Electrochemical Generation of Reactive Chlorine Species via Chloride Oxidation on –COOH-Modified Graphite Electrode to Attain Dye Degradation
Abstract
1. Introduction
2. Results and Discussion
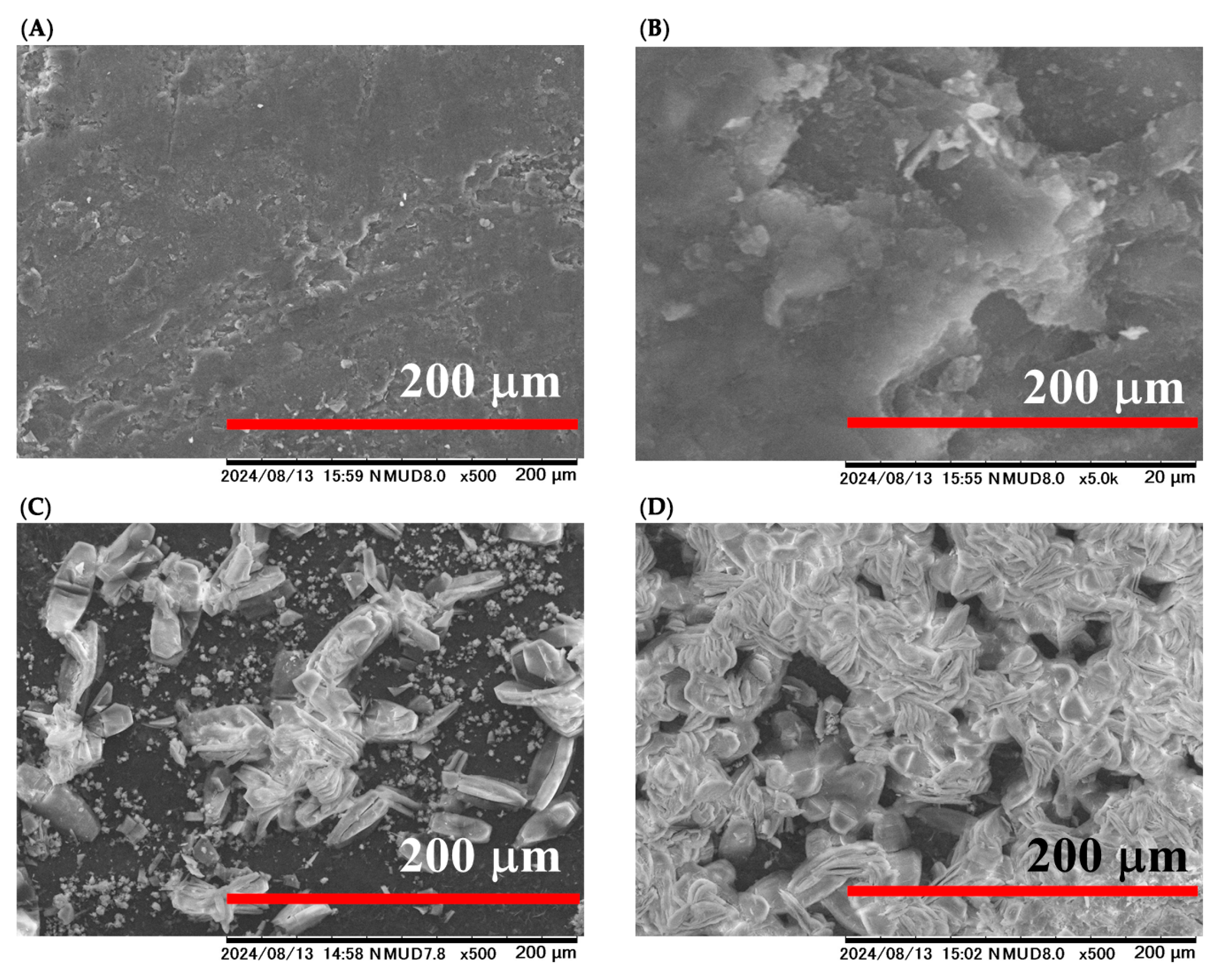
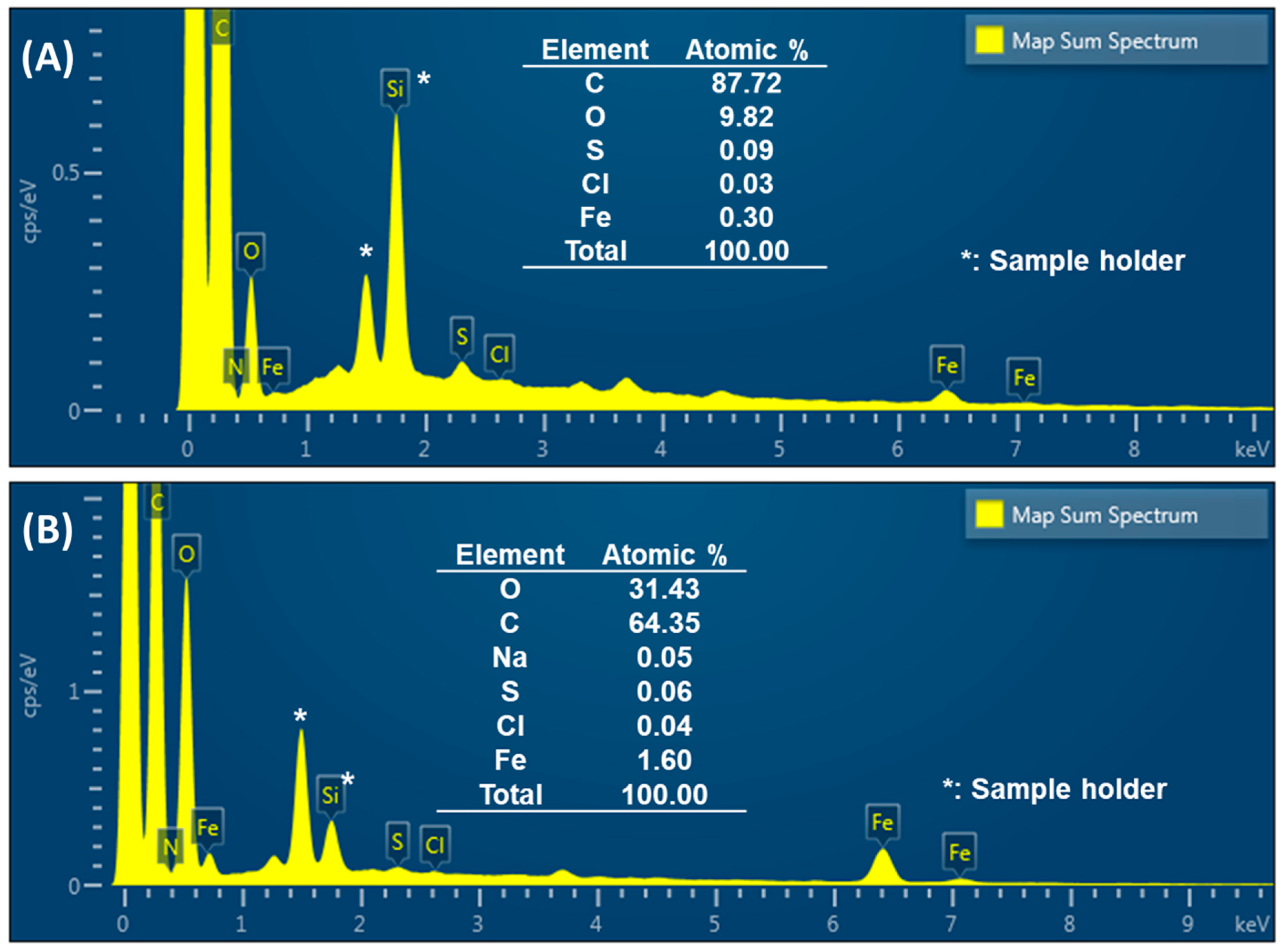
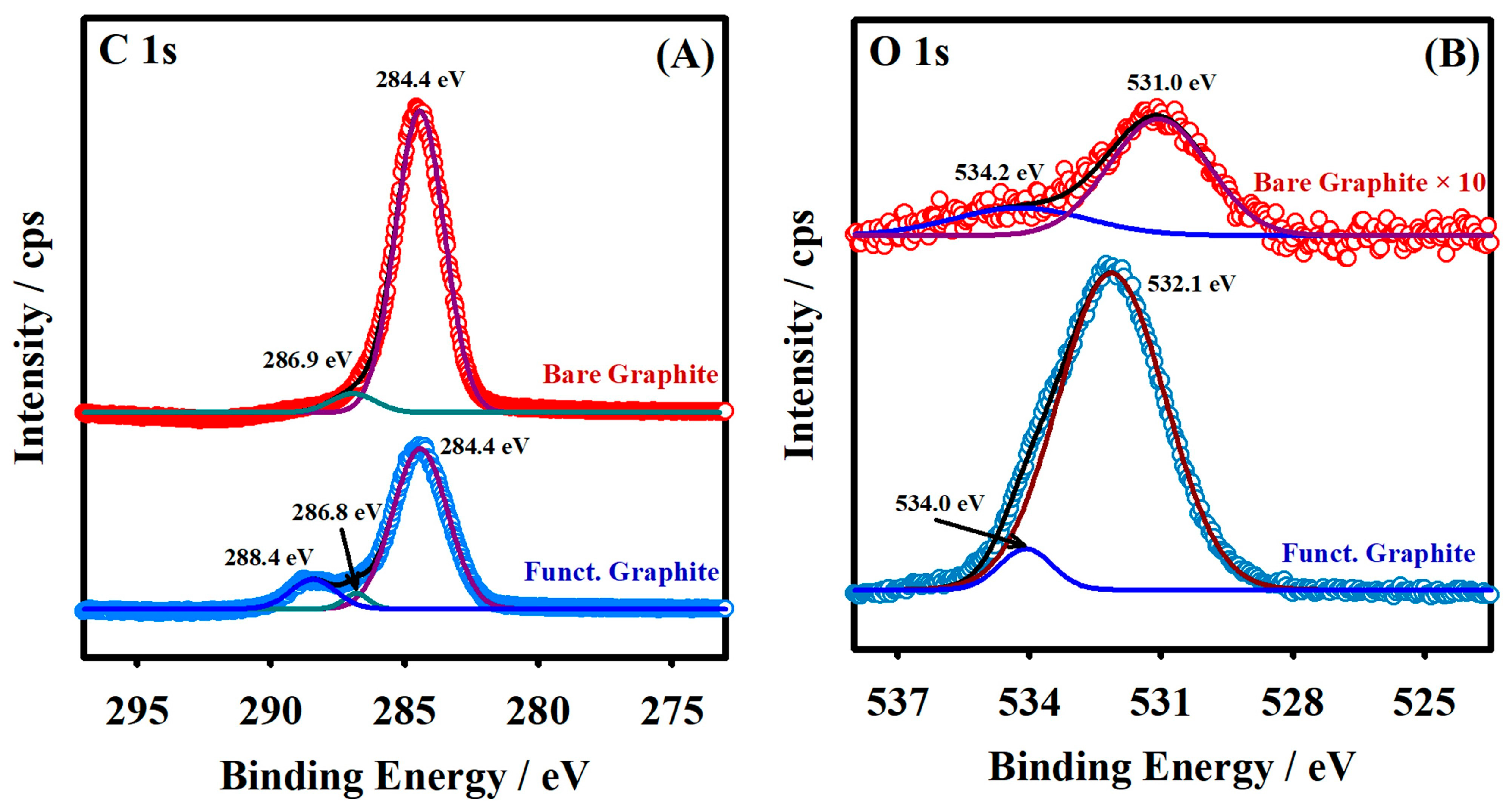
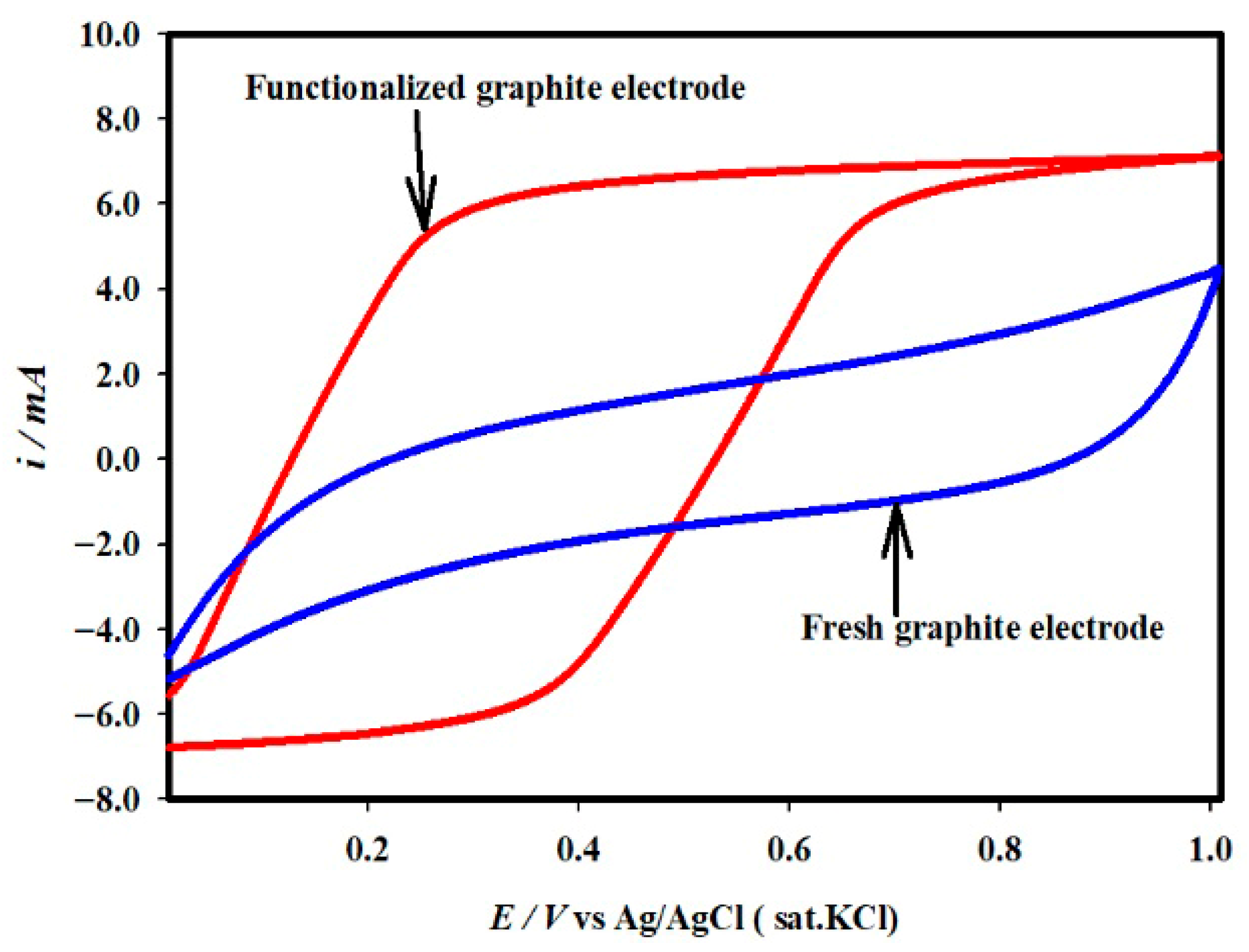
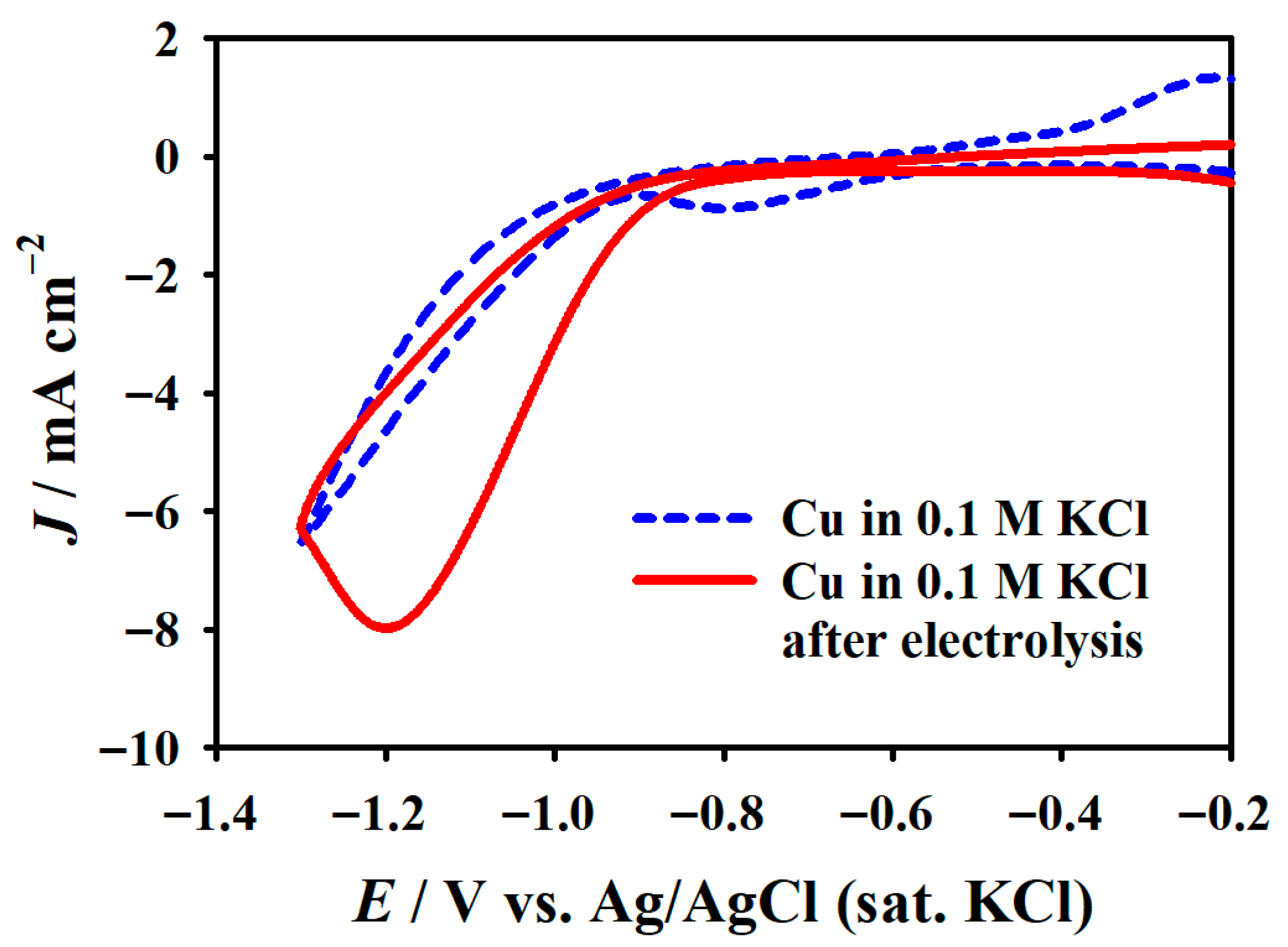
2.1. Fabrication of –COOH-Functionalized Pencil Graphite Electrode
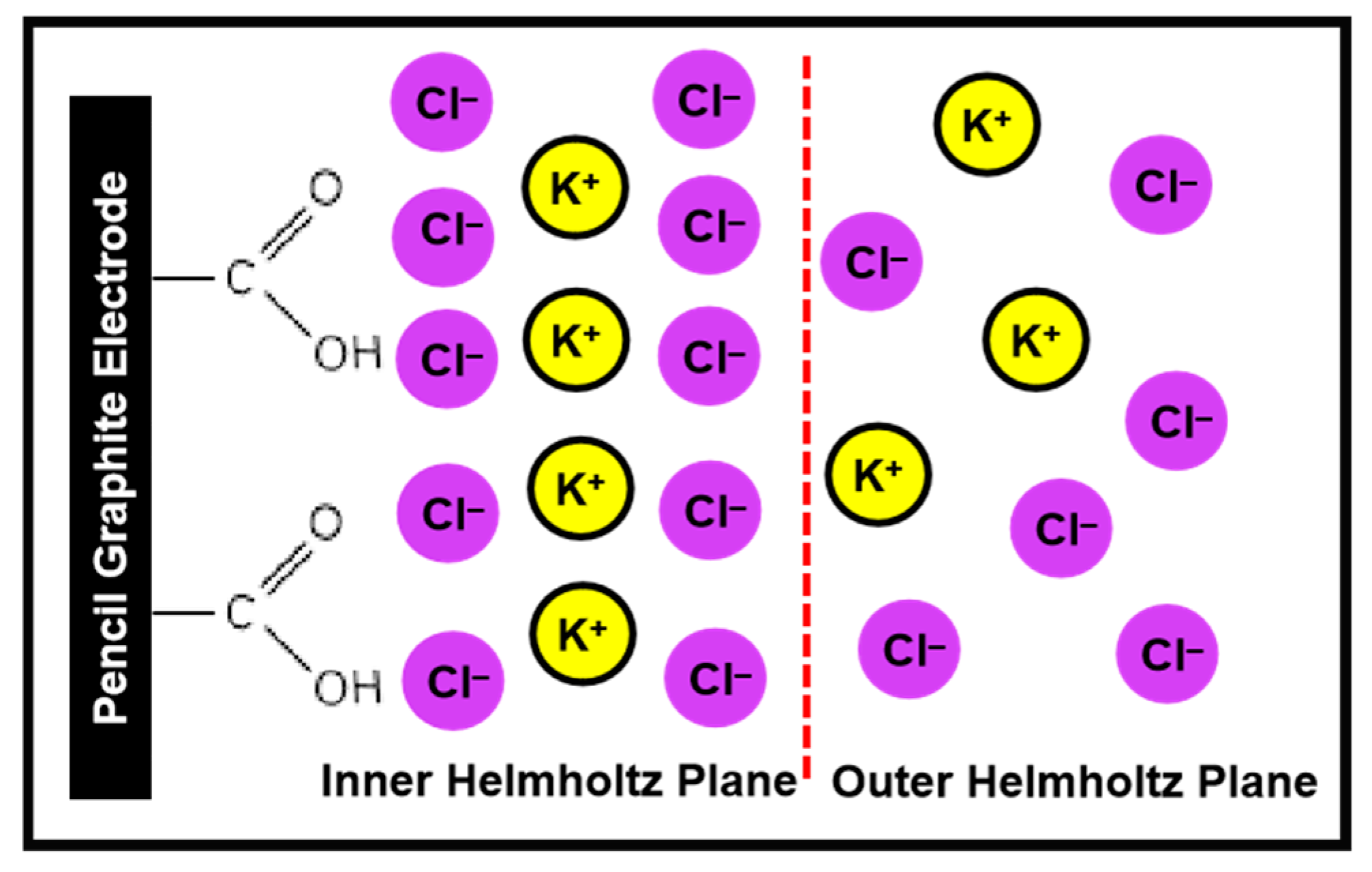
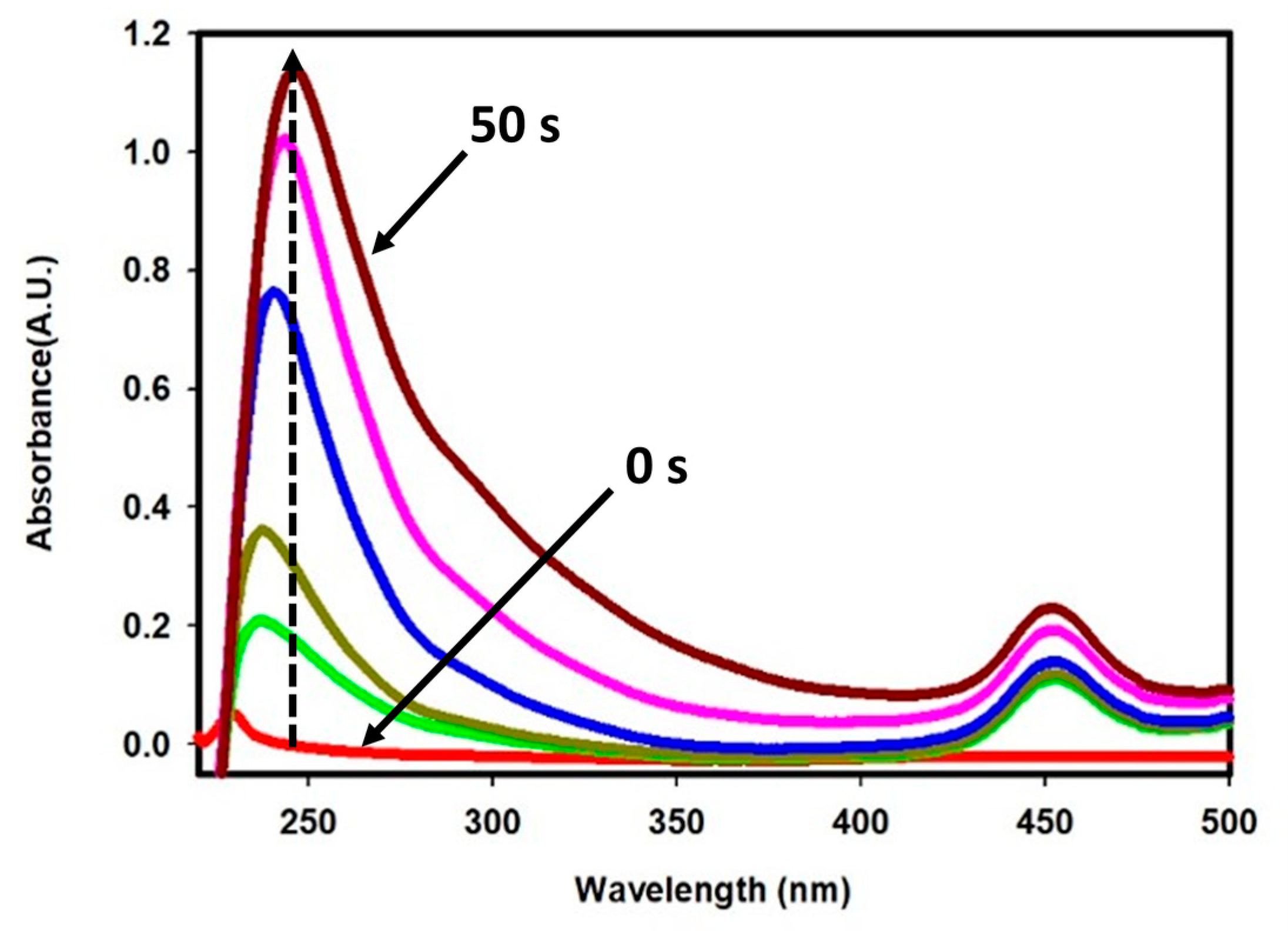
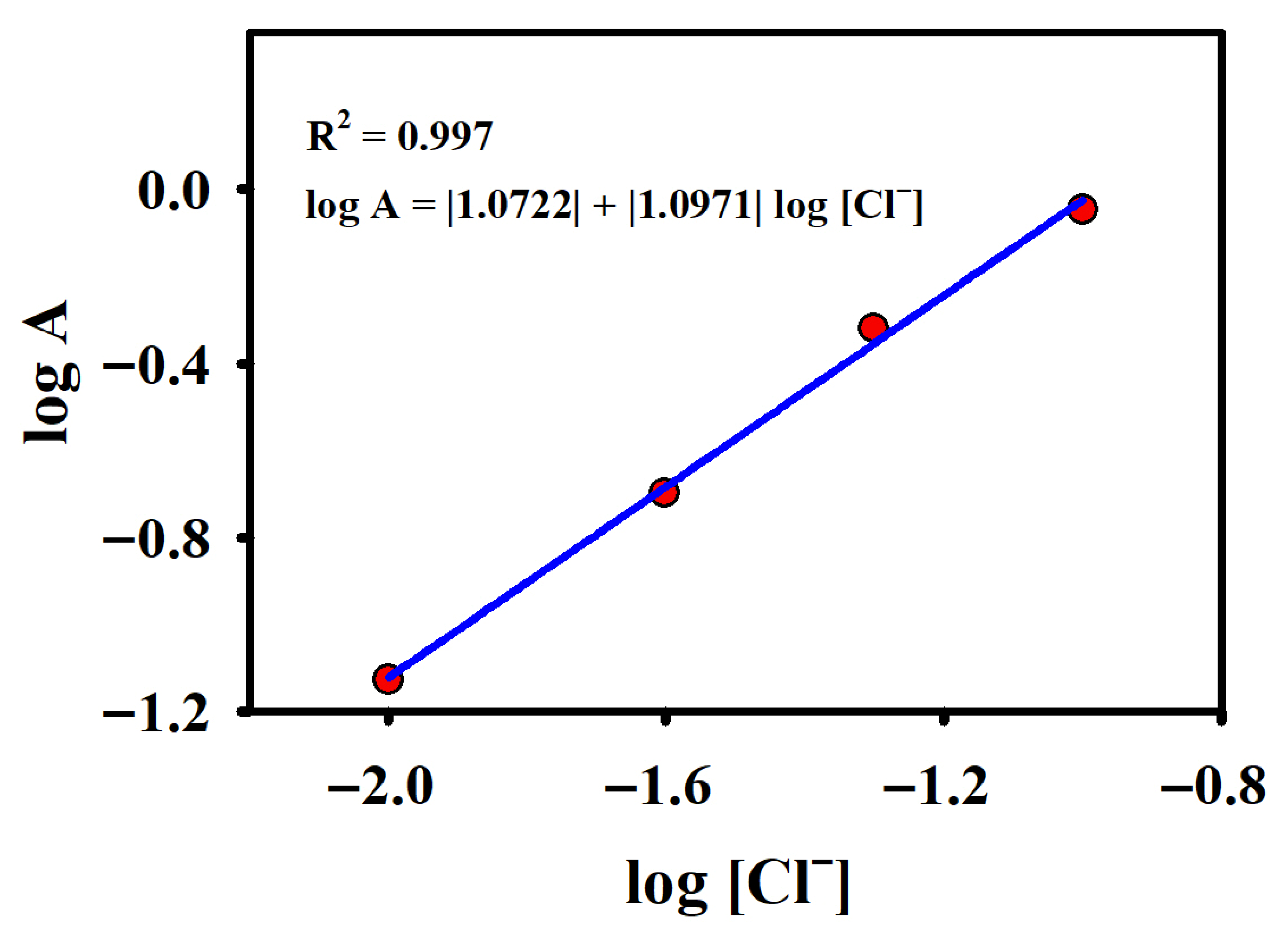
2.2. Oxidation of Chloride on CFG Electrode
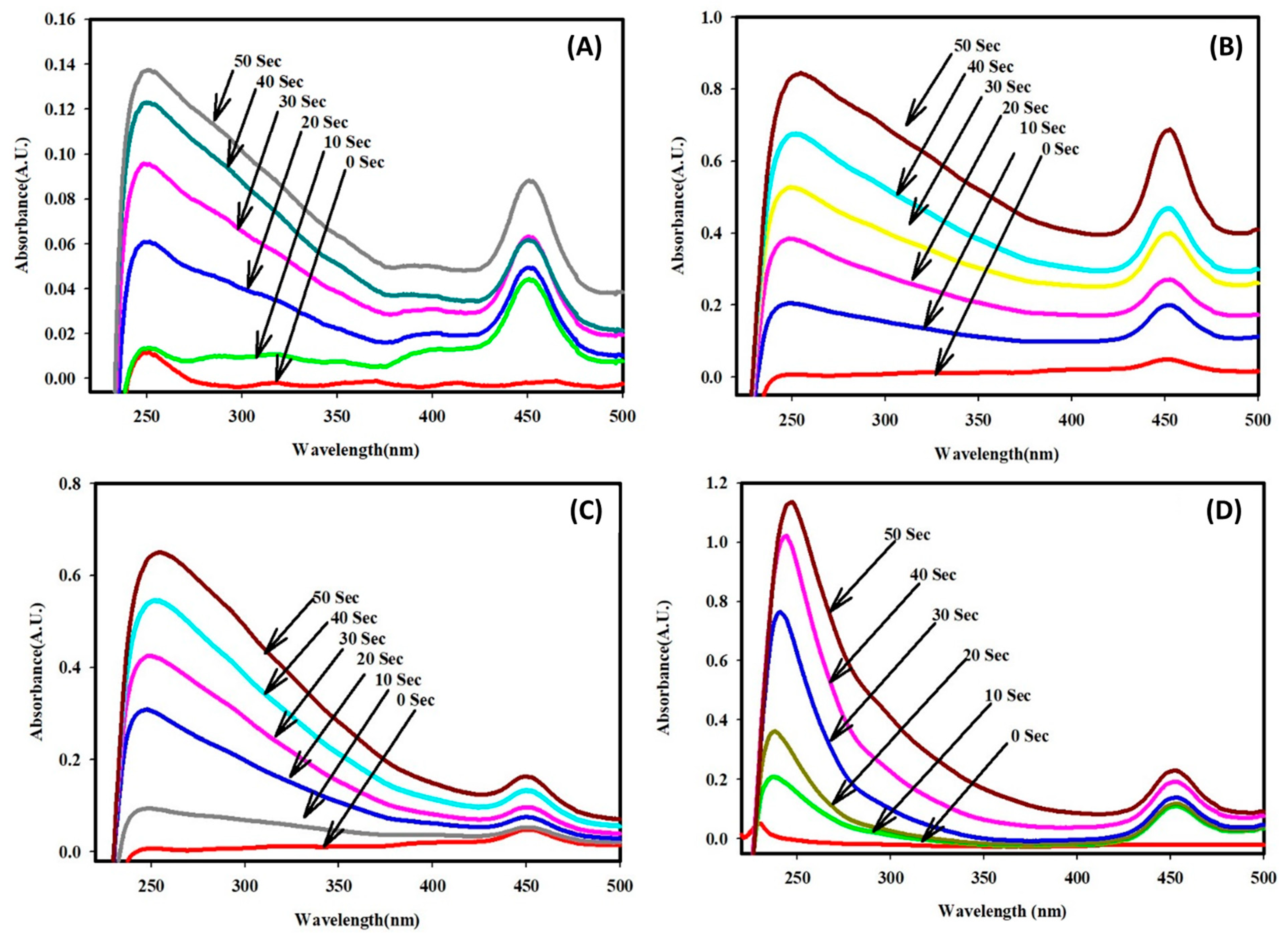
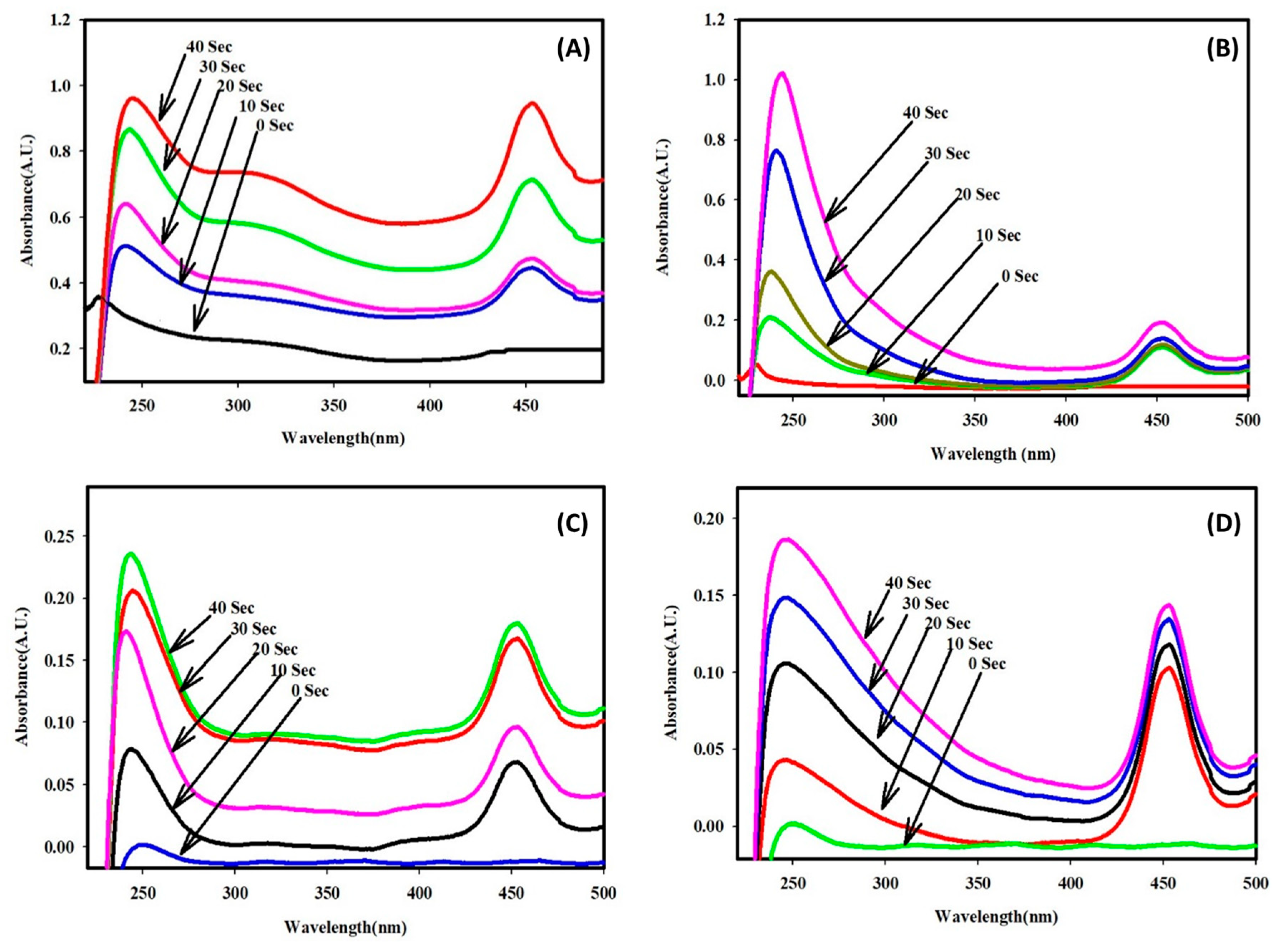
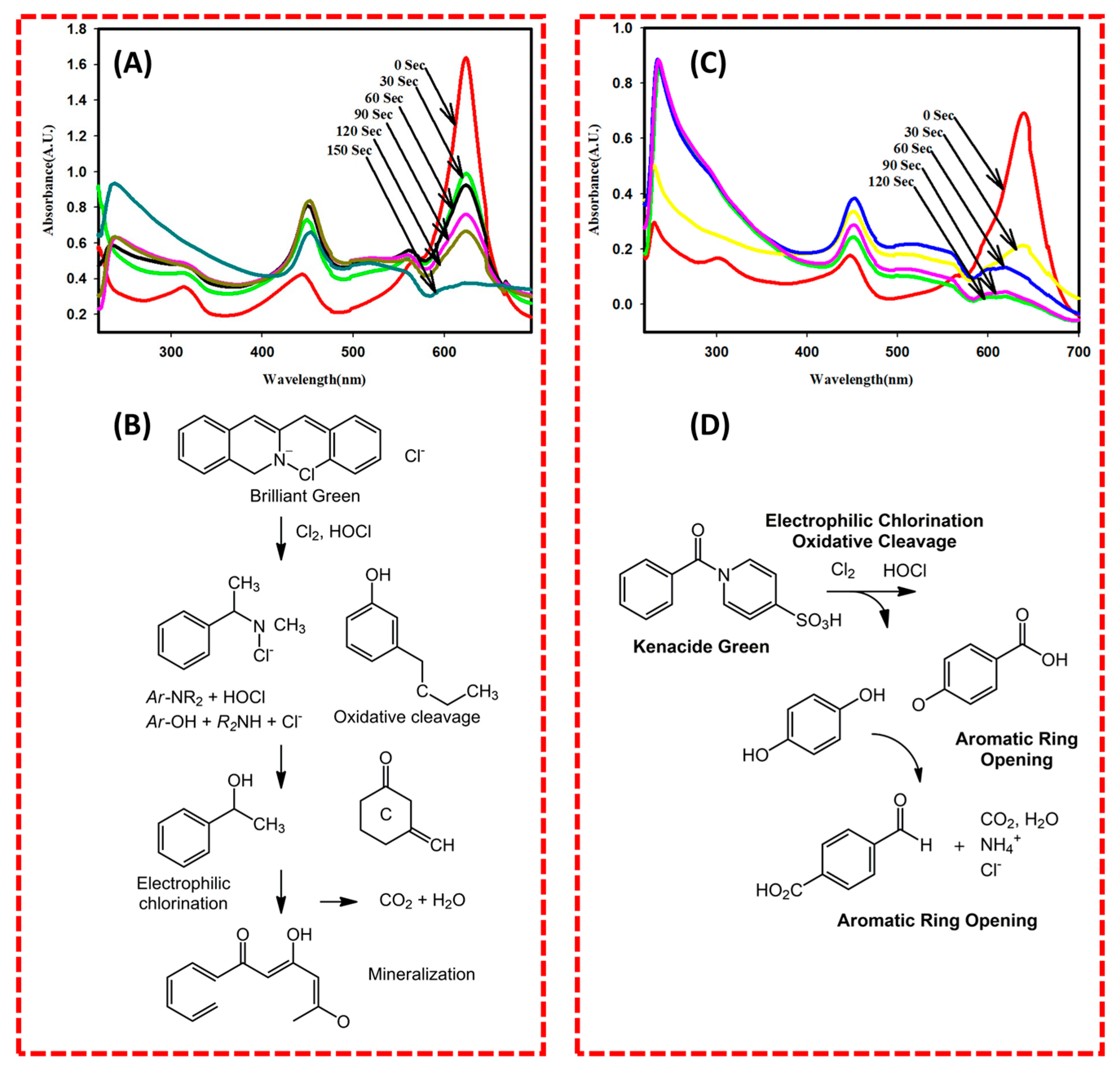
2.3. Electrocatalytic Dye Degradation
3. Experimental Section
3.1. Chemicals
3.2. Instruments and Equipment
3.3. Electrode Functionalization and Electrochemical Measurements
3.4. Chloride Ion (Cl−) Oxidation
3.5. Electrochemical Dye Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical Advanced Oxidation Processes: Today and Tomorrow. A