Degradation of Bisphenols by Air Micro-Nano Bubbles Activated Persulfate
Abstract
1. Introduction
2. Results and Discussion
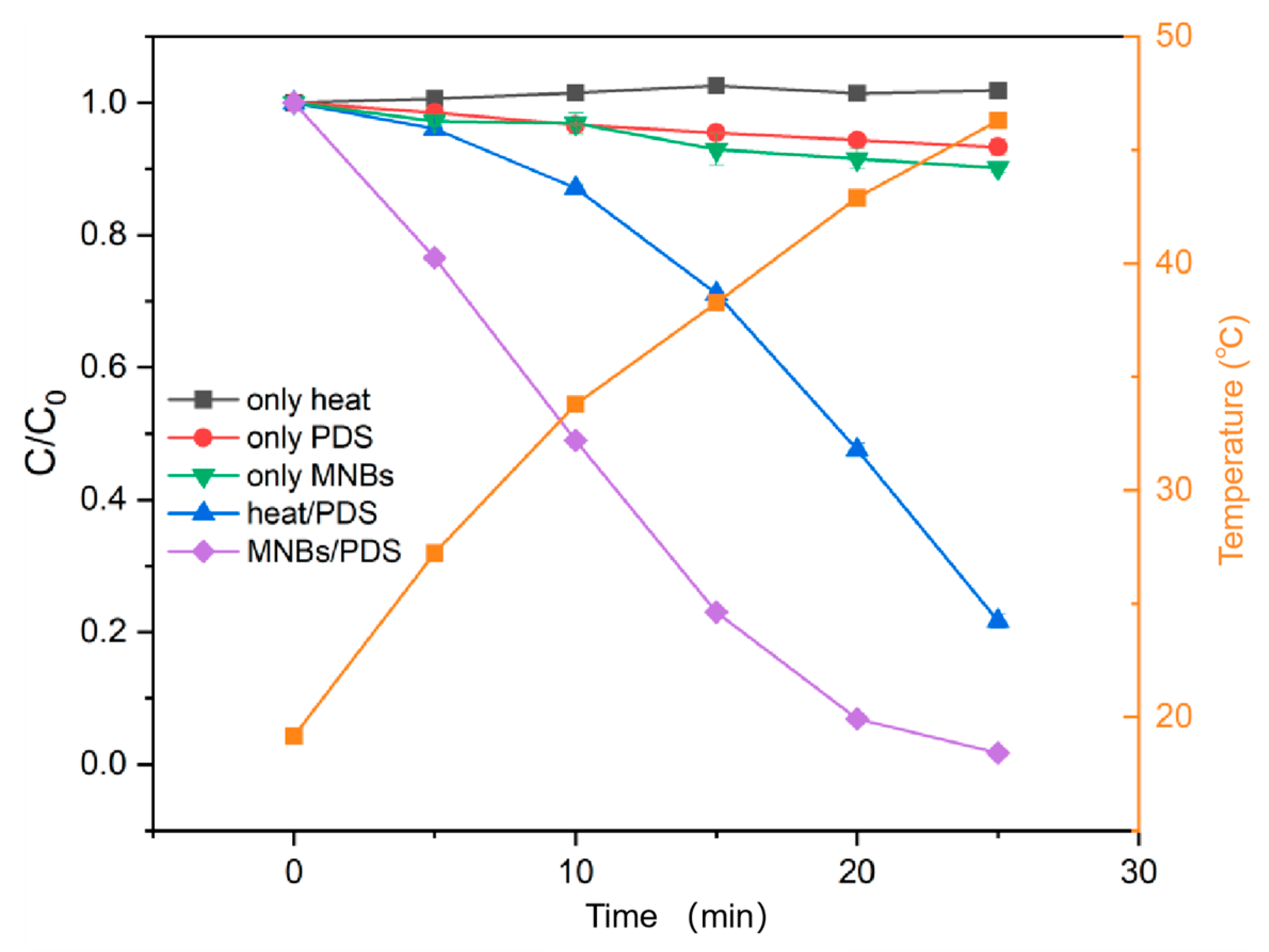
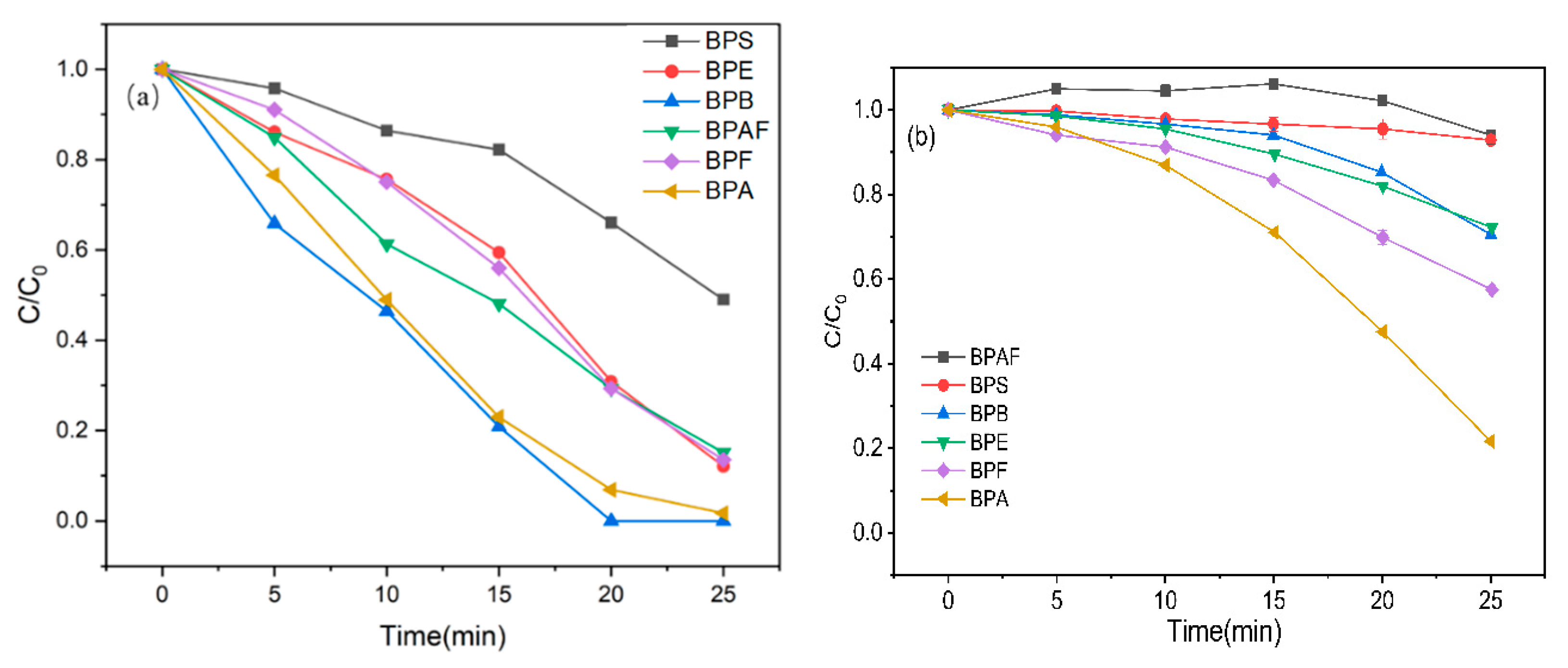
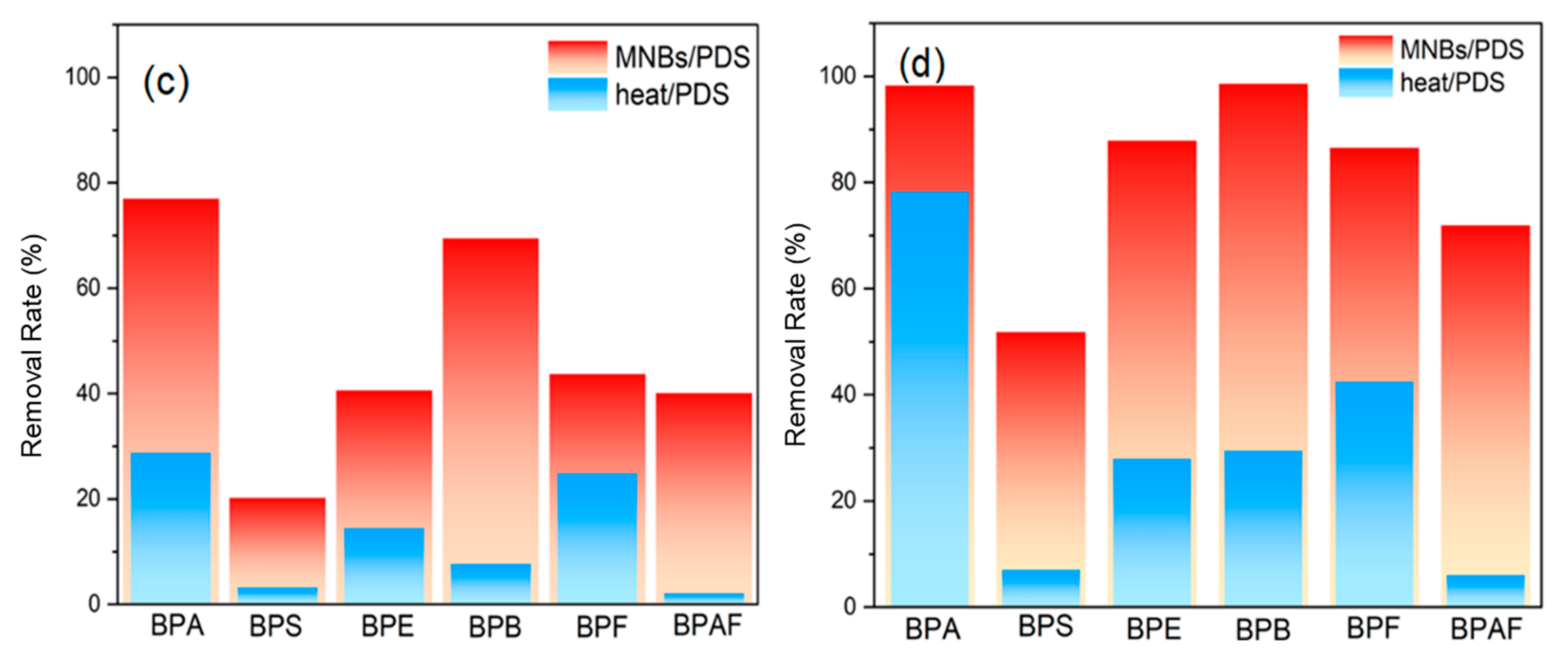
2.1. Degradation of BPA and Its Analogs by MNBs/PDS
2.2. Reaction Mechanisms in MNBs/PDS System
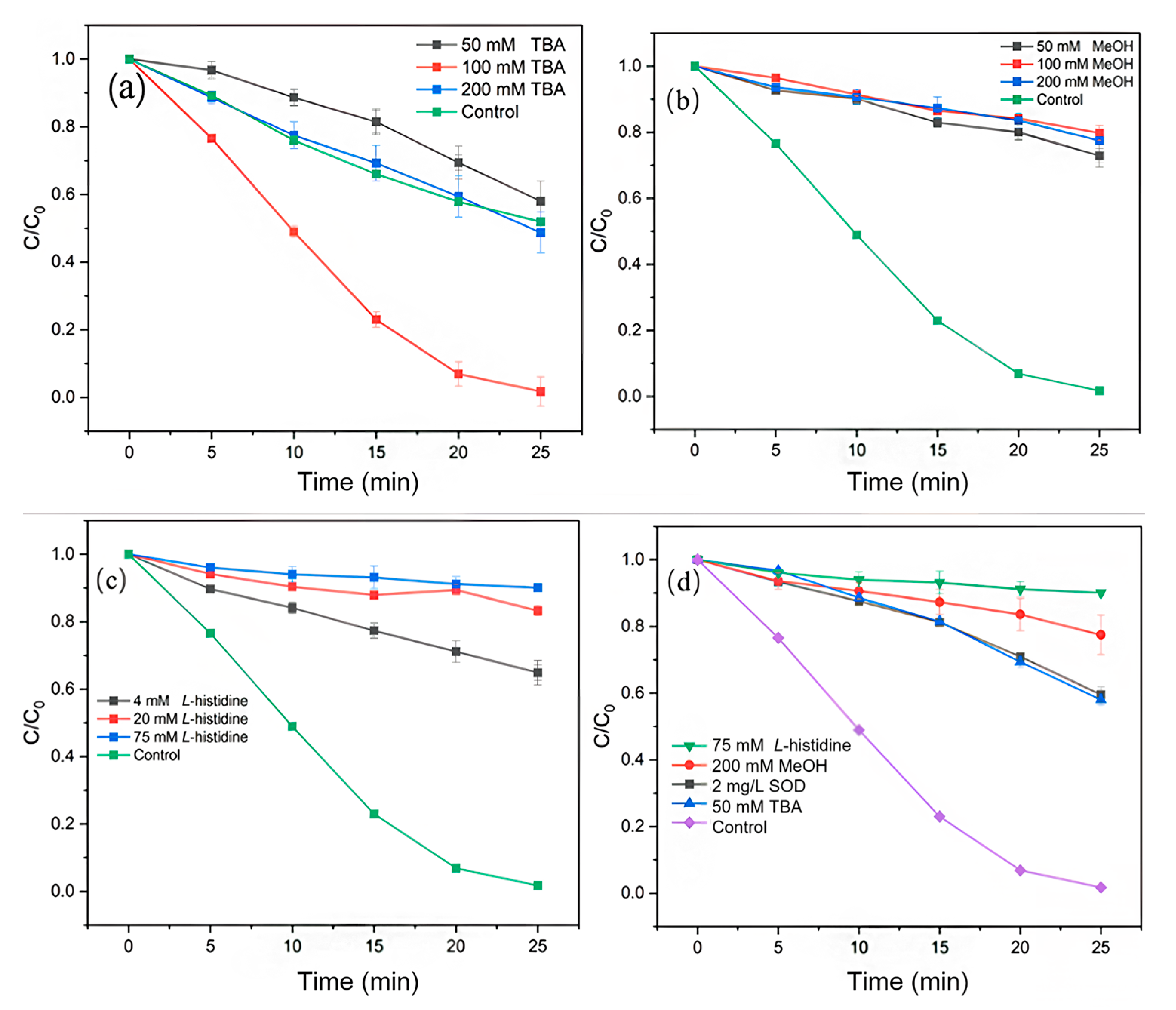
2.2.1. Quenching Experiment
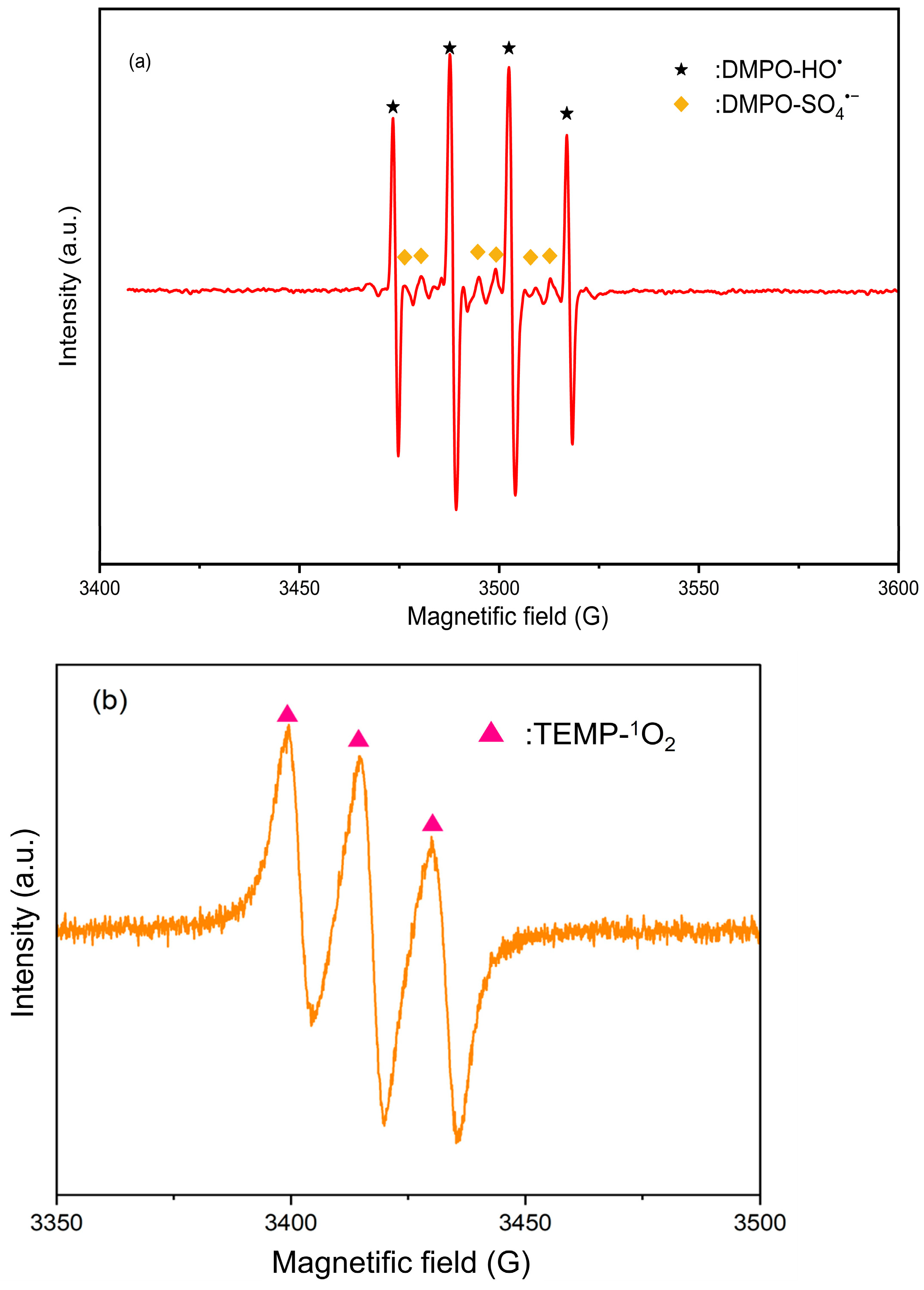
2.2.2. EPR Experiment
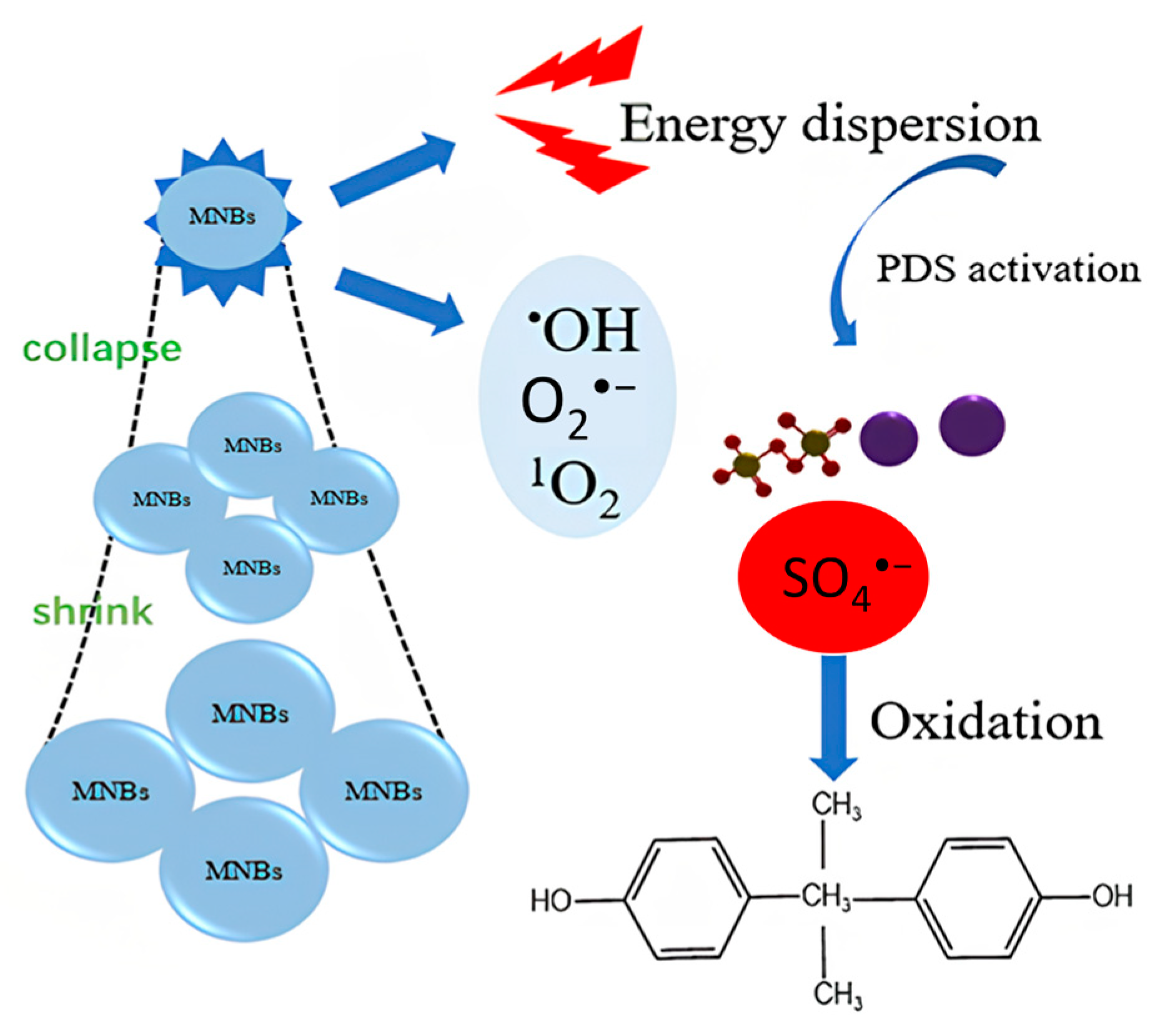
2.2.3. Reaction Mechanisms of MNBs/PDS
2.3. Influencing Factors of BPA Degradation by MNBs/PDS
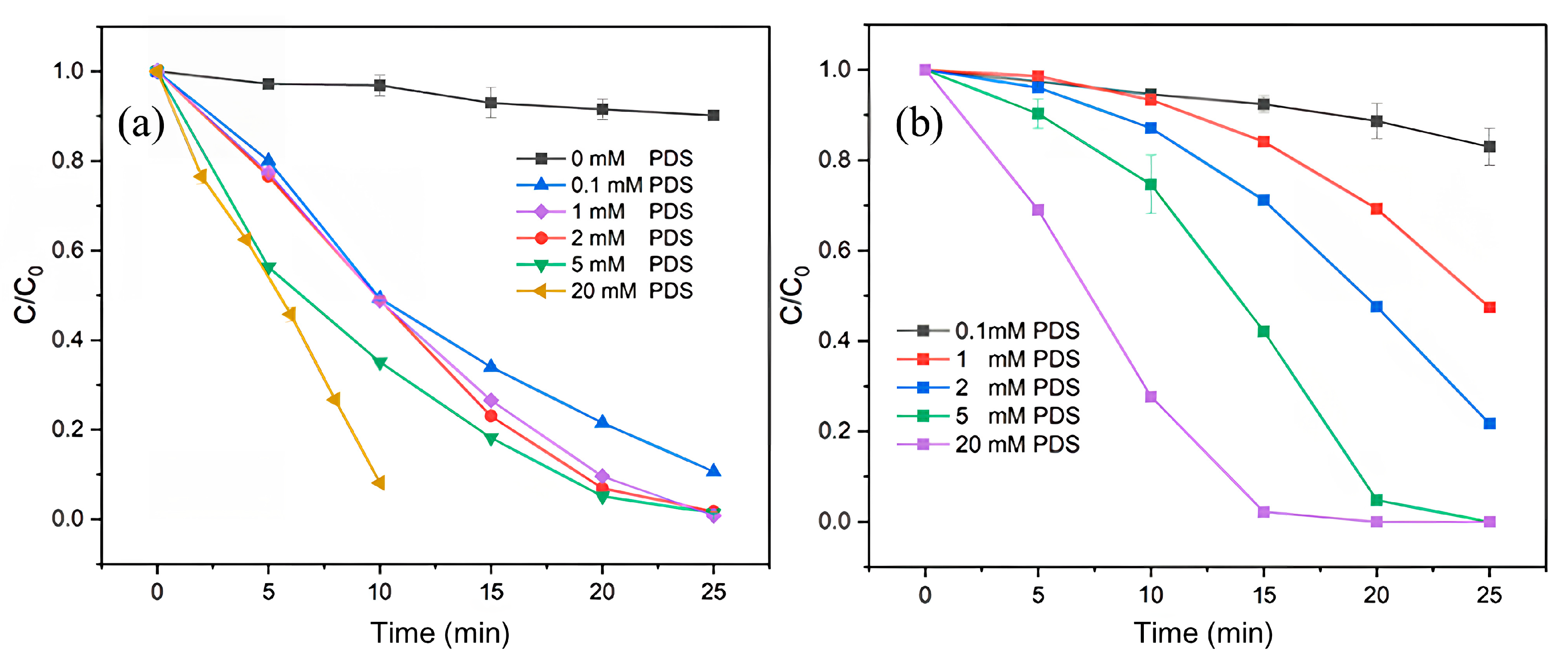
2.3.1. PDS Dosages
2.3.2. Air Intake and MNBs Generator Pressure
2.3.3. pH Value
2.3.4. Inorganic Ions and Natural Organic Matter (NOM)
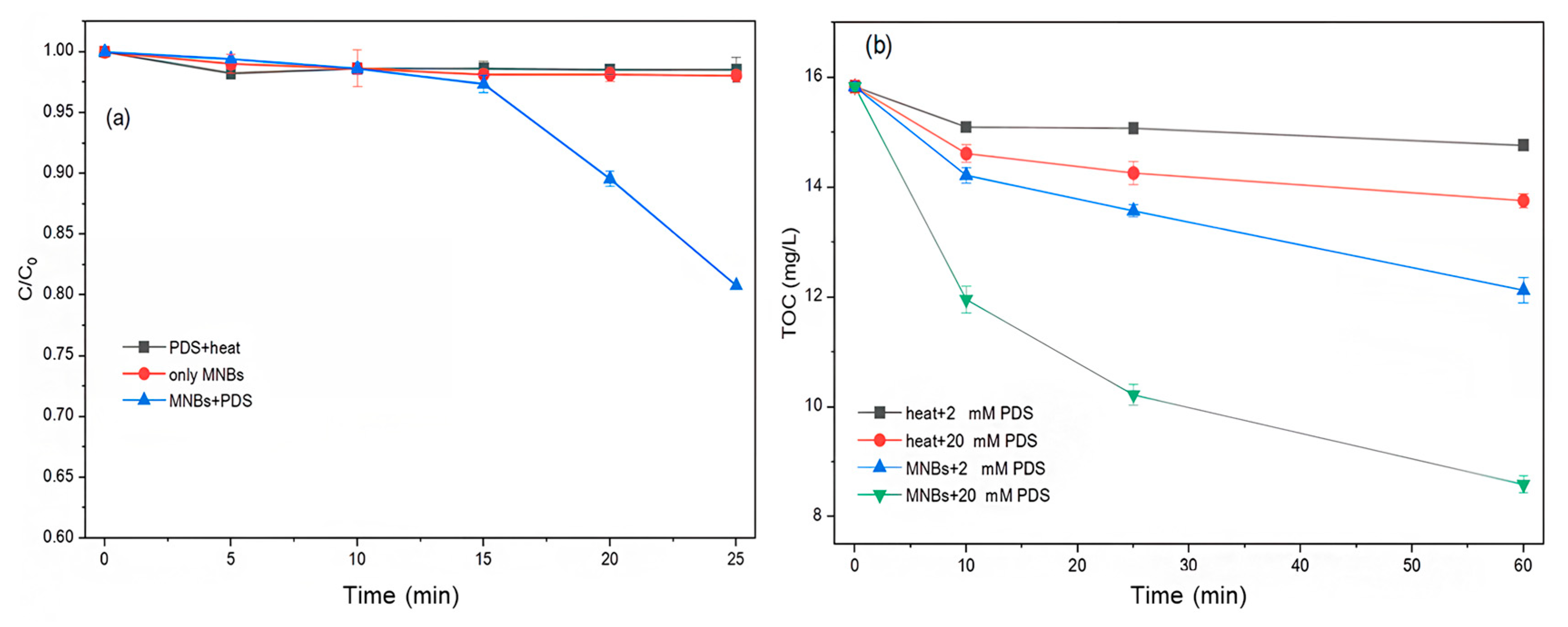
2.4. Advanced Treatment of Wastewater by MNBs/PDS
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Set-Up and Procedures
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shah, N.A.; Chen, X.; Javed, A.; Qu, J.; Zhang, Y. A Comparative Study on Degradation Kinetics and Toxicity Changes of BPA and BPS in UV-Based Advanced Oxidation Processes. Environ. Res. 2025, 284, 122223. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, K.; Zhu, R.; Wang, X.; Waigi, M.G.; Li, S. Biotransformation of Bisphenol A in Vivo and in Vitro by Laccase-Producing Trametes Hirsuta La-7: Kinetics, Products, and Mechanisms. Environ. Pollut. 2023, 321, 121155. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Park, B.J. Removal of Bisphenol A from Wastewater by Physical, Chemical and Biological Remediation Techniques. A Review. Environ. Chem. Lett. 2022, 20, 1801–1837. [Google Scholar] [CrossRef]
