Non-Metallic Doping of Multinary Metal Oxide Semiconductors for Energy Applications
Abstract
1. Introduction
2. Classification and Structural Characteristics of Multinary Metal Oxides
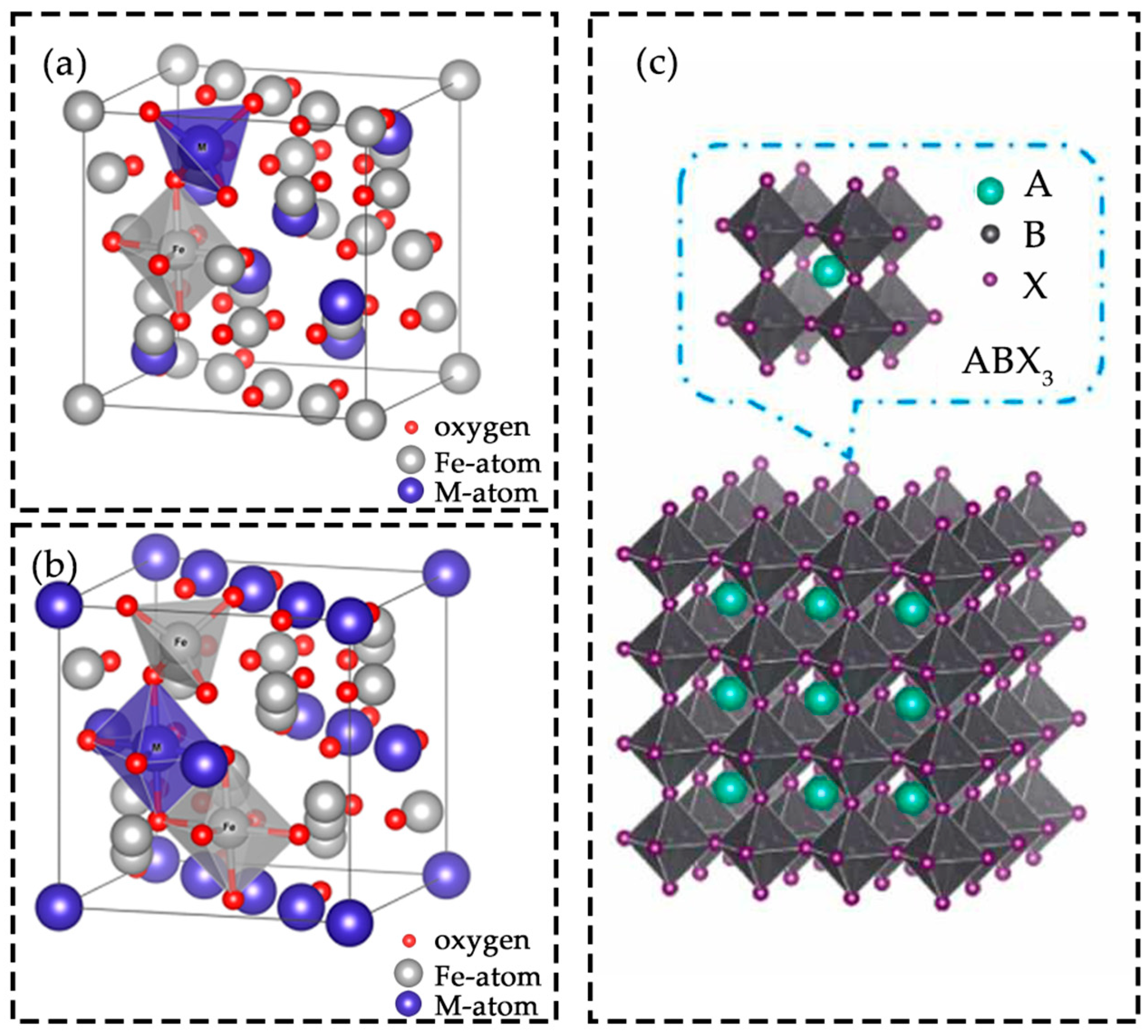
2.1. Spinel Structure
2.2. Perovskite Structure
2.3. Other
2.3.1. Other Representative Structures
2.3.2. Other Classification Criteria
3. Non-Metallic Doping
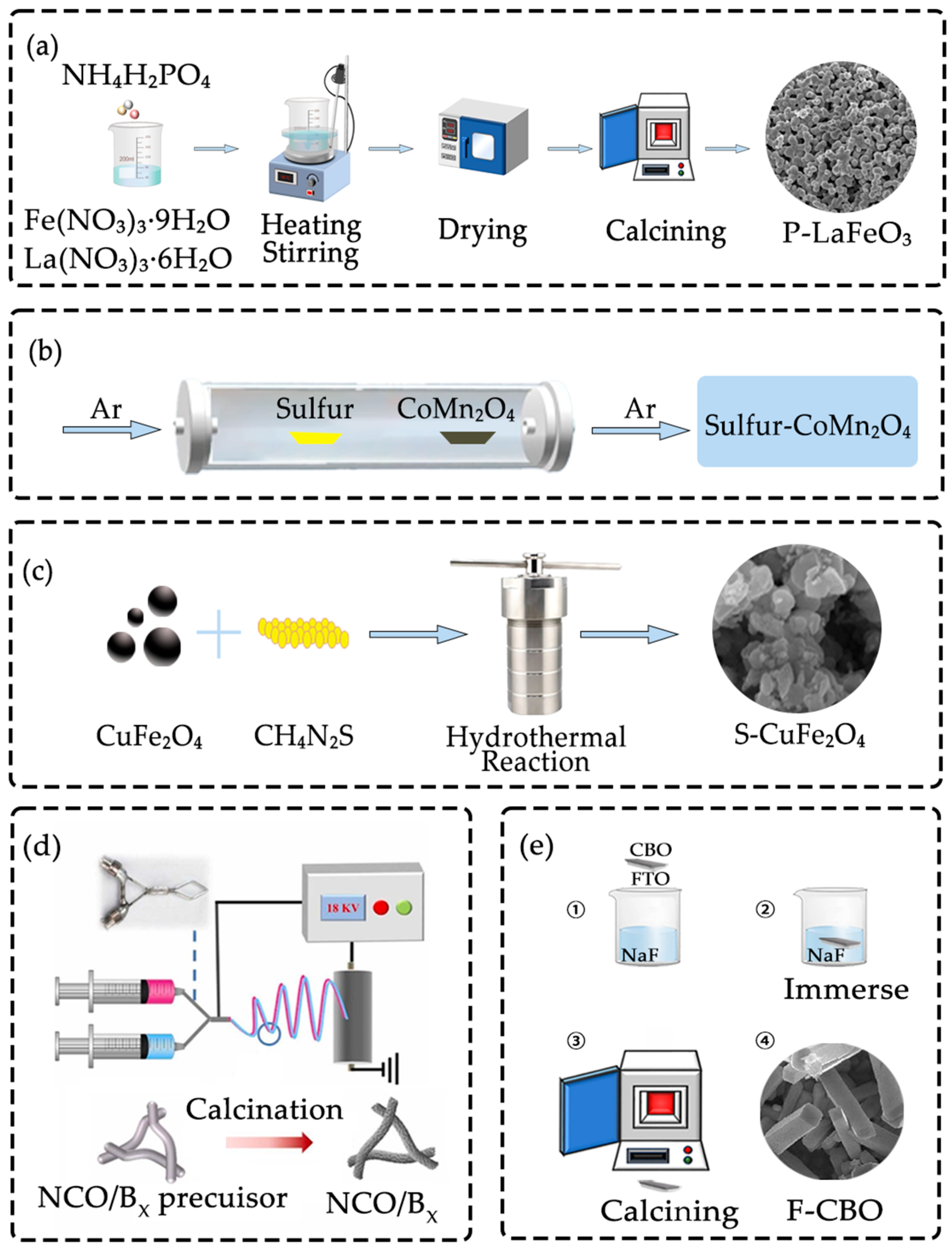
3.1. Doping Methods
3.1.1. Sol–Gel Method
3.1.2. Chemical Vapor Deposition (CVD)
3.1.3. Hydrothermal Method
3.1.4. Electrospinning Annealing Method
3.1.5. Impregnation Post Treatment Method
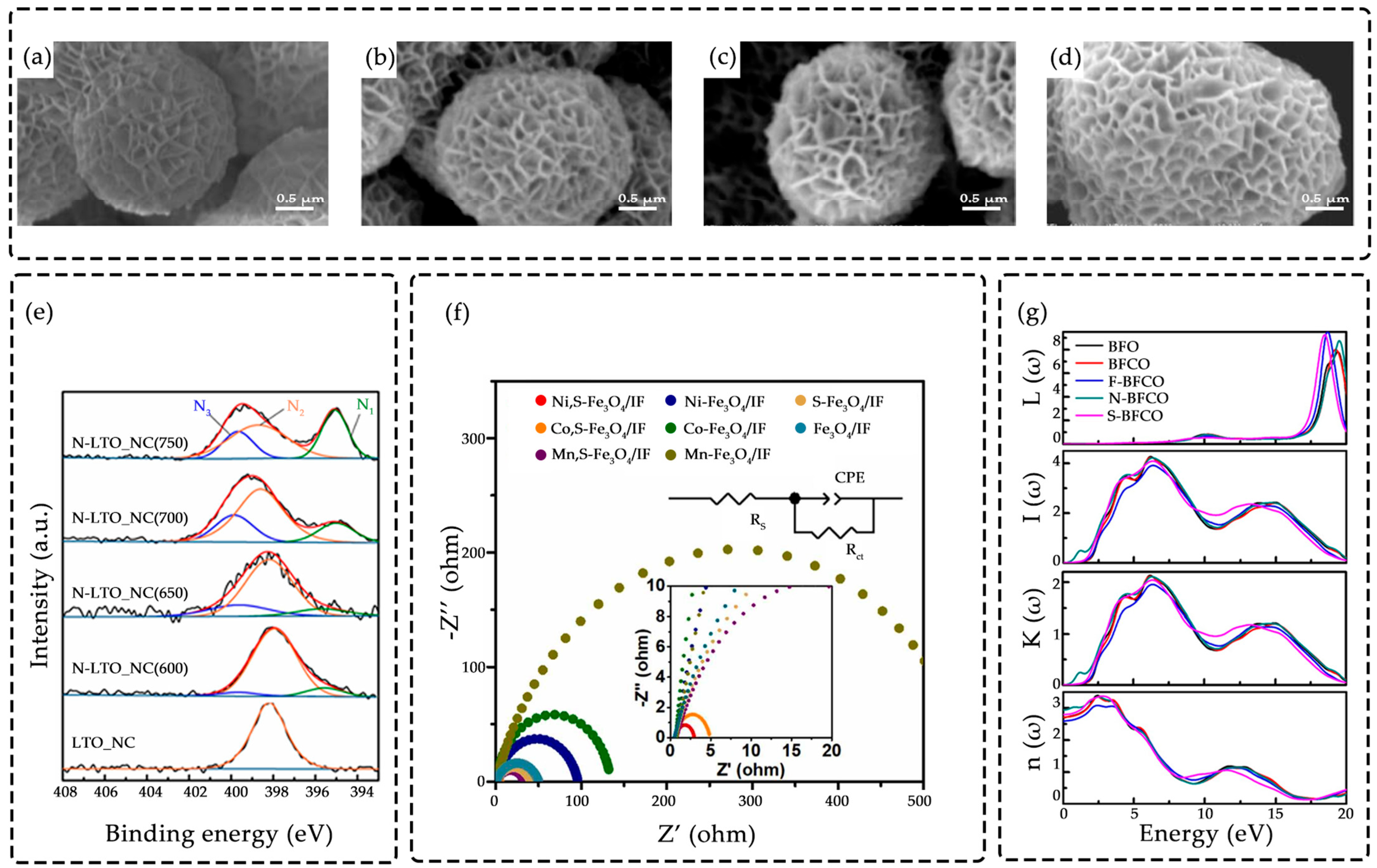
3.2. Systematic Characterization Techniques for Non-Metallic Doping
3.2.1. Characterization of Elemental Presence, Chemical States, and Distribution
3.2.2. Characterization of Effects on Crystal Structure and Defects
3.2.3. Characterization of Regulation of Electronic Structure and Optical Properties
3.2.4. Verification of Functional Performance
4. Regulation of Non-Metallic Doping
4.1. Doping Modes and Structural Effects
4.1.1. Oxygen Site Substitution
4.1.2. Cationic B Site Substitution
4.1.3. Interstitial Doping
4.1.4. Mixed Doping
4.1.5. Surface Confined Doping

4.2. Type of Nonmetallic Element

4.3. Doping Concentration
4.4. Co-Doping with Other Elements
5. Mechanisms of Structure and Electronic State Regulation by Non-Metallic Doping
5.1. Lattice Distortion
5.2. Crystallinity
5.3. Electronic Structure Reconfiguration
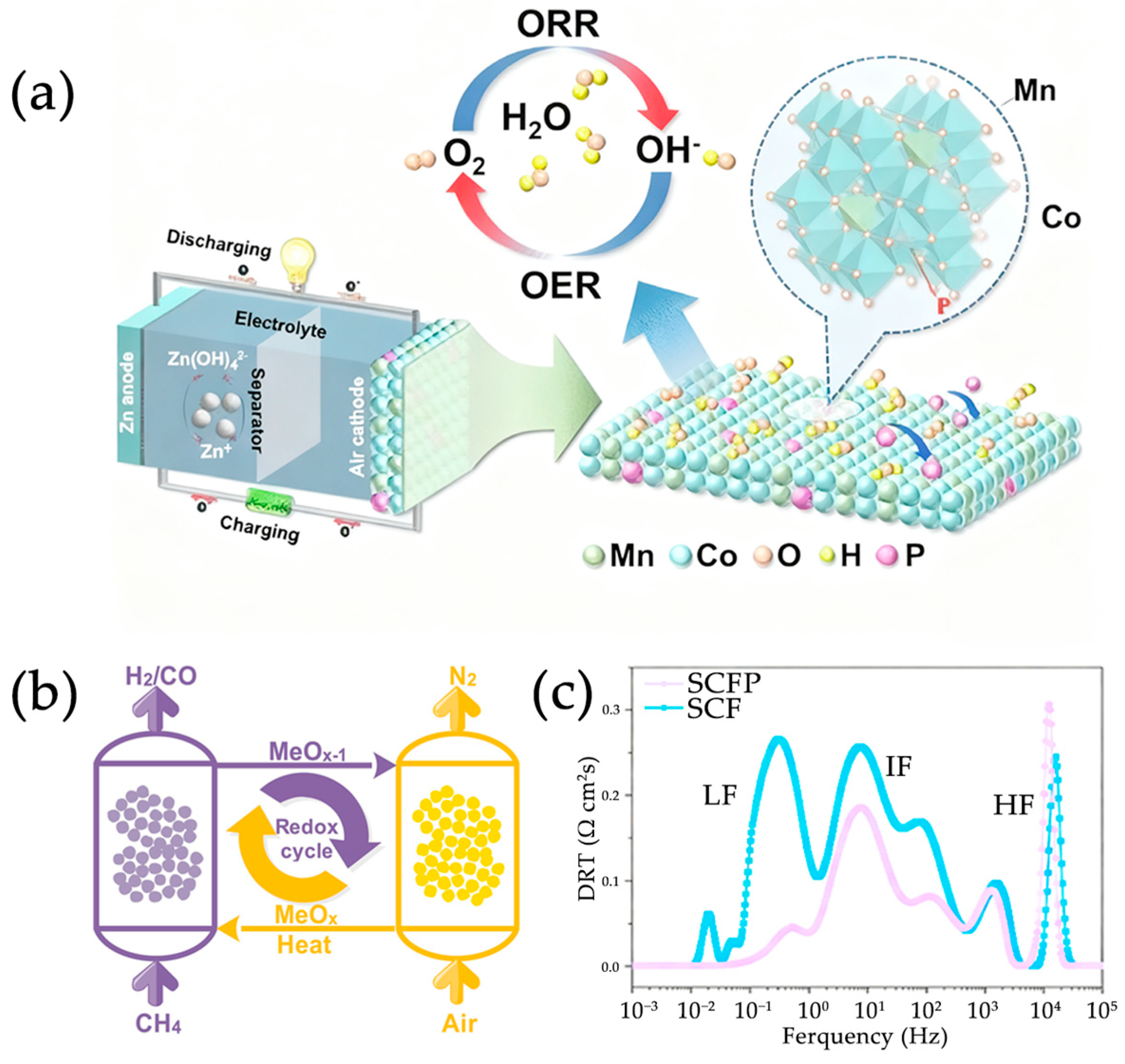
6. Application Fields of Non-Metallic Doping
6.1. Field of Energy
| Doping Element | Material | Application |
|---|---|---|
| B | MnFe2O4 | Seawater Electrolysis |
| CoFe2O4 | HER, OER | |
| C | CoFe2O4/Fe2O3 | Hydrogen Production |
| C,Fe | LaCoO3 | Photocatalytic CO2 Reduction |
| N | Li4Ti5O12 | LIBs |
| LaMnO3 | SFOF | |
| BiVO4 | OER | |
| F | Li4Ti5O12 | LIBs |
| SrFeO3−δ | Electrocatalytic | |
| La0.5Ba0.5FeO3−δ, La0.5Ba0.5Cu0.5FeO3−δ | SOFC | |
| LaCoO3 | ZIBs | |
| P | LaFeO3−δ | Metal-Air Battery |
| SrCo0.8Fe0.2O3−δ | PCFCs | |
| LaFeO3 | CLPOM | |
| SrFeO3−δ | SOFC | |
| MnCo2O4 | Supercapacitor | |
| CoMoO4 | Hydrogen Production | |
| NiMoO4 | HER, OER | |
| S | LaNiO3 | ORR, OER |
| NiCo2O4 | ZIBs | |
| S,Co | Li4Ti5O12 | LIBs |
| I | Li4Ti5O12 | LIBs |
6.2. Non-Metal Doping Strategies and Composite Engineering for Energy Material Performance Enhancement
7. Conclusion Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bui, T.H.; Shin, J.H. Perovskite Materials for Sensing Applications: Recent Advances and Challenges. Microchem. J. 2023, 191, 108924. [Google Scholar] [CrossRef]
- Yang, W.-D.; Zhao, R.-D.; Guo, F.-Y.; Xiang, J.; Loy, S.; Liu, L.; Dai, J.-Y.; Wu, F.-F. Interface Engineering of Hybrid ZnCo2O4@Ni2.5Mo6S6.7 Structures for Flexible Energy Storage and Alkaline Water Splitting. Chem. Eng. J. 2023, 454, 140458. [Google Scholar] [CrossRef]
- Park, J.-S.; Choi, S.; Yan, Y.; Yang, Y.; Luther, J.M.; Wei, S.-H.; Parilla, P.; Zhu, K. Electronic Structure and Optical Properties of α-CH3NH3PbBr3 Perovskite Single Crystal. J. Phys. Chem. Lett. 2015, 6, 4304–4308. [Google Scholar] [CrossRef]
- Zhuang, G.; Chen, Y.; Zhuang, Z.; Yu, Y.; Yu, J. Oxygen Vacancies in Metal Oxides: Recent Progress towards Advanced Catalyst Design. Sci. China Mater. 2020, 63, 2089–2118. [Google Scholar] [CrossRef]
- Shao, Z.; Wu, X.; Wu, X.; Feng, S.; Huang, K. Synthesis and Advantages of Spinel-Type Composites. Mater. Chem. Front. 2023, 7, 5288–5308. [Google Scholar] [CrossRef]
- Taffa, D.H.; Dillert, R.; Ulpe, A.C.; Bauerfeind, K.C.L.; Bredow, T.; Bahnemann, D.W.; Wark, M. Photoelectrochemical and Theoretical Investigations of Spinel Type Ferrites (MxFe3−xO4) for Water Splitting: A Mini-Review. J. Photonics Energy 2016, 7, 012009. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Meng, F. Spinel-Type Materials Used for Gas Sensing: A Review. Sensors 2020, 20, 5413. [Google Scholar] [CrossRef]