Review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Bulman, D.M.; Remucal, C.K.; Chaplin, B.P. Chlorinated Byproduct Formation during the Electrochemical Advanced Oxidation Process at Magnéli Phase Ti4O7 Electrodes. Environ. Sci. Technol. 2020, 54, 12673–12683. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Martínez-Huitle, C.A. Nature, Mechanisms and Reactivity of Electrogenerated Reactive Species at Thin-Film Boron-Doped Diamond (BDD) Electrodes During Electrochemical Wastewater Treatment. ChemElectroChem 2019, 6, 2379–2392. [Google Scholar] [CrossRef]
- Lee, K.-M.; Lee, H.-J.; Seo, J.; Lee, T.; Yoon, J.; Kim, C.; Lee, C. Electrochemical Oxidation Processes for the Treatment of Organic Pollutants in Water: Performance Evaluation Using Different Figures of Merit. ACS ES&T Eng. 2022, 2, 1797–1824. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green Synthesis of Platinum Nanoparticles Using Saudi’s Dates Extract and Their Usage on the Cancer Cell Treatment. Arab. J. Chem. 2019, 12, 330–349. [Google Scholar] [CrossRef]
- Zheng, S.; Qin, W.; Ji, H.; Guo, W.; Fang, J. Predicting Rate Constants of Reactive Chlorine Species toward Organic Compounds by Combining Machine Learning and Quantum Chemical Calculation. Environ. Sci. Technol. Lett. 2023, 10, 804–809. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Sui, Y.; Lu, J.; Hu, B.; Huang, Q. Formation of Chlorate and Perchlorate during Electrochemical Oxidation by Magnéli Phase Ti4O7 Anode: Inhibitory Effects of Coexisting Constituents. Sci. Rep. 2022, 12, 15880. [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.; Qi, L.; Wang, H.; Xian, G. Chlorine-Mediated Electrochemical Advanced Oxidation Process for Ammonia Removal: Mechanisms, Characteristics and Expectation. Sci. Total Environ. 2023, 896, 165169. [Google Scholar] [CrossRef]
- Yang, K.; He, Z. Formation and Control of Oxidation Byproducts in Electrochemical Wastewater Treatment: A Review. Chem. Eng. J. 2024, 499, 156160. [Google Scholar] [CrossRef]
- Goren, A.Y.; Recepoğlu, Y.K.; Edebalï, Ö.; Sahin, C.; Genisoglu, M.; Okten, H.E. Electrochemical Degradation of Methylene Blue by a Flexible Graphite Electrode: Techno-Economic Evaluation. ACS Omega 2022, 7, 32640–32652. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Zhang, C. Generation and Application of Reactive Chlorine Species by Electrochemical Process Combined with UV Irradiation: Synergistic Mechanism for Enhanced Degradation Performance. Sci. Total Environ. 2020, 712, 136501. [Google Scholar] [CrossRef]
- de Moura, D.C.; do Nascimento Brito, C.; Quiroz, M.A.; Pergher, S.B.C.; Martinez-Huitle, C.A. Cl-Mediated Electrochemical Oxidation for Treating an Effluent Using Platinum and Diamond Anodes. J. Water Process Eng. 2015, 8, e31–e36. [Google Scholar] [CrossRef]
- He, F.; Li, K.; Yin, C.; Wang, Y.; Tang, H.; Wu, Z. Single Pd Atoms Supported by Graphitic Carbon Nitride, a Potential Oxygen Reduction Reaction Catalyst from Theoretical Perspective. Carbon 2017, 114, 619–627. [Google Scholar] [CrossRef]
- Ohanele, E.; Oguzie, K.; Ezema, F.; Oguzie, E.; Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; et al. Corrigendum to “One-Pot Synthesis and Characterization of Polyynes End-Capped by Biphenyl Groups (α,ω-Biphenylpolyynes)” [Carbon 126 (2018) 232–240]. Carbon 2020, 162, 201. [Google Scholar] [CrossRef]
