Recycling of Waste PET into Terephthalic Acid in Neutral Media Catalyzed by the Cracking Zeolite/Alumina Binder Acidic Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Cracking Zeolite/γ-Al2O3-Based Catalysts
2.2. Cracking Zeolite/γ-Al2O3 Binder Mixtures: X-Ray Diffraction Analysis
2.3. Catalytic Performance of Cracking Zeolite and Its Alumina Binder Composite Catalysts
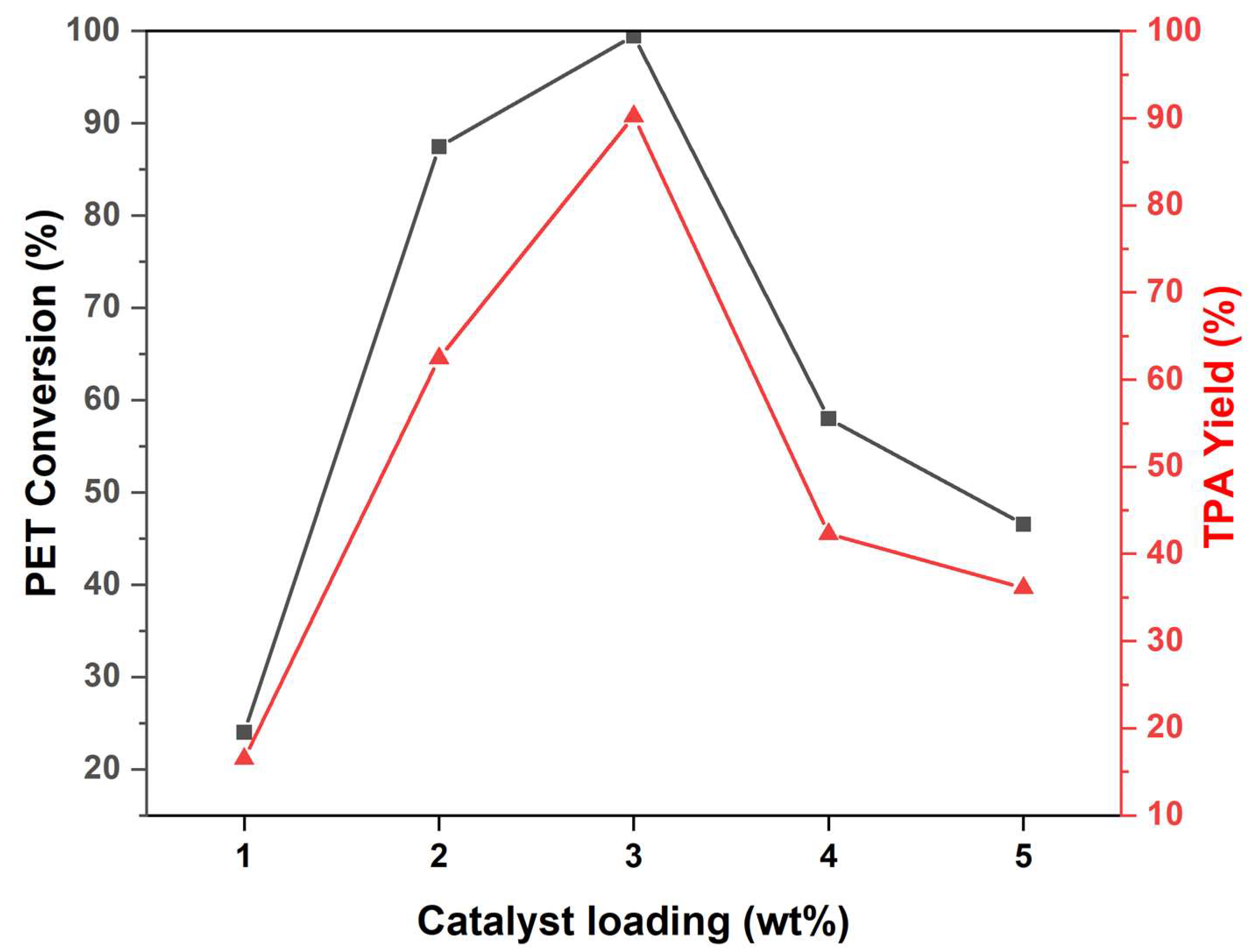
2.4. Impact of Catalyst Loading of Cat-90 @10%Alumina
2.5. Effect of Reaction Conditions
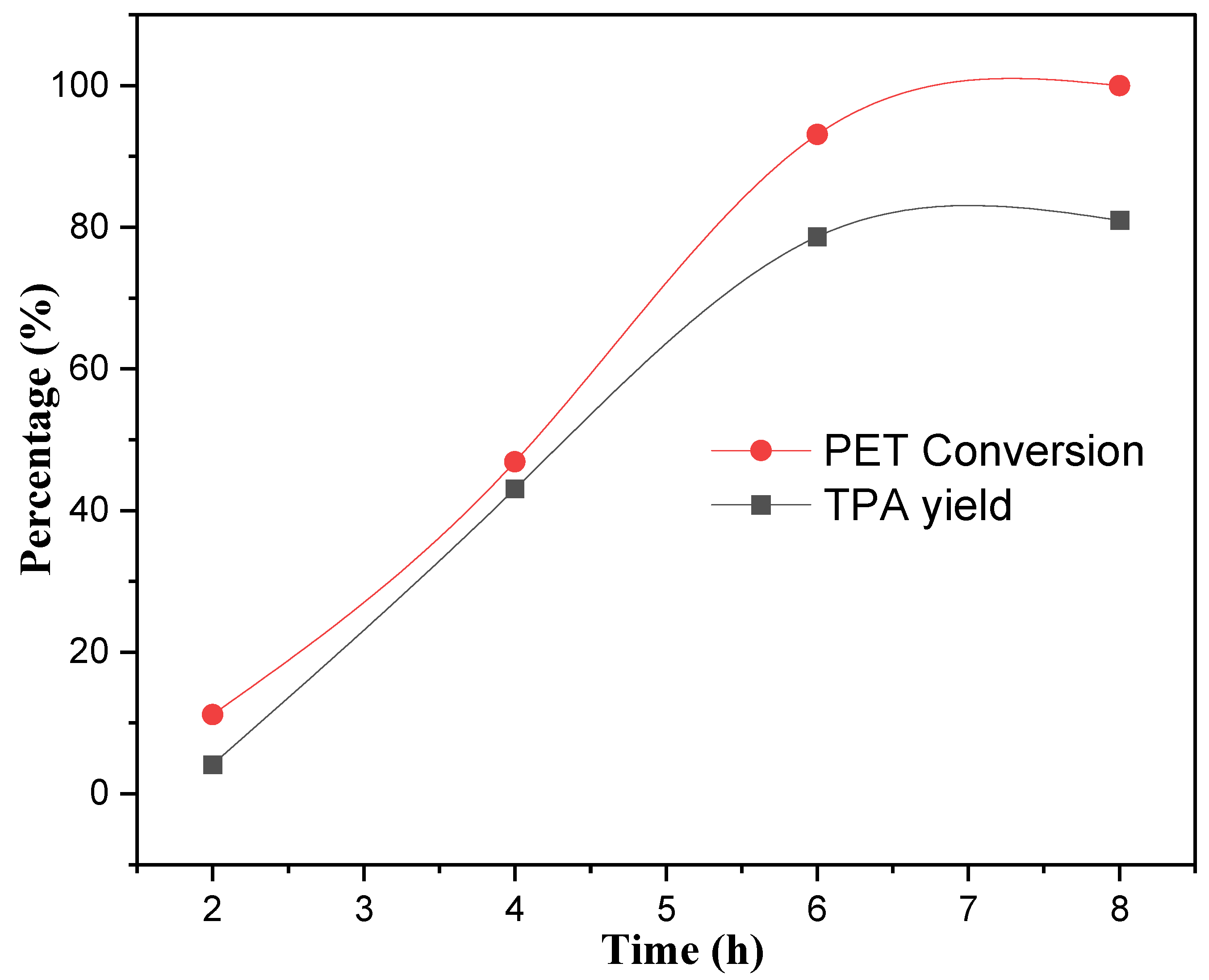
2.5.1. Effect of Reaction Time on Neutral Hydrolysis of PET
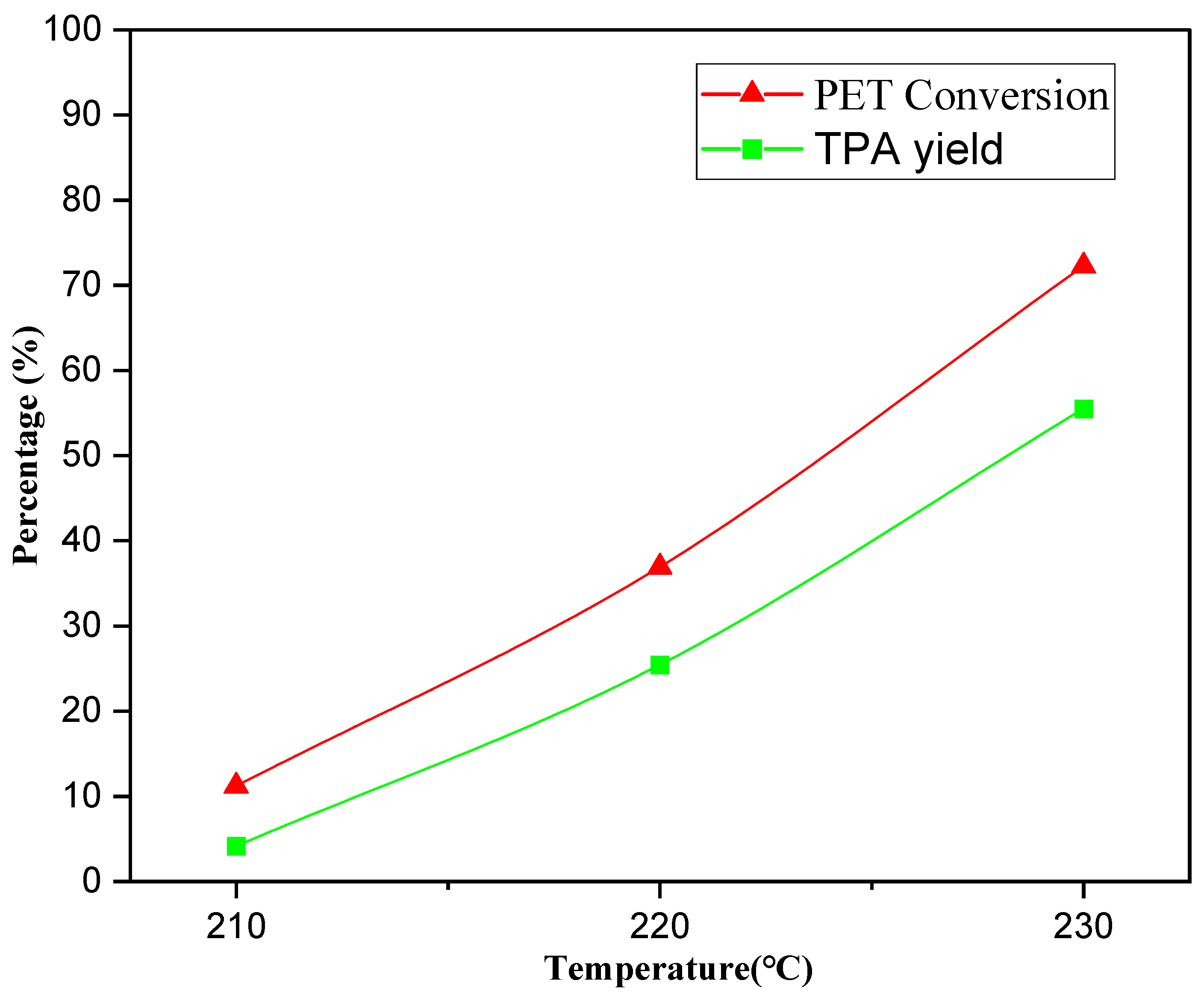
2.5.2. Effect of Temperature on Neutral Hydrolysis Reaction of Waste PET
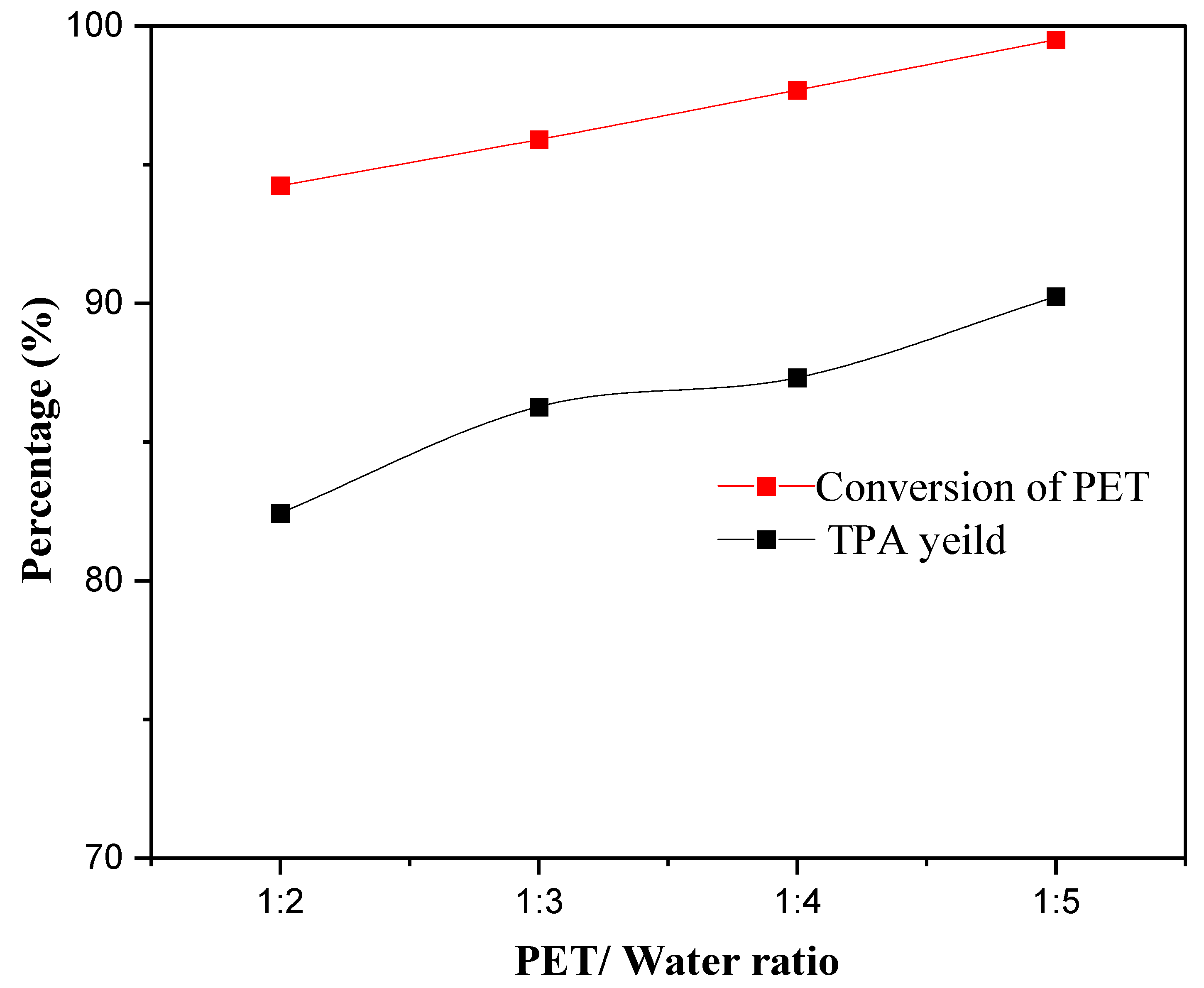
2.5.3. Effect of the PET/Water Ratio on the Conversion of PET and TPA Yield
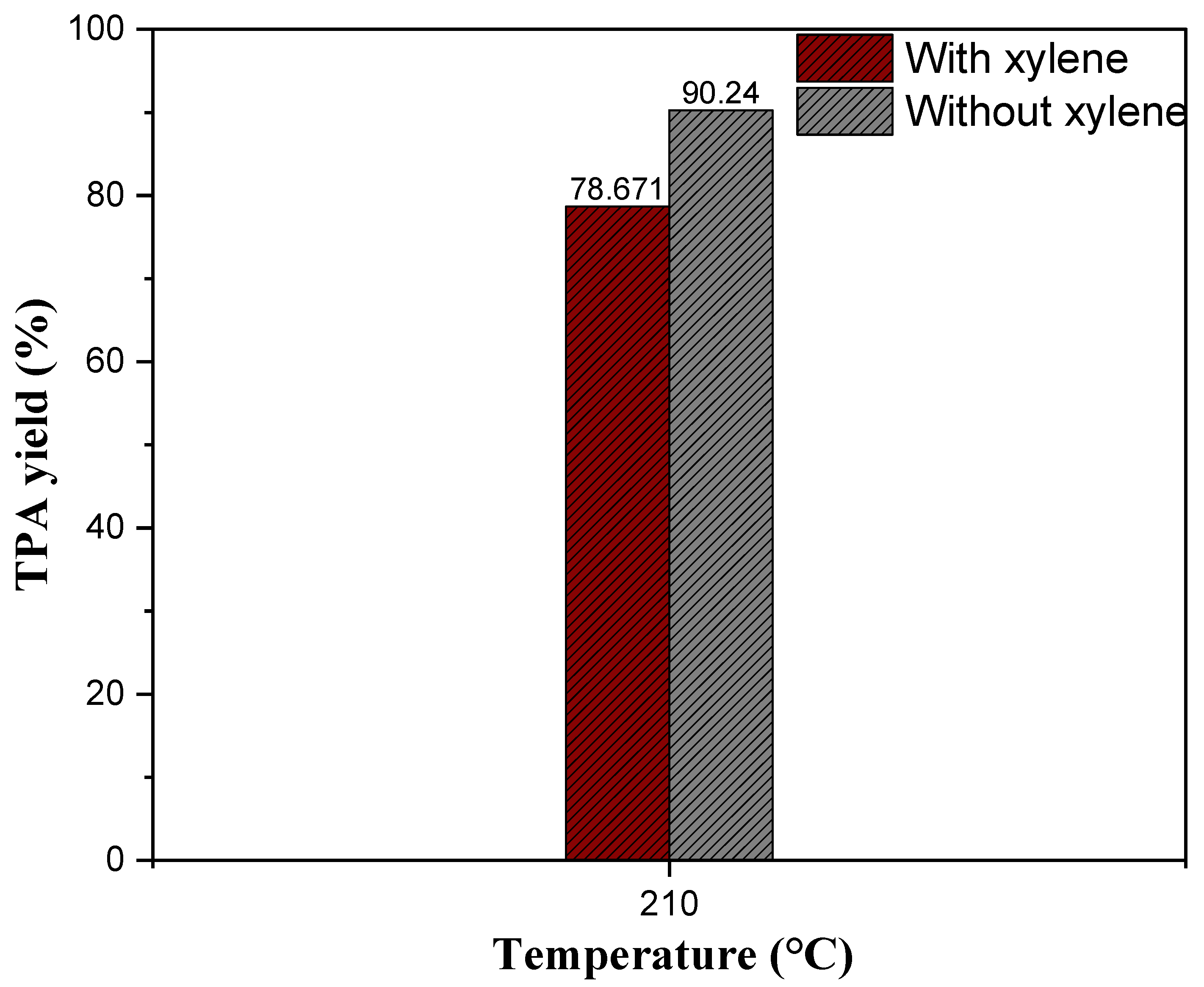
2.5.4. The Effect of Xylene as a Solvent on Neutral Hydrolysis of Waste PET
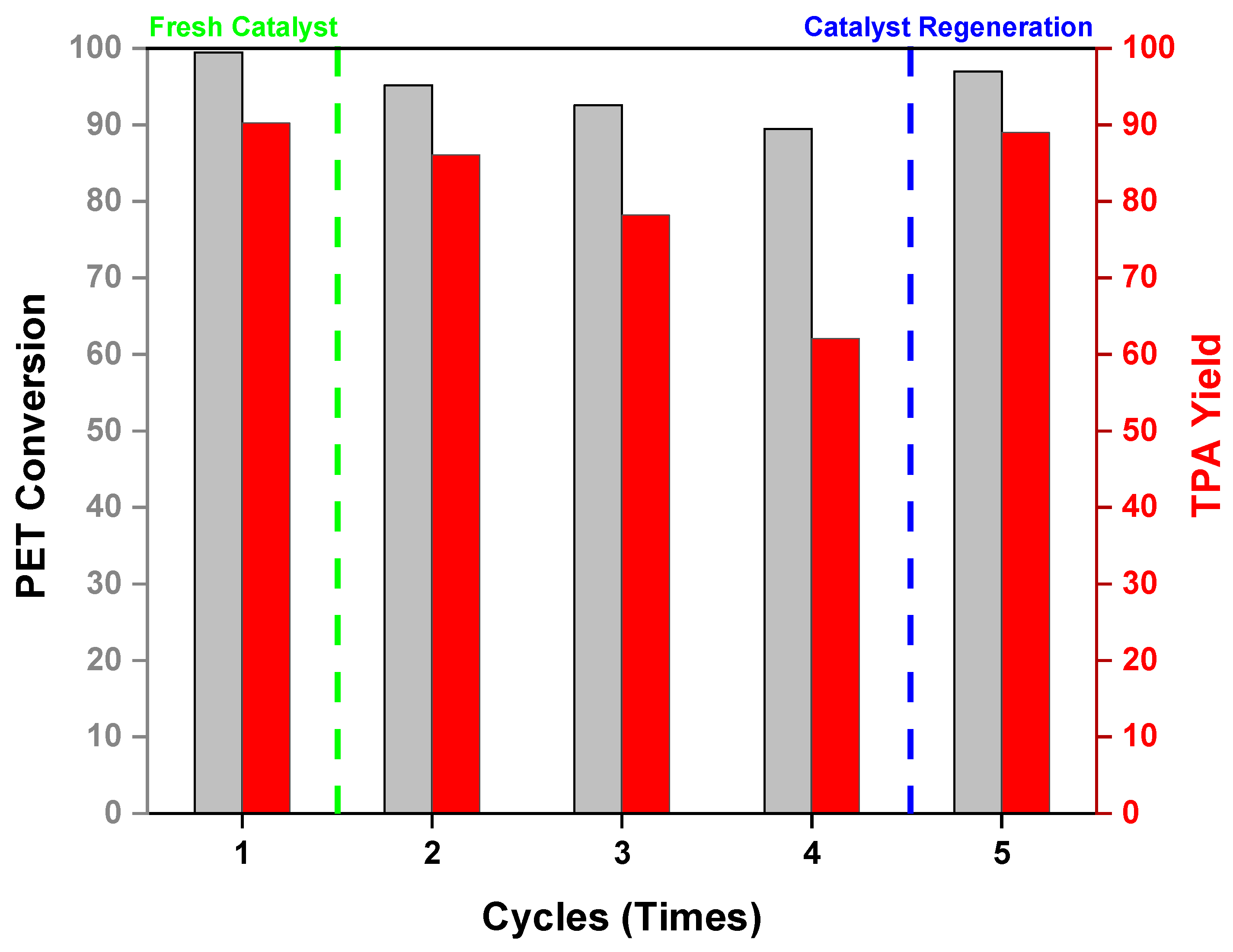
2.6. Catalytic Recyclability of Cat-90@10%Alumina
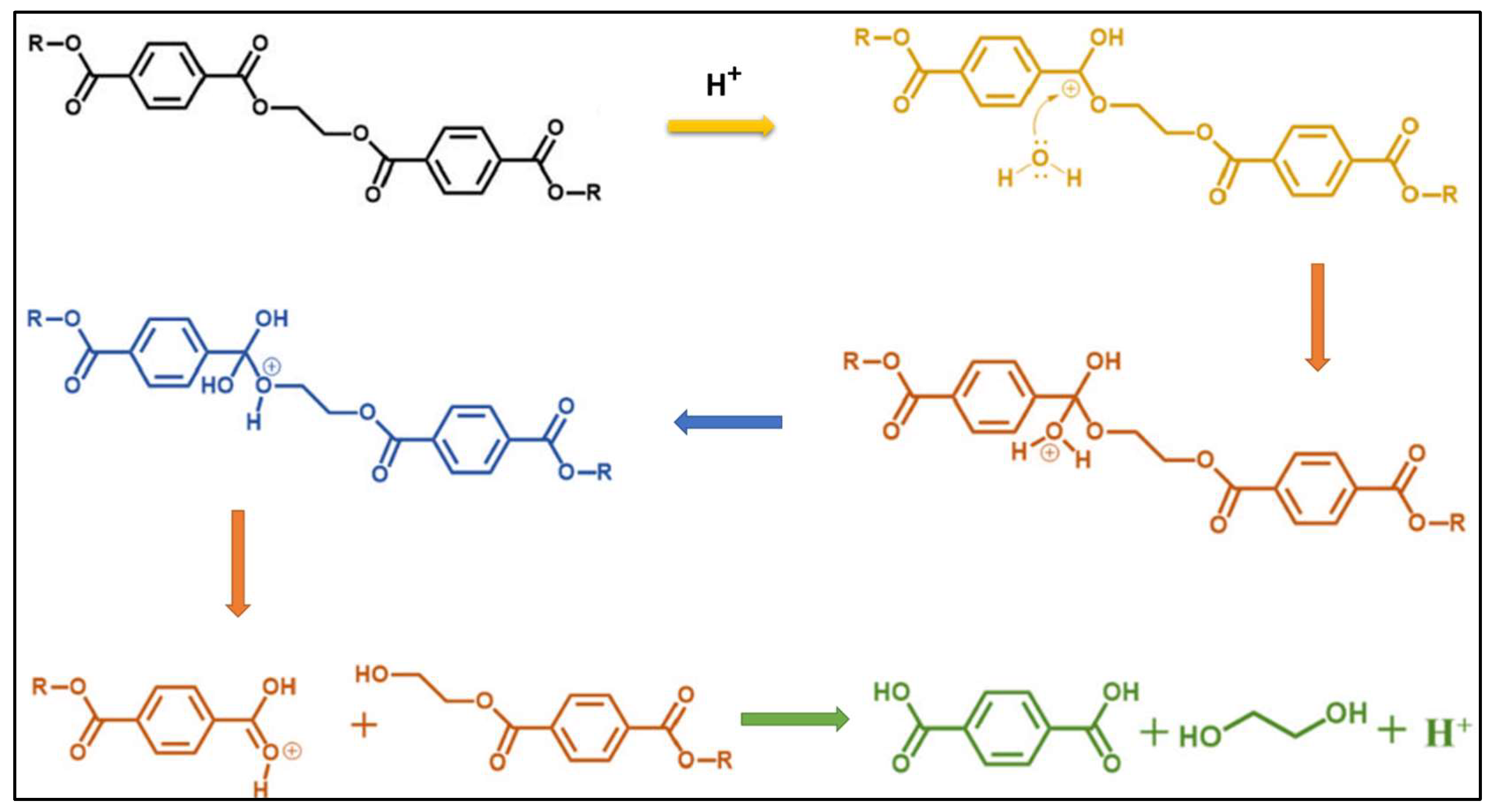
2.7. Reaction Mechanism of Neutral Catalytic Hydrolysis of PET
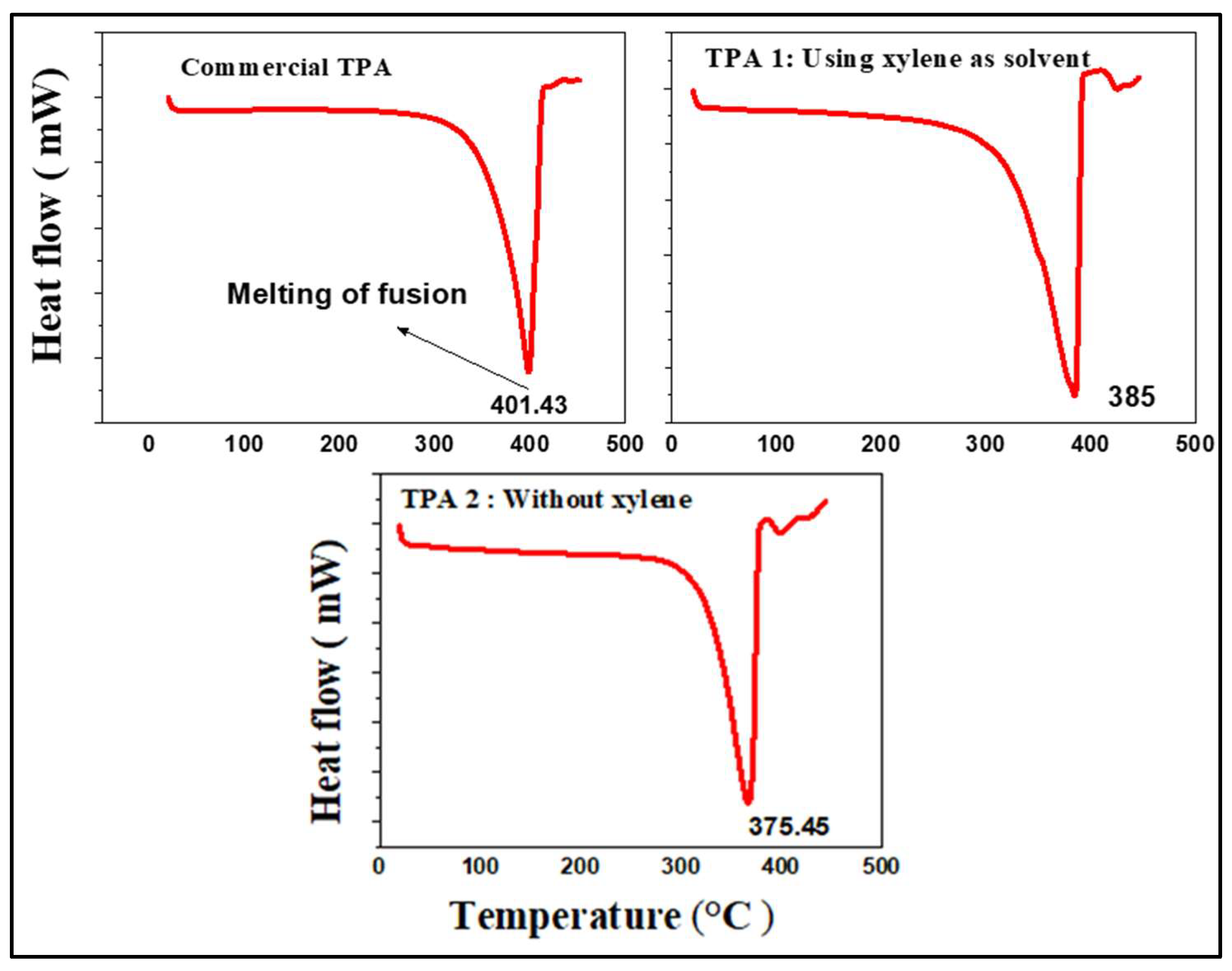
2.8. Comparison of the TPA Melting of Fusion Temperatures
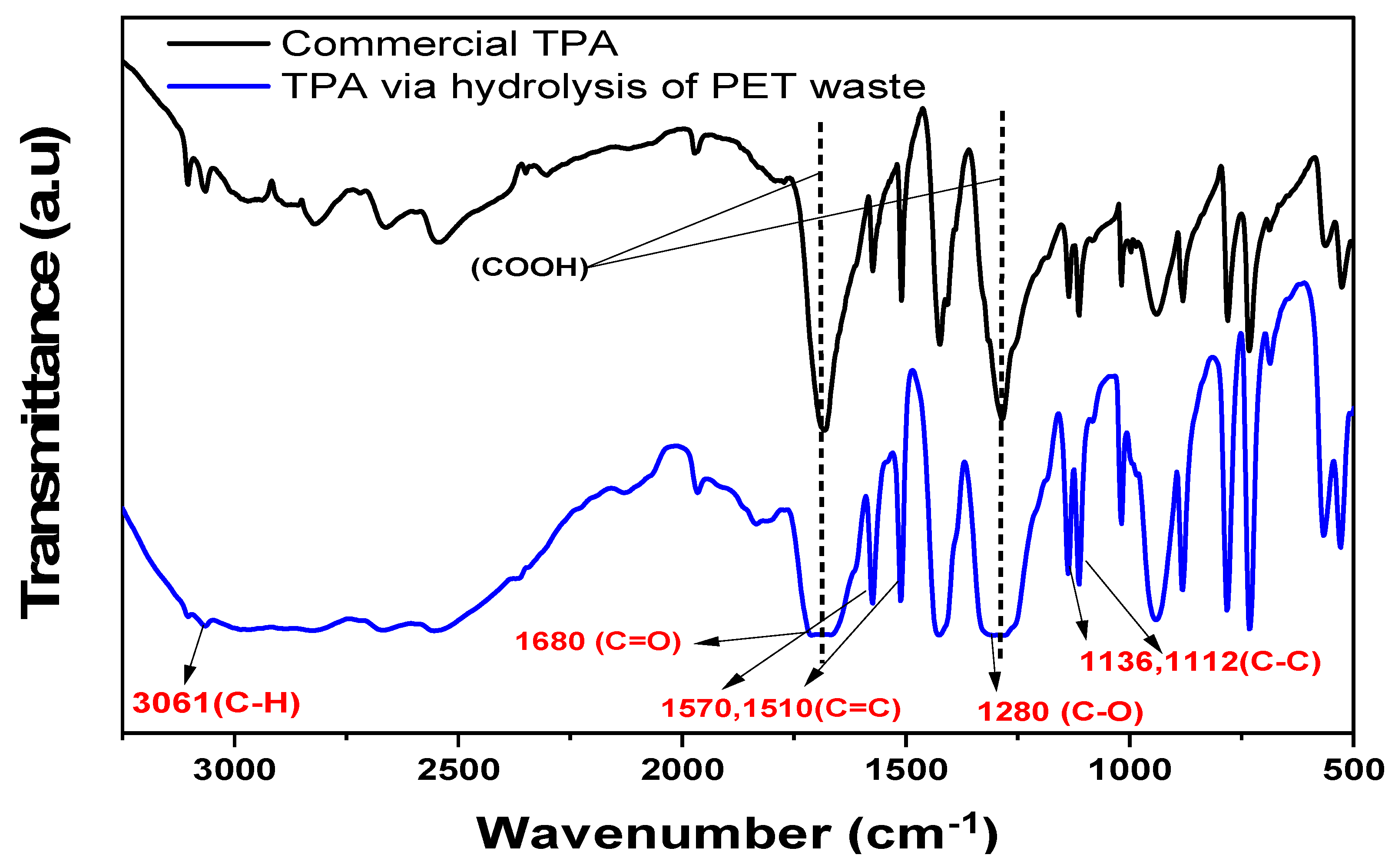
2.9. FTIR Spectroscopy (Comparison of Recycled TPA via Standard TPA)
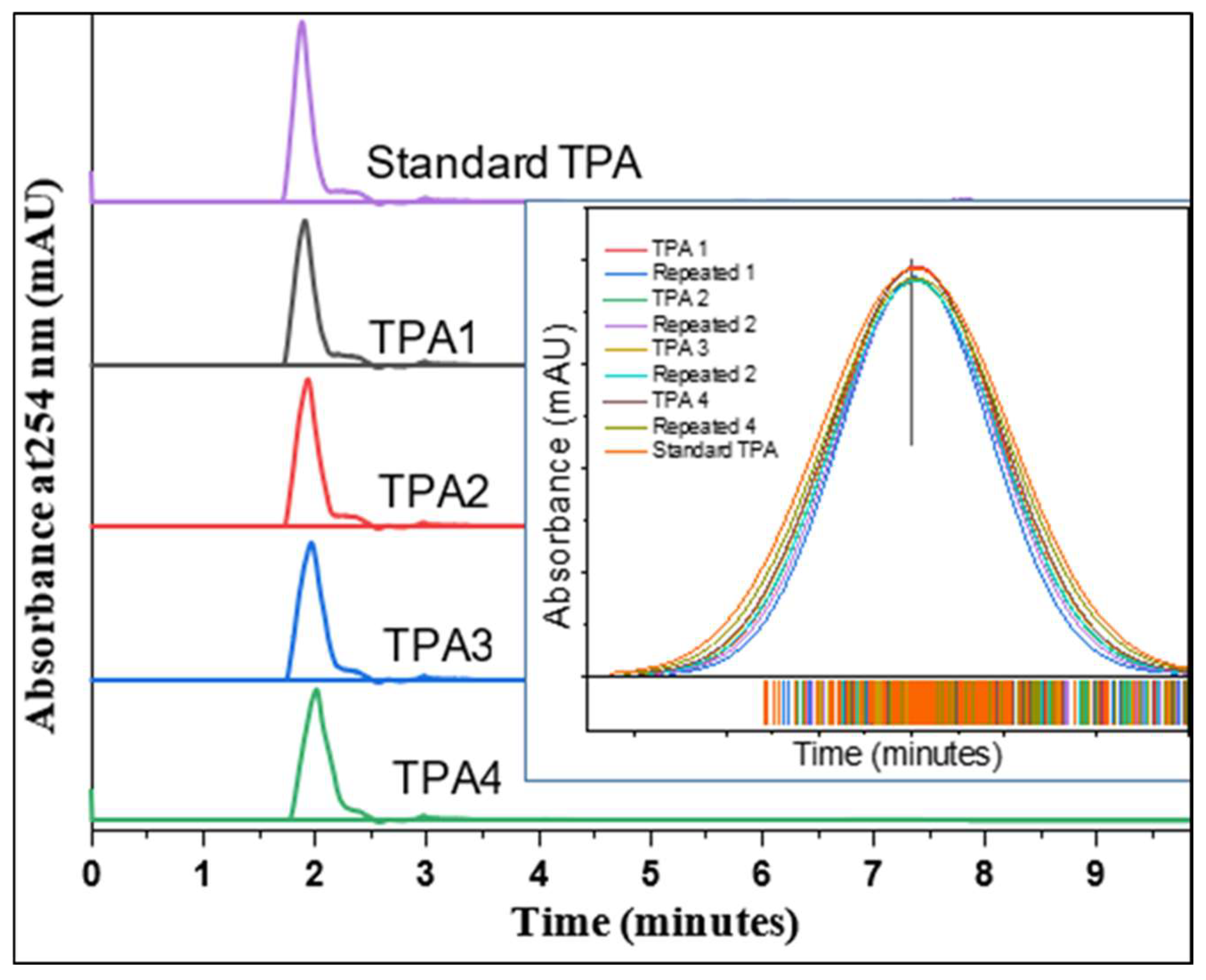
2.10. Purity Analysis of TPA by HPLC
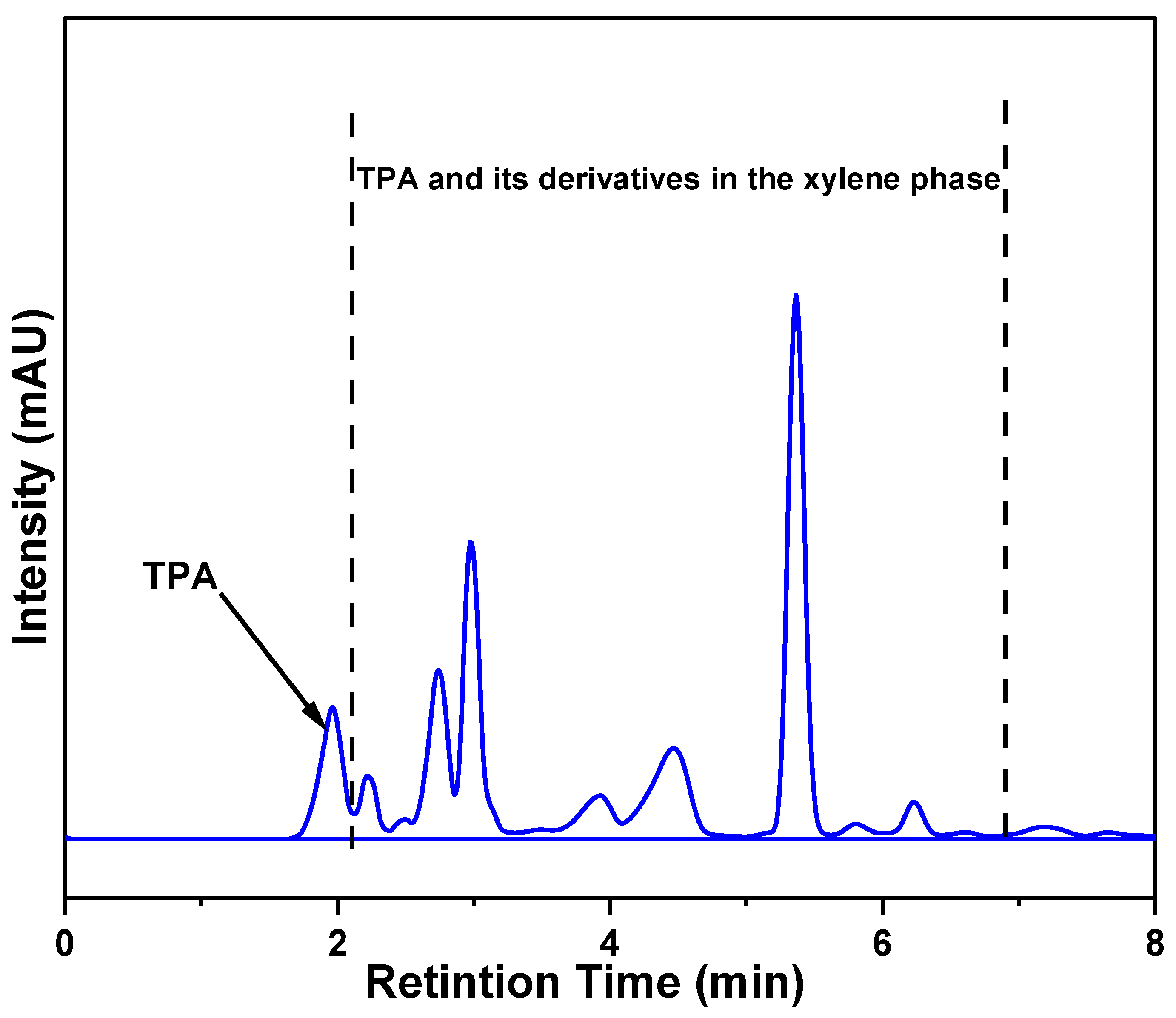
2.11. HPLC Analysis of Separated Xylene Phase
Effect on TPA Yield
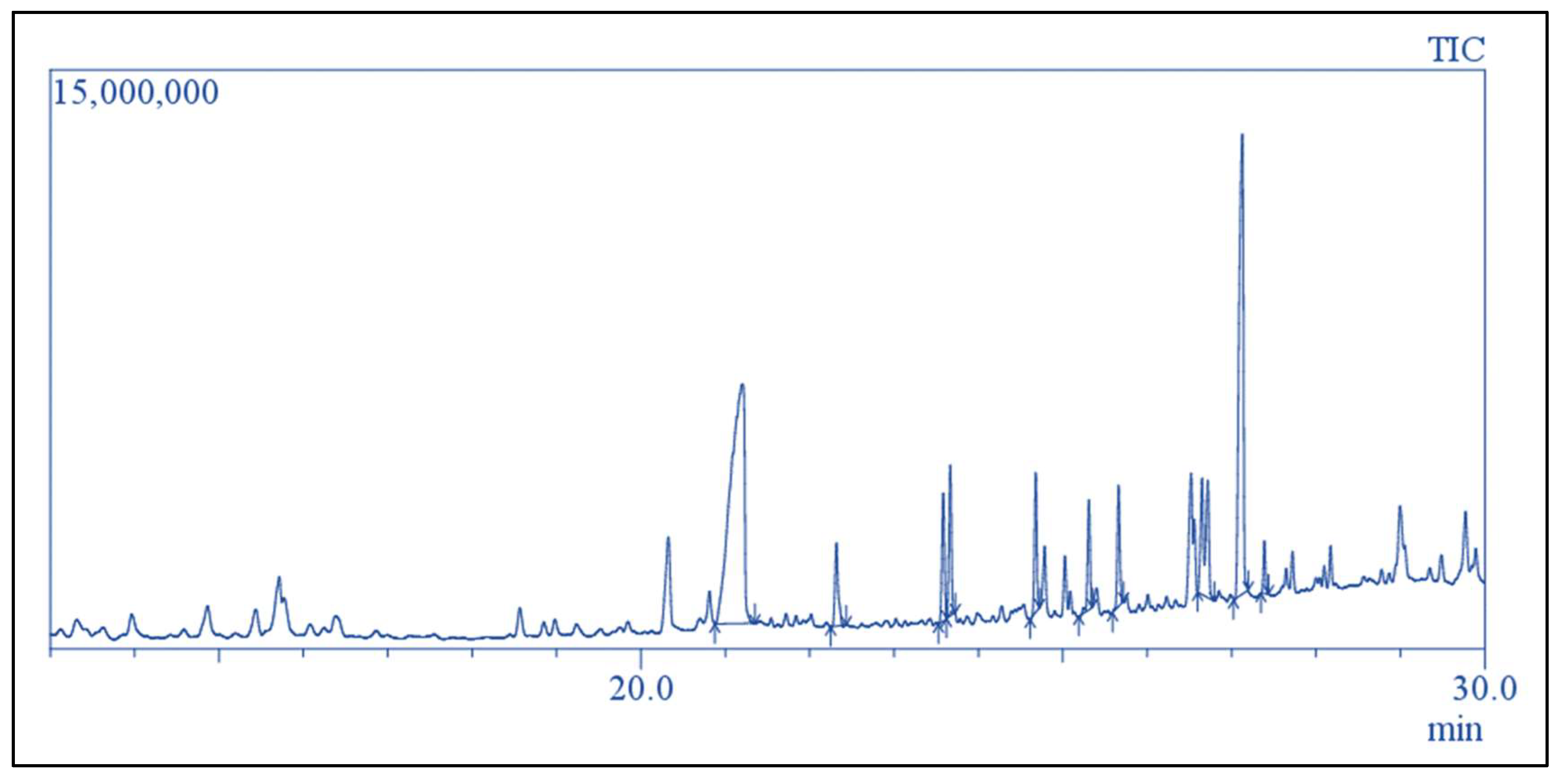
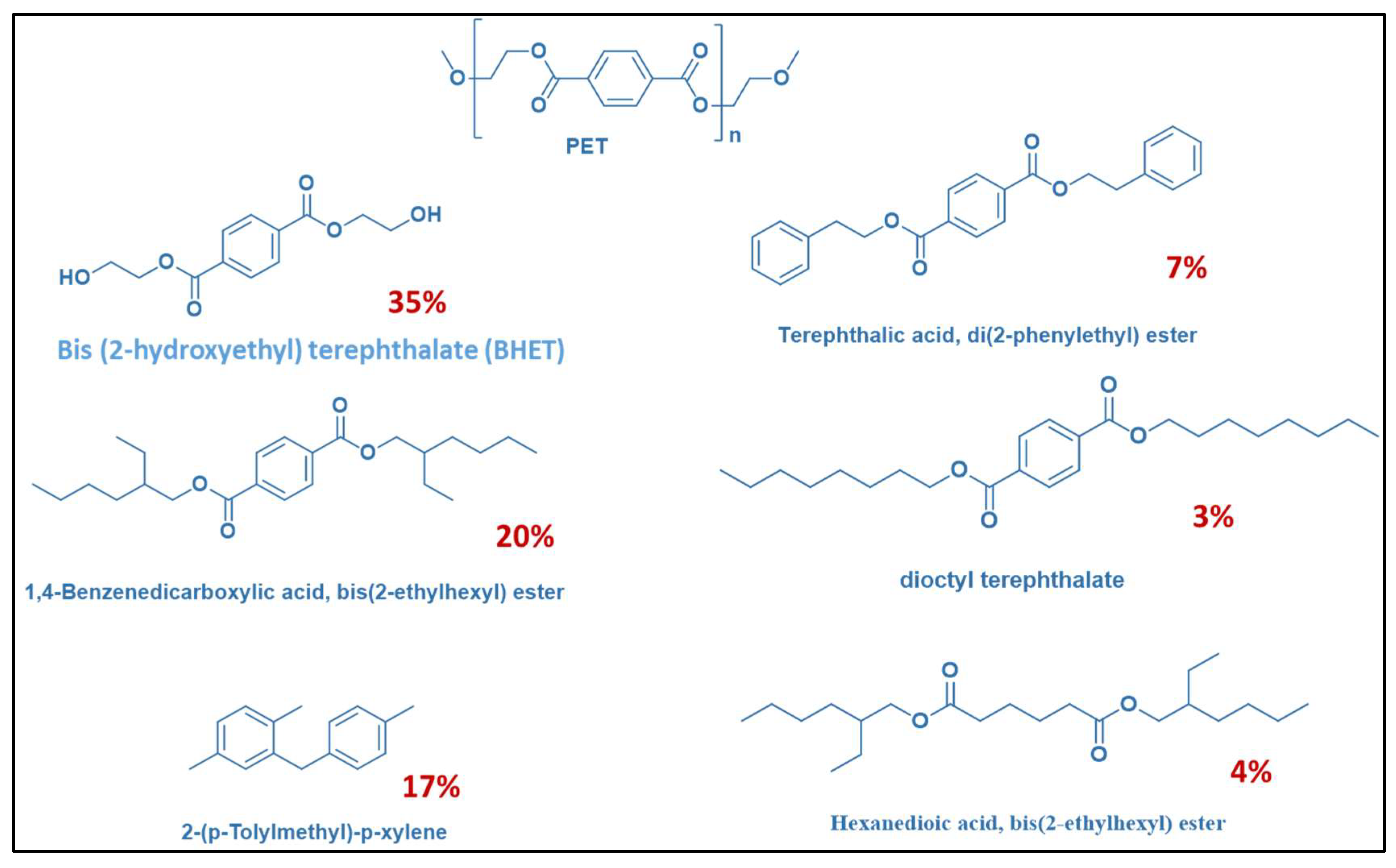
2.12. GC-MS Analysis of Separated Xylene Phase
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup
3.3. Poly (Ethylene Terephthalate) Flakes Preparation
3.4. Catalyst Preparation
3.5. Catalyst Loading Experiment Procedure
3.6. PET Recycling
3.7. Catalyst Reusability Assessment
3.8. TPA Recovery and Purification
3.9. Chemical Analysis
3.10. Calibration Curve
4. Analysis of Results
4.1. Quantitative Analysis
4.2. Analytical and Characterization Methods
4.2.1. Surface Area Analysis
4.2.2. X-Ray Diffraction (XRD)
4.2.3. Temperature Programmed Desorption (TPD)
4.2.4. Differential Scanning Calorimetry (DSC)
4.2.5. High-Performance Liquid Chromatography (HPLC)
4.2.6. Fourier Transform Infrared Spectroscopy (IR-FTIR)
