2 as an Efficient PMS Activator for Carbamazepine Degradation: Performance and Mechanism
Abstract
1. Introduction
2. Results and Discussion
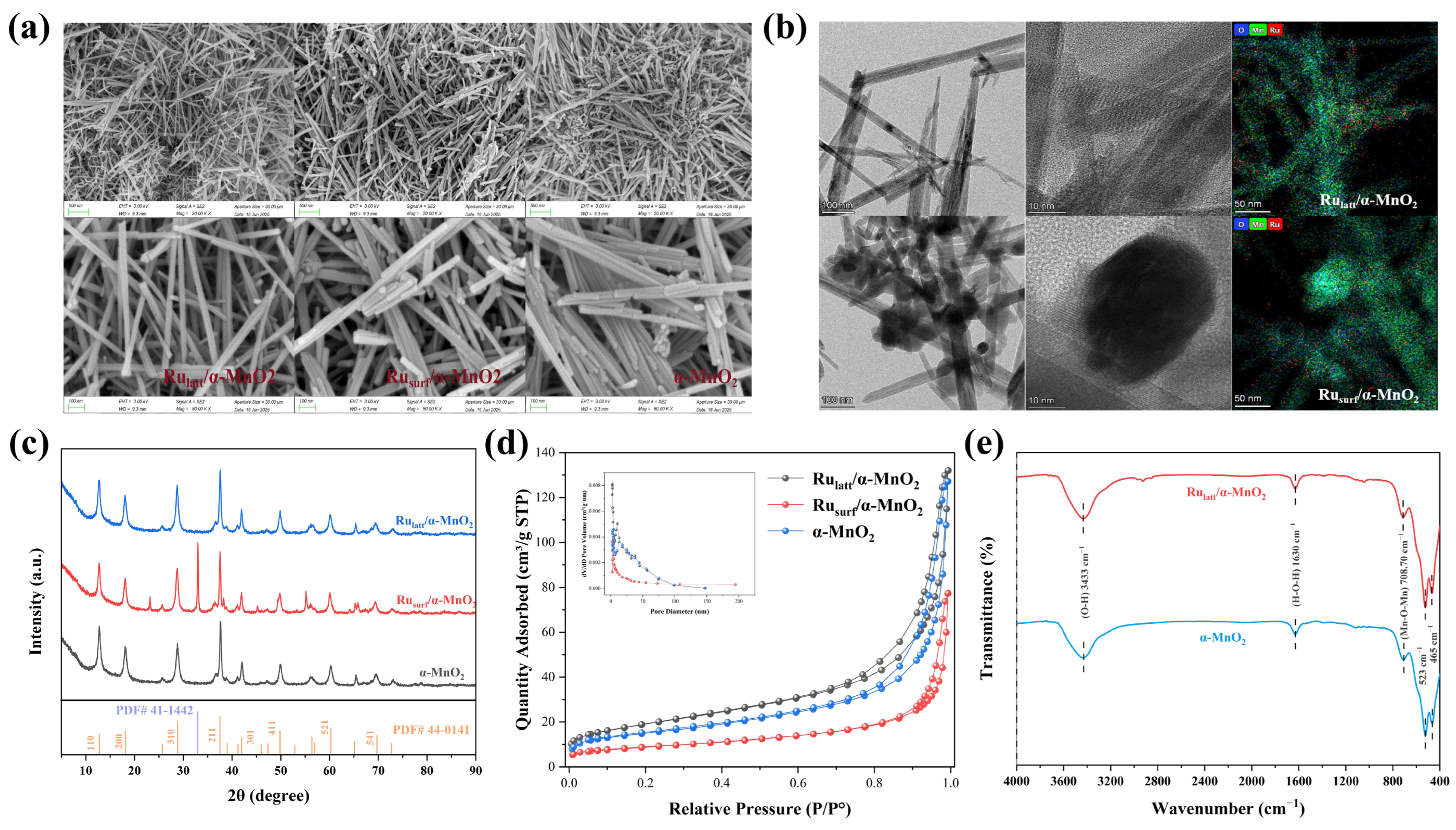
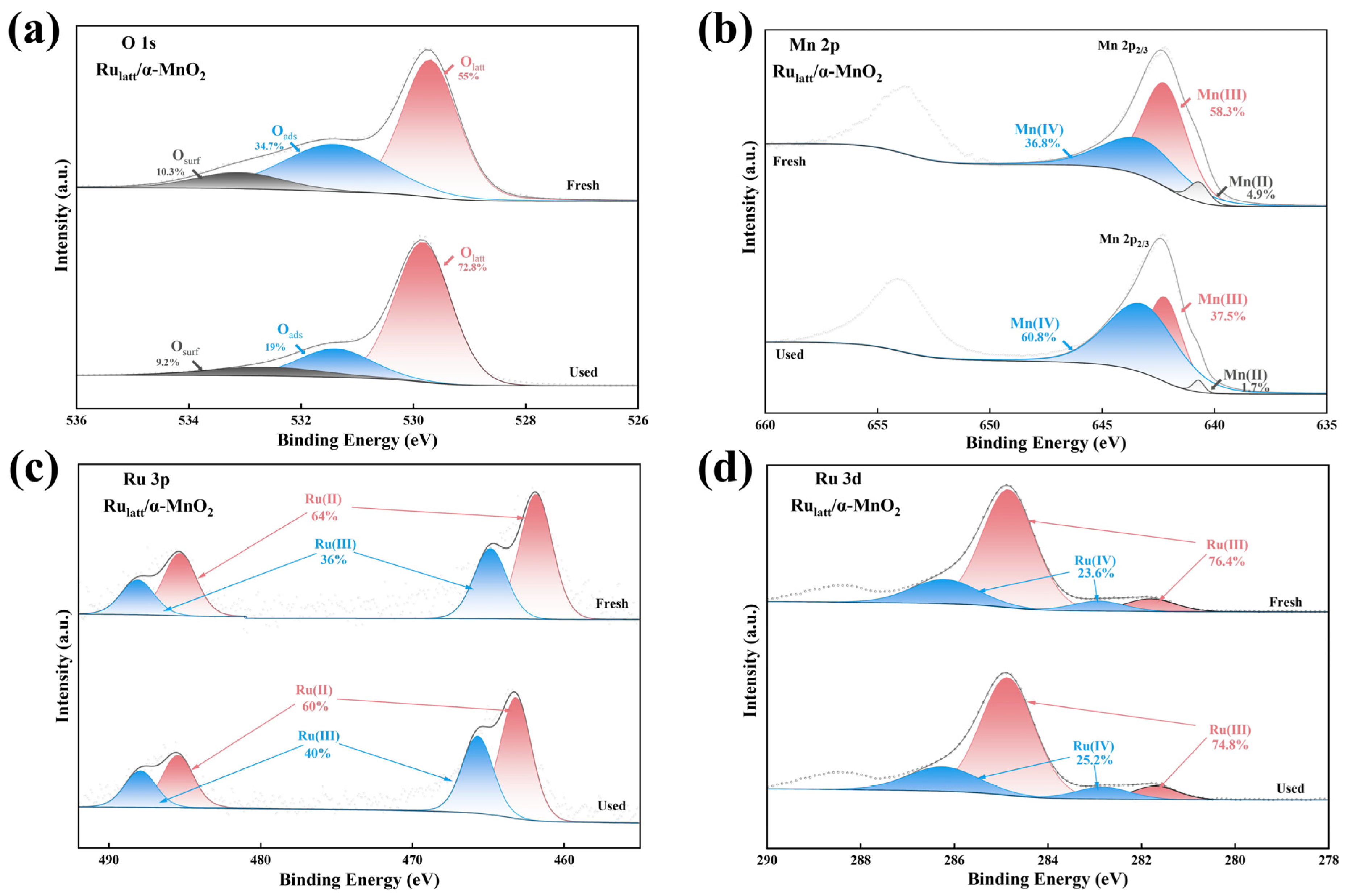
2.1. Catalyst Characterization
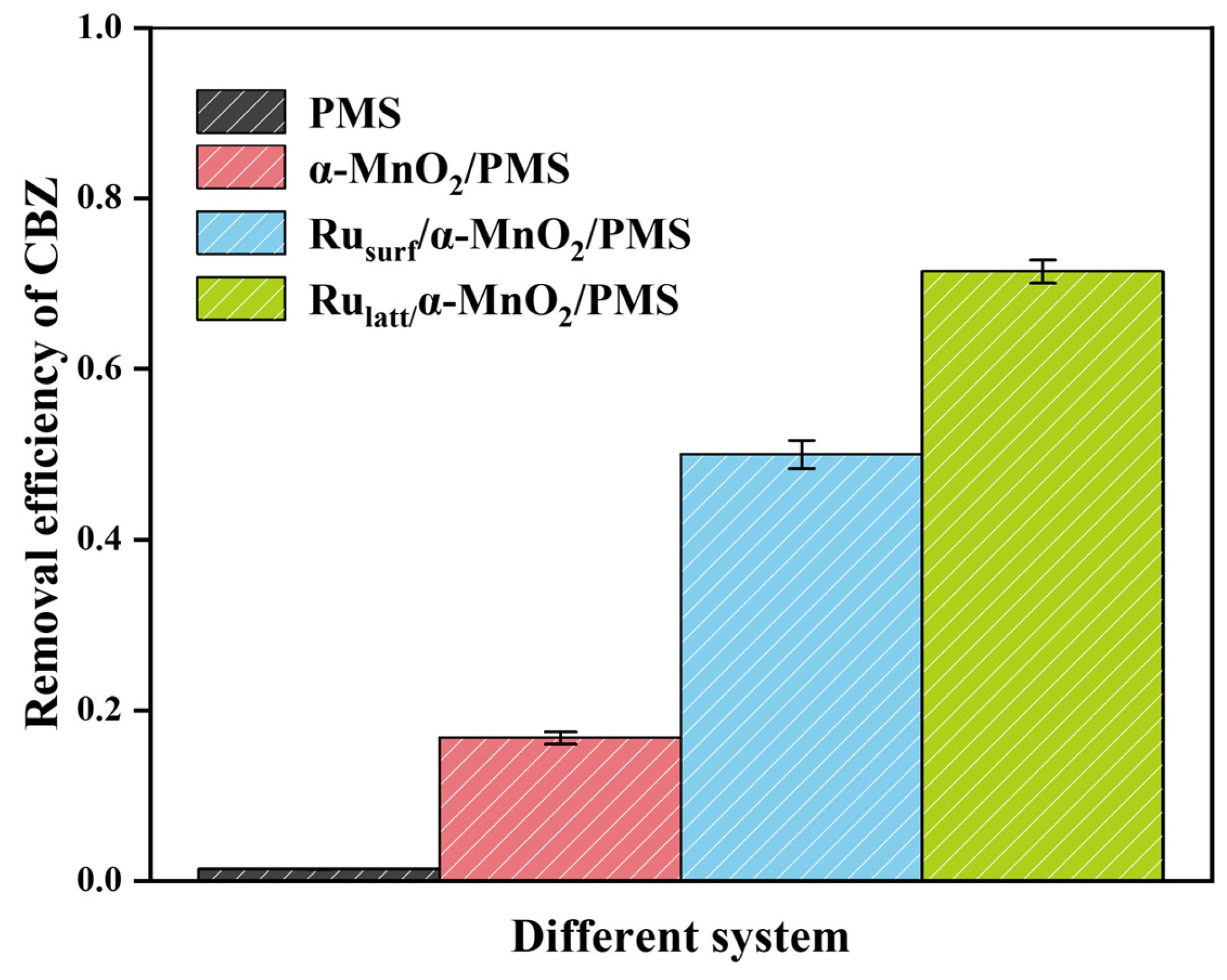
2.2. Evaluation of CBZ Degradation Efficiency Across Different Systems
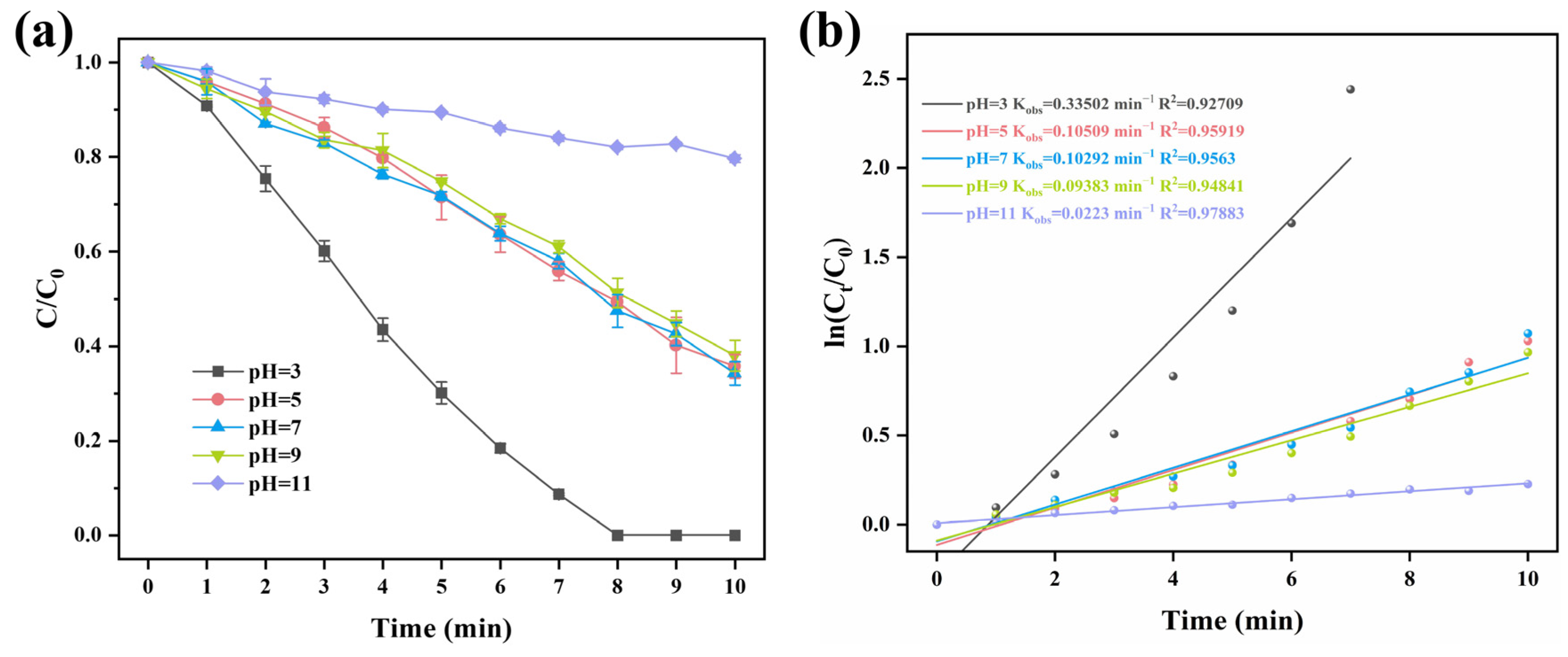
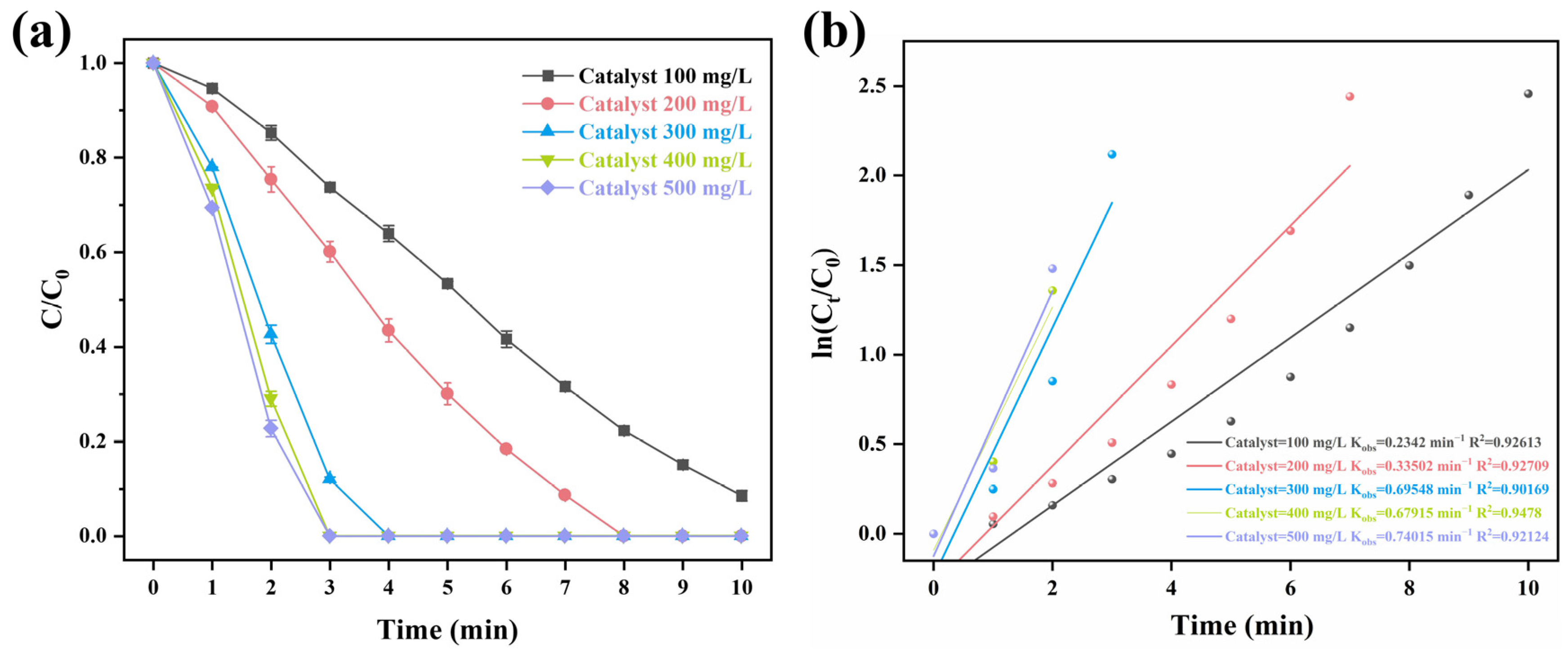
2.3. The Influence of Several Reaction Parameters on the Degradation of Carbamazepine
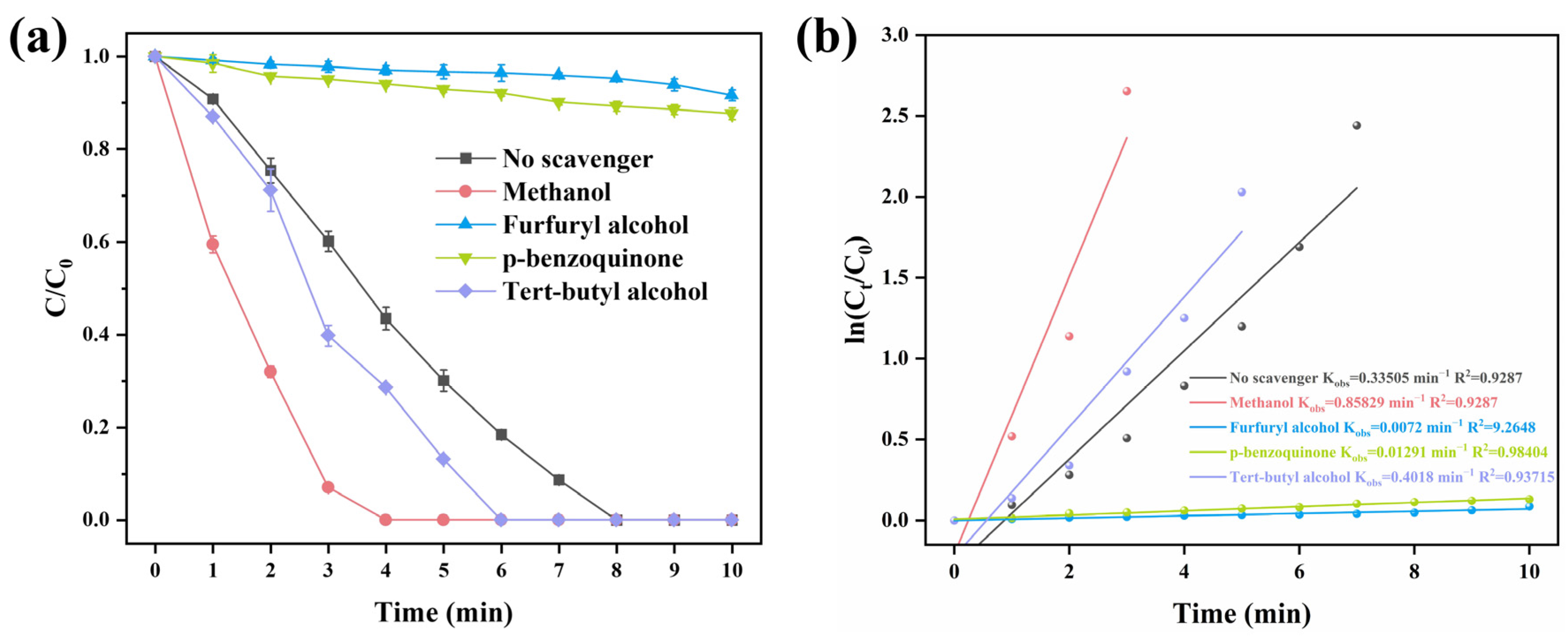
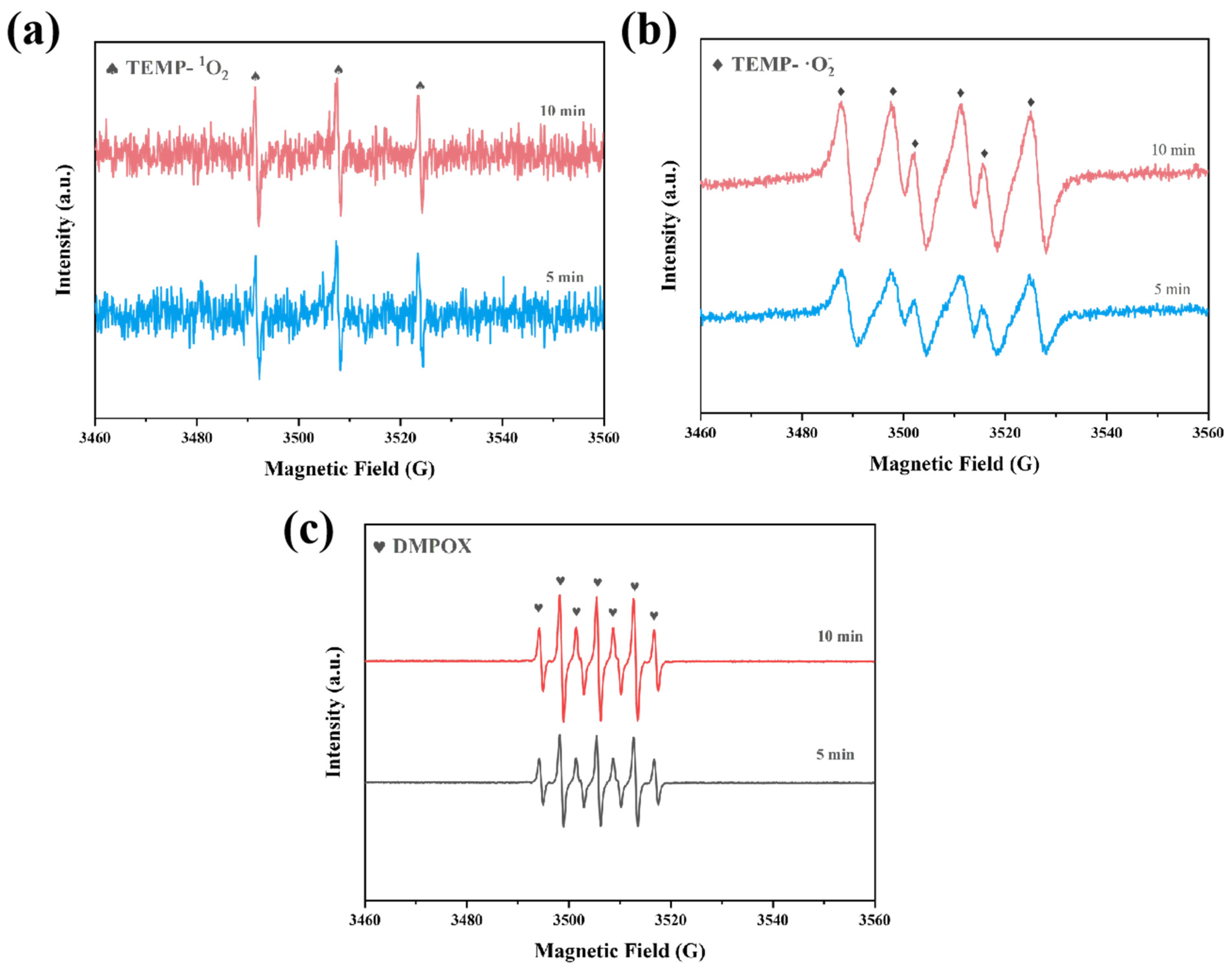
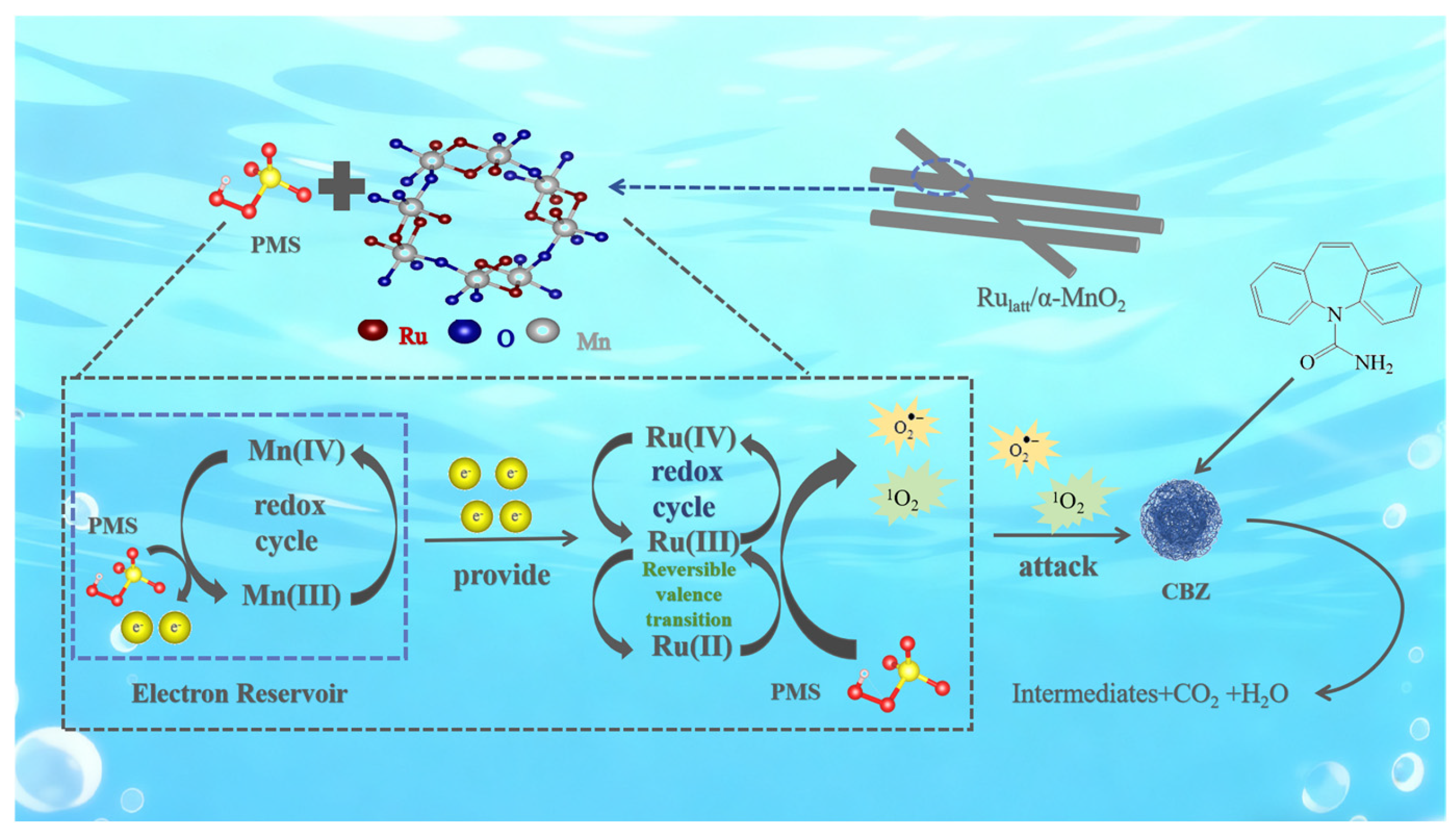
2.4. Catalytic Mechanism of Rulatt/α-MnO2
Identification of Reactive Species
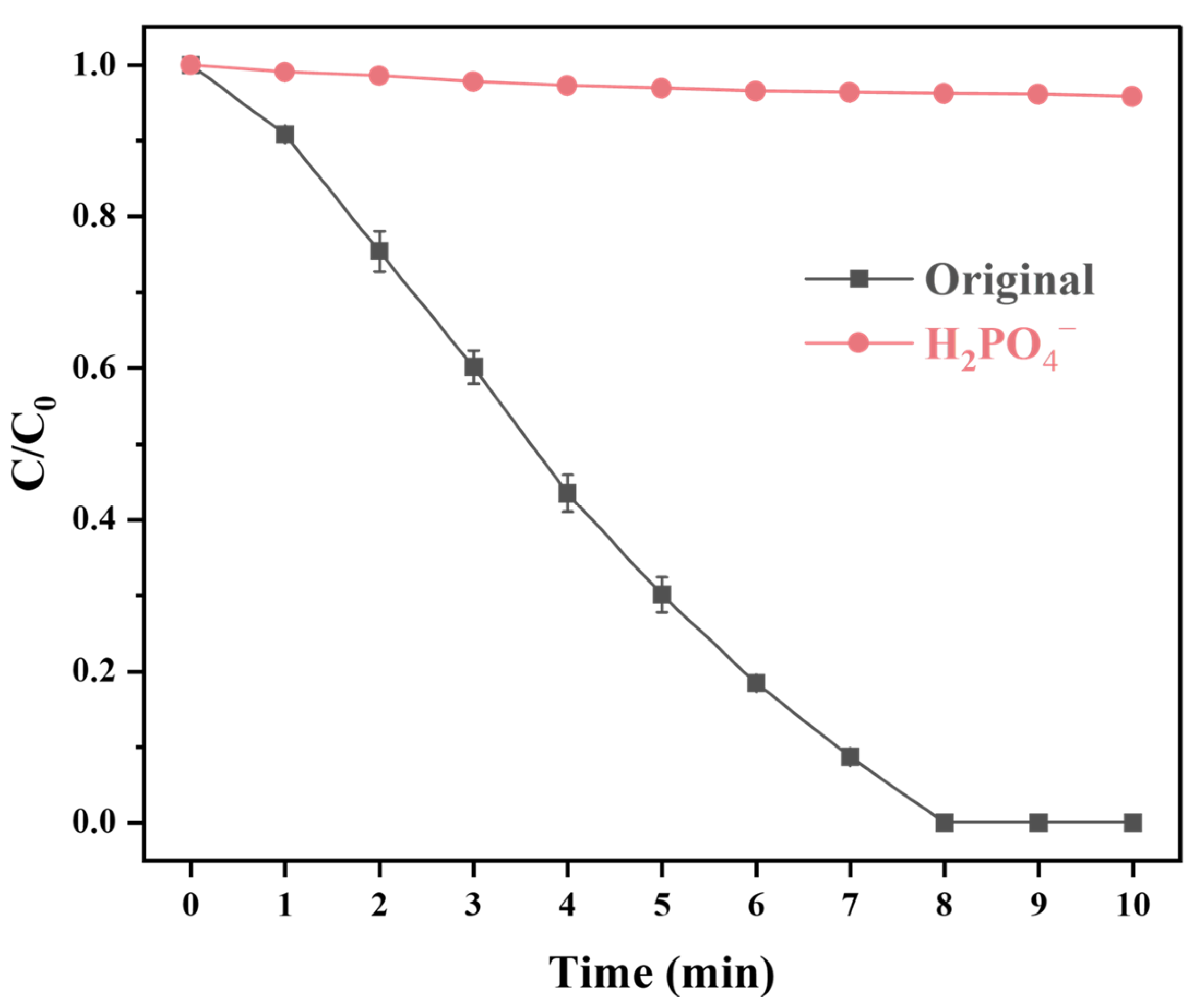
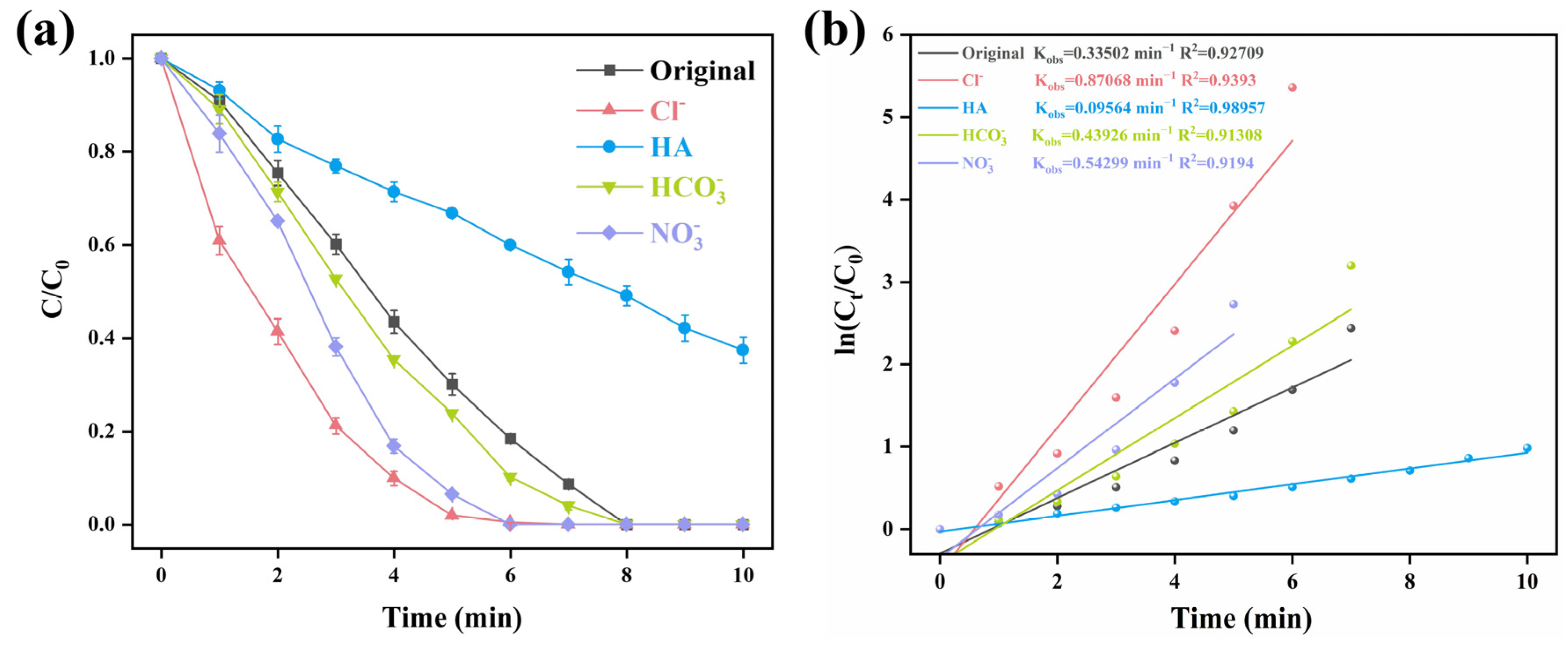
2.5. The Influence of Coexisting Anions and Natural Organic Matter
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Materials
3.3. Sample Characterizations
3.4. Test Apparatus and Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kujawska, A.; Kielkowska, U.; Atisha, A.; Yanful, E.; Kujawski, W. Comparative analysis of separation methods used for the elimination of pharmaceuticals and personal care products (PPCPs) from water-A critical review. Sep. Purif. Technol. 2022, 290, 36. [Google Scholar] [CrossRef]
- Wu, M.H.; Xiang, J.J.; Que, C.J.; Chen, F.F.; Xu, G. Occurrence and fate of psychiatric pharmaceuticals in the urban water system of Shanghai, China. Chemosphere 2015, 138, 486–493. [Google Scholar] [CrossRef]
- Gabet, A.; Guy, C.; Fazli, A.; Métivier, H.; de Brauer, C.; Brigante, M.; Mailhot, G. The ability of recycled magnetite nanoparticles to degrade carbamazepine in water through photo-Fenton oxidation at neutral pH. Sep. Purif. Technol. 2023, 317, 123877. [Google Scholar] [CrossRef]
- Alkahtani, M.Q.; Morabet, R.E.; Khan, R.A.; Khan, A.R. Pharmaceuticals removal from hospital wastewater by fluid-ized aerobic bioreactor in combination with tubesettler. Sci. Rep. 2024, 14, 24052. [Google Scholar] [CrossRef] [PubMed]
- Trognon, J.; Albasi, C.; Choubert, J.M. A critical review on the pathways of carbamazepine transformation products in oxidative wastewater treatment processes. J. Sci. Total Environ. 2024, 912, 169040. [Google Scholar] [CrossRef] [PubMed]