- Treviño-Reséndez, J.; Soto-Hernández, E.; Godínez, L.A.; Robles, I.; Meas Vong, Y.; García-Espinoza, J.D. Electrochemical Oxidation of Glyphosate Using Graphite Rod Electrodes: Impact of Acetic Acid Pretreatment on Degradation Efficiency. Processes 2024, 12, 2359. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef] [PubMed]
- Ohanele, E.; Oguzie, K.; Ezema, F.; Oguzie, E. Lead Oxide Modified Graphite Electrodes for Electrochemical Degradation of Congo Red Dye in Aqueous Solution. Period. Polytech. Chem. Eng. 2024, 68, 541–551. [Google Scholar] [CrossRef]
- Li, L.; Li, F. The Effect of Carbonyl, Carboxyl and Hydroxyl Groups on the Capacitance of Carbon Nanotubes. Carbon 2011, 49, 4610. [Google Scholar] [CrossRef]
- Dobrota, A.S.; Pašti, I.A.; Mentus, S.V.; Skorodumova, N.V. A General View on the Reactivity of the Oxygen-Functionalized Graphene Basal Plane. Phys. Chem. Chem. Phys. 2016, 18, 6580–6586. [Google Scholar] [CrossRef]
- Castillo-Cabrera, G.X.; Pliego-Cerdán, C.I.; Méndez, E.; Espinoza-Montero, P.J. Step-by-Step Guide for Electrochemical Generation of Highly Oxidizing Reactive Species on BDD for Beginners. Front. Chem. 2023, 11, 1298630. [Google Scholar] [CrossRef]