- De La Cruz, I.J.; Rodríguez, S.J.L.; Fuentes, I.; Tiznado, H.; Vazquez-Arce, J.L.; Romero-Ibarra, I.; Guzmán, C.J.I.; Gutiérrez, H.M. Effect of Crystalline Phase of MnO2 on the Degradation of Bisphenol A by Catalytic Ozonation. J. Environ. Chem. Eng. 2023, 11, 110753. [Google Scholar] [CrossRef]
- Slapničar, Š.; Žerjav, G.; Németh, M.; Zavašnik, J.; Pintar, A. Design and Evaluation of Plasmonic Metal-TiO2 Nanostructures for Photocatalytic Degradation of BPA as a Model Pollutant. J. Colloid Interface Sci. 2025, 700, 138361. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, J.; Knudsen, T.Š.; Pang, W.; Zhu, J.; Liu, B.; Li, H.; Li, M.; Su, J. Radical Chemistry, Degradation Mechanism and Toxicity Evolution of BPA in the UV/Chlorine and UV/H2O2. Chemosphere 2023, 312, 137169. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Muhamad, M.H.; Budi Kurniawan, S.; Buhari, J.; Husain Abuzeyad, O. Managing Bisphenol A Contamination: Advances in Removal Technologies and Future Prospects. Water 2023, 15, 3573. [Google Scholar] [CrossRef]
- Jasni, M.J.F.; Arulkumar, M.; Sathishkumar, P.; Mohd Yusoff, A.R.; Buang, N.A.; Gu, F.L. Electrospun Nylon 6,6 Membrane as a Reusable Nano-Adsorbent for Bisphenol A Removal: Adsorption Performance and Mechanism. J. Colloid Interface Sci. 2017, 508, 591–602. [Google Scholar] [CrossRef]
- AlDhawi, Z.A.; BinSharfan, I.I.; Abdulhamid, M.A. Carboxyl-Functionalized Polyimides for Efficient Bisphenol A Removal: Influence of Wettability and Porosity on Adsorption Capacity. Chemosphere 2023, 313, 137347. [Google Scholar] [CrossRef]