- Feijoo, S.; Kamali, M.; Dewil, R. A review of wastewater treatment technologies for the degradation of pharma-ceutically active compounds: Carbamazepine as a case study. J. Chem. Eng. J. 2023, 455, 140589. [Google Scholar] [CrossRef]
- Peng, Y.T.; Tang, H.M.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y.Y. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Dai, C.; You, X.; Liu, Q.; Han, Y.; Duan, Y.; Hu, J.; Li, J.; Li, Z.; Zhou, L.; Zhang, Y.; et al. Peroxymonosulfate activation by Ru/CeO2 for degradation of Triclosan: Efficacy, mechanisms and applicability in groundwater. Chem. Eng. J. 2023, 463, 142479. [Google Scholar] [CrossRef]
- Pan, Y.J.; Meng, F.Y.; Bai, J.X.; Song, B.; Cao, Q. Highly efficient peroxymonosulfate activation by CoFe2O4@attapulgite-biochar composites: Degradation properties and mechanism insights. J. Environ. Chem. Eng. 2024, 12, 112579. [Google Scholar] [CrossRef]
- Gao, P.; Fan, X.; Su, Y.; Wei, H.; Yang, C.; Wang, Q. Efficient peroxymonosulfate activation for sulfamethoxazole degradation by CoFe2O4 decorated mesoporous silica through a simple one-step grinding-calcination method. J. Environ. Chem. Eng. 2025, 13, 116890. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhu, M.S.; Dionysiou, D.D. What is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. 2021, 189, 116627. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, C.H.; Hu, J. Ultrasonic degradation of tetracycline combining peroxymonosulfate and BiVO4 microspheres. J. Water Process. Eng. 2023, 56, 104428. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, X.K.; Yin, Y.C.; Liu, Y.; Li, Y.; Hu, X.Y.; Zhang, L.; Zhang, X.W. Accelerated alkaline activation of peroxydisulfate by reduced rubidium tungstate nanorods for enhanced degradation of bisphenol A. Environ. Sci.-Nano 2020, 7, 3547–3556. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Lin, L.; Liu, S.S.; Chen, Y.Q.; Daniels, S.V.; Xu, Z.J.; Chen, Z.H.; Li, H.T.; Wu, Y.Q.; Guo, L.L.; et al. Unveiling the versatile performance of transition metal sulfides in peroxymonosulfate activation. Chem. Eng. J. 2024, 497, 154682. [Google Scholar] [CrossRef]
