Mechanistic Insights into Radical-Mediated Moxifloxacin Degradation Using Ultrasound-Assisted Persulfate Activation by Iron-Rich Soil
Abstract
1. Introduction
2. Results and Discussion
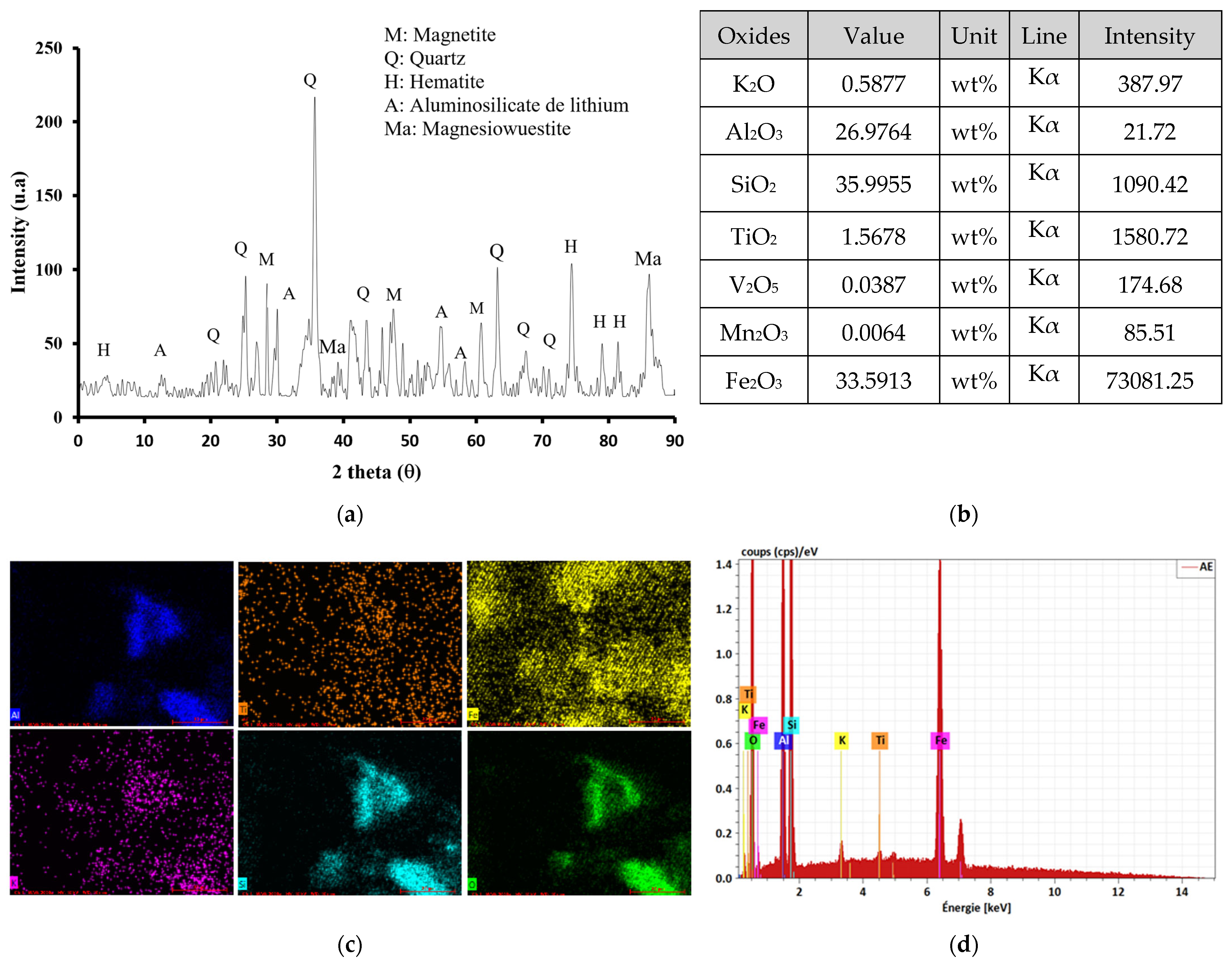
2.1. Laterite Characterization
2.1.1. Morphology and Composition
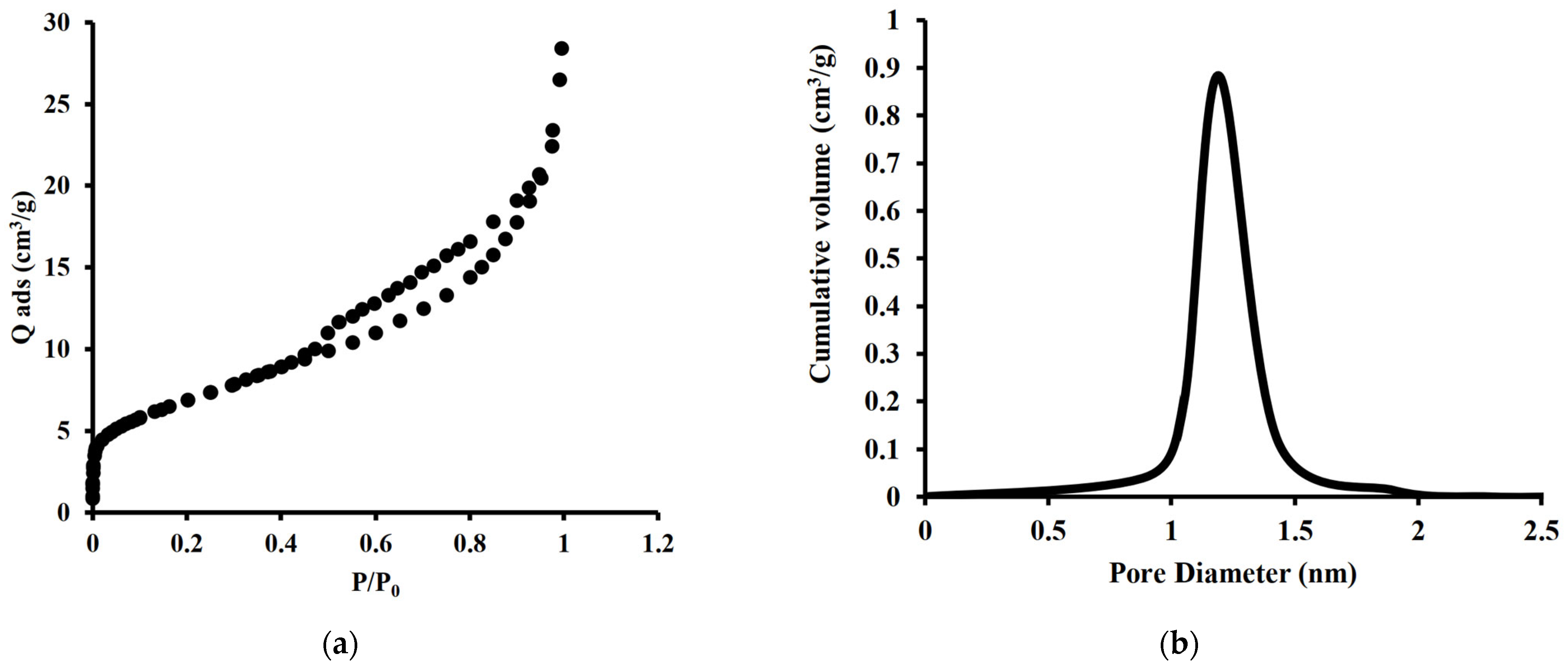
2.1.2. Surface Texture and Properties
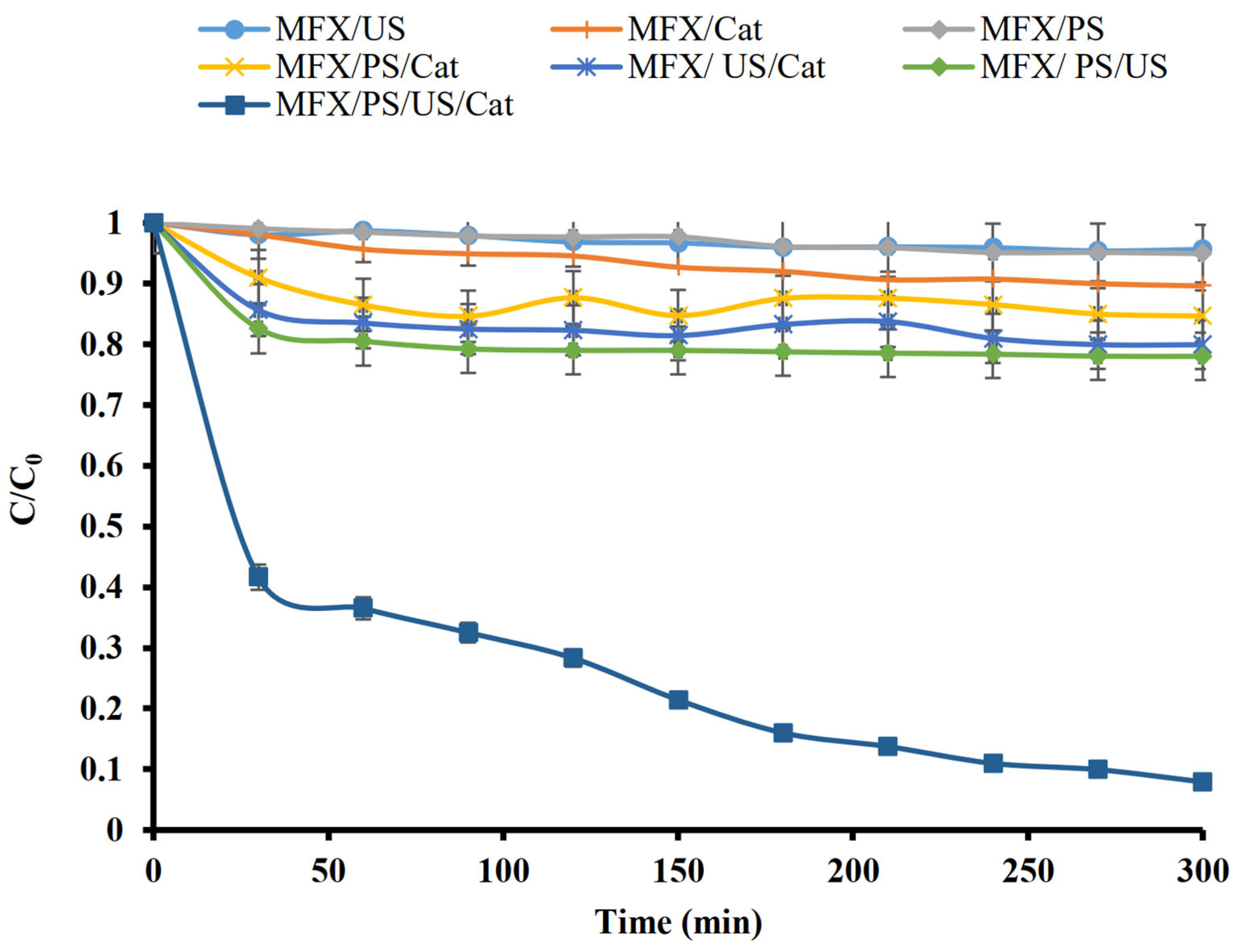
2.2. Oxidation and Adsorption Processes
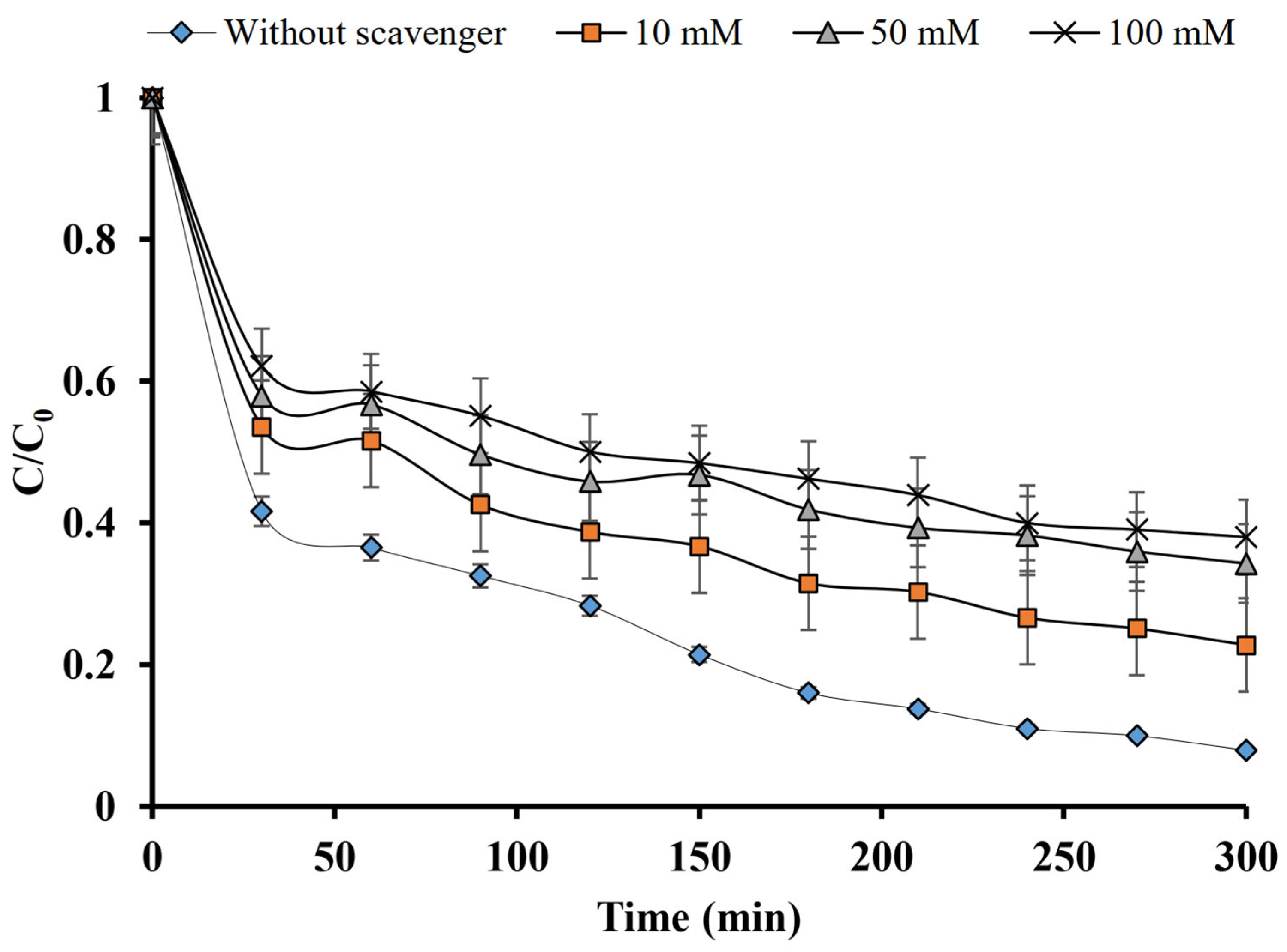
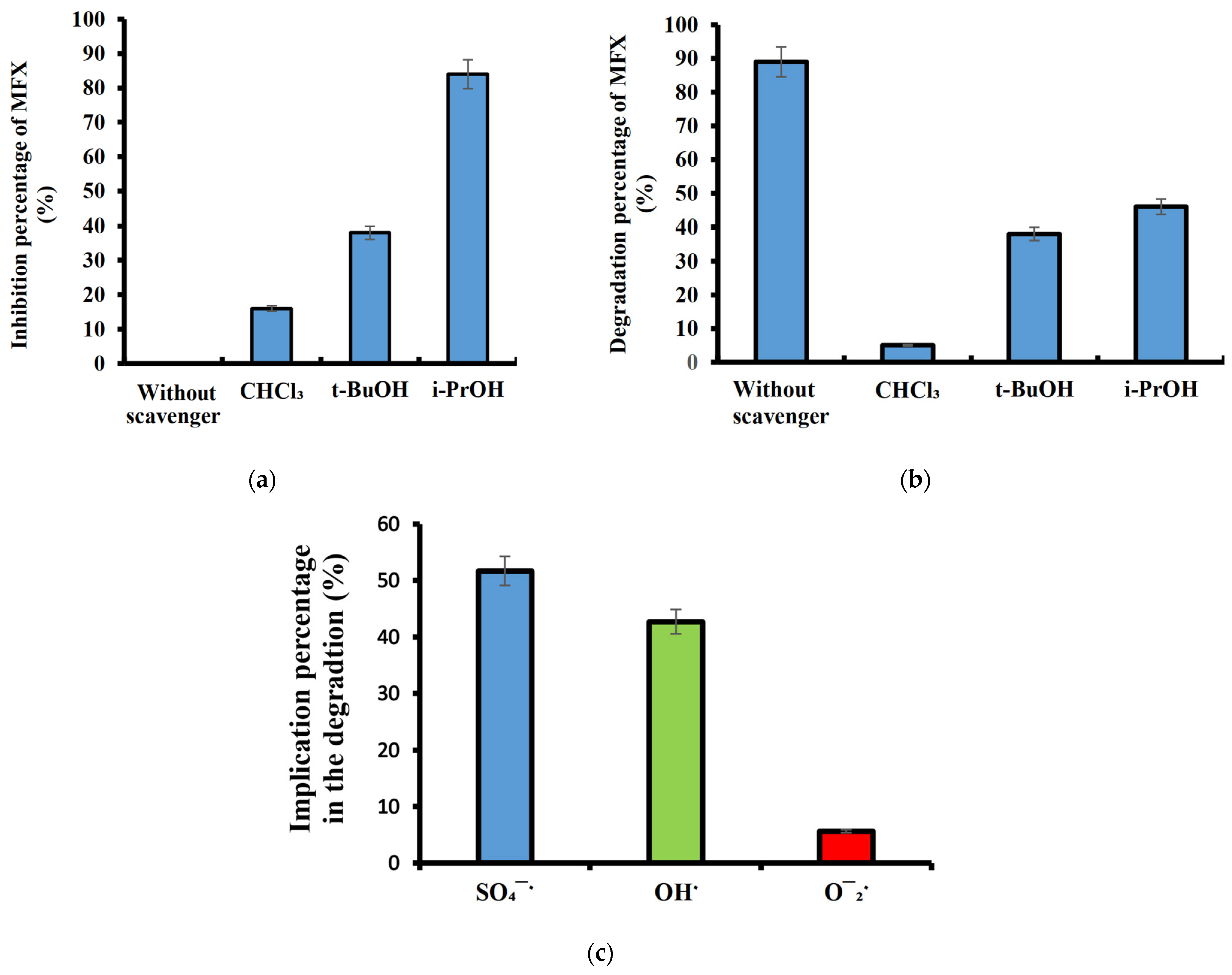
2.3. Chloroform Effect on MFX Degradation
2.4. Tert-Butanol Impact on MFX Degradation
2.5. Isopropanol Effect on MFX Degradation
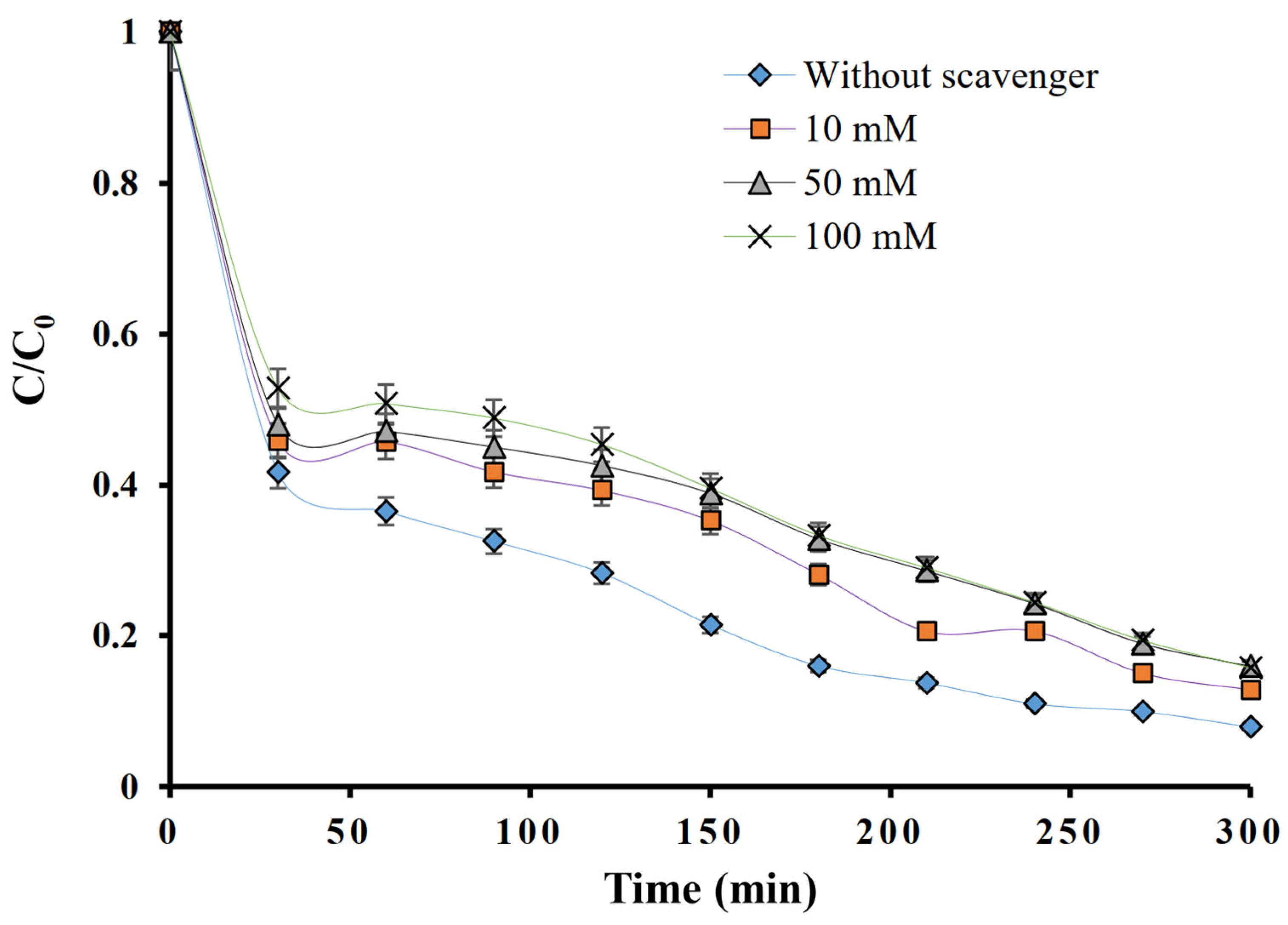
2.6. Scavenging Tests on MFX Degradation
2.7. Summary of Second-Order Rate Constants of ROS Relative to Some Scavengers
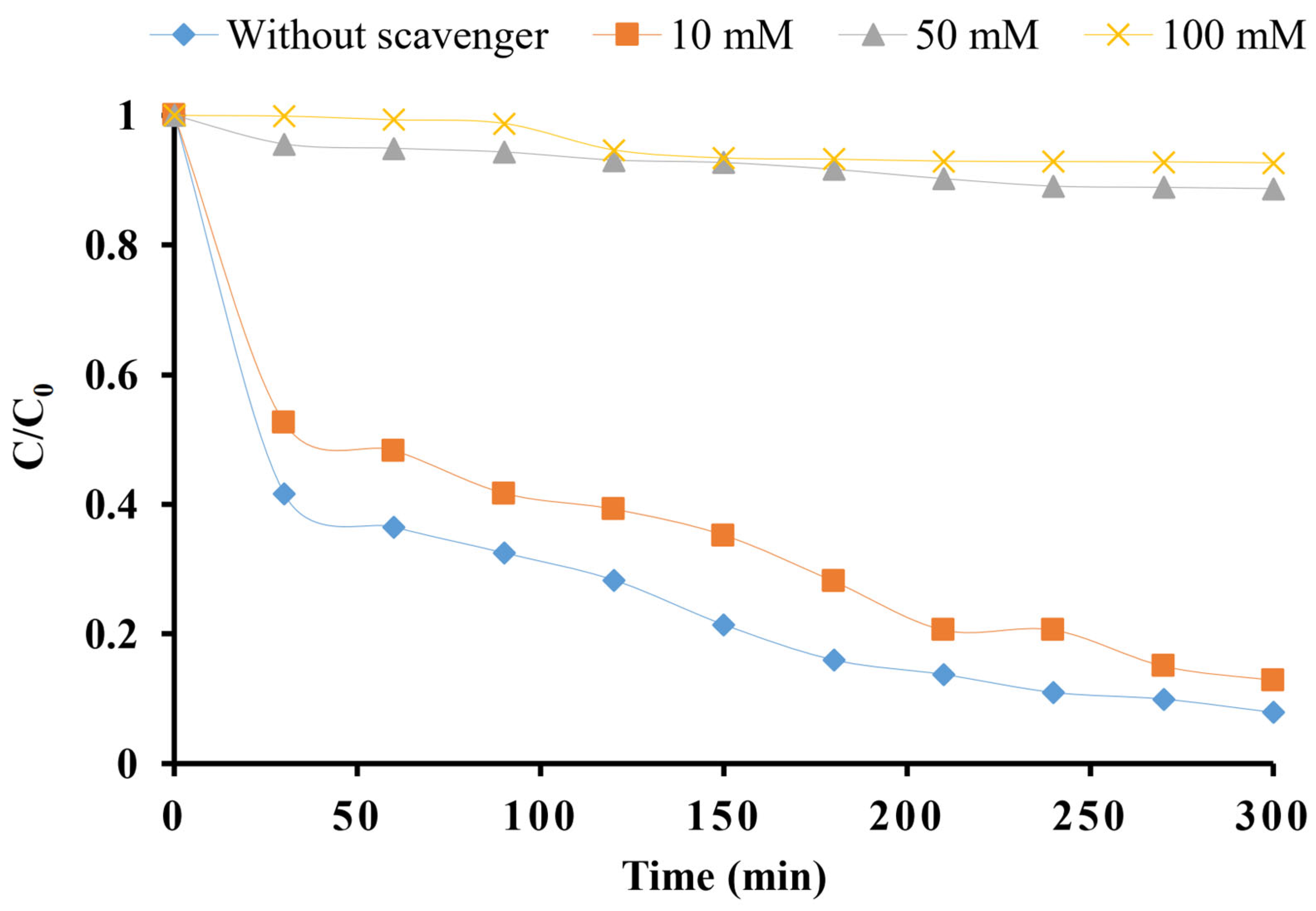
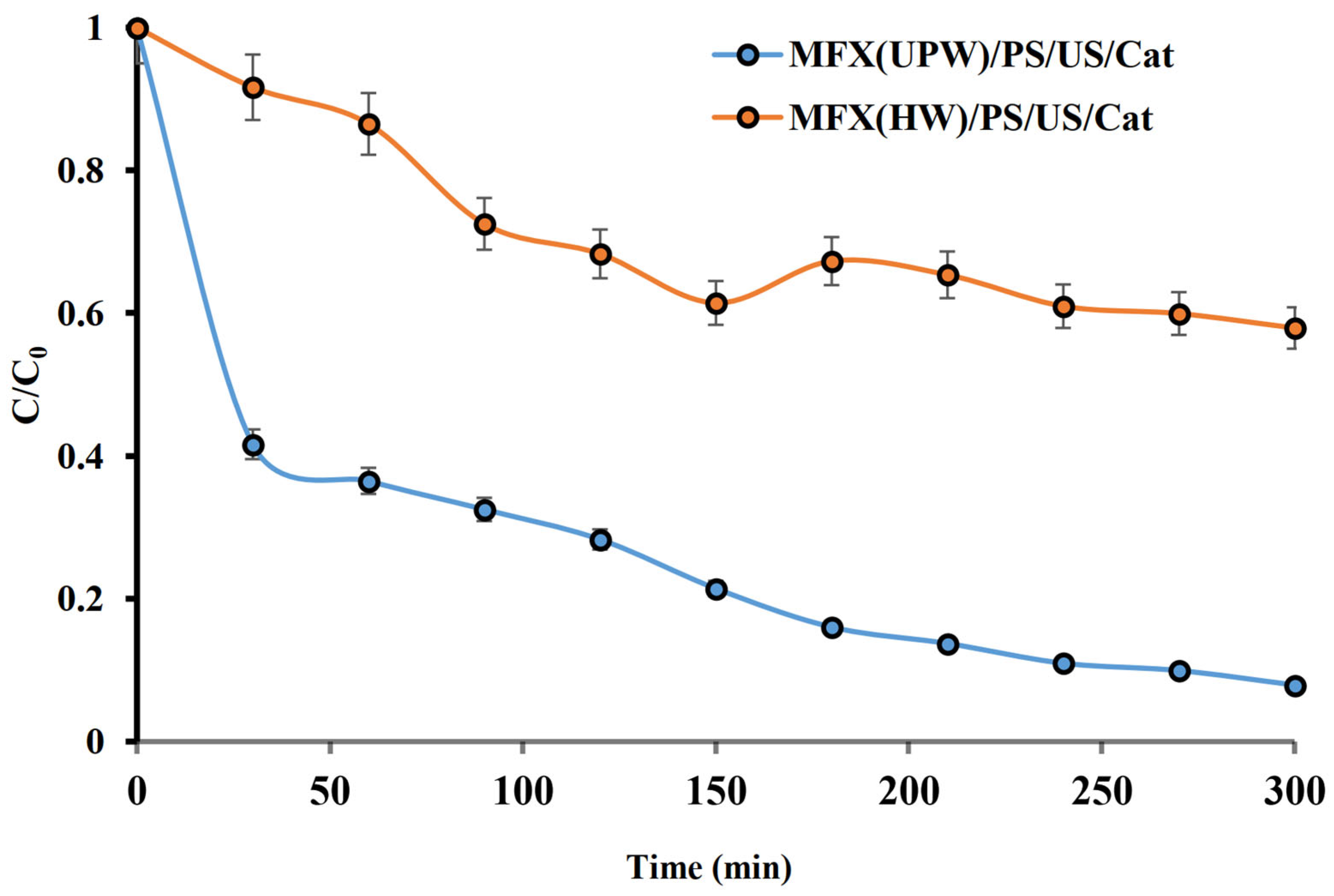
2.8. Effect of Hospital Wastewater Components on Removal Efficiency
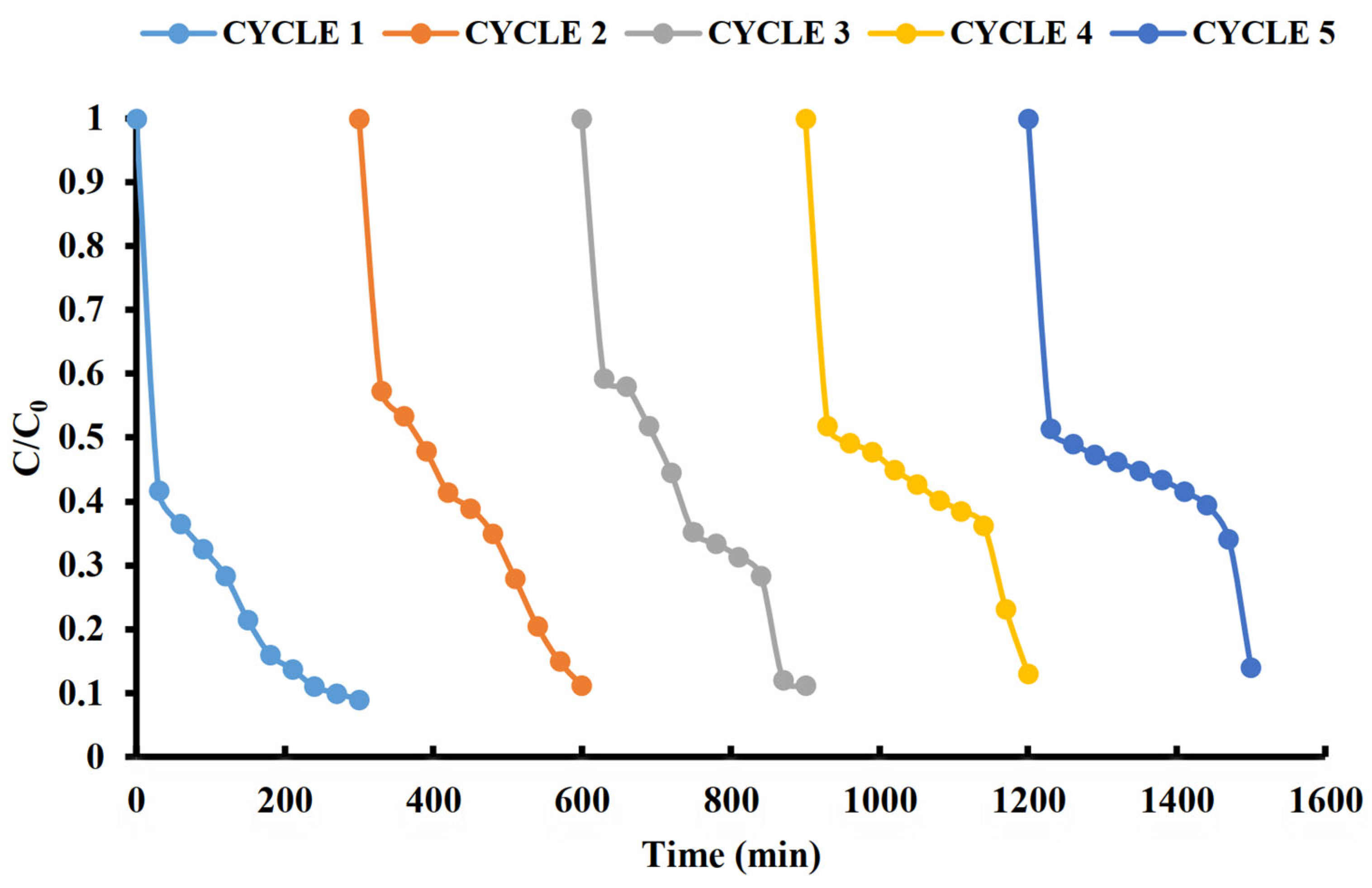
2.9. Stability and Reusability of the Catalyst
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Soil and Characterization Methods
3.3. Ultrasonic Oxidation Tests and Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ishaq, M.; Zafar, E.; Maqbool, M.F.; Mansha, M.; Khan, M.; Li, Y. Repurposing fluoroquinolones as cancer chemosensitizers: A way to overcome cancer therapeutic bottleneck. Naunyn. Schmiedebergs. Arch. Pharmacol. 2025. ahead of print. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef] [PubMed]
- Negrete-Paz, A.M.; Vázquez-Marrufo, G.; Rodríguez-Carlos, A.; Rivas-Santiago, B.; Vázquez-Garcidueñas, M.S. Whole-Genome Sequence-Based Diversity of Mycobacterium tuberculosis Strains Isolated from a Central Western Region of Mexico. Pathogens 2025, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.P. Irradiation of pharmaceuticals: A literature review. Radiat. Phys. Chem. 2022, 190, 109795. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Ishaque, F.; Ahn, Y.H. Fate of antibiotic resistant genes in wastewater environments and treatment strategies—A review. Chemosphere 2022, 298, 134671. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Bouallouche, R.; Kenfoud, H.; Khezami, L.; Assadi, A.A. High efficient Cefixime removal from water by the sillenite Bi12TiO20: Photocatalytic mechanism and degradation pathway. J. Clean. Prod. 2022, 330, 129934. [Google Scholar] [CrossRef]
- Bal, I.; Macit, M.; Alasiri, A.; Namli, O.C.; Arshad, M.S.; Ahmad, Z.; Duman, G.; Kucuk, I. Engineering Moxifloxacin-Encapsulated Liposome-Enriched Alginate Hydrogel Films. Gels 2025, 11, 448. [Google Scholar] [CrossRef]
- Kumar, K.S.; Mohanchand, G.; Gaddam, V.S.K.; Matli, C.S. Heterogeneous Photocatalysis of Fluoroquinolone Antibiotics: Scavenger Study and Determination of Residual Antibacterial Activity. Ozone Sci. Eng. 2025, 1–19. [Google Scholar] [CrossRef]
- Baaloudj, O.; Kenfoud, H.; Brienza, M.; El Jery, A.; Aldrdery, M.; Assadi, A.A. Exploring the Synthesis of Novel Sillenite Bi12SnO20: Effect of Calcination Temperature on the Phase Formation and Catalytic Performance. Catalysts 2024, 14, 650. [Google Scholar] [CrossRef]
- Kamagate, M.; Coulibaly, N.G.; Koffi, A.P.M.; Zie, O.; Coulibaly, L.; Assadi, A.A.; Elfalleh, W.; Hjiri, M.; Khezami, L.; Tahraoui, H.; et al. Enhancing Levofloxacin Degradation in Contaminated Water: Catalytic Performance of Pegmatite in a Sodium Percarbonate/Ultrasound System. Catalysts 2025, 15, 363. [Google Scholar] [CrossRef]
- Kabir, M.H.; Miah, M.J.; Mohiuddin, A.K.; Hossain, M.S.; Upoma, B.P.; Shaikh, M.A.A.; Pabel, M.Y.; Mojumder, F.; Mahmud, R.; Tanvir, N.I.; et al. Highly Effective Removal of Moxifloxacin from Aqueous Solutions Using Graphene Oxide Functionalized with Sodium Dodecyl Sulfate. ACS Sustain. Resour. Manag. 2025, 2, 256–266. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, W.; Wang, H.; Zhan, S.; Zhou, F. Triggering the efficient photocarriers separation of Bi8(CrO4)O11 under visible light greater than 550 nm via the synergistic effect of PMS and Cl−. Opt. Mater. 2024, 150, 1–19. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, T.; Yuan, S.; Zhu, C.; Yang, L.; Chen, Y.; Wang, X.; Yin, Y.; Chen, C.; Tang, L.; et al. Ultrathin TiO2(B) nanosheets-decorated hollow CoFeP cube as PMS activator for enhanced photocatalytic activity. Appl. Surf. Sci. 2024, 643, 158667. [Google Scholar] [CrossRef]
- Diao, Z.H.; Yan, L.; Dong, F.X.; Qian, W.; Deng, Q.H.; Kong, L.J.; Yang, J.W.; Lei, Z.X.; Du, J.J.; Chu, W. Degradation of 2,4-dichlorophenol by a novel iron based system and its synergism with Cd(II) immobilization in a contaminated soil. Chem. Eng. J. 2020, 379, 122313. [Google Scholar] [CrossRef]
- Kamagate, M.; Amin Assadi, A.; Kone, T.; Coulibaly, L.; Hanna, K. Activation of persulfate by irradiated laterite for removal of fluoroquinolones in multi-component systems. J. Hazard. Mater. 2018, 346, 159–166. [Google Scholar] [CrossRef]
- Zeghioud, H.; Kamagate, M.; Coulibaly, L.S.; Rtimi, S.; Assadi, A.A. Photocatalytic degradation of binary and ternary mixtures of antibiotics: Reactive species investigation in pilot scale. Chem. Eng. Res. Des. 2019, 144, 300–309. [Google Scholar] [CrossRef]
- Diao, Z.H.; Zhang, W.X.; Liang, J.Y.; Huang, S.T.; Dong, F.X.; Yan, L.; Qian, W.; Chu, W. Removal of herbicide atrazine by a novel biochar based iron composite coupling with peroxymonosulfate process from soil: Synergistic effect and mechanism. Chem. Eng. J. 2021, 409, 127684. [Google Scholar] [CrossRef]
