Abstract
The oxygen reduction reaction (ORR) is a crucial process in electrochemical systems, such as fuel cells, as it effectively converts oxygen into water, thereby contributing significantly to sustainable energy generation. In this study, copper oxide (CuO) thin films were deposited onto silver (Ag) substrates using a modified successive ionic layer adsorption and reaction (SILAR) method, followed by an investigation of their electrocatalytic performance toward ORR in an alkaline medium. Comprehensive electrochemical characterizations, including cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and open circuit potential (OCP), were employed to evaluate catalyst behaviour. Elemental analysis through energy-dispersive X-ray spectroscopy (EDX) confirmed the uniform distribution of CuO, while scanning electron microscopy (SEM) revealed a sponge-like surface morphology which potentially enhances catalytic efficiency. Moreover, EIS spectra revealed a lower charge transfer resistance for the CuO/Ag electrode (3.37 kΩ) compared to bare Ag (4.23 kΩ), reflecting improved ORR kinetics. Among different deposition cycles, 15 SILAR cycles yielded the highest current density of 0.8 mA cm−2 at 0.60 V. Kinetic analysis revealed that the reaction is irreversible, with a lower value of Tafel slope (32 mV dec−1) and high transfer coefficient (α = 0.45), indicating a concerted reduction mechanism. The ORR pathway was found to follow a four-electron (4e−) transfer process.
1. Introduction
Amidst the growing global energy demand and the push for sustainability, attention has shifted to green energy technologies. Even though coal is less polluting than natural gas, it still contributes to climate change, potentially increasing temperatures by 2 °C annually, leading to a worldwide push for clean energy and the advancement of electric mobility [1]. Despite being a zero-emission electricity source, nuclear energy faces challenges due to resource scarcity, high costs, safety concerns, and past catastrophes [2]. So, renewable energy sources, including solar, wind, hydropower, geothermal, and marine (wave) energy, are pivotal for achieving carbon-free electricity [3]. Given the dominance of fossil fuels, it is imperative to increase renewable energy to meet the global power requirement [4]. However, solar energy may fluctuate due to varying weather conditions and occasional disruptions, necessitating alternative energy storage methods [5]. In this context, electrochemical devices, especially those associated with the hydrogen economy, emerge as a feasible solution for storing and converting energy [6]. Surplus electricity can be used to produce hydrogen, which can then be effectively transformed back into electricity when needed using fuel cells. The idea of a hydrogen-based economy along with green and renewable energy has recently gained considerable interest from researchers [7,8].
The oxygen reduction reaction (ORR) plays a crucial role in electrochemical energy conversion for fuel cells, metal air batteries, and industrial processes [9]. Understanding the ORR remains challenging due to its intricate kinetics [10]. Numerous studies have been undertaken in recent years to create new catalysts for achieving ORR in both acidic and alkaline environments. Interestingly, the alkaline pH at the electrode surface boundary induces an outer sphere electron transfer process that is not reliant on the surface [11]. The reaction involves multiple-electron transfer and may encompass various basic steps with different reaction intermediates. Molecular oxygen (O2) reduction at the cathode typically proceeds via two distinct pathways. The first involves a two-electron transfer process in which O2 is partially reduced, yielding hydroperoxide (HO2−) as an intermediate, which can further react to form hydroxide ions (OH−). In contrast, the second pathway follows a direct four-electron transfer in which O2 is fully reduced to OH− without the formation of intermediate species such as HO2−. These distinct pathways play a crucial role in determining the efficiency and selectivity of the overall oxygen reduction process.
The ORR is highly dependent on both the electrode material and the electrolyte environment. To optimize ORR efficiency, a wide range of cathodic materials have been explored, including pure metals [12], metal oxides [13], and metallic alloys [14]. Despite ongoing progress, challenges such as high material costs, limited long–term stability, inconsistent reproducibility, and limited catalytic performance persist, motivating the pursuit of more effective alternatives [15]. While noble metals like platinum (Pt) [16,17], ruthenium (Ru) [18], gold (Au) [19], and silver (Ag) [20] have demonstrated significant catalytic performance in ORR, their high cost and resource scarcity pose limitations. In contrast, more abundant and cost-effective metals such as copper (Cu) [21], mercury (Hg) [22], and titanium (Ti) [23] have also shown promise, although generally with lower catalytic activity. Additionally, non-precious metal–carbon catalysts [24] and non-metallic carbon-based electrodes, such as glassy carbon (GC) [25], carbon black (CB) [26], and graphite (GP) [27], have garnered considerable attention due to their tunable surface properties and electronic conductivity. Recent advancements further highlight the potential of transition metals and their oxides, as well as mixed-metal systems, in enhancing ORR activity through synergistic effects and improved electron transfer dynamics [28,29,30,31,32,33].