- Tian, H.R.; Cui, K.P.; Chen, X.; Liu, J.; Zhang, Q. Size-matched hierarchical porous carbon materials anchoring single-atom Fe-N4 sites for PMS activation: An in-depth study of key active species and catalytic mechanisms. J. Hazard. Mater. 2024, 461, 132647. [Google Scholar] [CrossRef]
- Dai, C.M.; Huang, X.Y.; Liu, Q.; You, X.J.; Duan, Y.P.; Li, J.X.; Hu, J.J.; Zhang, Y.L.; Liu, S.G.; Fu, R.B. Peroxymonosulfate activation by ruthenium in homogeneous systems for degradation of triclosan: Comparison between Ru(II) and Ru(III). Sep. Purif. Technol. 2024, 332, 125820. [Google Scholar] [CrossRef]
- Chen, M.T.; Zhu, L.H.; Liu, S.G.; Li, R.; Wang, N.; Tang, H.Q. Efficient degradation of organic pollutants by low-level Co2+ catalyzed homogeneous activation of peroxymonosulfate. J. Hazard. Mater. 2019, 371, 456–462. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, S.S.; Xu, Y.X.; Xue, H.G.; Pang, H. Ruthenium based materials as electrode materials for supercapacitors. Chem. Eng. J. 2018, 333, 505–518. [Google Scholar] [CrossRef]
- Zell, T.; Langer, R. From Ruthenium to Iron and ManganeseA Mechanistic View on Challenges and Design Principles of Base-Metal Hydrogenation Catalysts. ChemCatChem 2018, 10, 1930–1940. [Google Scholar] [CrossRef]
- Bae, S.Y.; Mahmood, J.; Jeon, I.Y.; Baek, J.B. Recent advances in ruthenium-based electrocatalysts for the hydrogen evolution reaction. Nanoscale Horiz. 2020, 5, 43–56. [Google Scholar] [CrossRef]
- Sushma; Kumari, M.; Saroha, A.K. Performance of various catalysts on treatment of refractory pollutants in industrial wastewater by catalytic wet air oxidation: A review. J. Environ. Manag. 2018, 228, 169–188. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, Q.; Shang, Q.; Ai, J.; Yang, X.; Wang, D.; Liao, G. Ru doped graphitic carbon nitride mediated peroxymonosulfate activation for diclofenac degradation via singlet oxygen. Chem. Eng. J. 2022, 430, 133174. [Google Scholar] [CrossRef]
- Pereira Lopes, R.; Astruc, D. Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, M.; Shi, L.; Zhang, S.; Hirani, R.A.K.; Zhu, C.; Chen, C.; Yuan, A.; Duan, X.; Wang, S.; et al. Highly dispersive Ru confined in porous ultrathin g-C3N4 nanosheets as an efficient peroxymonosulfate activator for removal of organic pollutants. J. Hazard. Mater. 2022, 435, 128939. [Google Scholar] [CrossRef]