- Yan, J.; Yi, X.; Zhao, W.; Zheng, Y. Nonmetallic Phosphorus-Driven Oxygen Vacancy and Electronic Structure Modulation Enhancing Bifunctional Catalytic Activity of Cobalt-Based Spinel Air Electrode for Rechargeable Zn–Air Batteries. ACS Appl. Mater. Interfaces 2025, 17, 47057–47069. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Yang, Y.; Li, C.; Shi, Z.; Feng, S. Manipulation of Electrochemical Surface Reconstruction on Spinel Oxides for Boosted Water Oxidation Reaction. ACS Catal. 2025, 15, 8361–8389. [Google Scholar] [CrossRef]
- Wen, J.; Rong, K.; Jiang, L.; Wen, C.; Wu, B.; Sa, B.; Qiu, Y.; Ahuja, R. Copper-Based Perovskites and Perovskite-like Halides: A Review from the Perspective of Molecular Level. Nano Energy 2024, 128, 109802. [Google Scholar] [CrossRef]
- Grabowska, E. Selected Perovskite Oxides: Characterization, Preparation and Photocatalytic Properties—A Review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Chuangchote, S.; Roongraung, K. Perovskite Materials for Hydrogen Production. In Materials for Hydrogen Production, Conversion, and Storage; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 539–573. ISBN 978-1-119-82958-4. [Google Scholar]
- Liu, D.; Zhou, P.; Bai, H.; Ai, H.; Du, X.; Chen, M.; Liu, D.; Ip, W.F.; Lo, K.H.; Kwok, C.T.; et al. Development of Perovskite Oxide-Based Electrocatalysts for Oxygen Evolution Reaction. Small 2021, 17, 2101605. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sawada, S. The Study on Substances Having the Ilmenite Structure I. Physical Properties of Synthesized FeTiO3 and NiTiO3 Ceramics. J. Phys. Soc. Jpn. 1956, 11, 496–501. [Google Scholar] [CrossRef]
- Jo, H.J.; Kim, E.S. Dependence of Microwave Dielectric Properties on the Complex Substitution for Ti-Site of MgTiO3 Ceramics. Ceram. Int. 2017, 43, S326–S333. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, Y.; Zhang, R. Ilmenite Structure-Type β-CdSnO3 Used as an Ethanol Sensing Material. Mater. Lett. 1995, 23, 69–71. [Google Scholar] [CrossRef]
- Ermawati, F.U. The Use of Liquid Mixing Method to Prepare Ilmenite Structure-Based Ceramic Powders. J. Phys. Conf. Ser. 2020, 1491, 012004. [Google Scholar] [CrossRef]
- Hall, J.N.; Kropf, A.J.; Delferro, M.; Bollini, P. Kinetic and X-Ray Absorption Spectroscopic Analysis of Catalytic Redox Cycles over Highly Uniform Polymetal Oxo Clusters. ACS Catal. 2023, 13, 5406–5427. [Google Scholar] [CrossRef]
- Huang, Z.-W.; Hu, K.-Q.; Mei, L.; Wang, D.-G.; Wang, J.-Y.; Wu, W.-S.; Chai, Z.-F.; Shi, W.-Q. Encapsulation of Polymetallic Oxygen Clusters in a Mesoporous/Microporous Thorium-Based Porphyrin Metal–Organic Framework for Enhanced Photocatalytic CO2 Reduction. Inorg. Chem. 2022, 61, 3368–3373. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the Structural and Electrochemical Properties of Layered Li[NixCoyMnz]O2 (x= 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) Cathode Material for Lithium-Ion Batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, M.; Li, J.; Lu, Y.; Wang, L.; Wang, Z.; Zhang, X.; Ding, X. Modulation of Electronic Structure and Oxygen Vacancies of Perovskites SrCoO3−δ by Sulfur Doping Enables Highly Active and Stable Oxygen Evolution Reaction. Electrochim. Acta 2021, 390, 138872. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, J.; Lu, Y.; Li, S.; Han, S.; Liu, C.; Ma, Z. Non-Metallic Phosphorus-Induced Lattice Engineering in LaFeO3 Perovskite: Oxygen Vacancy Modulation for Enhanced Methane Conversion in Chemical Looping Partial Oxidation. Chem. Eng. J. 2025, 520, 165963. [Google Scholar] [CrossRef]
- Cai, F.; Sun, C.; Sun, Z.; Lai, Y.; Ding, H. Sulfur-Functionalized CoMn2O4 as a Fenton-like Catalyst for the Efficient Rhodamine B Degradation. Appl. Surf. Sci. 2023, 623, 157044. [Google Scholar] [CrossRef]
