Degradation of Atrazine to Cyanuric Acid by an Encapsulated Enzyme Cascade
Abstract
1. Introduction
2. Results and Discussion
2.1. Expression and Purification of Atrazine Degrading Enzymes
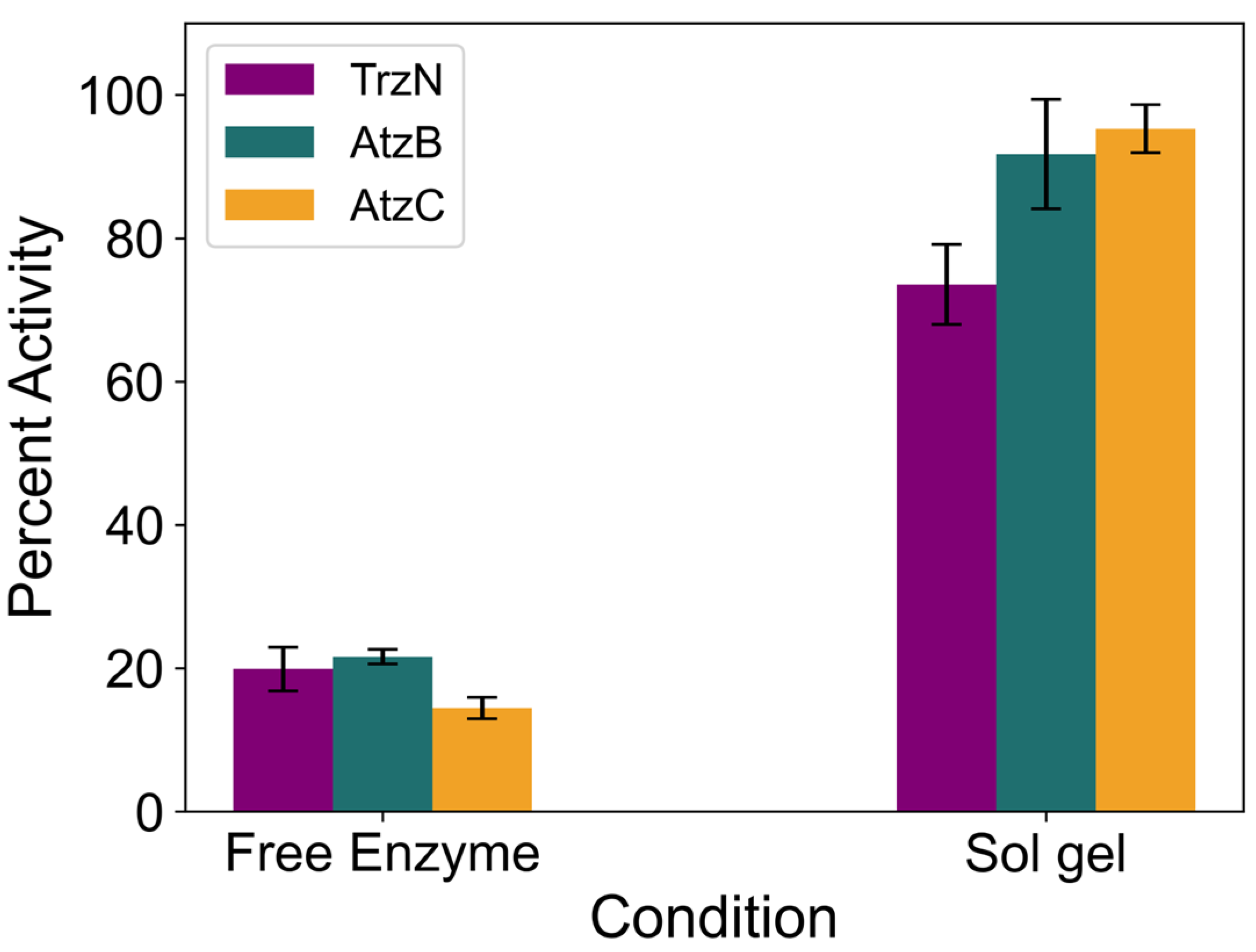
2.2. Encapsulation of TrzN, AtzB, and AtzC
2.3. Proteolytic Digestion of Free and Encapsulated TrzN, AtzB, and AtzC
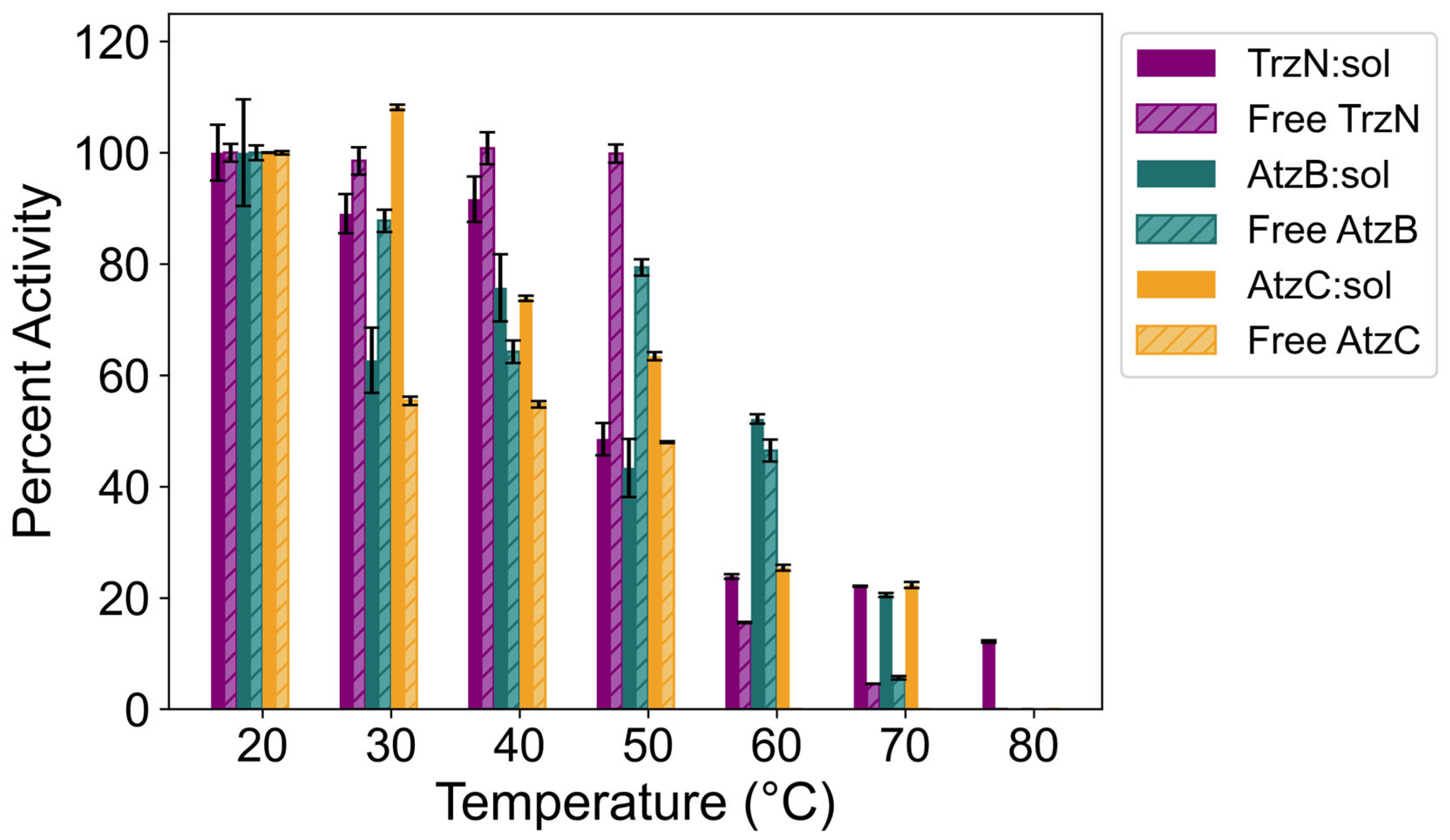
2.4. Thermostability of Free and Encapsulated TrzN, AtzB, and AtzC
2.5. pH Stability of Free and Encapsulated TrzN, AtzB, and AtzC
2.6. Reusability of Free and Encapsulated Enzyme
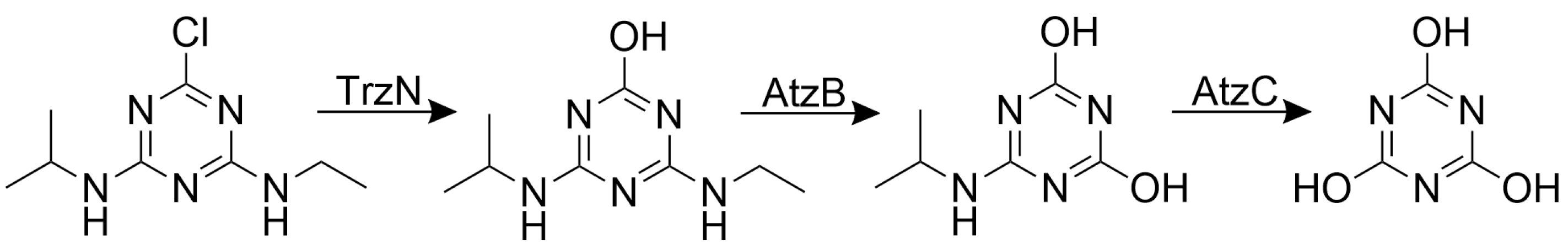
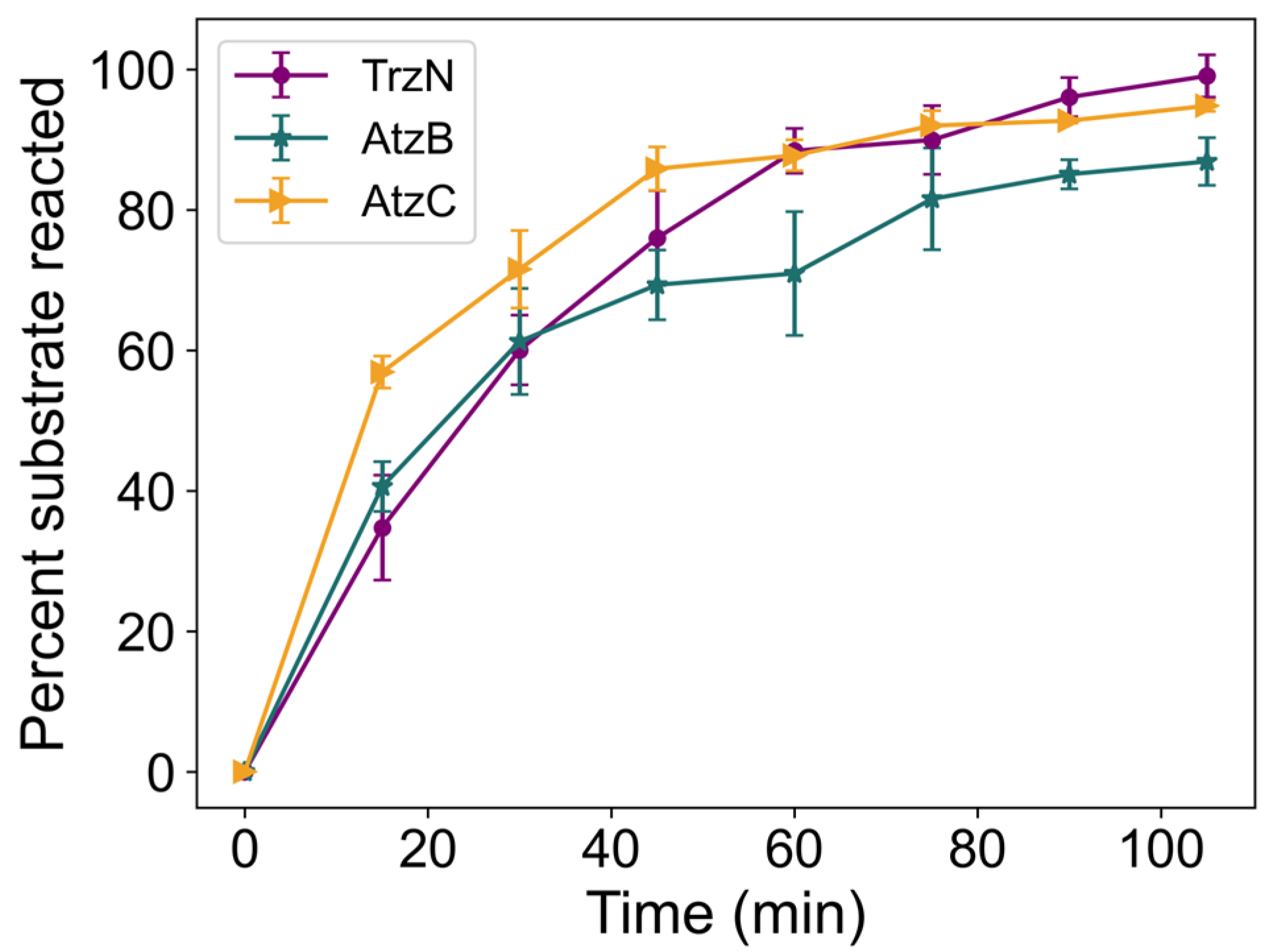
2.7. Degradation of Atrazine by an Encapsulated Enzyme Cascade
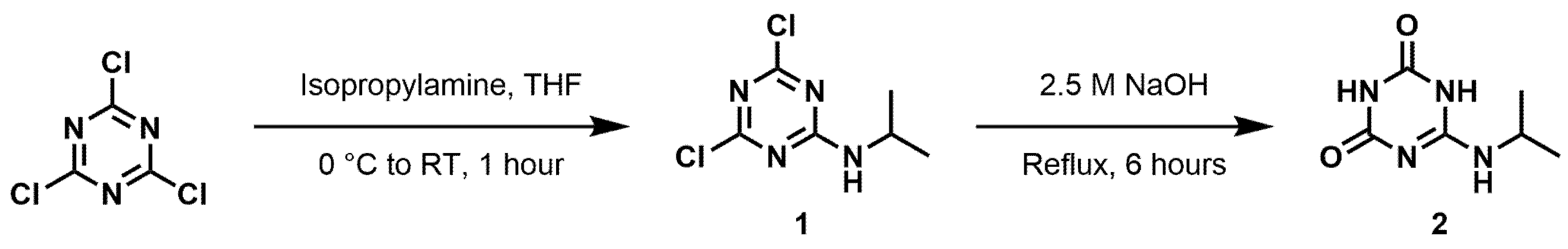
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TrzN | Triazine hydrolase; |
| AtzB | Hydroxyatrazine N-Ethylaminohydrolase; |
| AtzC | N-Isopropylammelide Amidohydrolase; |