4.2.7. GC-MS Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stanica-Ezeanu, D.; Matei, D. Natural depolymerization of waste poly(ethylene terephthalate) by neutral hydrolysis in marine water. Sci. Rep. 2021, 11, 4431. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.S. Strategic possibility routes of recycled pet. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Chiu, S.J.; Cheng, W.H. Thermal degradation and catalytic cracking of poly(ethylene terephthalate). Polym. Degrad. Stab. 1999, 63, 407–412. [Google Scholar] [CrossRef]
- Noritake, A.; Hori, M.; Shigematsu, M.; Tanahashi, M. Recycling of polyethylene terephthalate using high-pressure steam treatment. Polym. J. 2008, 40, 498–502. [Google Scholar] [CrossRef]
- Pudack, C.; Stepanski, M.; Fässler, P. PET Recycling—Contributions of Crystallization to Sustainability. Chem. Ing. Tech. 2020, 92, 452–458. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Deng, C.; Deng, J.; Shen, L.; Fu, Y. Acetolysis of waste polyethylene terephthalate for upcycling and life-cycle assessment study. Nat. Commun. 2023, 14, 3249. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Goje, A.S.; Thakur, S.A.; Patil, T.M.; Mishra, S. Glycolytic Aminolysis of Poly(ethylene terephthalate) Waste for Recovery of Value-Added Comonomer at Atmospheric Pressure. J. Appl. Polym. Sci. 2003, 90, 3437–3444. [Google Scholar] [CrossRef]
- Acar, I.; Bal, A.; Güçlü, G. The use of intermediates obtained from aminoglycolysis of waste poly(ethylene terephthalate) (PET) for the synthesis of water-reducible alkyd resin. Can. J. Chem. 2013, 91, 357–363. [Google Scholar] [CrossRef]
- Güçlü, G.; Orbay, M. Alkyd resins synthesized from postconsumer PET bottles. Prog. Org. Coat. 2009, 65, 362–365. [Google Scholar] [CrossRef]
- De Castro, R.E.N.; Vidotti, G.J.; Rubira, A.F.; Muniz, E.C. Depolymerization of poly(ethylene terephthalate) wastes using ethanol and ethanol/water in supercritical conditions. J. Appl. Polym. Sci. 2006, 101, 2009–2016. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Achilias, D.S. Chemical recycling of poly(ethylene terephthalate). Macromol. Mater. Eng. 2007, 292, 128–146. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Chatziavgoustis, A.P.; Achilias, D.S. Poly(ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv. Polym. Technol. 2002, 21, 250–259. [Google Scholar] [CrossRef]
- Mancini, S.D.; Zanin, M. Post Consumer Pet Depolymerization by Acid Hydrolysis. Polym.-Plast. Technol. Eng. 2007, 46, 135–144. [Google Scholar] [CrossRef]