- Muhamad, M.S.; Salim, M.R.; Lau, W.J.; Yusop, Z.; Hadibarata, T. The Removal of Bisphenol A in Water Treatment Plant Using Ultrafiltration Membrane System. Water Air Soil Pollut. 2016, 227, 250. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, M.; Deng, Y.; Liu, M.; Chen, Y.; Gao, N.; Du, E.; Chu, W.; Guo, H. Generality and Diversity on the Kinetics, Toxicity and DFT Studies of Sulfate Radical-Induced Transformation of BPA and Its Analogues. Water Res. 2022, 219, 118506. [Google Scholar] [CrossRef]
- Wang, C.; Ren, S.; Cao, T.; Wang, J.; Peng, R.; Lv, Z.; Zhu, X.; Song, Y.; Na, J.; Mao, Y. Sulfate Radicals-Based Advanced Oxidation Process (SR-AOPs): The Future Enabled by MOFs-Based Nanofibers. Chem. Eng. J. 2024, 496, 154352. [Google Scholar] [CrossRef]
- Xie, J.; Yang, C.; Li, X.; Wu, S.; Lin, Y. Generation and Engineering Applications of Sulfate Radicals in Environmental Remediation. Chemosphere 2023, 339, 139659. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Fu, C.; Wang, Z.; Zhou, Y.; Zhang, X.; Yang, J.; Wang, K.; Liu, Y.; Song, Q. Establishment of Sulfate Radical Advanced Oxidation Process Based on Fe2+/O2/Dithionite for Organic Contaminants Degradation. Chem. Eng. J. 2021, 410, 128204. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Zhang, C.; Lu, S.; Xi, Y.; Huang, Y.; Xue, Z.; Yang, T. Combining Ferrate(VI) with Thiosulfate to Oxidize Chloramphenicol: Influencing Factors and Degradation Mechanism. J. Environ. Chem. Eng. 2021, 9, 104625. [Google Scholar] [CrossRef]