- Li, Z.; Lv, L.; Wang, J.; Ao, X.; Ruan, Y.; Zha, D.; Hong, G.; Wu, Q.; Lan, Y.; Wang, C.; et al. Engineering Phosphorus-Doped LaFeO3−δ Perovskite Oxide as Robust Bifunctional Oxygen Electrocatalysts in Alkaline Solutions. Nano Energy 2018, 47, 199–209. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Wang, X.; Jia, L.; Jiang, H.; Huang, M.; Toghan, A. Nitrogen-Doped Sr2Fe1.5Mo0.5O6-δ Perovskite as an Efficient and Stable Catalyst for Hydrogen Evolution Reaction. Mater. Today Energy 2021, 20, 100695. [Google Scholar] [CrossRef]
- Aslam, A.; Abid, M.Z.; Rafiq, K.; Rauf, A.; Hussain, E. Tunable Sulphur Doping on CuFe2O4 Nanostructures for the Selective Elimination of Organic Dyes from Water. Sci. Rep. 2023, 13, 6306. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Xing, Y.; Liu, H.; Jin, X.; Kou, B.; Ni, G. Electronic Structure Modulation and Peroxymonosulfate Activation Mechanism of N-Doped MnCo2O4: A Study on the Efficient Catalytic Degradation of Sulfamethoxazole. Appl. Surf. Sci. 2025, 685, 161976. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Dual Strategies to Construct Electrospun NiCo2O4/Boron-Doped LaCoO3 Heterojunction Catalysts to Activate Peroxymonosulfate for Organic Pollutant Degradation. J. Alloys Compd. 2023, 968, 172184. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Hu, X.; Wu, X.; Chen, W.; Zhou, S.; Li, J.; Qi, C.; Ma, D.-K. Fluorine-Doped CuBi2O4 Nanorod Arrays for Enhanced Photoelectrochemical Oxygen Reduction Reaction toward H2O2 Production. J. Catal. 2022, 410, 339–346. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Zhao, Q.; Abudula, A.; Ma, Y.; Yan, K.; Guan, G. Tuning Octahedron Sites in MnFe2O4 Spinel by Boron Doping for Highly Efficient Seawater Splitting. Appl. Catal. B Environ. 2023, 330, 122577. [Google Scholar] [CrossRef]
- Kavitha, S.; Kurian, M. Acetylation of Glycerol for Triacetin Production over Metal/Non-Metal Doped Cobalt Ferrite Nanocatalysts. Braz. J. Chem. Eng. 2024, 41, 399–407. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Zhang, X.; Wang, J.; Nie, P.; Che, Q.; Ding, B. Nitrogen-Doped Carbon Coated Li4Ti5O12 Nanocomposite: Superior Anode Materials for Rechargeable Lithium Ion Batteries. J. Power Sources 2013, 221, 122–127. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Niu, B.; Wang, L.; Li, M.; Yao, W.; Zang, K.; Zhou, L.; Hu, X.; Zheng, Y. Lattice B-Doping Evolved Ferromagnetic Perovskite-like Catalyst for Enhancing Persulfate-Based Degradation of Norfloxacin. J. Hazard. Mater. 2022, 425, 127949. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Z.; Di, H.; Yang, M.; Yang, G.; Wang, W.; Ran, R.; Zhou, W. High-Performance Phosphorus-Doped SrCo0.8Fe0.2O3−δ Cathode for Protonic Ceramic Fuel Cells. Ceram. Int. 2024, 50, 40409–40416. [Google Scholar] [CrossRef]
- Han, X.; Liu, P.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Non-Metal Fluorine Doping in Ruddlesden–Popper Perovskite Oxide Enables High-Efficiency Photocatalytic Water Splitting for Hydrogen Production. Mater. Today Energy 2022, 23, 100896. [Google Scholar] [CrossRef]
- Środoń, J.; Andreoli, C.; Elsass, F.; Robert, M. Direct High-Resolution Transmission Electron Microscopic Measurement of Expandability of Mixed-Layer Illite/Smectite in Bentonite Rock. Clays Clay Miner. 1990, 38, 373–379. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, H.; Dai, X.; Nie, F.; Ren, Z.; Yin, X.; Gan, Y.; Wu, B.; Cao, Y.; Zhang, X. Phosphorus-Doping Induced Electronic Modulation of CoS2–MoS2 Hollow Spheres on MoO2 Film-Mo Foil for Synergistically Boosting Alkaline Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 33388–33396. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, H.; Xiao, Y.; Hu, H.; Cai, Y.; Liang, Y.; Sun, L.; Liu, Y.; Zheng, M. Three-Dimensional Nitrogen-Doped Graphene as Binder-Free Electrode Materials for Supercapacitors with High Volumetric Capacitance and the Synergistic Effect between Nitrogen Configuration and Supercapacitive Performance. Electrochim. Acta 2016, 218, 32–40. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect Engineering in Photocatalytic Materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Xie, S.; Sun, W.; Sun, J.; Wan, X.; Zhang, J. Apparent Symmetry Rising Induced by Crystallization Inhibition in Ternary Co-Crystallization-Driven Self-Assembly. Nat. Commun. 2023, 14, 6496. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Wu, H. Development of High Efficient Visible Light-Driven N, S-Codoped TiO2 Nanowires Photocatalysts. Appl. Surf. Sci. 2015, 328, 335–343. [Google Scholar] [CrossRef]