- Yates, K.; McClelland, R.A. Mechanisms of ester hydrolysis in aqueous sulfuric acids. J. Am. Chem. Soc. 1967, 89, 2686–2692. [Google Scholar] [CrossRef]
- Yoshioka, T.; Sato, T.; Okuwaki, A. Hydrolysis of waste PET by sulfuric acid at 150 °C for a chemical recycling. J. Appl. Polym. Sci. 1994, 52, 1353–1355. [Google Scholar] [CrossRef]
- Yoshioka, T.; Okayama, N.; Okuwaki, A. Kinetics of Hydrolysis of PET Powder in Nitric Acid by a Modified Shrinking-Core Model. Ind. Eng. Chem. Res. 1998, 37, 336–340. [Google Scholar] [CrossRef]
- Grancarić, A.M.; Kallay, N. Kinetics of polyester fiber alkaline hydrolysis: Effect of temperature and cationic surfactants. J. Appl. Polym. Sci. 1993, 49, 175–181. [Google Scholar] [CrossRef]
- Bohre, A.; Jadhao, P.R.; Tripathi, K.; Pant, K.K.; Likozar, B.; Saha, B. Chemical Recycling Processes of Waste Polyethylene Terephthalate Using Solid Catalysts. ChemSusChem 2023, 16, e202300142. [Google Scholar] [CrossRef]
- Woolery, G.L.; Kuehl, G.H.; Timken, H.C.; Chester, A.W.; Vartuli, J.C. On the nature of framework Brønsted and Lewis acid sites in ZSM-5. Zeolites 1997, 19, 288–296. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino reaction catalyzed by zeolites with Brønsted and Lewis acid sites for the production of γ-valerolactone from furfural. Angew. Chem. Int. Ed. 2013, 52, 8022–8025. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J.; Munnoch, A.L. A survey of the influence of binders in zeolite catalysis. Catal. Sci. Technol. 2013, 3, 1165. [Google Scholar] [CrossRef]
- Michels, N.-L.; Mitchell, S.; Pérez-Ramírez, J. Effects of Binders on the Performance of Shaped Hierarchical MFI Zeolites in Methanol-to-Hydrocarbons. ACS Catal. 2014, 4, 2409–2417. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, H.-K.; Ihm, S.-K. Influence of Catalyst Binders on the Acidity and Catalytic Performance of HZSM-5 Zeolites for Methanol-to-Propylene (MTP) Process: Single and Binary Binder System. Top. Catal. 2010, 53, 247–253. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Liu, S.; Xin, W.; Wang, Y.; Xie, S.; Xu, L. Influences of alkaline treatment on the structure and catalytic performances of ZSM-5/ZSM-11 zeolites with alumina as binder. J. Mol. Catal. A Chem. 2011, 336, 34–41. [Google Scholar] [CrossRef]
- Kasture, M.W.; Niphadkar, P.S.; Bokade, V.V.; Joshi, P.N. On the catalytic performance in isopropylation of benzene over H/β zeolite catalysts: Influence of binder. Catal. Commun. 2007, 8, 1003–1008. [Google Scholar] [CrossRef]