- Pei, L.; Jiang, W.; Ma, H.; Zhao, H.; An, J.; Ma, Y.; Zhan, S.; Zhou, F. Interfacial electric field driven photocatalytic activation of peroxymonosulfate over CoFe-LDH/Bi4O5Br2 for enhanced antibiotics removal across the full pH range. Appl. Surf. Sci. 2025, 692, 162754. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Gao, J.; Cai, A.; Wang, Y.; Li, Y.; Yuan, J. The constraints on oxidation of structural Fe(II) by aqueous Fe3+ and the pathways of electron transfer in clay minerals. Appl. Clay Sci. 2025, 276, 107940. [Google Scholar] [CrossRef]
- Coulibaly, L.S.; Akpo, S.K.; Yvon, J.; Coulibaly, L. Fourier transform infra-red (FTIR) spectroscopy investigation, dose effect, kinetics and adsorption capacity of phosphate from aqueous solution onto laterite and sandstone. J. Environ. Manage. 2016, 183, 1032–1040. [Google Scholar] [CrossRef]
- Kamagate, M.; Assadi, A.A.; Kone, T.; Giraudet, S.; Coulibaly, L.; Hanna, K. Use of laterite as a sustainable catalyst for removal of fluoroquinolone antibiotics from contaminated water. Chemosphere 2018, 195, 847–853. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Rao, Y.F.; Qu, L.; Yang, H.; Chu, W. Degradation of carbamazepine by Fe(II)-activated persulfate process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, H.; Xue, X. Ultrasound enhanced heterogeneous activation of peroxydisulfate by magnetite catalyst for the degradation of tetracycline in water. Sep. Purif. Technol. 2012, 84, 147–152. [Google Scholar] [CrossRef]
- Kianian, S.; Toulakani, R.M.; Zisti, F.; Balarak, D.; Siddiqui, S.H.; Saloot, M.K. Degradation of Antibiotics by Ultrasound-Assisted Heterogeneous Activation of Persulfate and Peroxymonosulfate: A Review. Int. J. Pharm. Investig. 2023, 14, 23–29. [Google Scholar] [CrossRef]
- Hayon, E.; Treinin, A.; Wilf, J. Electronic Spectra, Photochemistry, and Autoxidation Mechanism of the Sulfite-Bisulfite-Pyrosulfite Systems. the SO2-, SO3-, SO4-, and SO5- Radicals. J. Am. Chem. Soc. 1972, 94, 47–57. [Google Scholar] [CrossRef]