| TMOS | Tetramethyl orthosilicate; |
| IPTG | isopropyl ß-d-1-thiogalactopyranoside; |
| FPLC | Fast protein liquid chromatography. |
References
- de Albuquerque, F.P.; de Oliveira, J.L.; Moschini-Carlos, V.; Fraceto, L.F. An overview of the potential impacts of atrazine in aquatic environments: Perspectives for tailored solutions based on nanotechnology. Sci. Total. Environ. 2020, 700, 134868. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Jafari, S.; Moeini, Z.; Jaskulak, M.; Keshtgar, L.; Badeenezhad, A.; Azhdarpoor, A.; Rostami, M.; Zorena, K.; Dehghani, M. Current methods and technologies for degradation of atrazine in contaminated soil and water: A review. Environ. Technol. Innov. 2021, 24, 102019. [Google Scholar] [CrossRef]
- Ribaudo, M.; Bouzaher, A. Atrazine: Environmental Characteristics and Economics of Management; United States Department of Agriculture: Washington, DC, USA, 1994.
- Solomon, K.R.; Baker, D.B.; Richards, R.P.; Dixon, K.R.; Klaine, S.J.; La Point, T.W.; Kendall, R.J.; Weisskopf, C.P.; Giddings, J.M.; Giesy, J.P.; et al. Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 1996, 15, 31–76. [Google Scholar] [CrossRef]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef]
- Cragin, L.A.; Kesner, J.S.; Bachand, A.M.; Barr, D.B.; Meadows, J.W.; Krieg, E.F.; Reif, J.S. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ. Res. 2011, 111, 1293–1301. [Google Scholar] [CrossRef]
- Almberg, K.S.; Turyk, M.E.; Jones, R.M.; Rankin, K.; Freels, S.; Stayner, L.T. Atrazine Contamination of Drinking Water and Adverse Birth Outcomes in Community Water Systems with Elevated Atrazine in Ohio, 2006–2008. Int. J. Environ. Res. Public Health 2018, 15, 1889. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, F. The Economics of Atrazine. Int. J. Occup. Environ. Health 2007, 13, 437–445. [Google Scholar] [CrossRef]
- Bethsass, J.; Colangelo, A. European Union Bans Atrazine, While the United States Negotiates Continued Use. Int. J. Occup. Environ. Health 2006, 12, 260–267. [Google Scholar] [CrossRef]
- Donley, N.; Phillips, M. Endocrine-Disrupting Pesticide Atrazine to Be Banned in Hawaii, Five U.S. Territories, Prohibited on Conifers, Roadsides. Center for Biological Diversity 2020. Available online: https://biologicaldiversity.org/w/news/press-releases/endocrine-disrupting-pesticide-atrazine-be-banned-hawaii-five-us-territories-prohibited-conifers-roadsides-2020-09-23/ (accessed on 28 October 2025).
- Conley, M. Atrazine, an Endocrine-Disrupting Herbicide Banned in Europe, Is Widely Used in the U.S. U.S. Right to Knowx 2025. Available online: https://regenerationinternational.org/2023/09/15/atrazine-an-endocrine-disrupting-herbicide-banned-in-europe-is-widely-used-in-the-u-s/ (accessed on 28 October 2025).