Within this context, copper oxide (CuO) emerges as a particularly attractive candidate due to its environmental benignity, low-cost synthesis routes, and natural abundance [34]. CuO also demonstrates strong environmental resilience, stable electrochemical potentials, and favourable electrical and optical characteristics, making it a promising material for catalytic applications [35]. These attributes suggest that Cu-based catalysts may offer wide applicability in oxygen reduction processes [36]. A broad range of fabrication techniques have been employed for synthesizing CuO, including sol–gel methods [37], electrodeposition [38], thermal oxidation [39], sputtering [40], spray pyrolysis [41], and the successive ionic layer adsorption and reaction method (SILAR) [42]. Among these, SILAR stands out for its operational simplicity, cost efficiency, low-temperature processing, and precise control over film composition [43]. Additionally, by tuning experimental parameters such as deposition time, number of cycles, solution pH, and precursor concentration, the structural and functional properties of CuO can be finely adjusted for targeted applications [44]. On the other hand, Ag and Ag-modified electrodes [45] are well-regarded for their catalytic effectiveness, non-toxicity, ease of availability, and economic practicality. Their unique electronic configurations can contribute to enhanced reaction kinetics in ORR. Consequently, combining CuO with Ag via the SILAR approach presents a versatile platform for engineering efficient, tunable electrocatalysts for oxygen reduction.
In addition, Ag is a well-established catalyst for the alkaline ORR, and Ag–CuO composites have previously demonstrated improved activity and selectivity via interfacial synergy between metallic and oxide phases. However, most reported Ag–CuO systems have been fabricated using powder-based or high-temperature routes, which often lead to poorly defined interfaces, non-uniform morphologies, and limited long-term stability [46]. In contrast, the present work introduces a modified successive ionic layer adsorption and reaction (SILAR) approach to directly deposit a uniform CuO thin film onto Ag electrodes under mild conditions.
This strategy ensures strong interfacial adhesion and binder-free contact, forming a well-defined CuO/Ag heterojunction that promotes efficient charge transfer across the metal–semiconductor interface. The resulting continuous CuO film minimizes contact resistance and establishes uninterrupted electron transport pathways, in contrast to the discontinuous or particulate interfaces typically observed in powder-mixed or alloyed Ag–Cu systems [47].
Moreover, the cycle-controlled SILAR process enables precise tuning of CuO layer thickness and surface exposure, allowing systematic optimization of catalytic properties. Such control is difficult to achieve using conventional synthesis techniques such as pulsed laser deposition (PLD) or chemical reduction [48], where film morphology and interface quality are less predictable.
In this study, a modified SILAR approach was employed to deposit CuO thin films with controlled thickness directly onto Ag substrates, establishing a well-defined homogenous CuO/Ag electrode. This low-temperature and scalable method enable precise tuning of interfacial composition while ensuring strong adhesion and continuous charge transport pathways. The resulting electrode was systematically investigated to evaluate their ORR kinetics and stability, using cyclic voltammetry (CV), open circuit potential (OCP), and electrochemical impedance spectroscopy (EIS) analyses. The surface morphology and film uniformity were characterized by scanning electron microscopy (SEM), while energy-dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS) provided insights into elemental composition and chemical states. Overall, this work presents a novel and reproducible route for constructing CuO/Ag electrode with enhanced interfacial coupling and structural integrity, offering a promising platform for durable and efficient Ag-based electrocatalysts toward the alkaline ORR via a preferred 4e− pathway.
2. Results and Discussion
2.1. Electrochemical Characterization
The open circuit potential (OCP) provides insight into charge accumulation at the electrode–electrolyte interface in the absence of an applied current. To evaluate the influence of O2 on interfacial charge dynamics, OCP measurements were conducted on the synthesized CuO/Ag electrode in an alkaline medium under both the N2 and O2 saturation conditions.
As illustrated in Figure 1, linear polarization profiles were recorded for CuO/Ag in 0.1 M NaOH, comparing deoxygenated and oxygenated environments. In the presence of nitrogen, the system exhibited an OCP of approximately 0.16 V. However, upon saturation with molecular oxygen (O2), the OCP value shifted negatively to nearly 0.14 V, indicating the involvement of O2 species in modulating the surface electrochemical environment [49]. This suggests that some Ag particles on the Ag substrate may undergo partial oxidation to form Ag2O in the alkaline medium as per the following reaction (1):
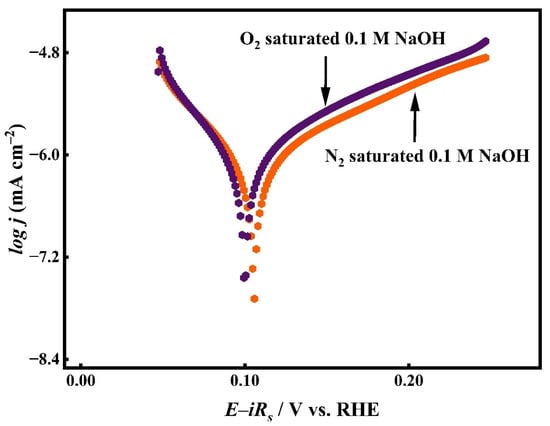
Figure 1.
Linear sweep polarization curves of CuO/Ag electrode recorded in N2 and O2-saturated 0.1 M NaOH, highlighting the effect of dissolved O2 on electrochemical behaviour.
Consequently, the resulting oxide particles formed on the Ag substrate was expected to drive ORR at a potential below OCP.