- Fang, G.D.; Dionysiou, D.D.; Al-Abed, S.R.; Zhou, D.M. Superoxide radical driving the activation of persulfate by magnetite nanoparticles: Implications for the degradation of PCBs. Appl. Catal. B Environ. 2013, 129, 325–332. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Yu, G.; Wang, Y. Revisiting the role of reactive oxygen species for pollutant abatement during catalytic ozonation: The probe approach versus the scavenger approach. Appl. Catal. B Environ. 2021, 280, 119418. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Wu, G.; Katsumura, Y.; Chu, G. Photolytic and radiolytic studies of SO4•− in neat organic solvents. Phys. Chem. Chem. Phys. 2000, 2, 5602–5605. [Google Scholar] [CrossRef]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e− transfer mechanisms. Appl. Catal. B Environ. 2010, 96, 290–298. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate Constants and Mechanism of Reaction of SO4 with Aromatic Compounds. J. Am. Chem. Soc. 1977, 99, 163–164. [Google Scholar] [CrossRef]
- Liang, C.; Lei, J. Identification of Active Radical Species in Alkaline Persulfate Oxidation. Water Environ. Res. 2015, 87, 656–659. [Google Scholar] [CrossRef]
- Petersen-Sonn, E.A.; Brigante, M.; Deguillaume, L.; Jaffrezo, J.L.; George, C. Tropospheric Multiphase Chemistry: Excited Triplet States Compete with OH Radicals and Singlet Molecular Oxygen. ACS Earth Space Chem. 2025, 9, 533–544. [Google Scholar] [CrossRef]
- Gao, J.; Alsaab, H.O.; Zare, M.H.; Shirazian, S. Magnesiothermal reduction and doping strategies in engineered TiO2 for pharmaceutical degradation and CO2 conversion. NPJ Clean Water 2025, 8, 76. [Google Scholar] [CrossRef]
- Wang, P.; Kang, F.; Huang, X.; Luo, Z.; Zou, J.; Yang, M.; Sun, M.; Yu, X.; Zeng, H. A pH-responsive production of hydroxyl radical in Fenton process. Environ. Sci. Ecotechnol. 2025, 25, 100566. [Google Scholar] [CrossRef]