- Etim, U.J.; Bai, P.; Wang, Y.; Subhan, F.; Liu, Y.; Yan, Z. Mechanistic insights into structural and surface variations in Y-type zeolites upon interaction with binders. Appl. Catal. A Gen. 2019, 571, 137–149. [Google Scholar] [CrossRef]
- Lefevere, J.; Protasova, L.; Mullens, S.; Meynen, V. 3D-printing of hierarchical porous ZSM-5: The importance of the binder system. Mater. Des. 2017, 134, 331–341. [Google Scholar] [CrossRef]
- Shihabi, D. Aluminum insertion into high-silica zeolite frameworks II. Binder activation of high-silica ZSM-5. J. Catal. 1985, 93, 471–474. [Google Scholar] [CrossRef]
- Maciver, D. Catalytic aluminas I. Surface chemistry of eta and gamma alumina. J. Catal. 1963, 2, 485–497. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Devadas, P.; Kinage, A.K.; Guisnet, M. Influence of binder on the acidity and performance of H-Gallosilicate (MFI) zeolite in propane aromatization. Appl. Catal. A Gen. 1997, 162, 223–233. [Google Scholar] [CrossRef]
- Wu, X.; Alkhawaldeh, A.; Anthony, R.G. Investigation on acidity of zeolites bound with silica and alumina. Stud. Surf. Sci. Catal. 2000, 143, 217–225. [Google Scholar]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- AlAbduljabbar, F.A.; Haider, S.; Ali, F.A.A.; Alghyamah, A.A.; Almasry, W.A.; Patel, R.; Mujtaba, I.M. Efficient Photocatalytic Degradation of Organic Pollutant in Wastewater by Electrospun Functionally Modified Polyacrylonitrile Nanofibers Membrane Anchoring TiO2 Nanostructured. Membranes 2021, 11, 785. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Si, Z.; Weng, D.; Wu, X.; Yang, J.; Wang, B. Modifications of CeO2-ZrO2 solid solutions by nickel and sulfate as catalysts for NO reduction with ammonia in excess O2. Catal. Commun. 2010, 11, 1045–1048. [Google Scholar] [CrossRef]
- Lu, P.; Sun, J.; Zhu, P.; Abe, T.; Yang, R.; Taguchi, A.; Vitidsant, T.; Tsubaki, N. Sputtered Nano-Cobalt on H-Usy Zeolite for Selectively Converting Syngas to Gasoline. J. Energy Chem. 2015, 24, 637–641. [Google Scholar] [CrossRef]
- Yan, T.; Mengting, Z.; Liu, R.; Dai, W.; Guan, N.; Li, L. Acetone–Butanol–Ethanol Catalytic Upgrading Into Aromatics Over Ga-Modified HZSM-5 Zeolites. Acs Catal. 2023, 13, 7087–7102. [Google Scholar] [CrossRef]
- Onwucha, C.N.; Ehi-Eromosele, C.O.; Ajayi, S.O.; Schaefer, M.; Indris, S.; Ehrenberg, H. Uncatalyzed Neutral Hydrolysis of Waste PET Bottles into Pure Terephthalic Acid. Ind. Eng. Chem. Res. 2023, 62, 6378–6385. [Google Scholar] [CrossRef]