- Lin, B.Y.; Wu, Y.Y.; Fang, B.Y.; Li, C.Y.; Ni, J.; Wang, X.Y.; Lin, J.X.; Jiang, L.L. Ru surface density effect on ammonia synthesis activity and hydrogen poisoning of ceria-supported Ru catalysts. Chin. J. Catal. 2021, 42, 1712–1723. [Google Scholar] [CrossRef]
- Luo, X.; Liang, H.; Qu, F.; Ding, A.; Cheng, X.; Tang, C.Y.; Li, G. Free-standing hierarchical alpha-MnO2@CuO membrane for catalytic filtration degradation of organic pollutants. Chemosphere 2018, 200, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.X.; Zhao, J.J.; Rahaman, M.H.; Zhu, M.; Zhai, J. Enhanced peroxymonosulfate activation for carbamazepine degradation under strongly alkaline conditions using Cu-doped Mn3O4 catalyst: Characterization, catalytic performance, and mechanism insights. J. Clean. Prod. 2023, 429, 139600. [Google Scholar] [CrossRef]
- He, C.; Liao, Y.H.; Chen, C.; Xia, D.H.; Wang, Y.Y.; Tian, S.H.; Yang, J.L.; Shu, D. Realizing a redox-robust Ag/MnO2 catalyst for efficient wet catalytic ozonation of S-VOCs: Promotional role of Ag(0)/Ag(I)-Mn based redox shuttle. Appl. Catal. B-Environ. Energy 2022, 303, 120881. [Google Scholar] [CrossRef]
- Boyom-Tatchemo, F.W.; Devred, F.; Ndiffo-Yemeli, G.; Laminsi, S.; Gaigneaux, E.M. Plasma-induced redox reactions synthesis of nanosized α-, γ- and δ-MnO2 catalysts for dye degradation. Appl. Catal. B-Environ. 2020, 260, 118159. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, X.; Zhao, Q.; Jiang, W.; Wang, J.; He, L.; Ma, Y.; Yang, L.; Chen, Z. Understanding the nonradical activation of peroxymonosulfate by different crystallographic MnO2: The pivotal role of Mn(III) content on the surface. J. Hazard. Mater. 2022, 439, 129613. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.-H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230, 115877. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Guan, X.H.; Wang, H.; Bao, H.L.; Huang, Y.Y.; Qiao, J.L.; Zhou, G.M. Ruthenium Nanoparticles Supported on CeO2 for Catalytic Permanganate Oxidation of Butylparaben. Environ. Sci. Technol. 2013, 47, 13011–13019. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, Z.Q.; Cullen, D.A.; Hu, W.H.; Huang, J.E.; Yao, L.B.; Peng, Z.M.; Liao, P.L.; Wang, R.G. Distribution and Valence State of Ru Species on CeO2 Supports: Support Shape Effect and Its Influence on CO Oxidation. ACS Catal. 2019, 9, 11088–11103. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Mahanty, S. Interconnected Network of MnO2 Nanowires with a “Cocoonlike” Morphology: Redox Couple-Mediated Performance Enhancement in Symmetric Aqueous Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 10754–10762. [Google Scholar] [CrossRef]
- Ma, M.D.; Zhu, Q.; Jiang, Z.Y.; Jian, Y.F.; Chen, C.W.; Liu, Q.Y.; He, C. Achieving toluene efficient mineralization over K/α-MnO2 via oxygen vacancy modulation. J. Colloid. Interface Sci. 2021, 598, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Ushani, U.; Lu, X.Q.; Wang, J.H.; Zhang, Z.Y.; Dai, J.J.; Tan, Y.J.; Wang, S.S.; Li, W.J.; Niu, C.X.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the-art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Cai, H.Y.; Zou, J.; Lin, J.N.; Li, J.W.; Huang, Y.X.; Zhang, S.Y.; Yuan, B.L.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Ding, C.L.; Liu, Z.; Pan, S.Y.; Zhao, C.; Wang, Z.W.; Gao, B.Y.; Li, Q. Activation of peroxydisulfate via Fe@sulfur-doped carbon-supported nanocomposite for degradation of norfloxacin: Efficiency and mechanism. Chem. Eng. J. 2023, 460, 141729. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Rodriguez, V.; Montero, E.; Romero, A.; Santos, A. Methanol-enhanced degradation of carbon tetrachloride by alkaline activation of persulfate: Kinetic model. Sci. Total Environ. 2019, 666, 631–640. [Google Scholar] [CrossRef]
- Wu, W.T.; Huang, Z.; Liu, Y.Y.; Hong, J.M.; Zhang, Q. Novel Co-MOF/GO triggered peroxymonosulfate activation: Insight into singlet oxygen generation mechanism from peroxymonosulfate self-decomposition and secondary radical conversion. Chem. Eng. J. 2023, 476, 146365. [Google Scholar] [CrossRef]