- Wen, C.Z.; Hu, Q.H.; Guo, Y.N.; Gong, X.Q.; Qiao, S.Z.; Yang, H.G. From Titanium Oxydifluoride (TiOF2) to Titania (TiO2): Phase Transition and Non-Metal Doping with Enhanced Photocatalytic Hydrogen (H2) Evolution Properties. Chem. Commun. 2011, 47, 6138–6140. [Google Scholar] [CrossRef]
- Chaitra, U. Impact of Defect Sites on the Raman Scattering Properties of Nitrogen Doped ZnO Thin Films. Inorg. Chem. Commun. 2021, 131, 108784. [Google Scholar] [CrossRef]
- Bartasyte, A.; Chaix-Pluchery, O.; Kreisel, J.; Santiso, J.; Margueron, S.; Boudard, M.; Jimenez, C.; Abrutis, A.; Weiss, F. Raman Spectroscopy and X-Ray Diffraction Studies of Stress Effects in PbTiO/Sub 3/Thin Films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 2623–2631. [Google Scholar] [CrossRef]
- Yu, X.; Han, P.; Wei, Z.; Huang, L.; Gu, Z.; Peng, S.; Ma, J.; Zheng, G. Boron-Doped Graphene for Electrocatalytic N2 Reduction. Joule 2018, 2, 1610–1622. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-Free Synthesis of 2D Porous Ultrathin Nonmetal-Doped g-C3N4 Nanosheets with Highly Efficient Photocatalytic H2 Evolution from Water under Visible Light. Appl. Catal. B Environ. 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Cui, K.; Liu, Y.; Lv, L. Mn and P Co-Doped Biochar Catalyst for Persulfate Efficient Degradation of Tetracycline hydrochloride: Process and Mechanism. J. Environ. Chem. Eng. 2024, 12, 114228. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Huang, Y.; Zhou, Z.; Shen, S. Reduction of Oxygen Vacancy and Enhanced Efficiency of Perovskite Solar Cell by Doping Fluorine into TiO2. J. Alloys Compd. 2016, 681, 191–196. [Google Scholar] [CrossRef]
- Jia, L.; Li, J.; Fang, W. Enhanced Visible-Light Active C and Fe Co-Doped LaCoO3 for Reduction of Carbon Dioxide. Catal. Commun. 2009, 11, 87–90. [Google Scholar] [CrossRef]
- Xu, M.; Wang, W.; Liu, Y.; Zhong, Y.; Xu, X.; Sun, Y.; Wang, J.; Zhou, W.; Shao, Z. An Intrinsically Conductive Phosphorus-Doped Perovskite Oxide as a New Cathode for High-Performance Dye-Sensitized Solar Cells by Providing Internal Conducting Pathways. Sol. RRL 2019, 3, 1900108. [Google Scholar] [CrossRef]
- Helmy, E.T.; Nemr, A.E.; Arafa, E.; Eldafrawy, S.; Mousa, M. Photocatalytic Degradation of Textile Dyeing Wastewater under Visible Light Irradiation Using Green Synthesized Mesoporous Non-Metal-Doped TiO2. Bull. Mater. Sci. 2021, 44, 30. [Google Scholar] [CrossRef]
- Naik, S.S.; Lee, S.J.; Yeon, S.; Yu, Y.; Choi, M.Y. Pulsed Laser-Assisted Synthesis of Metal and Nonmetal-Codoped ZnO for Efficient Photocatalytic Degradation of Rhodamine B under Solar Light Irradiation. Chemosphere 2021, 274, 129782. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Goswami, T.; Yadav, D.K.; Bhatt, H.; Kaur, G.; Shukla, A.; Babu, K.J.; Ghosh, H.N. Defect-Mediated Slow Carrier Recombination and Broad Photoluminescence in Non-Metal-Doped ZnIn2S4 Nanosheets for Enhanced Photocatalytic Activity. J. Phys. Chem. Lett. 2021, 12, 5000–5008. [Google Scholar] [CrossRef]
- El-Hosainy, H.M.; El-Sheikh, S.M.; Ismail, A.A.; Hakki, A.; Dillert, R.; Killa, H.M.; Ibrahim, I.A.; Bahnemann, D.W. Highly Selective Photocatalytic Reduction of O-Dinitrobenzene to o-Phenylenediamine over Non-Metal-Doped TiO2 under Simulated Solar Light Irradiation. Catalysts 2018, 8, 641. [Google Scholar] [CrossRef]