- Li, J.; Cassol, G.S.; Zhao, J.; Sato, Y.; Jing, B.; Zhang, Y.; Shang, C.; Yang, X.; Ao, Z.; Chen, G.; et al. Superfast Degradation of Micropollutants in Water by Reactive Species Generated from the Reaction between Chlorine Dioxide and Sulfite. Water Res. 2022, 222, 118886. [Google Scholar] [CrossRef] [PubMed]
- Madihi-Bidgoli, S.; Asadnezhad, S.; Yaghoot-Nezhad, A.; Hassani, A. Azurobine Degradation Using Fe2O3@multi-Walled Carbon Nanotube Activated Peroxymonosulfate (PMS) under UVA-LED Irradiation: Performance, Mechanism and Environmental Application. J. Environ. Chem. Eng. 2021, 9, 106660. [Google Scholar] [CrossRef]
- Peng, W.; Dong, Y.; Fu, Y.; Wang, L.; Li, Q.; Liu, Y.; Fan, Q.; Wang, Z. Non-Radical Reactions in Persulfate-Based Homogeneous Degradation Processes: A Review. Chem. Eng. J. 2021, 421, 127818. [Google Scholar] [CrossRef]
- Wen, Y.; Huang, C.-H.; Ashley, D.C.; Meyerstein, D.; Dionysiou, D.D.; Sharma, V.K.; Ma, X. Visible Light-Induced Catalyst-Free Activation of Peroxydisulfate: Pollutant-Dependent Production of Reactive Species. Environ. Sci. Technol. 2022, 56, 2626–2636. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y. Response Surface Methodology for Optimization of Bisphenol A Degradation Using Fe3O4-Activated Persulfate. Catalysts 2023, 13, 128. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Fan, Z.; Wang, X.; Wu, Z.; Akram, M.; Wang, L.; Liu, J.; Pan, J.; Gao, B. Unique Role of Doped Mo into Fe-Based Catalyst to Intensify Peroxydisulfate Activation for Micropollutants Degradation: Promote the Conversion of SO4•− to FeIV = O. Chem. Eng. J. 2024, 500, 157255. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, C.; Zhang, Z.; Lu, L.; Chen, J.; Chen, D. Enhancing Biodegradation of Gaseous Chlorobenzene by Introducing Micro-Nano Bubbles (MNBs): Performance and Mechanisms. J. Hazard. Mater. 2025, 494, 138745. [Google Scholar] [CrossRef]
- Dong, J.; Yao, J.; Tao, J.; Shi, X.; Wei, F. Degradation of Methyl Orange by Ozone Microbubble Process with Packing in the Bubble Column Reactor. Environ. Technol. 2023, 44, 2512–2524. [Google Scholar] [CrossRef]
- Xia, Z.; Hu, L.; Kusaba, S.; Song, D. Remediation of TCE Contaminated Site by Ozone Micro-Nano-Bubbles. In Proceedings of the 8th International Congress on Environmental Geotechnics, Hangzhou, China, 28 October–1 November 2018. [Google Scholar]
- Hu, L.; Chen, B.; Ma, J. Micro-/Nano- Bubbles Ozonation for Effective Industrial Wastewater Remediation: From Lab to Pilot-Scale Application. J. Environ. Chem. Eng. 2023, 11, 110807. [Google Scholar] [CrossRef]
- Tang, L.; Huang, J.; Zhuang, C.; Zhou, S.; Sun, L.; Lu, H. Micronano-Bubble Ozonation as an Efficient Pretreatment Technology for Raw Oxytetracycline Production Wastewater Discharged to Biological Treatment. Chem. Eng. J. 2023, 476, 146518. [Google Scholar] [CrossRef]
- Fan, W.; Cui, J.; Li, Q.; Huo, Y.; Xiao, D.; Yang, X.; Yu, H.; Wang, C.; Jarvis, P.; Lyu, T.; et al. Bactericidal Efficiency and Photochemical Mechanisms of Micro/Nano Bubble–Enhanced Visible Light Photocatalytic Water Disinfection. Water Res. 2021, 203, 117531. [Google Scholar] [CrossRef]