- Yu, S.Y.; Xie, Z.H.; Wu, X.; Zheng, Y.Z.; Shi, Y.; Xiong, Z.K.; Zhou, P.; Liu, Y.; He, C.S.; Pan, Z.C.; et al. Review of advanced oxidation processes for treating hospital sewage to achieve decontamination and disinfection. Chin. Chem. Lett. 2024, 35, 108714. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Z.; Aruffo, E.; Dai, X.; Zhao, Z.; Ye, Z. Kinetics of Different Substituted Phenolic Compounds’ Aqueous OH Oxidation in Atmosphere. Atmosphere 2025, 16, 567. [Google Scholar] [CrossRef]
- Huang, B.; Wu, Z.; Zhang, J.; Shi, Y.; He, C.-S.; Xiong, Z.; Lai, B. Is the choice of quencher appropriate for exploring reactive oxygen species in advanced oxidation processes? Chin. Chem. Lett. 2025, 111537. [Google Scholar] [CrossRef]
- Hwang, S.; Huling, S.G.; Ko, S. Fenton-like degradation of MTBE: Effects of iron counter anion and radical scavengers. Chemosphere 2010, 78, 563–568. [Google Scholar] [CrossRef]
- Galeas, S.; Guerrero, V.H.; Pontón, P.I.; Goetz, V. Visible Light-Driven Phenol Degradation via Advanced Oxidation Processes with Ferrous Oxalate Obtained from Black Sands: A Kinetics Study. Molecules 2025, 30, 2059. [Google Scholar] [CrossRef]
- Mo, F.; Liu, Y.; Xu, Y.; He, Q.; Sun, P.; Dong, X. Photocatalytic elimination of moxifloxacin by two-dimensional graphitic carbon nitride nanosheets: Enhanced activity, degradation mechanism and potential practical application. Sep. Purif. Technol. 2022, 292, 121067. [Google Scholar] [CrossRef]
- Joohyun, K.; Coulibaly, G.N.; Yoon, S.; Assadi, A.A.; Hanna, K.; Bae, S. Red mud-activated peroxymonosulfate process for the removal of fluoroquinolones in hospital wastewater. Water Res. 2020, 184, 116171. [Google Scholar] [CrossRef]
- Khare, N.; Hesterberg, D.; Martin, J.D. XANES investigation of phosphate sorption in single and binary systems of iron and aluminum oxide minerals. Environ. Sci. Technol. 2005, 39, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Ma, L.; An, W.; Shen, P. Stability optimization of iron-based catalysts: Application of Cr-enriched alloy steel catalyst in ozonation pharmaceutical wastewater. Sep. Purif. Technol. 2025, 354, 129007. [Google Scholar] [CrossRef]