- Wu, L.; Garg, S.; Dai, Y.; Lv, S.; Wang, Y.; Waite, T.D. Pilot-Scale Electrochemical Advanced Oxidation (EAOP) System for the Treatment of Ni-EDTA-Containing Wastewater. J. Hazard. Mater. 2024, 474, 134840. [Google Scholar] [CrossRef] [PubMed]
- Blyth, R.I.R.; Buqa, H.; Netzer, F.P.; Ramsey, M.G.; Besenhard, J.O.; Golob, P.; Winter, M. XPS Studies of Graphite Electrode Materials for Lithium Ion Batteries. Appl. Surf. Sci. 2000, 167, 99–106. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water Purification from Chlorobenzenes Using Heteroatom-Functionalized Carbon Nanofibers Produced on Self-Organizing Ni-Pd Catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Karim, M.R.; Rahman, M.M.; Asiri, A.M. Termination of Structural Deformation and Proton-Electron Conductive Inflection of Graphene Oxide in Six Years. ACS Appl. Electron. Mater. 2020, 2, 1304–1312. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, Z.; Fan, W.; Peng, G.; Qu, M. Effects of Functional Groups of Graphene Oxide on the Electrochemical Performance of Lithium-Ion Batteries. RSC Adv. 2015, 5, 90041–90048. [Google Scholar] [CrossRef]