Electrochemical impedance spectroscopy (EIS) serves as a powerful diagnostic tool for probing interfacial charge dynamics and evaluating the catalytic efficiency of electrode materials in electrochemical reactions. To investigate the potential of CuO as an ORR electrocatalyst, the impedance characteristics of the modified CuO/Ag electrode were compared with those of bare (unmodified) Ag. EIS analyses were carried out in 0.1 M NaOH, with measurements taken at selected potentials below the OCP [50].
The OCP represents the natural equilibrium state of an electrode in a given environment, making it an ideal condition for studying its intrinsic surface properties. Measuring EIS at the OCP ensures that the system remains in a true equilibrium state, where no concentration gradients or diffusion layers are formed at the electrode–electrolyte interface. Under these conditions, the applied alternating current (AC) signal becomes the sole influencing potential, allowing for an accurate evaluation of intrinsic surface properties [51,52].
The Nyquist plots recorded at 0.12 V in the O2-saturated environment are shown in Figure 2, while the extracted fitting parameters are detailed in Table 1.
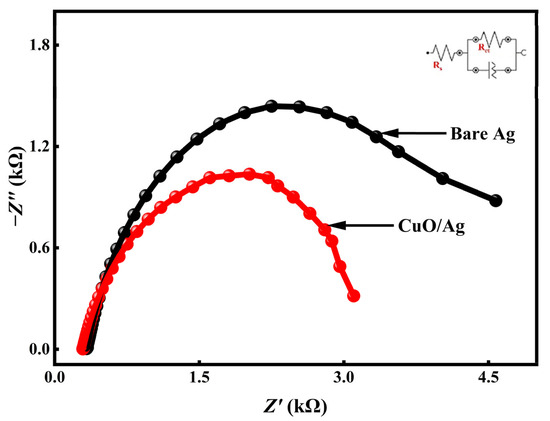
Figure 2.
Nyquist plots for bare Ag and CuO/Ag electrode measured in O2-saturated 0.1 M NaOH at an applied potential of 0.12 V.

Table 1.
Impedance parameters related to ORR at bare Ag and CuO/Ag electrode, obtained at an applied potential of 0.12 V in the experimental setup.
The findings indicate that the charge transfer resistance (Rct) of bare Ag under the O2-saturated environment in 0.1 M NaOH was reduced from 4.23 kΩ to 3.37 kΩ upon CuO modification. This notable reduction in Rct reflects enhanced catalytic activity and more efficient charge transfer dynamics. Furthermore, the solution resistance (Rs), determined from the intercept on the real axis of the Nyquist plot, was also lower for the modified CuO/Ag electrode compared to the bare Ag electrode. This observation suggests improved electrical conductivity and a greater electrochemically active surface area [53]. Overall, these results demonstrate that the CuO/Ag electrode exhibits superior electrocatalytic performance toward ORR relative to unmodified Ag. Collectively, the EIS data confirm that CuO/Ag facilitates more efficient charge transfer and offers enhanced catalytic activity for the oxygen reduction reaction.
The double-layer capacitance (Cdl) represents the capacity of the electrical double layer formed at the interface between an electrode and the electrolyte to store charge. Measuring Cdl provides insight into the electrochemically active surface area (ECSA) of the electrode, which is a key factor in determining its catalytic performance toward the ORR [54].
To assess Cdl, cyclic voltammograms (CVs) were recorded at different scan rates ranging from 0.005 to 0.030 Vs−1 within the non-faradaic potential region (1.06–0.96 V vs. RHE) using target electrodes. Figure 3A,B show the non-faradaic CV responses of bare Ag and modified CuO/Ag, respectively. The difference between anodic (ia) and cathodic (ic) capacitive currents at 1.01 V was plotted against the scan rate, as shown in Figure 3C. This analysis was based on Equation (2).
where is the scan rate (v). According to this relationship, a linear plot of capacitive current versus scan rate yields a slope corresponding to the Cdl value [55].
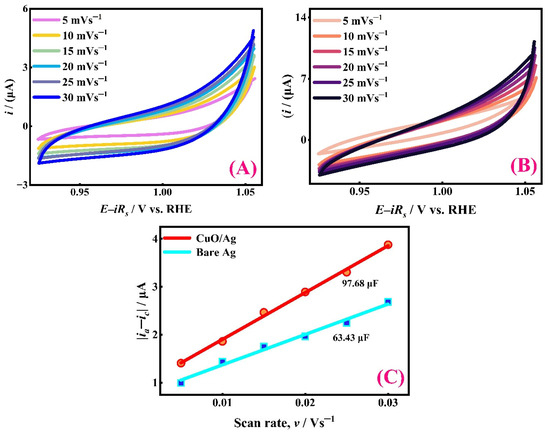
Figure 3.
Investigation of double-layer capacitance in 0.1 M NaOH (A) CVs recorded with bare Ag electrode at various scan rates and (B) CVs recorded with CuO/Ag electrode at various scan rates. (C) Dependency of capacitive currents on variable scan rates.
The measured Cdl values were 63.43 μF for bare Ag and 97.68 μF for the modified CuO/Ag electrode. Notably, the Cdl value for the modified electrode was approximately 1.5 times greater than that of the unmodified Ag, indicating a significantly enhanced electrochemical surface area and catalytic activity, which is assumed to enhance catalytic activity for some reactions like ORR [56,57].