- Wang, G.L.; Liu, Y.C.; Dong, X.L.; Zhang, X.F. Transforming radical to non-radical pathway in peroxymonosulfate activation on nitrogen doped carbon sphere for enhanced removal of organic pollutants: Combined effect of nitrogen species and carbon structure. J. Hazard. Mater. 2022, 437, 129357. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.W.; Zhang, D.D.; Jun, Y.S. Does Tert-Butyl Alcohol Really Terminate the Oxidative Activity of •OH in Inorganic Redox Chemistry? J. Environ. Sci. Technol. 2021, 55, 10442–10450. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Guo, J.Y.; Li, S.R.; Pu, L.; Huang, L. Insight in sulfadiazine degradation by peroxymonosulfate activated by polydopamine-derived nitrogen-doped carbon supported CoFe2O4: Co leaching inhibition and degradation enhancement. Ecotox. Environ. Saf. 2024, 285, 117126. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Wu, C.; Qiu, X.H.; Tanaka, K.; Ohnuki, T.; Yu, Q.Q. New insight of Mn(III) in δ-MnO2 for peroxymonosulfate activation reaction: Via direct electron transfer or via free radical reactions. Environ. Res. 2023, 217, 114874. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, J.; Shang, B.; Zhai, J. Carbamazepine degradation through peroxymonosulfate activation by α-MnO2 with the different exposed facets: Enhanced radical oxidation by facet-dependent surface effect. J. Environ. Chem. Eng. 2022, 10, 108532. [Google Scholar] [CrossRef]
- Fan, J.H.; Wang, Q.Q.; Yan, W.; Chen, J.B.; Zhou, X.F.; Xie, H.J. Mn3O4-g-C3N4 composite to activate peroxymonosulfate for organic pollutants degradation: Electron transfer and structure-dependence. J. Hazard. Mater. 2022, 434, 128818. [Google Scholar] [CrossRef]
- Cai, T.; Huang, H.; Deng, W.; Dai, Q.G.; Liu, W.; Wang, X.Y. Catalytic combustion of 1,2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts. Appl. Catal. B-Environ. Energy 2015, 166, 393–405. [Google Scholar] [CrossRef]
- He, C.; Wang, Y.C.; Li, Z.Y.; Huang, Y.J.; Liao, Y.H.; Xia, D.H.; Lee, S.C. Facet Engineered α-MnO2 for Efficient Catalytic Ozonation of Odor CH3SH: Oxygen Vacancy-Induced Active Centers and Catalytic Mechanism. Environ. Sci. Technol. 2020, 54, 12771–12783. [Google Scholar] [CrossRef]