- Acar, I.; Bal, A.; Güçlü, G. The effect of xylene as aromatic solvent to aminoglycolysis of post consumer PET bottles. Polym. Eng. Sci. 2013, 53, 2429–2438. [Google Scholar] [CrossRef]
- Mancini, S.D.; Zanin, M. Optimization of Neutral Hydrolysis Reaction of Post-consumer PET for Chemical Recycling. Prog. Rubber Plast. Recycl. Technol. 2004, 20, 117–132. [Google Scholar] [CrossRef]
- Achilias, D.S.; Tsintzou, G.P.; Nikolaidis, A.K.; Bikiaris, D.N.; Karayannidis, G.P. Aminolytic depolymerization of poly(ethylene terephthalate) waste in a microwave reactor. Polym. Int. 2011, 60, 500–506. [Google Scholar] [CrossRef]
- Wu, W.; Wan, C.; Zhang, Y. Morphology and mechanical properties of ethylene-vinyl acetate rubber/polyamide thermoplastic elastomers. J. Appl. Polym. Sci. 2013, 130, 338–344. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.G.; Dubois, P. Modification of Poly(ethylene 2,5-furandicarboxylate) with Biobased 1,5-Pentanediol: Significantly Toughened Copolyesters Retaining High Tensile Strength and O2 Barrier Property. Biomacromolecules 2019, 20, 353–364. [Google Scholar] [CrossRef]
- Ahmed, J.; Zhang, Y. Effect of electron beam irradiation on the thermal and mechanical properties of ethylene-vinyl acetate copolymer/polyamide blends. Polym. Polym. Compos. 2021, 29, 714–723. [Google Scholar] [CrossRef]
- Bolis, R.M.; Morard, G.; Vinci, T.; Ravasio, A.; Bambrink, E.; Guarguaglini, M.; Koenig, M.; Musella, R.; Remus, F.; Bouchet, J.; et al. Decaying shock studies of phase transitions in MgO-SiO2 systems: Implications for the super-Earths’ interiors. Geophys. Res. Lett. 2016, 43, 9475–9483. [Google Scholar] [CrossRef]
- Mahadevan Subramanya, S.; Mu, Y.; Savage, P.E. Effect of Cellulose and Polypropylene on Hydrolysis of Polyethylene Terephthalate for Chemical Recycling. ACS Eng. Au 2022, 2, 507–514. [Google Scholar] [CrossRef]
- Valh, J.V.; Vončina, B.; Lobnik, A.; Zemljič, L.F.; Škodič, L.; Vajnhandl, S. Conversion of polyethylene terephthalate to high-quality terephthalic acid by hydrothermal hydrolysis: The study of process parameters. Text. Res. J. 2020, 90, 1446–1461. [Google Scholar] [CrossRef]
- Pereira, P.; Savage, P.E.; Pester, C.W. Neutral Hydrolysis of Post-Consumer Polyethylene Terephthalate Waste in Different Phases. ACS Sustain. Chem. Eng. 2023, 11, 7203–7209. [Google Scholar] [CrossRef]