- Sun, Z.; Xia, F.; Lou, Z.; Chen, X.; Zhu, N.; Yuan, H.; Shen, Y. Innovative Process for Total Petroleum Hydrocarbons Reduction on Oil Refinery Sludge through Microbubble Ozonation. J. Clean. Prod. 2020, 256, 120337. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Chen, C.; Fang, J. UV/Chlorine Process: An Efficient Advanced Oxidation Process with Multiple Radicals and Functions in Water Treatment. Accounts Chem. Res. 2022, 55, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zheng, Y.; Shang, C.; Yin, R. Concentration-Dependent Chloride Effect on Radical Distribution and Micropollutant Degradation in the Sulfate Radical-Based AOPs. J. Hazard. Mater. 2022, 430, 128450. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, Z.; Qiu, G.; Wan, Y.; Song, W.; Zeng, H.; Yang, F.; Zhao, D.; Yuan, W.; Ju, P.; et al. Generation of Reactive Oxygen Species through Dissolved Oxygen Activation on Defected Porous Carbon for Efficient Degradation of Antibiotics. Chem. Eng. J. 2023, 455, 140602. [Google Scholar] [CrossRef]
- Naz, H. Thermal Stability of Purified Superoxide Dismutase from Liver of Commercially Valuable Fish, Labeo Rohita Exposed to Pb+Cr Mixture. Pak. J. Agric. Sci. 2021, 58, 1191–1196. [Google Scholar] [CrossRef]
- Han, M.; Wang, H.; Jin, W.; Chu, W.; Xu, Z. The Performance and Mechanism of Iron-Mediated Chemical Oxidation: Advances in Hydrogen Peroxide, Persulfate and Percarbonate Oxidation. J. Environ. Sci. 2023, 128, 181–202. [Google Scholar] [CrossRef]
- Cerrón-Calle, G.A.; Flores, M.; Roldan, M.A.; Flores, K.; Brillas, E.; Dos Santos, A.J.; Garcia-Segura, S. Electrochemical Persulfate Activation for Degradation of BPA Using a Novel Self-Standing CuOx/CoOx Cathode. Chem. Eng. J. 2025, 519, 164960. [Google Scholar] [CrossRef]