- Hoy-Benítez, J.A.; Colina-Ruiz, R.A.; Lezama-Pacheco, J.S.; Mustre de León, J.; Espinosa-Faller, F.J. Local Atomic Structure and Lattice Defect Analysis in Heavily Co-Doped ZnS Thin Films Using X-Ray Absorption Fine Structure Spectroscopy. J. Phys. Chem. Solids 2020, 136, 109154. [Google Scholar] [CrossRef]
- Avashthi, G.; Maktedar, S.S.; Singh, M. Scrutinizing XAFS Spectroscopy and Biocompatibility of N-Doped Edge-Functionalized Graphene Oxide. Acta Crystallogr. Sect. Found. Adv. 2017, 73, C690. [Google Scholar] [CrossRef]
- Wang, D.; Guo, X.; Zhang, G.; Liu, Y.; Liu, S.; Zhang, Z.; Chai, Y.; Chen, Y.; Zhang, J.; Sun, B. SnO2 Electron Transport Layer Modified by F/N-Doped Graphdiyne and in Situ XRD and in Situ XAFS Exploration on Its Effect on Perovskite Active Layer. Nano Today 2023, 50, 101852. [Google Scholar] [CrossRef]
- Wen, J.; Li, N.; Shi, Q.; Wu, H.; Feng, X.; Wang, C.; Zhang, J. First-Principles Calculations to Investigate Electronic Structures and Magnetic Regulation of Non-Metallic Elements Doped BP with Point Defects. J. Mol. Graph. Model. 2023, 118, 108370. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; He, X.; Wei, Q.; Zhang, J.; Wei, B.; Li, M. Effects of Non-Metal Doping in Graphene-like Structure: Engineering Narrow Bandgaps and Reserved Carrier Mobility. J. Alloys Compd. 2025, 1035, 181578. [Google Scholar] [CrossRef]
- Fan, C.; Zang, Z.; Zhang, X. Non-Metal Doping Regulation in Transition Metal and Their Compounds for Electrocatalytic Water Splitting. Int. J. Hydrogen Energy 2024, 56, 1273–1283. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; He, S.; Tjiu, W.W.; Pan, J.; Xia, Y.-Y.; Liu, T. Nitrogen-Doped Graphene Nanoribbons as Efficient Metal-Free Electrocatalysts for Oxygen Reduction. ACS Appl. Mater. Interfaces 2014, 6, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, B.; Yang, Y.; Zhao, M.; Fang, Y.; Cui, Y.; Tian, J. Enhanced Electrocatalytic Performance of P-Doped MoS2/rGO Composites for Hydrogen Evolution Reactions. Molecules 2025, 30, 1205. [Google Scholar] [CrossRef]
- Preetha, R.; Govinda raj, M.; Vijayakumar, E.; Narendran, M.G.; Varathan, E.; Neppolian, B.; Jeyapaul, U.; Bosco, A.J. Promoting Photocatalytic Interaction of Boron Doped Reduced Graphene Oxide Supported BiFeO3 Nanocomposite for Visible-Light-Induced Organic Pollutant Degradation. J. Alloys Compd. 2022, 904, 164038. [Google Scholar] [CrossRef]
- Angineni, R.; Venkataswamy, P.; Veldurthi, N.K.; Ramaswamy, K.; Sudheera, M.; Vithal, M. Photocatalytic Degradation Studies of Carbon and Sulfur-Doped K2Ta2O6. J. Mater. Sci. Mater. Electron. 2023, 34, 633. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-Doped Graphene and Its Electrochemical Applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Hu, Q.; Li, G.; Han, Z.; Wang, Z.; Huang, X.; Yang, H.; Zhang, Q.; Liu, J.; He, C. Nonmetal Doping as a Robust Route for Boosting the Hydrogen Evolution of Metal-Based Electrocatalysts. Chem.–Eur. J. 2020, 26, 3930–3942. [Google Scholar] [CrossRef]
- Noerochim, L.; Wibowo, A.T.; Widyastuti; Subhan, A.; Prihandoko, B.; Caesarendra, W. Direct Double Coating of Carbon and Nitrogen on Fluoride-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries. Batteries 2022, 8, 5. [Google Scholar] [CrossRef]
- Sun, N.; Zeng, Z.; Zhang, L.; Li, L.; Song, C.; Yang, J. Boron-Doping P2/O3 Biphasic Na0.95Mn0.6Ni0.4O2 for High Performance Cathode Materials of Sodium-Ion Batteries. J. Energy Storage 2025, 135, 118379. [Google Scholar] [CrossRef]
- Mahara, Y.; Oka, H.; Nonaka, T.; Kosaka, S.; Takahashi, N.T.; Kondo, Y.; Makimura, Y. Activation and Stabilization of Mn-Based Positive Electrode Materials by Doping Nonmetallic Elements. Adv. Energy Mater. 2023, 13, 2301843. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, S.; Li, X.; Shi, J.; Mao, Y.; Wang, M.; Tan, F. P-Doped NiMn2O4 Hollow Tubular Nanofiber Spinel Composites for Electrocatalytic Dechlorination. ACS Appl. Nano Mater. 2024, 7, 1713–1722. [Google Scholar] [CrossRef]