2.2. ORR Electrocatalysis
A series of CuO-modified Ag electrodes were prepared by varying the SILAR cycles. The modified Ag electrodes were fabricated using 10, 15, 20, and 25 SILAR cycles and are denoted as SC10 CuO/Ag, SC15 CuO/Ag, SC20 CuO/Ag, and SC25 CuO/Ag, respectively. In comparison to a pure Ag substrate, an electrode prepared using 10 SILAR cycles (SC10 CuO/Ag) exhibited improved ORR activity in an alkaline medium in terms of reduction current, as shown in Figure 4A. This enhancement indicates that CuO particles deposited on a Ag substrate have catalytic effects pertaining to ORR in an alkaline medium.
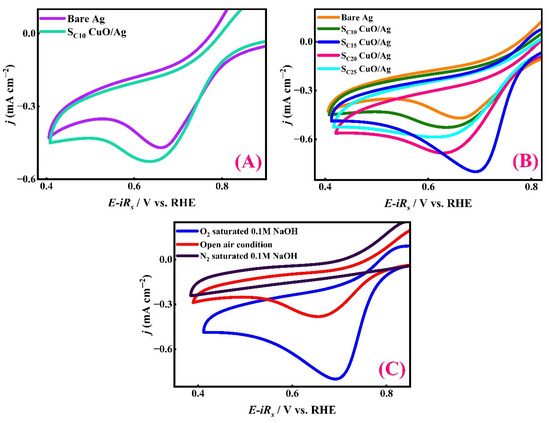
Figure 4.
(A) Comparative ORR activity of bare Ag and CuO-modified Ag (SC10 CuO/Ag) in 0.1 M NaOH solution under oxygen saturation. (B) Effect of CuO deposition cycles (10, 15, 20, and 25) on ORR performance. (C) CVs in 0.1 M NaOH obtained using the modified CuO/Ag electrode under N2-saturated, ambient air, and O2-saturated environments. In all cases, the scan rate was 100 mV s−1.
To further optimize the catalytic efficiency of CuO particles, other CuO/Ag electrodes prepared with 15, 20, and 25 SILAR cycles were employed to check ORR activity (Figure 4B). Among them, the SC15 CuO/Ag electrode demonstrated the maximum cathodic current density, highlighting 15 SILAR cycles as the optimal deposition condition. CV conducted in oxygen-saturated 0.1 M NaOH revealed that the SC15 CuO/Ag electrode exhibited a pronounced cathodic peak current density of 0.800 mA cm−2 at a potential of 0.602 V, as presented in Table 2. Notably, further increasing the number of SILAR cycles to more than 15 resulted in decreased activity, likely due to elevated charge transfer resistance or reduced accessibility of active sites.

Table 2.
Electrocatalytic performance of modified CuO/Ag electrodes fabricated via SILAR with varying deposition cycles toward ORR in O2-saturated 0.1 M NaOH.
To confirm the specificity of the electrocatalytic activity of SC15 CuO/Ag toward ORR, a comparative electrochemical analysis was conducted under different atmospheric conditions, namely O2 saturation, N2 saturation, and ambient air, in 0.1 M NaOH solution (Figure 4C). A pronounced increase in cathodic current was observed with O2 saturation, whereas the current notably decreased in the N2-saturated environment. This confirms that the cathodic response originates predominantly from oxygen reduction rather than background or non-faradaic processes. The open-air condition displayed an intermediate current, consistent with the lower dissolved oxygen concentration compared to the O2-saturated medium [58,59,60].
Additionally, to enable a direct and reliable assessment of electrocatalytic performance, the ORR activity of the benchmark Pt/C electrode was measured under the same carefully controlled experimental conditions as the optimized SC15 CuO/Ag electrode, with detailed comparative results provided in the Supplementary Materials (Figure S1 and Table S1).
Collectively, these findings validate that the optimized CuO loading on Ag significantly enhances ORR activity, with the SC15 CuO/Ag configuration offering the most efficient catalytic performance under alkaline conditions. Consequently, all the subsequent experiments were performed using the SC15 CuO/Ag electrode.
2.3. ORR Kinetics
The response of an electrocatalyst at variable scan rates in CV is a useful tool in elucidating a faradaic process. An electrocatalyst with superior intrinsic activity can facilitate faradaic reactions even at high scan rates. This behaviour stems from a reduced diffusion layer thickness at higher scan rates, which promotes more efficient mass transport. As a result, electrodes with high intrinsic activity exhibit increased current densities due to their ability to maintain charge transfer kinetics despite the presence of a large number of reactive species on the surface. In contrast, electrodes with limited activity show reduced current responses, constrained by slower reaction kinetics [61]. Thus, to further explore the kinetic characteristics and surface-controlled processes of the SC15 CuO/Ag electrode, CV measurements were performed in an O2-saturated alkaline medium at varying scan rates ranging from 0.01 to 0.90 V s−1. The results are presented in Figure 5A–D.