- Han, H.; Chen, M.; Sun, C.; Han, Y.; Xu, L.; Zhao, Y. Synergistic Enhancement in Hydrodynamic Cavitation Combined with Peroxymonosulfate Fenton-like Process for Bpa Degradation: New Insights into the Role of Cavitation Bubbles in Regulation Reaction Pathway. Water Res. 2025, 268, 122666. [Google Scholar] [CrossRef]
- Soyluoglu, M.; Kim, D.; Zaker, Y.; Karanfil, T. Removal Mechanisms of Geosmin and MIB by Oxygen Nanobubbles during Water Treatment. Chem. Eng. J. 2022, 443, 136535. [Google Scholar] [CrossRef]
- Huang, W.; Bianco, A.; Brigante, M.; Mailhot, G. UVA-UVB Activation of Hydrogen Peroxide and Persulfate for Advanced Oxidation Processes: Efficiency, Mechanism and Effect of Various Water Constituents. J. Hazard. Mater. 2018, 347, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, M.; Zoh, K. Effect of Nitrate, Carbonate/Bicarbonate, Humic Acid, and H2O2 on the Kinetics and Degradation Mechanism of Bisphenol-A during UV Photolysis. Chemosphere 2018, 204, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wojnárovits, L.; Tóth, T.; Takács, E. Rate Constants of Carbonate Radical Anion Reactions with Molecules of Environmental Interest in Aqueous Solution: A Review. Sci. Total. Environ. 2020, 717, 137219. [Google Scholar] [CrossRef] [PubMed]
- Abdul, L.; Si, X.; Sun, K.; Si, Y. Degradation of Bisphenol A in Aqueous Environment Using Peroxymonosulfate Activated with Carbonate: Performance, Possible Pathway, and Mechanism. J. Environ. Chem. Eng. 2021, 9, 105419. [Google Scholar] [CrossRef]