- Lee, D.W.; De Los Santos, V.L.; Seo, J.W.; Felix, L.L.; Bustamante, D.A.; Cole, J.M.; Barnes, C.H.W. The Structure of Graphite Oxide: Investigation of Its Surface Chemical Groups. J. Phys. Chem. B 2010, 114, 5723–5728. [Google Scholar] [CrossRef]
- Oxygen|XPS Periodic Table|Thermo Fisher Scientific—BD. Available online: https://www.thermofisher.com/bd/en/home/materials-science/learning-center/periodic-table/non-metal/oxygen.html (accessed on 21 October 2025).
- Finney, A.R.; McPherson, I.J.; Unwin, P.R.; Salvalaglio, M. Electrochemistry, Ion Adsorption and Dynamics in the Double Layer: A Study of NaCl(Aq) on Graphite. Chem. Sci. 2021, 12, 11166–11180. [Google Scholar] [CrossRef]
- Groenendijk, D.J.; van Wunnik, J.N.M. Surfactant Adsorption and Ion Exchange on Calcite Surfaces. Energy Fuels 2021, 35, 8763–8772. [Google Scholar] [CrossRef]
- Sun, Z.; Chai, L.; Shu, Y.; Li, Q.; Liu, M.; Qiu, D. Chemical Bond between Chloride Ions and Surface Carboxyl Groups on Activated Carbon. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 530, 53–59. [Google Scholar] [CrossRef]
- Hypochlorous Acid—Wikipedia. Available online: https://en.wikipedia.org/wiki/Hypochlorous_acid (accessed on 1 September 2025).