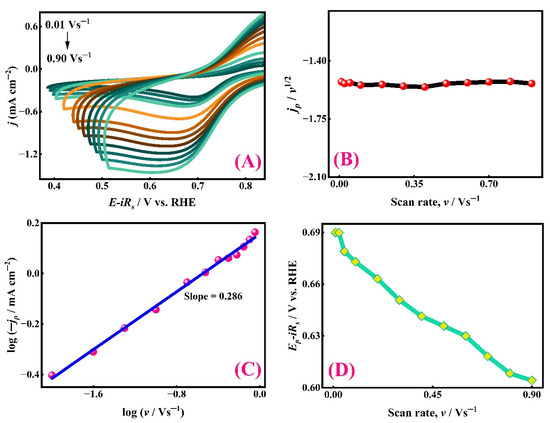
Figure 5.
Kinetic investigation of the ORR on the SC15 CuO/Ag electrode surface in 0.1 M NaOH solution under the O2 saturation condition. (A) CVs recorded at variable scan rates (0.01 V s−1 to 0.90 V s−1) using the modified CuO/Ag electrode. Plots showing (B) the variation of jp/v1/2 with the scan rate (v), (C) the logarithmic relationship between current density (jp) and the scan rate (v), and (D) the dependence of peak potential (Ep) on the scan rate (v).
Figure 5A displays the CV curves at different scan rates. A gradual increase in both anodic and cathodic peak currents with an increasing scan rate is evident, reflecting enhanced electron transfer kinetics at the catalyst surface. Notably, the cathodic peak current density (jp) increases progressively, while the peak potential (Ep) shifts toward more negative values with higher scan rates, indicating kinetic irreversibility. The increasing separation between anodic and cathodic peaks with the scan rate further signifies quasi-reversible behaviour, often associated with surface-adsorption-controlled electrocatalysis [62].
To better understand the mass transport dynamics, the relationship between (jp) and the square root of the scan rate (v1/2) was examined using the Randles–Sevcik-like expression (Equation (3)), as shown in (Figure 5B) [63].
Here, n denotes the number of electrons involved in the heterogeneous charge transfer, α is the cathodic charge transfer coefficient, A is the electroactive surface area, C = 9 × 10−7 mol cm−3 is the bulk concentration of O2, D0 = 1.9 × 10−5 cm2 s−1 represents the diffusion coefficient of O2 in 0.1 M NaOH, and v is the scan rate in V s−1. The linear dependence of jp on v1/2 confirms that oxygen mass transport plays a significant role in the observed current response.
Additionally, the plot of current density at a fixed potential versus the scan rate (Figure 5B) shows a relatively constant response, suggesting either capacitive behaviour or a rapid equilibrium between surface-adsorbed intermediates and the bulk electrolyte.
A logarithmic relation between jp and v1/2 (Figure 5C) led to the following empirical relationship (4) [64]:
The slope of this plot is approximately 0.286, substantially lower than the theoretical value of 0.5 expected for diffusion-controlled processes [65]. This deviation implies that the ORR on the SC15 CuO/Ag electrode is predominantly governed by adsorption-controlled kinetics, where strong interactions between oxygen intermediates and the catalyst surface dominate the overall rate. This observation points to a surface-controlled mechanism, potentially involving pseudo-capacitive effects or adsorption-limited electron transfer.
Figure 5D illustrates the variation in peak potential (Ep) as a function of the increasing scan rate (v). As the scan rate increases, Ep shifts more negatively, indicating increased overpotential and polarization resistance. This shift highlights the kinetic hindrance of electron transfer at higher scan rates.
To gain deeper insights into the kinetics of ORR on the SC15 CuO/Ag electrode surface, Tafel slope analysis was conducted at various scan rates. One of the critical parameters in such analysis is the cathodic charge transfer coefficient (α) [66], which provides information on the electron transfer mechanism and its efficiency. To determine α values, Tafel analysis was performed in the kinetically controlled potential range between 0.75 and 0.71 V vs. RHE, using CV data acquired at scan rates of 0.1, 0.2, and 0.3 V s−1 (Figure 3) and applying Equation (5) [67].
As illustrated in Figure 6, the Tafel slopes derived from the corresponding curves were approximately 32, 34, and 35 mV dec−1, respectively. These relatively consistent slope values yield an average α of 0.45 ± 0.02, indicating minimal variation across the scan rates. A charge transfer coefficient below 0.5 typically reflects a concerted single-step electron transfer, whereas values exceeding 0.5 are indicative of a multi-step process [68,69,70]. Hence, the obtained α value confirms that ORR on the SC15 CuO/Ag surface proceeds via a concerted charge transfer mechanism. Furthermore, low Tafel slopes within the range of 30–40 mV dec−1 are often associated with a direct four-electron ORR pathway, which bypasses peroxide (H2O2) intermediates by promoting direct O–O bond cleavage. The findings suggest that the rate-determining step likely involves the initial electron transfer to O2, enhanced by strong interfacial electronic coupling between CuO and Ag species. This synergy not only improves the density of active catalytic sites but also optimizes the binding energies of key oxygenated intermediates (*O2, *OOH), leading to efficient charge transfer kinetics. Thus, the comprehensive scan rate and Tafel slope analyses conclusively indicate that the ORR process on the SC15 CuO/Ag surface is predominantly an adsorption-controlled process in the activation region, with a kinetically favourable four-electron pathway with minimal peroxide formation. A long-term bulk electrolysis experiment conducted later also evidenced that no measurable H2O2 was generated, which also validates that the ORR process over the CuO/Ag electrode involved a 4e− transfer process.