- Hu, F.; Zhan, P.; Long, L.; Zhu, J.; Xu, G.; Peng, X. Activation of Peroxymonosulfate by ZIFs Derived Fe/Cu Encapsulated N-Doped Carbon for Bisphenol A Degradation: The Role of N Doping. Chemosphere 2022, 293, 133455. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Yuan, J.; Zhang, L.; Qian, W.; Bian, G.; Song, Y.; Gao, X.; Mao, H. Degradation of Bisphenol A during Heat-Activated Peroxodisulfate Treatment: Kinetics, Mechanism, and Transformation Products. RSC Adv. 2025, 15, 28949–28958. [Google Scholar] [CrossRef] [PubMed]
- Wahba, N.; El Asmar, M.F.; El Sadr, M.M. Iodometric Method for Determination of Persulfates. Anal. Chem. 1959, 31, 1870–1871. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Lu, C.; Li, X.; Tang, L.; Al-Anazi, A.; Duan, X. Degradation of Bisphenols by Air Micro-Nano Bubbles Activated Persulfate. Catalysts 2025, 15, 1048. https://doi.org/10.3390/catal15111048
Niu X, Lu C, Li X, Tang L, Al-Anazi A, Duan X. Degradation of Bisphenols by Air Micro-Nano Bubbles Activated Persulfate. Catalysts. 2025; 15(11):1048. https://doi.org/10.3390/catal15111048
Chicago/Turabian StyleNiu, Xiaoxiao, Can Lu, Xinjuan Li, Liang Tang, Abdulaziz Al-Anazi, and Xiaodi Duan. 2025. "Degradation of Bisphenols by Air Micro-Nano Bubbles Activated Persulfate" Catalysts 15, no. 11: 1048. https://doi.org/10.3390/catal15111048
APA StyleNiu, X., Lu, C., Li, X., Tang, L., Al-Anazi, A., & Duan, X. (2025). Degradation of Bisphenols by Air Micro-Nano Bubbles Activated Persulfate. Catalysts, 15(11), 1048. https://doi.org/10.3390/catal15111048