- Shapir, N.; Osborne, J.P.; Johnson, G.; Sadowsky, M.J.; Wackett, L.P. Purification, Substrate Range, and Metal Center of AtzC: The N -Isopropylammelide Aminohydrolase Involved in Bacterial Atrazine Metabolism. J. Bacteriol. 2002, 184, 5376–5384. [Google Scholar] [CrossRef]
- Wang, L.; Samac, D.A.; Shapir, N.; Wackett, L.P.; Vance, C.P.; Olszewski, N.E.; Sadowsky, M.J. Biodegradation of atrazine in transgenic plants expressing a modified bacterial atrazine chlorohydrolase (atzA) gene. Plant Biotechnol. J. 2005, 3, 475–486. [Google Scholar] [CrossRef]
- Sajjaphan, K.; Shapir, N.; Wackett, L.P.; Palmer, M.; Blackmon, B.; Tomkins, J.; Sadowsky, M.J. Arthrobacter aurescens TC1 Atrazine Catabolism Genes trzN, atzB, and atzC Are Linked on a 160-Kilobase Region and Are Functional in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 4402–4407. [Google Scholar] [CrossRef]
- Busch, M.R.; Drexler, L.; Mahato, D.R.; Hiefinger, C.; Osuna, S.; Sterner, R. Retracing the Rapid Evolution of an Herbicide-Degrading Enzyme by Protein Engineering. ACS Catal. 2023, 13, 15558–15571. [Google Scholar] [CrossRef] [PubMed]
- Avgoulas, D.I.; Festa, D.; Petala, M.; Marra, D.; Zabulis, X.; Karamaounas, P.; Giannios, M.; Kostoglou, M.; Caserta, S.; Karapantsios, T.D. Flow geometry effect on Pseudomonas fluorescens SBW25 biofilm structure. Colloids Surfaces B Biointerfaces 2025, 256, 115048. [Google Scholar] [CrossRef]
- Bischoff, K. Melamine and Cyanuric Acid. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2017; pp. 493–501. [Google Scholar] [CrossRef]