- Liu, H.-J.; Zhang, S.; Fan, R.-Y.; Liu, B.; Lv, R.-Q.; Chai, Y.-M.; Dong, B. Activated M,S Co-Doping (M = Ni, Co, Mn) Inverse Spinel Oxides with Mixed Mechanisms for Water Oxidation. Appl. Catal. B Environ. 2024, 343, 123567. [Google Scholar] [CrossRef]
- Yuan, B.; Lai, F.; Yan, R.; Yu, Q.; Wang, T.; Gao, H.; Hao, R. Nonmetallic Boron-Doped LaMnO3 with Synergistic Enhancement in Oxidation Activity and Sulfur Resistance for Elemental Mercury Removal. Ceram. Int. 2025, 51, 43626–43636. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, Y.; Jing, Y.; Yao, Y.; Ma, J.; Sun, J. Effect of Non-Metal Elements (B, C, N, F, P, S) Mono-Doping as Anions on Electronic Structure of SrTiO3–ScienceDirect. Comput. Mater. Sci. 2013, 79, 69–74. [Google Scholar] [CrossRef]
- Xing, T.; Ouyang, Y.; Chen, Y.; Zheng, L.; Wu, C.; Wang, X. P-Doped Ternary Transition Metal Oxide as Electrode Material of Asymmetric Supercapacitor. J. Energy Storage 2020, 28, 101248. [Google Scholar] [CrossRef]
- Wang, J.; Asakura, Y.; Hasegawa, T.; Yin, S. High-Concentration N-Doped La2Ti2O7 Nanocrystals: Effects of Nano-Structuration and Doping Sites on Enhancing the Photocatalytic Activity. Chem. Eng. J. 2021, 423, 130220. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, Y.; Wang, Z.; Mao, W.; Weng, Y.; Zhang, J.; Pu, Y.; Yang, J.; Li, X. Effect of Non-Metallic X(X=F, N, S) and Cr Co-Doping on Properties of BiFeO3: A First-Principles Study. Phys. Lett. A 2019, 383, 383–388. [Google Scholar] [CrossRef]
- Matsoso, B.J.; Ranganathan, K.; Mutuma, B.K.; Lerotholi, T.; Jones, G.; Coville, N.J. Time-Dependent Evolution of the Nitrogen Configurations in N-Doped Graphene Films. RSC Adv. 2016, 6, 106914–106920. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Zhao, Y.; Zeng, X. Nb/N Co-Doped Layered Perovskite Sr2TiO4: Preparation and Enhanced Photocatalytic Degradation Tetracycline under Visible Light. Int. J. Mol. Sci. 2022, 23, 10927. [Google Scholar] [CrossRef]
- Quyyum, U.; Rafiq, K.; Abid, M.Z.; Ahmad, F.; Rauf, A.; Hussain, E. Tunable Sulphur Doping in CuFe2O4 for the Efficient Removal of Arsenic through Arsenomolybdate Complex Adsorption: Kinetics, Isothermal and Mechanistic Studies. Environ. Sci. Water Res. Technol. 2023, 9, 1147–1160. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, C.; Zhao, S.-N.; Wu, Y.; Liu, C.; Huang, J.; Xue, L.; Sun, J.; Zhang, W.; Wang, X.; et al. A Dual-Site Doping Strategy for Developing Efficient Perovskite Oxide Electrocatalysts towards Oxygen Evolution Reaction. Nano Energy 2022, 99, 107344. [Google Scholar] [CrossRef]
- Bu, X.; Li, J.; Wang, J.; Li, Y.; Zhang, G. Boosting Charge Transfer Promotes Photocatalytic Peroxymonosulfate Activation of S-Doped CuBi2O4 Nanorods for Ciprofloxacin Degradation: Key Role of Ov–Cu–S and Mechanism Insight. Chem. Eng. J. 2024, 494, 153075. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, M.; Qin, J.; Zhou, H.; Ding, Z. Reinforced Photocatalytic Reduction of CO2 to CO by a Ternary Metal Oxide NiCo2O4. Phys. Chem. Chem. Phys. 2015, 17, 16040–16046. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xiang, X.; Liu, Y.; Yang, X.; Shi, N.; Xu, D.; Zhou, C.; Han, M.; Bao, J.; Huang, W. Defect-Rich High-Entropy Spinel Oxide as an Efficient and Robust Oxygen Evolution Catalyst for Seawater Electrolysis. SusMat 2025, 5, e70010. [Google Scholar] [CrossRef]
- Noerochim, L.; Prabowo, R.S.; Widyastuti, W.; Susanti, D.; Subhan, A.; Idris, N.H. Enhanced High-Rate Capability of Iodide-Doped Li4Ti5O12 as an Anode for Lithium-Ion Batteries. Batteries 2023, 9, 38. [Google Scholar] [CrossRef]
- Ballal, R.S.; Kalubarme, R.S.; Jayswal, M.S.; Chothe, U.P.; Kulkarni, M.V.; Kale, B.B. N-Doped Lithium Titanium Oxide for High Power Li-Ion Rechargeable Batteries with Excellent Cyclability. Inorg. Chem. Commun. 2024, 161, 112013. [Google Scholar] [CrossRef]