- Lu, Z.C.; Zhang, P.; Hu, C.; Li, F. Insights into singlet oxygen generation and electron-transfer process induced by a single-atom Cu catalyst with saturated Cu-N4 sites. iScience 2022, 25, 104930. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.N.; Hu, B.; Wang, Q.X.; Asif, A.H.; Shi, L.; Zhang, S.; Sun, H.Q.; Cui, S. Strengthened carbamazepine degradation via producing 1O2 with Ru-doped hollow tubular MoS2 under peroxymonosulfate activation. J. Environ. Chem. Eng. 2025, 13, 115328. [Google Scholar] [CrossRef]
- Lin, X.N.; Ge, Q.; Zhou, X.B.; Wang, Y.; Zhu, C.Y.; Liu, K.Y.; Wan, J.Q. Enhancement of Electron Transfer Between Fe/Mn Promotes Efficient Activation of Peroxomonosulfate by FeMn-NBC. Water 2025, 17, 1700. [Google Scholar] [CrossRef]
- Wei, H.X.; Zhao, J.J.; Rahaman, M.H.; Wang, Q.F.; Zhai, J. The catalytic activity of different Mn(III) species towards peroxymonosulfate activation for carbamazepine degradation. Catal. Commun. 2023, 173, 106563. [Google Scholar] [CrossRef]
- Yun, E.T.; Lee, J.H.; Kim, J.; Park, H.D.; Lee, J. Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation Versus Mediated Electron Transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ya, C.; Ge, Y.J.; Cheng, Y.Q.; Chen, Y.J.; Xu, M.Y.; Wang, H.Y. Activation of peroxymonosulfate by metal (Fe, Mn, Cu and Ni) doping ordered mesoporous Co3O4 for the degradation of enrofloxacin. RSC Adv. 2018, 8, 2338–2349. [Google Scholar] [CrossRef]
- Li, X.G.; Guo, Y.X.; Yan, L.G.; Yan, T.; Song, W.; Feng, R.; Zhao, Y.W. Enhanced activation of peroxymonosulfate by ball-milled MoS2 for degradation of tetracycline: Boosting molybdenum activity by sulfur vacancies. Chem. Eng. J. 2022, 429, 132234. [Google Scholar] [CrossRef]
- Chen, Y.L.; Bai, X.; Ji, Y.T.; Shen, T. Reduced graphene oxide-supported hollow Co3O4@N-doped porous carbon as peroxymonosulfate activator for sulfamethoxazole degradation. Chem. Eng. J. 2022, 430, 132951. [Google Scholar] [CrossRef]
- Wang, S.Z.; Liu, Y.; Wang, J.L. Peroxymonosulfate Activation by Fe-Co-O-Codoped Graphite Carbon Nitride for Degradation of Sulfamethoxazole. Environ. Sci. Technol. 2020, 54, 10361–10369. [Google Scholar] [CrossRef]