- Abedsoltan, H. A focused review on recycling and hydrolysis techniques of polyethylene terephthalate. Polym. Eng. Sci. 2023, 63, 2651–2674. [Google Scholar] [CrossRef]
- Mrigwani, A.; Thakur, B.; Guptasarma, P. Counter-intuitive enhancement of degradation of polyethylene terephthalate through engineering of lowered enzyme binding to solid plastic. Proteins Struct. Funct. Bioinforma. 2023, 91, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, Y.; Huo, F.; Liu, J.; Hou, W.; Wang, N.; Zhou, Q.; Xu, J.; Lu, X. PET pyrolysis and hydrolysis mechanism in the fixed pyrolyzer. Can. J. Chem. Eng. 2023, 101, 4395–4408. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Wan, B.-Z.; Cheng, W.-H. Kinetics of Hydrolytic Depolymerization of Melt Poly(ethylene terephthalate). Ind. Eng. Chem. Res. 1998, 37, 1228–1234. [Google Scholar] [CrossRef]
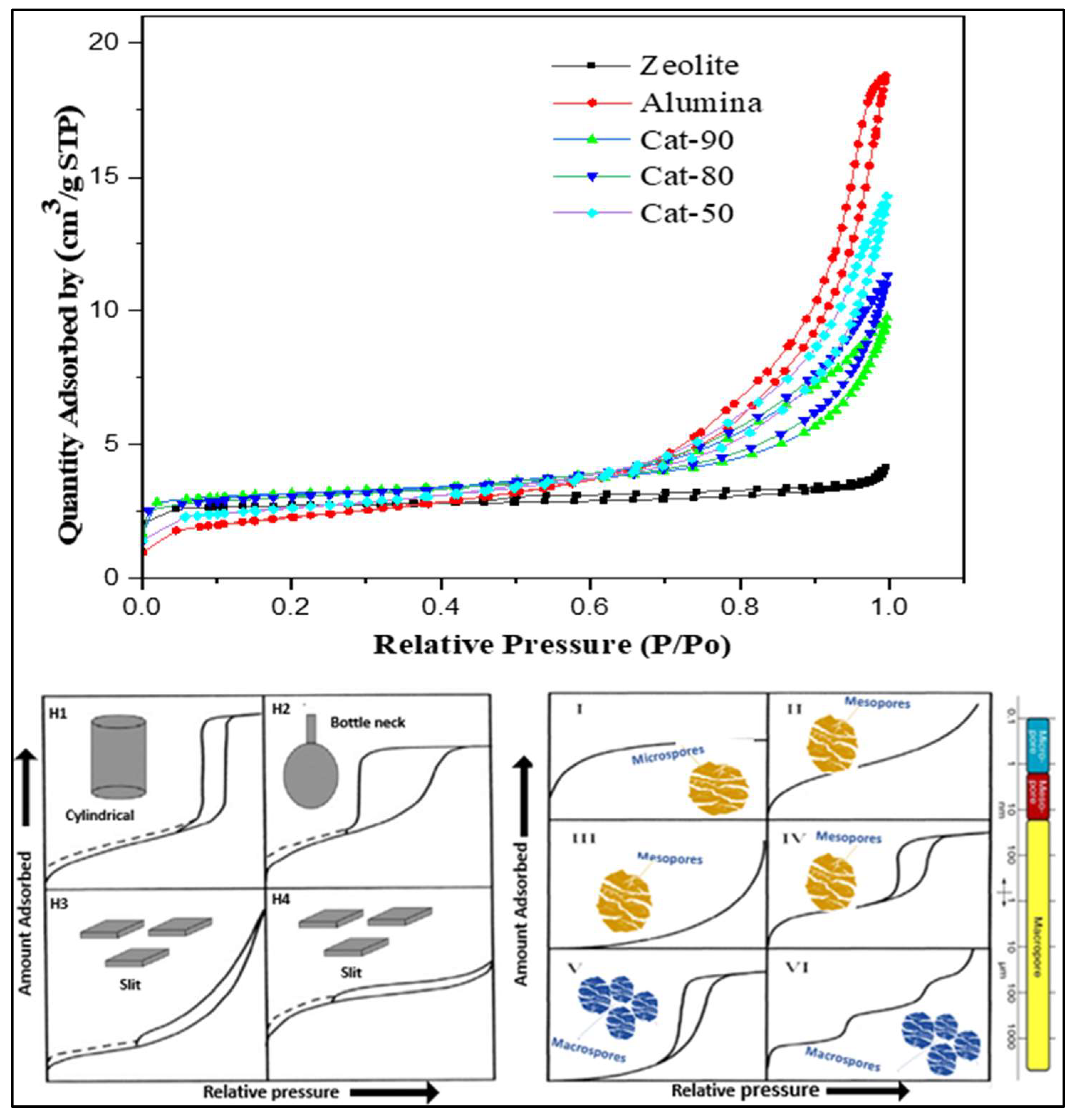
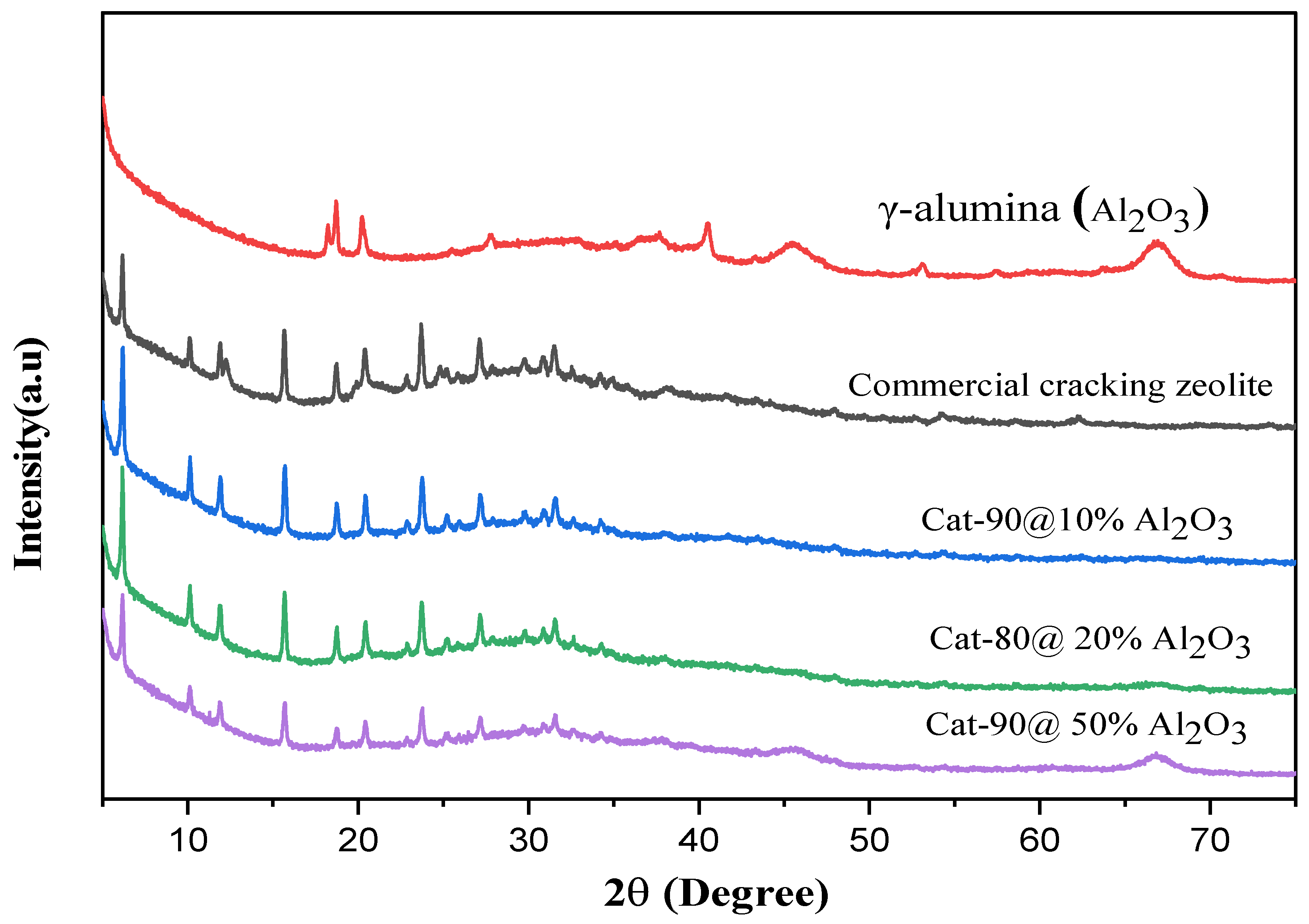
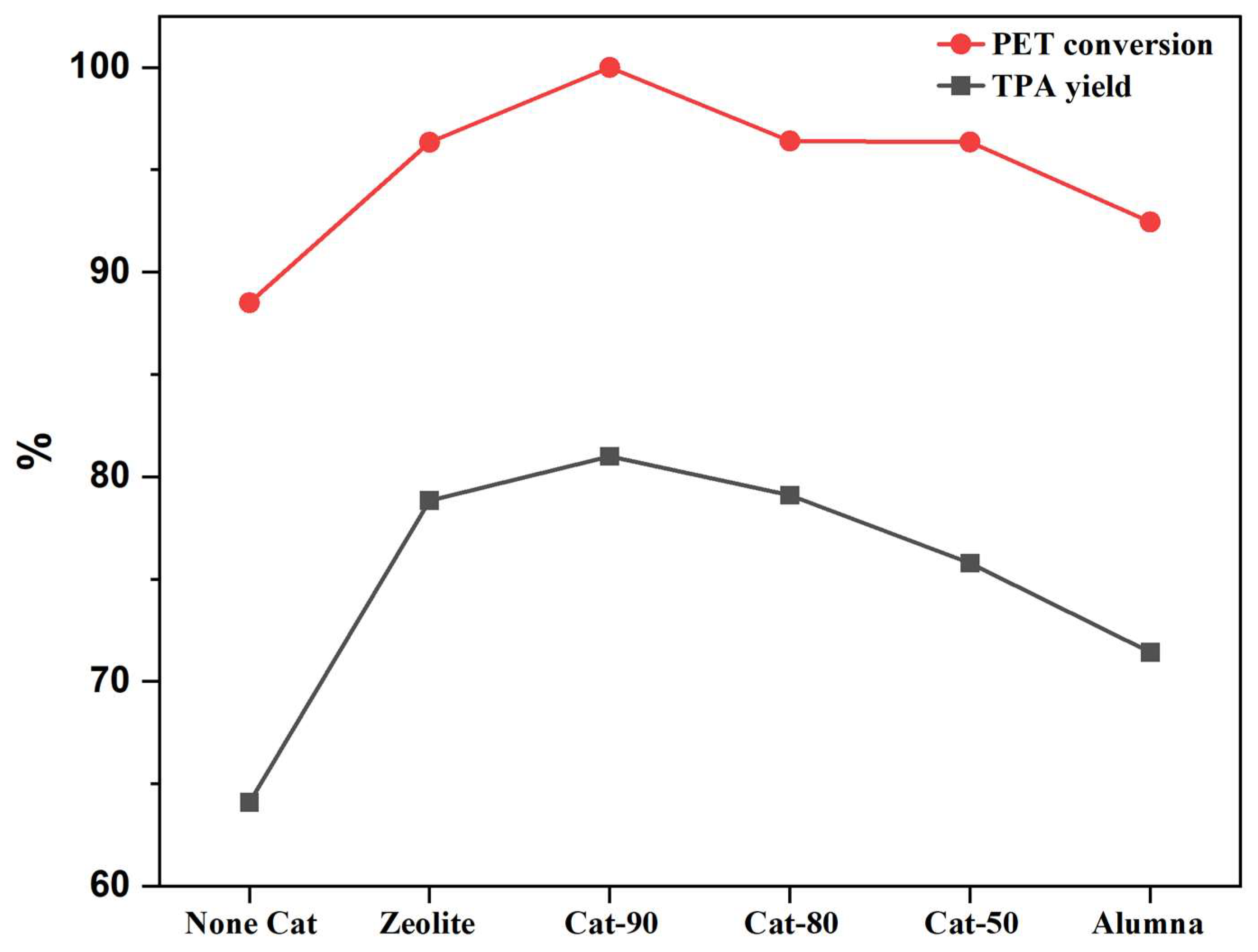
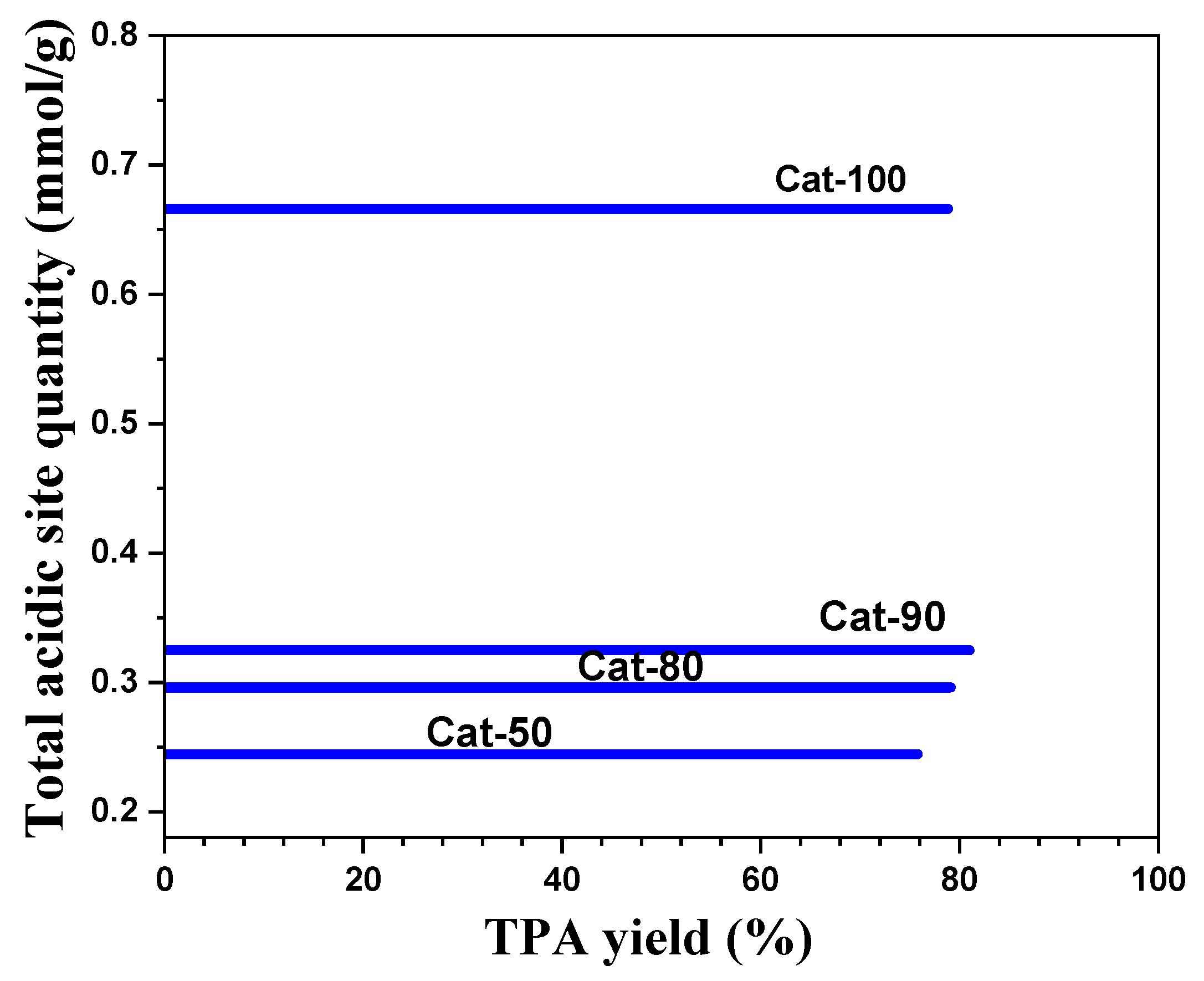
| Run. | Temp (°C) | Xylene | Time (h) | Catalyst | Cat-Load (g) | Cat-Style | PET/Water Ratio (wt.%) | PET (%) | TPA (%) | TAP (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 210 | ✓ | 8 | None | 0.143 | Fresh | 1:5 | 88.5 | 64.09 | 2.755 |
| 2 | 210 | ✓ | 8 | Cat-100 (Zeolite) | 0.143 | Fresh | 1:5 | 96.34 | 78.83 | 3.407 |
| 3 | 210 | ✓ | 8 | Cat-0 (Alumina) | 0.143 | Fresh | 1:5 | 92.45 | 71.4 | 3.085 |
| 4 | 210 | ✓ | 8 | Cat-90@10%Alumina | 0.143 | Fresh | 1:5 | 100 | 81 | 3.50 |
| 5 | 210 | ✓ | 8 | Cat-80@20%Alumina | 0.143 | Fresh | 1:5 | 96.4 | 79.1 | 3.417 |
| 6 | 210 | ✓ | 8 | Cat-50@50%Alumina | 0.143 | Fresh | 1:5 | 96.36 | 75.78 | 3.275 |
| 7 | 210 | ✓ | 2 | Cat-90@10%Alumina | 0.143 | Fresh | 1:5 | 11.2 | 4.164 | 0.18 |
| 8 | 210 | ✓ | 4 | Cat-90@10%Alumina | 0.143 | Fresh | 1:5 | 46.86 | 43.04 | 1.86 |
| 9 | 210 | ✓ | 6 | Cat-90@10%Alumina | 0.143 | Fresh | 1:5 | 93.09 | 78.67 | 3.40 |
| 10 | 210 | No | 6 | Cat-90@10%Alumina | 0.143 | Fresh | 1:2 | 94.24 | 82.44 | 3.563 |
| 11 | 210 | No | 6 | Cat-90@10%Alumina | 0.143 | Fresh | 1:3 | 95.9 | 86.27 | 3.728 |
| 12 | 210 | No | 6 | Cat-90@10%Alumina | 0.143 | Fresh | 1:4 | 97.68 | 87.32 | 3.774 |
| 13 | 210 | No | 6 | Cat-90@10%Alumina | 0.143 | Fresh | 1:5 | 99.5 | 90.24 | 3.90 |
| 14 | 220 | ✓ | 2 | Cat-90@10%Alumina | 0.143 | Fresh | 1:5 | 36.94 | 25.45 | 1.10 |
| 15 | 230 | ✓ | 2 | Cat-90@10%Alumina | 0.143 | Fresh | 1:5 | 72.26 | 55.53 | 2.40 |