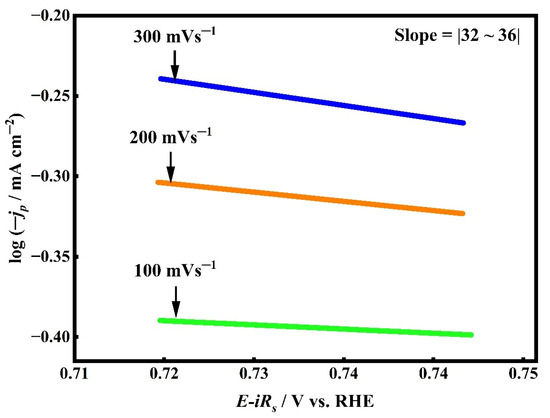
Figure 6.
Tafel plots derived from CV data at scan rates of 100, 200, and 300 mV s−1 for SC15 CuO/Ag electrode in O2-saturated 0.1 M NaOH. The linear regions show consistent slopes (~32–36 mV dec−1), indicating stable charge transfer kinetics across varying scan rates.
To further elucidate the oxygen reduction reaction (ORR) mechanism, both rotating disc electrode (RDE) and rotating ring–disc electrode (RRDE) measurements were conducted.
The linear sweep voltammograms (LSVs) and the corresponding H2O2 oxidation currents recorded at the Pt ring during RRDE ORR experiments for the SC15 CuO/Ag catalyst are shown in Figure 7A. The ring currents are negligible across the measured potential window, indicating very low peroxide formation and suggesting that the catalyst follows the four-electron (4e−) ORR pathway to H2O as the predominant product. Using Equations (7) and (8), the number of electrons transferred (n) and the H2O2 selectivity were calculated and are presented in Figure 7B,C. Over the potential range of 0.70–0.30 V vs. RHE, the SC15 CuO/Ag electrocatalyst exhibits an average electron transfer number of 3.94 (≈4) and a mean H2O2 yield of 6.23%.
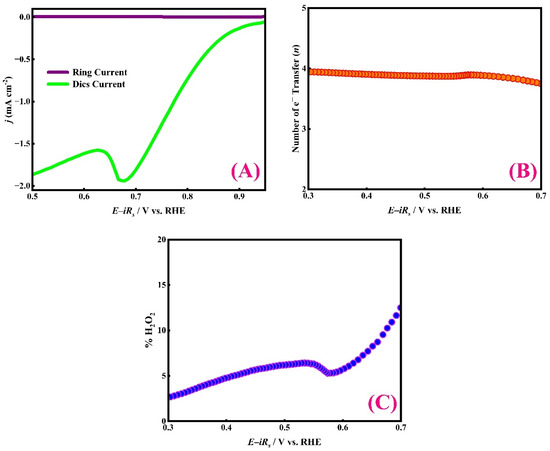
Figure 7.
ORR performance of the SC15 CuO/Ag electrode in 0.1 M NaOH under O2 saturation conditions. (A) RRDE LSVs obtained at 600 rpm and a scan rate of 0.05 V s−1, with the corresponding H2O2 oxidation current detected at the Pt ring (1.3 V vs. RHE). (B) Electron transfer number (n). (C) Corresponding H2O2 yield (%H2O2), determined from RRDE analysis.
The low peroxide yield (≈6% or less) indicates efficient O–O bond cleavage at the electrode interface, consistent with an optimal oxygen-binding affinity at the active sites and possible synergistic interactions between CuO and Ag that enhance O2 activation and proton/electron transfer [71]. Low H2O2 production is beneficial for device stability and desirable for fuel cells and metal–air battery applications.
In addition, in the supportive information, Figure S2A presents the linear sweep voltammograms (LSVs) recorded under hydrodynamic conditions at different electrode rotation speeds (100 to 1000 rpm) to investigate the ORR kinetics on the catalyst surface. With increasing rotation speed, the diffusion layer thickness decreases, resulting in higher limiting current densities, which is a typical characteristic of diffusion-controlled electrochemical processes [72]. The angular rotation rate (ω, rad s−1) is related to the electrode rotation speed (f, rpm) through the following expression:
To further assess the ORR mechanism, the kinetic analysis was performed using the Koutecky–Levich (K–L) equation [73]:
Here, j is the measured current density, jk is the kinetic current density, n represents the number of electrons transferred per O2 molecule, F is the Faraday constant, CO2 is the bulk O2 concentration ( mol cm−3 in 0.1 M NaOH), D0 is the O2 diffusion coefficient ( cm2 s−1), and the kinematic viscosity of the electrolyte is cm2 s1 [74].
The K–L plots shown in Figure S2B display linear and nearly parallel lines across different potentials (0.65–1.15 V vs. RHE), signifying a consistent electron transfer mechanism and confirming that the ORR on this catalyst surface is predominantly diffusion-limited. The good linearity also suggests first-order reaction kinetics with respect to the dissolved O2 concentration.
The slope of each K–L line was used to calculate the average number of electrons transferred (n), yielding a value of 3.94 ± 0.01, which indicates that the ORR proceeds mainly via a four-electron (4e–) pathway [75], producing water as an end product.