- Shao, P.H.; Tian, J.Y.; Yang, F.; Duan, X.G.; Gao, S.S.; Shi, W.X.; Luo, X.B.; Cui, F.Y.; Luo, S.L.; Wang, S.B. Identification and Regulation of Active Sites on Nanodiamonds: Establishing a Highly Efficient Catalytic System for Oxidation of Organic Contaminants. Adv. Funct. Mater. 2018, 28, 1705295. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, C.; Shao, P.H.; Luo, X.B.; Zhang, H.; Wang, S.B.; Duan, X.G. Origins of Electron-Transfer Regime in Persulfate-Based Nonradical Oxidation Processes. Environ. Sci. Technol. 2022, 56, 78–97. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.F.; Niu, C.G.; Tang, N.; Guo, H.; Wen, X.J.; Liang, C.; Zeng, G.M. Enhanced activation of peroxymonosulfate by magnetic Co3MnFeO6 nanoparticles for removal of carbamazepine: Efficiency, synergetic mechanism and stability. Chem. Eng. J. 2019, 362, 851–864. [Google Scholar] [CrossRef]
- Wu, J.X.; Cagnetta, G.; Wang, B.; Cui, Y.Z.; Deng, S.B.; Wang, Y.J.; Huang, J.; Yu, G. Efficient degradation of carbamazepine by organo-montmorillonite supported nCoFe2O4-activated peroxymonosulfate process. Chem. Eng. J. 2019, 368, 824–836. [Google Scholar] [CrossRef]
- Yang, J.C.E.; Zhu, M.P.; Dionysiou, D.D.; Yuan, B.L.; Fu, M.L. Interplay of bicarbonate and the oxygen-containing groups of carbon nanotubes dominated the metal-free activation of peroxymonosulfate. Chem. Eng. J. 2022, 430, 133102. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Qin, L.; Feng, M.; Cheng, Y.; Tang, P.; Xin, B.; Song, W.; Wang, Q.; Zhao, J. Ru-Modified α-MnO2 as an Efficient PMS Activator for Carbamazepine Degradation: Performance and Mechanism. Catalysts 2025, 15, 1085. https://doi.org/10.3390/catal15111085
Hu P, Qin L, Feng M, Cheng Y, Tang P, Xin B, Song W, Wang Q, Zhao J. Ru-Modified α-MnO2 as an Efficient PMS Activator for Carbamazepine Degradation: Performance and Mechanism. Catalysts. 2025; 15(11):1085. https://doi.org/10.3390/catal15111085
Chicago/Turabian StyleHu, Panfeng, Long Qin, Manman Feng, Yuanling Cheng, Pan Tang, Beibei Xin, Wei Song, Quanfeng Wang, and Jujiao Zhao. 2025. "Ru-Modified α-MnO2 as an Efficient PMS Activator for Carbamazepine Degradation: Performance and Mechanism" Catalysts 15, no. 11: 1085. https://doi.org/10.3390/catal15111085
APA StyleHu, P., Qin, L., Feng, M., Cheng, Y., Tang, P., Xin, B., Song, W., Wang, Q., & Zhao, J. (2025). Ru-Modified α-MnO2 as an Efficient PMS Activator for Carbamazepine Degradation: Performance and Mechanism. Catalysts, 15(11), 1085. https://doi.org/10.3390/catal15111085