- Moreno-Bermedo, L.; Correa-Puerta, J.; González-Fuentes, C.; Escalona, N.; Onfray, C.; Thiam, A. Tea Waste as a Sustainable Catalyst Support for Enhanced Removal of Contaminants of Emerging Concern via the Electro-Fenton Process: A Circular Economy Approach. Appl. Sci. 2025, 15, 1418. [Google Scholar] [CrossRef]
- Zhang, K.X.; Tunyaz, A.; Zhou, G.N.; Ding, R.R.; Zheng, P.W.; Liu, X.C.; Yang, H.Y.; Mu, Y. Carbon encapsulated Fe0/Fe oxides for heterogeneous electro-Fenton process: Promoting Fe(II) regeneration under nanoconfinement. Chem. Eng. J. 2025, 519, 165674. [Google Scholar] [CrossRef]
- Fahim, A.M.; Abas, K.M. Properties and computational insights of catalysts based on amide linked polymer for photo-Fenton remediation of Rhodamine B dye. Sci. Rep. 2025, 15, 30566. [Google Scholar] [CrossRef]
- Maxime, G.; Aymen Amine, A.; Abdelkrim, B.; Dominique, W. Removal of gas-phase ammonia and hydrogen sulfide using photocatalysis, nonthermal plasma, and combined plasma and photocatalysis at pilot scale. Environ. Sci. Pollut. Res. 2014, 21, 13127–13137. [Google Scholar] [CrossRef]
- Assadi, A.A.; Palau, J.; Bouzaza, A.; Penya-Roja, J.; Martinez-Soriac, V.; Wolbert, D. Abatement of 3-methylbutanal and trimethylamine with combined plasma and photocatalysis in a continuous planar reactor. J. Photochem. Photobiol. A Chem. 2014, 282, 1–8. [Google Scholar] [CrossRef]

| Compounds | Formular | pKa | MW | M−1 s−1 | ||
|---|---|---|---|---|---|---|
| kO2•− | kOH• | kSO4•− | ||||
| Chloroform | CHCl3 | 15.5 | 119.37 | 2.3 × 108 | 5.4 × 107 | 1.5 × 105 |
| Isopropanol | C3H80 | 16.5 | 60.10 | 1.0 × 106 | 1.9 × 109 | 7.42 × 107 |
| Terbutanol | C4H10O | 16.54 | 74.12 | - | (3.8–7.6) × 108 | (4–9.1) × 105 |
| Methanol | CH3OH | 15.54 | 32.04 | - | 9.7 × 108 | 2.5 × 107 |
| Phenol | C6H6O | 10.0 | 94.11 | - | 6.0 × 108 | - |