| 16 | 210 | No | 6 | Cat-90@10%Alumina | 0.05 | Fresh | 1:5 | 24 | 16.5 | 0.713 |
| 17 | 210 | No | 6 | Cat-90@10%Alumina | 0.1 | Fresh | 1:5 | 87.46 | 62.47 | 2.7 |
| 18 | 210 | No | 6 | Cat-90@10%Alumina | 0.2 | Fresh | 1:5 | 58 | 42.3 | 1.83 |
| 19 | 210 | No | 6 | Cat-90@10%Alumina | 0.25 | Fresh | 1:5 | 46.52 | 36.1 | 1.56 |
| 20 | 210 | No | 6 | Cat-90@10%Alumina | 0.143 | Cycle 2 | 1:5 | 95.2 | 86.1 | 3.72 |
| 21 | 210 | No | 6 | Cat-90@10%Alumina | 0.143 | Cycle 3 | 1:5 | 92.58 | 78.2 | 3.38 |
| 22 | 210 | No | 6 | Cat-90@10%Alumina | 0.143 | Cycle 4 | 1:5 | 89.47 | 62.1 | 2.68 |
| 23 | 210 | No | 6 | Cat-90@10%Alumina | 0.143 | Regenerated | 1:5 | 97.32 | 89 | 3.86 |
| Catalyst | Zeolite: Alumina Ratio | BET Surface Area (m2/g) | Micropore Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|---|---|
| Cat-100 (Zeolite) | 100:0 | 187.8831 | 155.1992 | 0.057467 | 8.37 |
| Cat-90 | 90:10 | 228.0894 | 170.0267 | 0.246529 | 16.04 |
| Cat-80 | 80:20 | 221.4337 | 153.4648 | 0.312032 | 16.56 |
| Cat-50 | 50:50 | 198.3791 | 115.7011 | 0.450898 | 15.6 |
| Cat-0 (γ-Al2O3) | 0:100 | 178.7842 | 70.0541 | 0.648641 | 17.14 |
| Catalysts | γ-Al2O3 Content (wt.%) | Total Acidic Site Quantity (mmol/g) | Lewis Acidic Site | Bronsted Acidic Site | |||
|---|---|---|---|---|---|---|---|
| Tm (°C) | Quantity (mmol/g) | Tm (°C) | Quantity (mmol/g) | BAS/LAS | |||
| Cat-100 | 0 | 0.666 | 123 269 | 0.027 0.14366 | 508 723 | 0.4885 0.00715 | 2.9 |
| Cat-90 | 10 | 0.3248 | 116 283 | 0.192 0.0434 | 685 | 0.0894 | 0.38 |
| Cat-80 | 20 | 0.2961 | 118 291 | 0.1146 0.109 | 681 | 0.0725 | 0.324 |
| Cat-50 | 50 | 0.2446 | 121 283 | 0.1619 0.02822 | 504 720 | 0.0145 0.040 | 0.2866 |
| Assignment | Peak (cm−1) | Structure | Products |
|---|---|---|---|
| C-H | 3061 | 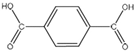 | Terephthalic acid |
| C-O | 1280 | ||
| C=O | 1680 | ||
| C-C | 1136, 1112 | ||
| Hydroxyl stretching vibrations (O-H) | 2500–3400 | 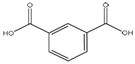 | Isophthalic acid |
| C=O | 1690 | ||
| C=C | 1570, 1510 | ||
| The para-substituted arene group of TPA | 732 | ||
| C-O | 1287 |
| Sample No. | Peak Area | Concentration (mg/mL) | TPA Purity (%) | Actual TPA Yield (g) by HPLC (%) |
|---|---|---|---|---|
| 1 | 1,158,692 | 43.71 | 90.21 | 2.485165 |
| 2 | 1,235,232 | 46.59 | 96.16 | 3.276319 |
| 3 | 1,265,258 | 47.73 | 98.50 | 3.447567 |
| 4 | 1,247,277 | 47.05 | 97.10 | 3.317978 |
| 5 | 1,287,940 | 48.58 | 95.68 | 3.133628 |