Overall, the combined LSVs from RDE and K–L analyses clearly demonstrate that the ORR on the studied electrode follows a diffusion-controlled mechanism with a dominant 4e− reduction pathway and exhibits enhanced intrinsic activity compared to commercial Pt-based catalysts. So, the linear sweep voltammograms (LSVs) obtained from RDE and RRDE are given in the Supplementary Materials (Figure 7), revealing the average electron transfer number to be 3.94 ± 0.01 and suggesting a predominant four-electron (4e−) pathway.
It can thus be said that the enhanced reduction current, low Tafel slopes, and stable α, along with the RDE and RRDE measurements, collectively validate the excellent catalytic performance of SC15 CuO/Ag in alkaline media, making it a promising candidate for energy conversion devices, such as alkaline fuel cells and metal–air batteries.
2.4. Optical Characterization
To examine the surface morphology of the synthesized CuO/Ag catalyst, SEM images were captured at different magnifications, as illustrated in Figure 8. The micrographs at 30 µm, 20 µm, and 10 µm scales reveal a uniform and densely packed granular surface, indicating successful deposition of copper oxide onto the silver substrate via the modified SILAR method.
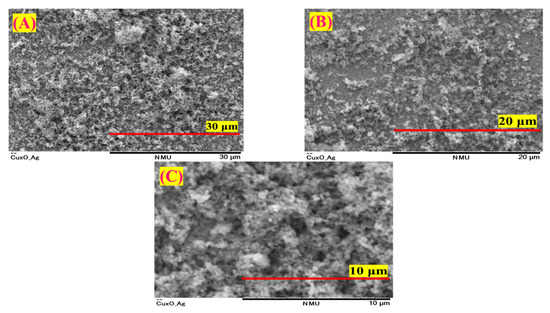
Figure 8.
SEM images of the CuO thin film on the Ag surface at scales of (A) 30 μm, (B) 20 μm, and (C) 10 μm.
Figure 8 shows that at lower magnification (30 µm), the surface is rough and textured with grain clusters, indicating a high ECSA beneficial for electrocatalysis. Higher magnifications (20 µm and 10 µm) reveal a clearer, porous nanoparticle structure, which supports efficient reactant diffusion during ORR. Moreover, the cross-sectional SEM images (Figure S3) clearly show the uniform growth of CuO films on an Ag electrode surface, with thicknesses varying depending on the SILAR cycle number. The measured film thicknesses were approximately 62.5 μm (SC10 CuO/Ag), 81.2 μm (SC15 CuO/Ag, before reaction), 70.3 μm (SC15 CuO/Ag, after reaction), 98.9 μm (SC20 CuO/Ag), and 117.3 μm (SC25 CuO/Ag), confirming the progressive layer-by-layer deposition with increasing SILAR cycles.
Furthermore, post-mortem SEM (Figure S3B,C) revealed that the CuO film remained continuous and well-adhered to the Ag substrate after electrochemical cycling, with a slight decrease in thickness from 81.2 μm to 70.3 μm. This minor reduction suggests limited surface restructuring or partial dissolution of the outer CuO layer, while the bulk film integrity was largely preserved, demonstrating the mechanical stability of the SILAR-grown CuO/Ag electrode.
Moreover, the effect of CuO layer thickness on the electrochemical performance of the CuO/Ag electrodes was systematically examined by varying the number of SILAR deposition cycles (10, 15, 20, and 25) (see Supplementary Materials section). The corresponding CuO film thicknesses, estimated from cross-sectional imaging, were correlated with the electrochemically active surface area (ECSA) and kinetic current density, as presented in Figure S4A,B. Both parameters exhibited a non-linear dependence on film thickness, with the SC15CuO/Ag sample (~81.2 µm) showing the highest ECSA and kinetic current density. This behaviour suggests that moderate CuO growth promotes a greater density of accessible active sites and efficient interfacial charge transfer between CuO and Ag. In contrast, thicker films (≥100 µm in SC25CuO/Ag) likely introduce higher charge transport resistance and restrict ion diffusion, while thinner coatings (<70 µm) provide insufficient catalytic coverage. Consequently, an optimal CuO layer thickness of approximately 81.2 µm (SC15CuO/Ag) achieves the best compromise between active surface area and electrical conductivity, leading to enhanced ORR kinetics and overall catalytic efficiency.
The progressive magnified views confirm the homogeneous distribution of copper oxide over the silver surface without significant cracks or voids, indicating good mechanical stability and surface adhesion of the active material [76]. These morphological characteristics collectively support the improved performance of CuO/Ag as an efficient electrocatalyst for ORR.
To elucidate the surface chemical states and electronic interactions in the CuO/Ag electrocatalyst, X-ray photoelectron spectroscopy (XPS) was carried out in high-resolution. The results are presented in three regions, namely Cu 2p, O 1s, and Ag 3d, as shown in Figure 9A, Figure 9B, and Figure 9C, respectively.
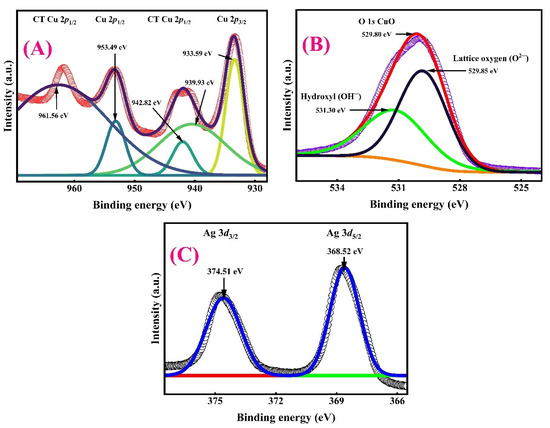
Figure 9.