- Dorne, J.L.; Doerge, D.R.; Vandenbroeck, M.; Fink-Gremmels, J.; Mennes, W.; Knutsen, H.K.; Vernazza, F.; Castle, L.; Edler, L.; Benford, D. Recent advances in the risk assessment of melamine and cyanuric acid in animal feed. Toxicol. Appl. Pharmacol. 2013, 270, 218–229. [Google Scholar] [CrossRef]
- Shapir, N.; Pedersen, C.; Gil, O.; Strong, L.; Seffernick, J.; Sadowsky, M.J.; Wackett, L.P. TrzN from Arthrobacter aurescens TC1 Is a Zinc Amidohydrolase. J. Bacteriol. 2006, 188, 5859–5864. [Google Scholar] [CrossRef]
- Somu, P.; Narayanasamy, S.; Gomez, L.A.; Rajendran, S.; Lee, Y.R.; Balakrishnan, D. Immobilization of enzymes for bioremediation: A future remedial and mitigating strategy. Environ. Res. 2022, 212, 113411. [Google Scholar] [CrossRef]
- Bhandari, S.; Poudel, D.K.; Marahatha, R.; Dawadi, S.; Khadayat, K.; Phuyal, S.; Shrestha, S.; Gaire, S.; Basnet, K.; Khadka, U.; et al. Microbial Enzymes Used in Bioremediation. J. Chem. 2021, 2021, 8849512. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Diviesti, K.; Holz, R.C. Catalytic Biomaterials for Atrazine Degradation. Catalysts 2023, 13, 140. [Google Scholar] [CrossRef]
- Seffernick, J.L.; Aleem, A.; Osborne, J.P.; Johnson, G.; Sadowsky, M.J.; Wackett, L.P. Hydroxyatrazine N -Ethylaminohydrolase (AtzB): An Amidohydrolase Superfamily Enzyme Catalyzing Deamination and Dechlorination. J. Bacteriol. 2007, 189, 6989–6997. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.; Coppin, C.W.; Carr, P.D.; Aleksandrov, A.; Wilding, M.; Sugrue, E.; Ubels, J.; Paks, M.; Newman, J.; Peat, T.S.; et al. 300-Fold Increase in Production of the Zn 2+ -Dependent Dechlorinase TrzN in Soluble Form via Apoenzyme Stabilization. Appl. Environ. Microbiol. 2014, 80, 4003–4011. [Google Scholar] [CrossRef]