| Benzoquinone | C6H4O2 | 9.91 | 108.10 | 9.6 × 108 | 7.4 × 106 | - |
| Potassium iodide | KI | 0.06 | 166 | - | 1.1 × 1010 | >1.0 × 1010 |
| Parameters | Values |
|---|---|
| pH | 6.91 ± 0.2 |
| Turbidity (NTU) | 186 ± 2 |
| Conductivity (µS Cm−1) | 1432 ± 5 |
| Total Dissolved Solids (TDS) (mg L−1) | 270 ± 5 |
| Total Suspended Solids (TSS) (mg L−1) | 110 ± 2 |
| Carbon Oxygen Demand (COD) (mg O2 L−1) | 196 ± 2 |
| Nitrates (mg L−1) | 69 ± 2 |
| Chloride (mg L−1) | 72 ± 2 |
| Sulfate (mg L−1) | 47 ± 3 |
| Phosphate (mg L−1) | 179 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamagate, M.; Ahmed Ali, F.A.; Lancine, T.; Gnougon Nina, C.; Assadi, A.A.; Lacina, C.; Lanciné, G.D.; Baaloudj, O. Mechanistic Insights into Radical-Mediated Moxifloxacin Degradation Using Ultrasound-Assisted Persulfate Activation by Iron-Rich Soil. Catalysts 2025, 15, 1056. https://doi.org/10.3390/catal15111056
Kamagate M, Ahmed Ali FA, Lancine T, Gnougon Nina C, Assadi AA, Lacina C, Lanciné GD, Baaloudj O. Mechanistic Insights into Radical-Mediated Moxifloxacin Degradation Using Ultrasound-Assisted Persulfate Activation by Iron-Rich Soil. Catalysts. 2025; 15(11):1056. https://doi.org/10.3390/catal15111056
Chicago/Turabian StyleKamagate, Mahamadou, Fekri Abdulraqeb Ahmed Ali, Traore Lancine, Coulibaly Gnougon Nina, Amine Aymen Assadi, Coulibaly Lacina, Goné Droh Lanciné, and Oussama Baaloudj. 2025. "Mechanistic Insights into Radical-Mediated Moxifloxacin Degradation Using Ultrasound-Assisted Persulfate Activation by Iron-Rich Soil" Catalysts 15, no. 11: 1056. https://doi.org/10.3390/catal15111056
APA StyleKamagate, M., Ahmed Ali, F. A., Lancine, T., Gnougon Nina, C., Assadi, A. A., Lacina, C., Lanciné, G. D., & Baaloudj, O. (2025). Mechanistic Insights into Radical-Mediated Moxifloxacin Degradation Using Ultrasound-Assisted Persulfate Activation by Iron-Rich Soil. Catalysts, 15(11), 1056. https://doi.org/10.3390/catal15111056