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Keller-Rudek, H.; Moortgat, G.K.; Sander, R.; Sörensen, R. The MPI-Mainz UV/VIS Spectral Atlas of Gaseous Molecules of Atmospheric Interest. Earth Syst. Sci. Data 2013, 5, 365–373. [Google Scholar] [CrossRef]
- Kishimoto, N. State of the Art of UV/Chlorine Advanced Oxidation Processes: Their Mechanism, Byproducts Formation, Process Variation, and Applications. J. Water Environ. Technol. 2019, 17, 302–335. [Google Scholar] [CrossRef]
- Jungkamp, T.P.W.; Kirchner, U.; Schmidt, M.; Schindler, R.N. UV Absorption Cross-Section Data for the Hypochlorites ROCl (R H, CH3, C2H5, i-C3H7, Tert-C4H9). J. Photochem. Photobiol. A Chem. 1995, 91, 1–6. [Google Scholar] [CrossRef]
- Maric, D.; Burrows, J.P.; Meller, R.; Moortgat, G.K. A Study of the UV—Visible Absorption Spectrum of Molecular Chlorine. J. Photochem. Photobiol. A Chem. 1993, 70, 205–214. [Google Scholar] [CrossRef]
- Sant’Anna, R.T.P.; Santos, C.M.P.; Silva, G.P.; Ferreira, R.J.R.; Oliveira, A.P.; Côrtes, C.E.S.; Faria, R.B. Kinetics and Mechanism of Chlorate-Chloride Reaction. J. Braz. Chem. Soc. 2012, 23, 1543–1550. [Google Scholar] [CrossRef]
- Deborde, M.; von Gunten, U. Reactions of Chlorine with Inorganic and Organic Compounds during Water Treatment—Kinetics and Mechanisms: A Critical Review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef]
- Czarnetzki, L.; Janssen, L.J.J. Janssen Electrochemical Oxidation of Hypochlorite at Platinum Anodes. Electrochim. Acta 1988, 33, 561–566. [Google Scholar] [CrossRef][Green Version]
- Dong, H.; Yu, W.; Hoffmann, M.R. Mixed Metal Oxide Electrodes and the Chlorine Evolution Reaction. J. Phys. Chem. C 2021, 125, 20745–20761. [Google Scholar] [CrossRef]
- Seymour, I.; O’Sullivan, B.; Lovera, P.; Rohan, J.F.; O’Riordan, A. Electrochemical Detection of Free-Chlorine in Water Samples Facilitated by in-Situ PH Control Using Interdigitated Microelectrodes. Sens. Actuators B Chem. 2020, 325, 128774. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamental and Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Mostafa, E.; Reinsberg, P.; Garcia-Segura, S.; Baltruschat, H. Chlorine Species Evolution during Electrochlorination on Boron-Doped Diamond Anodes: In-Situ Electrogeneration of Cl2, Cl2O and ClO2. Electrochim. Acta 2018, 281, 831–840. [Google Scholar] [CrossRef]
- Jasper, J.T.; Yang, Y.; Hoffmann, M.R. Toxic Byproduct Formation during Electrochemical Treatment of Latrine Wastewater. Environ. Sci. Technol. 2017, 51, 7111–7119. [Google Scholar] [CrossRef]
- Ballesteros Martín, M.M.; Sánchez Pérez, J.A.; Casas López, J.L.; Oller, I.; Malato Rodríguez, S. Degradation of a Four-Pesticide Mixture by Combined Photo-Fenton and Biological Oxidation. Water Res. 2009, 43, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Malinović, B.N.; Markelj, J.; Žgajnar Gotvajn, A.; Kralj Cigić, I.; Prosen, H. Electrochemical Treatment of Wastewater to Remove Contaminants from the Production and Disposal of Plastics: A Review. Environ. Chem. Lett. 2022, 20, 3765–3787. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Wang, J.; Lin, J.; Sun, X.; Mao, S. The Effect of Various Electrolyte Cations on Electrochemical Performance of Polypyrrole/RGO Based Supercapacitors. Phys. Chem. Chem. Phys. 2015, 17, 28666–28673. [Google Scholar] [CrossRef]
- Seery, D.J.; Britton, D. The Continuous Absorption Spectra of Chlorine, Bromine, Bromine Chloride, Iodine Chloride, and Iodine Bromide. J. Phys. Chem. 1964, 68, 2263–2266. [Google Scholar] [CrossRef]
- Shin, S.-J.; Kim, D.H.; Bae, G.; Ringe, S.; Choi, H.; Lim, H.-K.; Choi, C.H.; Kim, H. On the Importance of the Electric Double Layer Structure in Aqueous Electrocatalysis. Nat. Commun. 2022, 13, 174. [Google Scholar] [CrossRef]
- Lima, L.F.; Vieira, A.L.; Mukai, H.; Andrade, C.M.G.; Fernandes, P.R.G. Electric Impedance of Aqueous KCl and NaCl Solutions: Salt Concentration Dependence on Components of the Equivalent Electric Circuit. J. Mol. Liq. 2017, 241, 530–539. [Google Scholar] [CrossRef]
- Gan, W.; Ge, Y.; Zhong, Y.; Yang, X. The Reactions of Chlorine Dioxide with Inorganic and Organic Compounds in Water Treatment: Kinetics and Mechanisms. Environ. Sci. Water Res. Technol. 2020, 6, 2287–2312. [Google Scholar] [CrossRef]
- Koo, M.S.; Han, S.; Cho, K.; Choi, W. Quantitative Photoelectrochemical Conversion of Ammonium to Dinitrogen Using a Bromide-Mediated Redox Cycle. ACS ES&T Eng. 2021, 1, 1287–1297. [Google Scholar] [CrossRef]
- Wanngård, J.; Wildlock, M. The Catalyzing Effect of Chromate in the Chlorate Formation Reaction. Chem. Eng. Res. Des. 2017, 121, 438–447. [Google Scholar] [CrossRef]
- Agarwala, R.; Mulky, L. Adsorption of Dyes from Wastewater: A Comprehensive Review. ChemBioEng Rev. 2023, 10, 326–335. [Google Scholar] [CrossRef]
- Dominguez, M.A.; Etcheverry, M.; Zanini, G.P. Evaluation of the Adsorption Kinetics of Brilliant Green Dye onto a Montmorillonite/Alginate Composite Beads by the Shrinking Core Model. Adsorption 2019, 25, 1387–1396. [Google Scholar] [CrossRef]
- Inoue, K.; Nojiri, H. Structure and Function of Aromatic-Ring Hydroxylating Dioxygenase System. In Biodegradative Bacteria: How Bacteria Degrade, Survive, Adapt, and Evolve; Nojiri, H., Tsuda, M., Fukuda, M., Kamagata, Y., Eds.; Springer: Tokyo, Japan, 2014; pp. 181–205. ISBN 978-4-431-54520-0. [Google Scholar]
- Li, M.; Mei, Q.; Wei, B.; An, Z.; Sun, J.; Xie, J.; He, M. Mechanism and Kinetics of ClO-Mediated Degradation of Aromatic Compounds in Aqueous Solution: DFT and QSAR Studies. Chem. Eng. J. 2021, 412, 128728. [Google Scholar] [CrossRef]
- Oturan, M.A.; Pimentel, M.; Oturan, N.; Sirés, I. Reaction Sequence for the Mineralization of the Short-Chain Carboxylic Acids Usually Formed upon Cleavage of Aromatics during Electrochemical Fenton Treatment. Electrochim. Acta 2008, 54, 173–182. [Google Scholar] [CrossRef]
- Su, Y.; Fu, K.; Pang, C.; Zheng, Y.; Song, C.; Ji, N.; Ma, D.; Lu, X.; Liu, C.; Han, R.; et al. Recent Advances of Chlorinated Volatile Organic Compounds’ Oxidation Catalyzed by Multiple Catalysts: Reasonable Adjustment of Acidity and Redox Properties. Environ. Sci. Technol. 2022, 56, 9854–9871. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.S.; Hossain, M.I.; Malek, M.A.; Singha, N.R.; Khan, M.; Rahaman, M.; Uddin, J.; Hasnat, M.A. Electrochemical Generation of Reactive Chlorine Species via Chloride Oxidation on –COOH-Modified Graphite Electrode to Attain Dye Degradation. Catalysts 2025, 15, 1046. https://doi.org/10.3390/catal15111046
Alam MS, Hossain MI, Malek MA, Singha NR, Khan M, Rahaman M, Uddin J, Hasnat MA. Electrochemical Generation of Reactive Chlorine Species via Chloride Oxidation on –COOH-Modified Graphite Electrode to Attain Dye Degradation. Catalysts. 2025; 15(11):1046. https://doi.org/10.3390/catal15111046
Chicago/Turabian StyleAlam, Md. Saiful, Mohammad Imran Hossain, Md Abdul Malek, Nayan Ranjan Singha, Merajuddin Khan, Mostafizur Rahaman, Jamal Uddin, and Mohammad A. Hasnat. 2025. "Electrochemical Generation of Reactive Chlorine Species via Chloride Oxidation on –COOH-Modified Graphite Electrode to Attain Dye Degradation" Catalysts 15, no. 11: 1046. https://doi.org/10.3390/catal15111046
APA StyleAlam, M. S., Hossain, M. I., Malek, M. A., Singha, N. R., Khan, M., Rahaman, M., Uddin, J., & Hasnat, M. A. (2025). Electrochemical Generation of Reactive Chlorine Species via Chloride Oxidation on –COOH-Modified Graphite Electrode to Attain Dye Degradation. Catalysts, 15(11), 1046. https://doi.org/10.3390/catal15111046