- Manea, M.; Mező, G.; Hudecz, F.; Przybylski, M. Mass spectrometric identification of the trypsin cleavage pathway in lysyl-proline containing oligotuftsin peptides. J. Pept. Sci. 2007, 13, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Zhao, H.; Liu, Y.; Cui, Z.; Beattie, D.; Gu, Y.; Wang, Q. Design, Synthesis, and Biological Activities of Arylmethylamine Substituted Chlorotriazine and Methylthiotriazine Compounds. J. Agric. Food Chem. 2011, 59, 11711–11717. [Google Scholar] [CrossRef]
- Talebian, A.; Ghiorghis, A.; Hammer, C.F.; Murril, E.A.; Pallas, F. Synthesis, purification and spectroscopic characterization of potential impurities of hexamethylmelamine. J. Heterocycl. Chem. 1992, 29, 979–984. [Google Scholar] [CrossRef]
- Shapir, N.; Rosendahl, C.; Johnson, G.; Andreina, M.; Sadowsky, M.J.; Wackett, L.P. Substrate Specificity and Colorimetric Assay for Recombinant TrzN Derived from Arthrobacter aurescens TC1. Appl. Environ. Microbiol. 2005, 71, 2214–2220. [Google Scholar] [CrossRef]
- Balotra, S.; Warden, A.C.; Newman, J.; Briggs, L.J.; Scott, C.; Peat, T.S. X-Ray Structure and Mutagenesis Studies of the N-Isopropylammelide Isopropylaminohydrolase, AtzC. PLoS ONE 2015, 10, e0137700. [Google Scholar] [CrossRef]
- Mowery-Evans, M.; Diviesti, K.; Holz, R.C. Degradation of Chlorothalonil by Catalytic Biomaterials. Catalysts 2024, 14, 805. [Google Scholar] [CrossRef]
- Hervey, W.J.; Strader, M.B.; Hurst, G.B.; Hervey, I.W.J. Comparison of Digestion Protocols for Microgram Quantities of Enriched Protein Samples. J. Proteome Res. 2007, 6, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