- Zhu, S.; Du, C.; Zhang, J.; Zhang, J. The Effects of Fluorine Doping on the Structure, Interface and Electrochemical Properties of Lithium Titanate. Ceram. Int. 2024, 50, 25095–25102. [Google Scholar] [CrossRef]
- Bai, L.; Pan, B.; Song, F.; Chen, Q. Co/S Co-Doped Li4Ti5O12 as Lithium-Ion Batteries Anode for High-Rate. J. Energy Storage 2023, 73, 109155. [Google Scholar] [CrossRef]
- Huth, N. New Synthetic Pathways for Carbon-Doped Oxides for Battery and Related Applications. Ph.D. Thesis, The University of St Andrews, St. Andrews, Scotland, 2024. [Google Scholar]
- Liu, D.; Xu, W.; Zheng, D.; Wang, Y.; Wang, F.; Zhou, L.; Nie, Z.; Lu, X. Sulfur Doped NiCo2O4 Nanosheets as Advanced Cathode for Flexible Alkaline Zn Batteries. J. Power Sources 2023, 571, 233088. [Google Scholar] [CrossRef]
- Ran, J.; Wang, L.; Si, M.; Liang, X.; Gao, D. Tailoring Spin State of Perovskite Oxides by Fluorine Atom Doping for Efficient Oxygen Electrocatalysis. Small Weinh. Bergstr. Ger. 2023, 19, e2206367. [Google Scholar] [CrossRef]
- Mondal, S.; Sarkar, S.; Riyaz, M.; Kar, M.; Fortuin, A.C.; Vashishth, S.; Das, R.; Eswaramoorthy, M.; Kramer, D.; Peter, S.C. Nitrogen Doping-Induced Structural Distortion in LaMnO3 Enhances Oxygen Reduction and Oxygen Evolution Reactions. ACS Energy Lett. 2024, 9, 3440–3447. [Google Scholar] [CrossRef]
- Huan, D.; Zhang, L.; Zhu, K.; Li, X.; Peng, R.; Ding, D.; Xia, C. Improving Ruddlesden-Popper Electrocatalysts through Interstitial Fluorination-Driven Rearrangements of Local Coordination Environment. Sustain. Mater. Technol. 2023, 38, e00754. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; He, C.; Wang, Y.; Ni, J.; Ni, C. Unraveling the Microstructure and Defect Chemistry in Fluorine-Doped Cathode for a Protonic Solid Oxide Fuel Cell. Chem. Eng. J. 2025, 505, 159774. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, C.; Zhang, Z.; Xia, B.; Zhang, H.; Ding, J.; Zhang, L.; Zhang, W.; Lang, X.; Cai, K. Non-Metal Doping Enhsances Oxygen Reduction Kinetics and CO2 Tolerance of SrFeO3−δ Perovskite as High-Performance Cathodes for Solid Oxide Fuel Cells. Fuel 2024, 378, 132917. [Google Scholar] [CrossRef]
- Feng, W.; Pu, W.; Zheng, Y.; Wu, H.; Li, L.; Wei, X. Excellent Rate Capability Supercapacitor Electrodes with Highly Hydroxyl Ion Adsorption Capacity Enabled by P-Doped MnCo2O4 Nanotube Arrays. Appl. Surf. Sci. 2022, 599, 153908. [Google Scholar] [CrossRef]
- Farooq, A.; Khalil, S.; Basha, B.; Habib, A.; Al-Buriahi, M.S.; Warsi, M.F.; Yousaf, S.; Shahid, M. Electrochemical Investigation of C-Doped CoFe2O4/Fe2O3 Nanostructures for Efficient Electrochemical Water Splitting. Int. J. Hydrogen Energy 2024, 51, 1318–1332. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Chen, H.; Yang, Y.; Wang, D.; Shang, H.; Zhang, B. Phosphorus and Sulfur Co-Doped Nickel Molybdate with Rich-Oxygen Vacancies for Efficient Water Splitting. J. Colloid Interface Sci. 2025, 677, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wen, B.; Dong, Q.; Wang, P.; Lyu, X. Boron Doped CoFe2O4 with Enriched Oxygen Vacancy as Bifunctional Electrocatalysts for Overall Water Splitting. Int. J. Hydrogen Energy 2025, 105, 556–564. [Google Scholar] [CrossRef]
- Li, R.; Long, J.; Li, M.; Du, D.; Ren, L.; Zhou, B.; Zhao, C.; Xu, H.; Wen, X.; Zeng, T.; et al. Sulfur-Doped LaNiO3 Perovskite Oxides with Enriched Anionic Vacancies and Manipulated Orbital Occupancy Facilitating Oxygen Electrode Reactions in Lithium-Oxygen Batteries. Mater. Today Chem. 2022, 24, 100889. [Google Scholar] [CrossRef]