| 6 | 1,250,587 | 47.17 | 97.36 | 3.368647 |
| 7 | 1,154,576 | 43.55 | 89.89 | 0.161793 |
| 8 | 1,265,360 | 47.73 | 98.51 | 1.832283 |
| 9 | 1,192,022 | 44.96 | 92.80 | 3.155213 |
| 10 | 1,268,380 | 47.85 | 98.74 | 3.518283 |
| 11 | 1,218,256 | 45.95 | 94.84 | 3.535738 |
| 12 | 1,249,403 | 47.13 | 97.27 | 3.670878 |
| 13 | 1,242,065 | 46.85 | 96.70 | 3.771156 |
| 14 | 1,240,664 | 46.80 | 96.59 | 1.06246 |
| 15 | 1,229,213 | 46.37 | 95.70 | 2.296698 |
| Zeolite | Channel Dimension | Channel Diameter (nm) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | XRF |
|---|---|---|---|---|---|
| (Si/Al) Ratio | |||||
| Cracking | 3D | 8.37 | 187.8831 | 0.0574 | 1.436 |
| Abbreviation | Zeolite Type | Zeolite: γ-Al2O3 | γ-Al2O3 Content (wt.%) |
|---|---|---|---|
| Cat-100 (Zeolite) | Cracking | 100:0 | 0 |
| Cat-90 | Cracking | 90:10 | 10 |
| Cat-80 | Cracking | 80:20 | 20 |
| Cat-90 | Cracking | 50:50 | 50 |
| V (µL) | m (mg) | C (mg/L) | Peak Area |
|---|---|---|---|
| 10 | 0.01 | 10 | 319,421 |
| 20 | 0.02 | 20 | 572,893 |
| 30 | 0.03 | 30 | 825,481 |
| 40 | 0.04 | 40 | 1,054,455 |
| 50 | 0.05 | 50 | 1,284,501 |
| 60 | 0.06 | 60 | 1,654,632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamedi, S.S.; Al-Masry, W.; Al-Fatesh, A.S.; Haider, S.; Mahmood, A.; Blidi, L.E.; Bin Jumah, A. Recycling of Waste PET into Terephthalic Acid in Neutral Media Catalyzed by the Cracking Zeolite/Alumina Binder Acidic Catalyst. Catalysts 2025, 15, 1072. https://doi.org/10.3390/catal15111072
Alhamedi SS, Al-Masry W, Al-Fatesh AS, Haider S, Mahmood A, Blidi LE, Bin Jumah A. Recycling of Waste PET into Terephthalic Acid in Neutral Media Catalyzed by the Cracking Zeolite/Alumina Binder Acidic Catalyst. Catalysts. 2025; 15(11):1072. https://doi.org/10.3390/catal15111072
Chicago/Turabian StyleAlhamedi, Shaddad S., Waheed Al-Masry, Ahmed S. Al-Fatesh, Sajjad Haider, Asif Mahmood, Lahssen El Blidi, and Abdulrahman Bin Jumah. 2025. "Recycling of Waste PET into Terephthalic Acid in Neutral Media Catalyzed by the Cracking Zeolite/Alumina Binder Acidic Catalyst" Catalysts 15, no. 11: 1072. https://doi.org/10.3390/catal15111072
APA StyleAlhamedi, S. S., Al-Masry, W., Al-Fatesh, A. S., Haider, S., Mahmood, A., Blidi, L. E., & Bin Jumah, A. (2025). Recycling of Waste PET into Terephthalic Acid in Neutral Media Catalyzed by the Cracking Zeolite/Alumina Binder Acidic Catalyst. Catalysts, 15(11), 1072. https://doi.org/10.3390/catal15111072