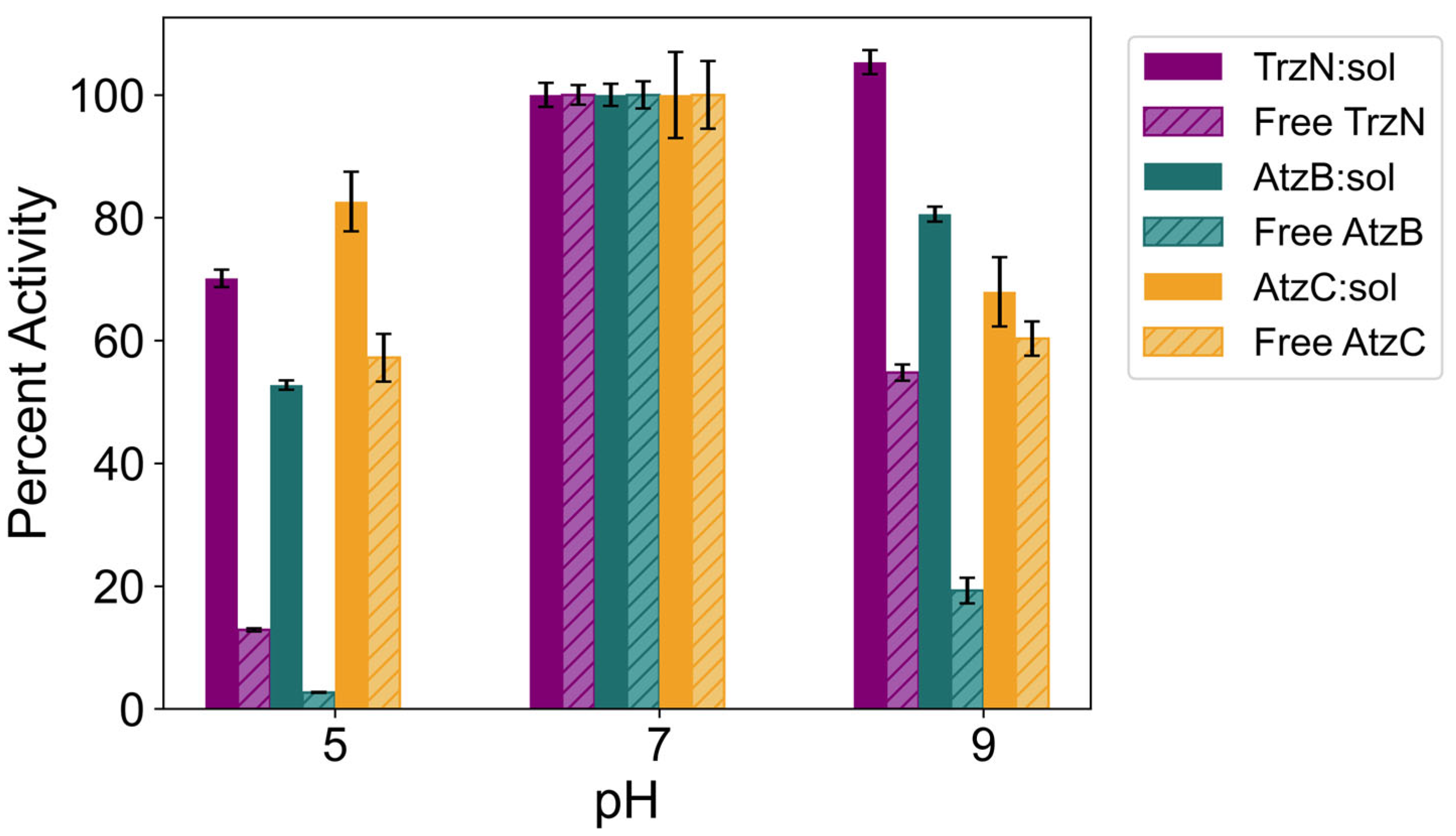
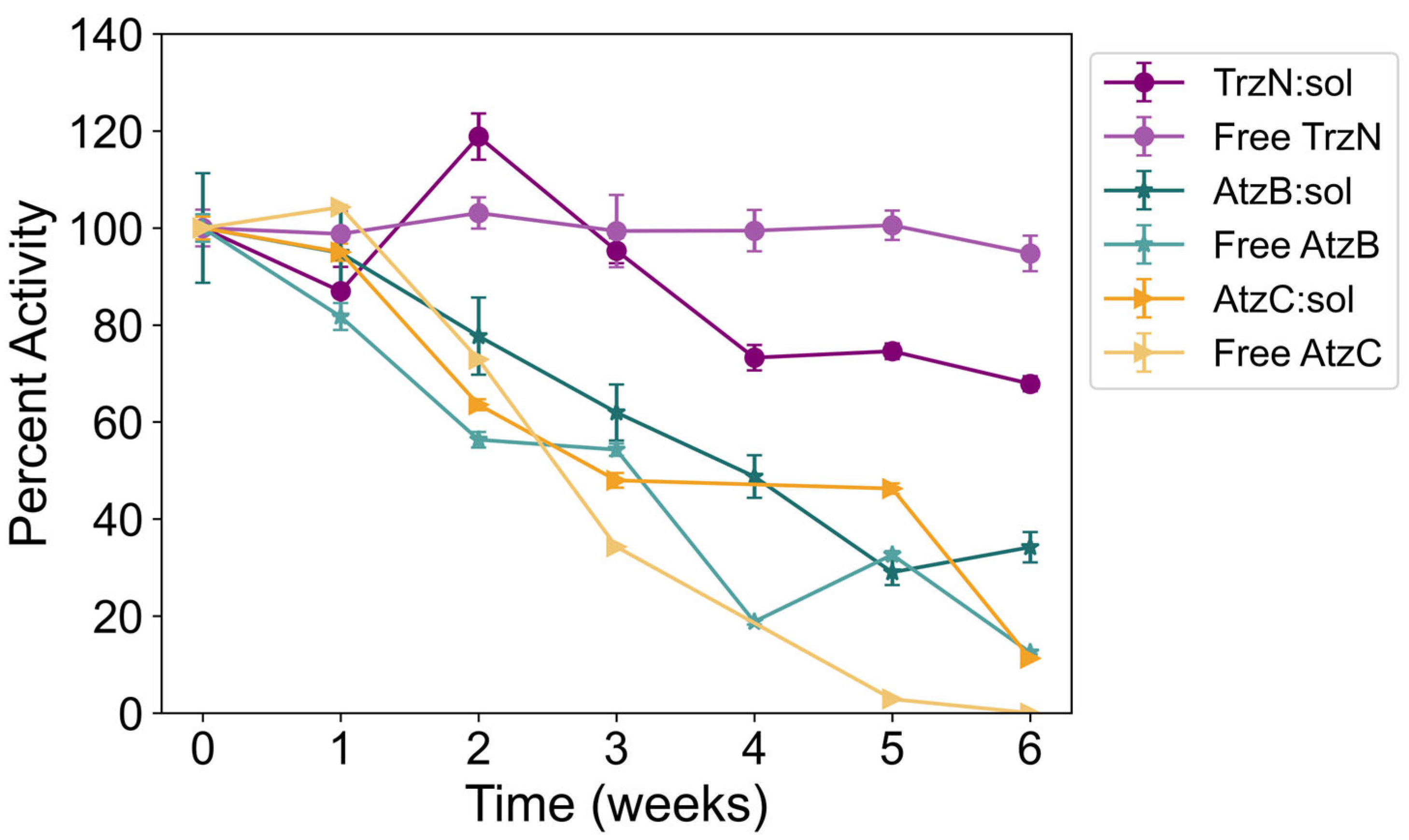
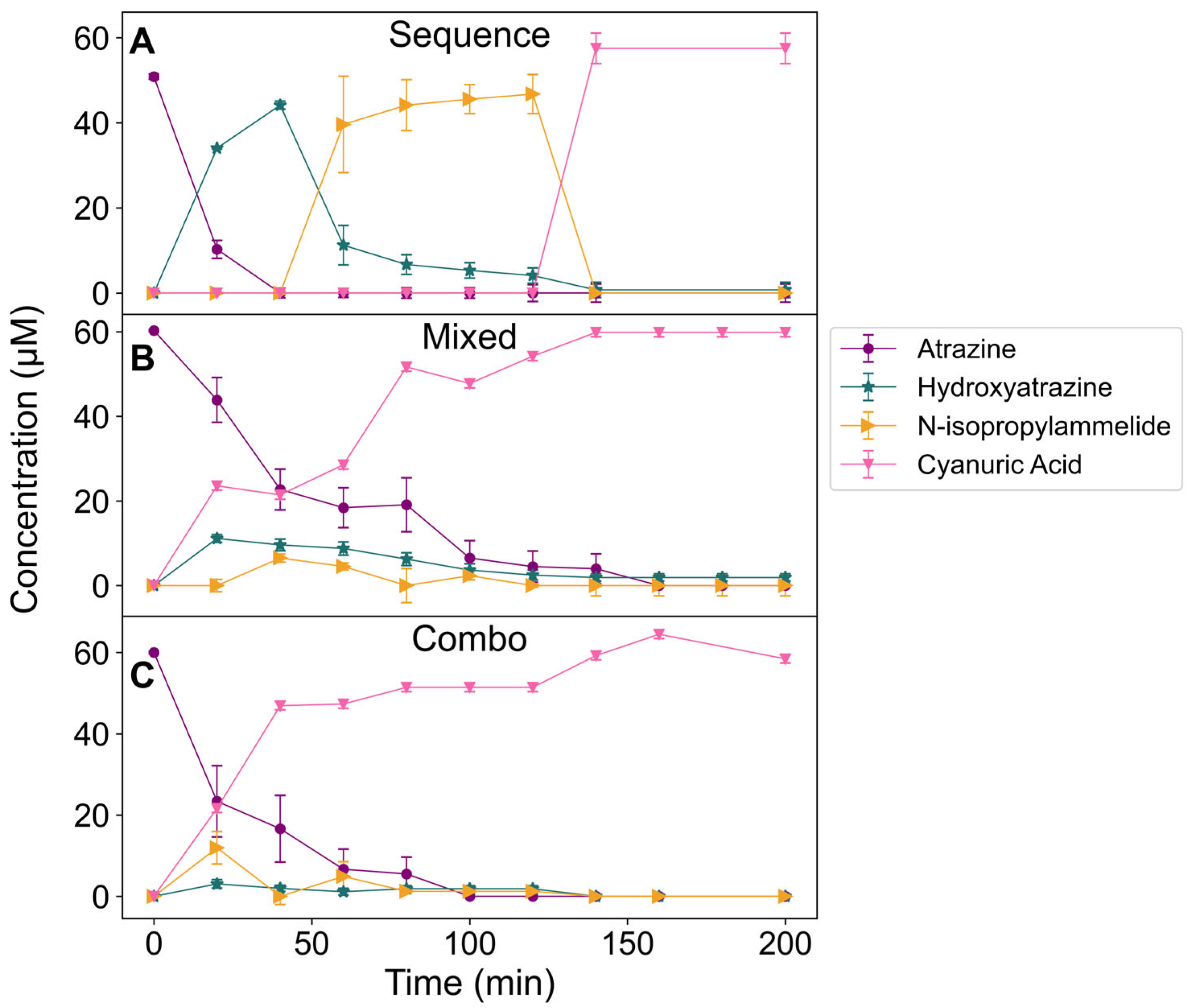
| Percent Activity | ||||||
|---|---|---|---|---|---|---|
| Enzyme | Material | After 18 h Digestion | pH 5 | pH 9 | 70 °C | 6 Weeks |
| TrzN | Soluble | 20 ± 3% | 13 ± 1% | 55 ± 1% | 5 ± 1% | 95 ± 4% |
| Sol–gel | 74 ± 6% | 70 ± 1% | 102 ± 3% | 22 ± 11% | 68 ± 2% | |
| AtzB | Soluble | 21 ± 1% | 2 ± 1% | 19 ± 2% | 6 ± 1% | 13 ± 4% |
| Sol–gel | 92 ± 8% | 53 ± 1% | 81 ± 3% | 21 ± 1% | 34 ± 3% | |
| AtzC | Soluble | 14 ± 2% | 57 ± 4% | 60 ± 3% | 0% | 0% |
| Sol–gel | 95 ± 3% | 83 ± 4% | 68 ± 5% | 22 ± 1% | 11 ± 1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mowery-Evans, M.; Benzie, E.; Alansari, N.; Melville, M.; Domaille, D.; Holz, R.C. Degradation of Atrazine to Cyanuric Acid by an Encapsulated Enzyme Cascade. Catalysts 2025, 15, 1055. https://doi.org/10.3390/catal15111055
Mowery-Evans M, Benzie E, Alansari N, Melville M, Domaille D, Holz RC. Degradation of Atrazine to Cyanuric Acid by an Encapsulated Enzyme Cascade. Catalysts. 2025; 15(11):1055. https://doi.org/10.3390/catal15111055
Chicago/Turabian StyleMowery-Evans, Maya, Emma Benzie, Noha Alansari, Michael Melville, Dylan Domaille, and Richard C. Holz. 2025. "Degradation of Atrazine to Cyanuric Acid by an Encapsulated Enzyme Cascade" Catalysts 15, no. 11: 1055. https://doi.org/10.3390/catal15111055
APA StyleMowery-Evans, M., Benzie, E., Alansari, N., Melville, M., Domaille, D., & Holz, R. C. (2025). Degradation of Atrazine to Cyanuric Acid by an Encapsulated Enzyme Cascade. Catalysts, 15(11), 1055. https://doi.org/10.3390/catal15111055