- Liang, Q.; Ouhbi, H.; Österbacka, N.; Ambrosio, F.; Wiktor, J. Defect Engineering in BiVO4 Photoanodes: The Synergistic Role of Nitrogen Doping and Oxygen Vacancy for Oxygen Evolution Reaction. J. Phys. Energy 2025, 7, 045030. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, C.; Chen, P.; Chu, X.; Zhu, L. Enhanced Photocatalytic Performance of Boron Doped Bi2WO6 Nanosheets under Simulated Solar Light Irradiation. J. Hazard. Mater. 2013, 254–255, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, N.; Liao, L.; Luo, Y.; Wang, S.; Cao, F.; Zhou, W.; Huang, D.; Chen, H. Doping β-CoMoO4 Nanoplates with Phosphorus for Efficient Hydrogen Evolution Reaction in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 37038–37045. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Zhang, M.; Li, M.; Li, M.; Li, J.; Zhi, L. Phosphorus-Doped Nickel Cobalt Oxide (NiCo2O4) Wrapped in 3D Hierarchical Hollow N-Doped Carbon Nanoflowers as Highly Efficient Bifunctional Electrocatalysts for Overall Water Splitting. J. Colloid Interface Sci. 2024, 668, 243–251. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kang, S.H.; Roh, K.C.; An, G.-H. Enhancing Lithium Titanate Anode Performance through Surface Modification with Fluorine and Nitrogen Co-Doped Carbon Nanotubes. J. Alloys Compd. 2024, 1004, 175768. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Wen, C.; Dong, L.; Tan, C.; Li, B.; Fan, M.; He, H.; Chen, Z. Nitrogen-Doped MoO2/Cu Heterojunction Electrocatalyst for Highly Efficient Alkaline Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2024, 66, 103–109. [Google Scholar] [CrossRef]
- He, B.; Song, J.-J.; Li, X.-Y.; Xu, C.-Y.; Li, Y.-B.; Tang, Y.-W.; Hao, Q.-L.; Liu, H.-K.; Su, Z. A Nitrogen-Doped NiCo2S4/CoO Hollow Multi-Layered Heterostructure Microsphere for Efficient Oxygen Evolution in Zn–Air Batteries. Nanoscale 2021, 13, 810–818. [Google Scholar] [CrossRef]
| Characterization Dimension | Core Characterization Technique | Key Detection Targets and Evidential Role |
|---|---|---|
| Elemental Existence, Chemical State and Distribution | XPS | Detect the presence of doped elements, identify chemical states, and analyze changes in electronic structure and surface oxygen vacancy concentration. |
| SEM/TEM-EDS Mapping | Visually characterize elemental distribution at the microscale, verify uniformity, and rule out elemental segregation or formation of secondary phases. | |
| Crystal Structure and Defect Effects | XRD | Determine lattice expansion, contraction, and distortion through peak shifting and broadening. |
| HRTEM | Observe lattice fringe distortion at the atomic scale. | |
| SAED | Verify whether crystal symmetry changes due to doping. | |
| Raman | Analyze local structure and lattice defects, supplementing information from XRD | |
| EPR/ESR | Detect paramagnetic defects. | |
| Regulation of Electronic Structure and Optical Properties | UV-Vis DRS | Analyze absorption edge redshift and band gap narrowing, and correlate structural modification with enhanced light absorption. |
| TRPL | Monitor fluorescence quenching and changes in carrier lifetime. | |
| XAFS | Investigate the local coordination environment of doped elements. | |
| DFT | Simulate experimental phenomena and reveal the doping mechanism from the atomic and electronic levels. | |
| Functional Performance Verification | LSV | Measure overpotential and current density to quantify performance. |
| EIS | Analyze charge transfer resistance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Gao, J.; Kuang, Y. Non-Metallic Doping of Multinary Metal Oxide Semiconductors for Energy Applications. Catalysts 2025, 15, 1062. https://doi.org/10.3390/catal15111062
Wu Z, Gao J, Kuang Y. Non-Metallic Doping of Multinary Metal Oxide Semiconductors for Energy Applications. Catalysts. 2025; 15(11):1062. https://doi.org/10.3390/catal15111062
Chicago/Turabian StyleWu, Zhihua, Jing Gao, and Yongbo Kuang. 2025. "Non-Metallic Doping of Multinary Metal Oxide Semiconductors for Energy Applications" Catalysts 15, no. 11: 1062. https://doi.org/10.3390/catal15111062
APA StyleWu, Z., Gao, J., & Kuang, Y. (2025). Non-Metallic Doping of Multinary Metal Oxide Semiconductors for Energy Applications. Catalysts, 15(11), 1062. https://doi.org/10.3390/catal15111062