Deconvoluted XPS fine spectra of (A) Cu 2p orbitals obtained from CuO deposited on Ag, (B) O 1s orbital for the CuO/Ag surface, and (C) Ag 3d orbital recorded from the Ag electrode.
In Figure 9A, the Cu 2p spectrum displays two prominent spin–orbit split peaks at 933.59 eV (Cu 2p3/2) and 953.49 eV (Cu 2p1/2), confirming the presence of Cu2+ species indicative of CuO. Additionally, satellite peaks located at 942.82 eV and 961.56 eV are characteristic of Cu2+ shake-up features, further validating the formation of CuO. A peak at 939.93 eV suggests partial reduction or the presence of mixed valence states, possibly Cu+ or Cu(0), implying partial electronic interaction with the Ag substrate. Charge transfer (CT) features observed near both Cu 2p peaks indicate strong metal–support interactions [77].
In Figure 9B, the O 1s core-level spectrum exhibits a prominent peak centred at 529.80 eV. The peak at 529.85 eV corresponds to lattice oxygen (O2−), while the signal observed at 531.30 eV is attributed to the surface hydroxyl group (OH−) peak in CuO. The sharpness and strong intensity of these peaks confirm the formation of a well-defined CuO phase. Additionally, the slight shoulder or asymmetry in the spectrum suggests the presence of surface-adsorbed oxygen species or hydroxyl groups, which are typically recognized as catalytically active sites for ORR [78].
Figure 9C presents the Ag 3d spectrum featuring two distinct peaks at 368.52 eV (Ag 3d5/2) and 374.51 eV (Ag 3d3/2), consistent with Ag0 and/or Ag+ states. The absence of peak shifting or satellite structures implies that Ag remains largely in its metallic state and is not oxidized, although its close interface with CuO may still facilitate electronic exchange. The presence of Ag confirms its structural and potentially synergistic role in enhancing electron conductivity and promoting ORR activity through interfacial effects [79].
The coexistence of Cu2+ and Ag0, along with strong Cu–O and Ag–Cu interfacial interactions, suggests the formation of a heterostructure that can enhance oxygen adsorption, facilitate charge transfer, and promote efficient 4e− ORR pathways. The well-defined lattice oxygen (O 1s) and Cu2+ species are likely key contributors to catalytic activity via the generation of active oxygen intermediates [80].
To analyze the composition of the deposited CuO on the Ag surface, EDX analysis was carried out, and the corresponding elemental percentages are presented in Figure 10.
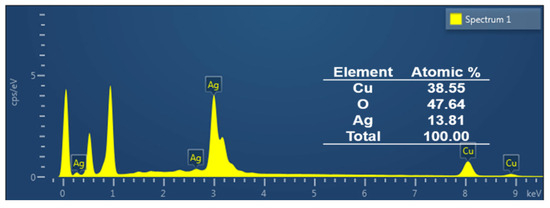
Figure 10.
EDX spectrum of developed catalyst CuO on Ag surface.
The atomic ratio of Cu to O is 1:1.23, indicating a composition close to CuO, though it is slightly oxygen rich. This deviation from ideal stoichiometry suggests the presence of defects, adsorbed oxygen, or surface overoxidation [81]. Elemental analysis further reveals surface atomic percentages of Cu (38.55%), O (47.64%), and Ag (13.81%), confirming a uniform and homogeneous CuO coating on the Ag substrate achieved via the modified SILAR method.
3. Materials and Methods
3.1. Materials
A 0.3 M amount of Cu(II) stock solution was prepared by dissolving an accurately weighed amount of copper(II) sulphate pentahydrate (, Merck KGaA, 64271, Darmstadt, Germany) in ultrapure Milli-Q water (resistivity < 18 MΩ cm) (Smart-Q30UT deionized water system, Qingdao, China). In a similar way, a 0.1 M amount of sodium hydroxide (NaOH) solution was prepared using the same grade of water. Additional reagents, including hydrochloric acid (HCl), NaOH, O2 gas (from Linde Inc. Dhaka, Bangladesh), ethanol (EtOH), and acetone and alumina powder (Al2O3), were obtained from Aldrich Chemical Co. Inc., Darmstadt, Germany. All the chemicals utilized in this work were of analytical grade and were used without further purification.
3.2. Electrode Fabrication
A plate-shaped silver electrode was thoroughly cleaned by successive sonication in acetone and ethanol for several hours to effectively remove surface contaminants and ensure a clean electrochemical interface. Subsequently, copper oxide (CuO) thin films were deposited onto the Ag electrode using a modified successive ionic layer adsorption and reaction (SILAR) technique [82]. In this process, CuSO4.5H2O served as the cation source, while NaOH provided the anions necessary for CuO thin film formation.
The deposition involved alternating immersion of the Ag substrate into the cationic and anionic solutions, with each complete cycle consisting of the following six sequential steps: (i) dipping the electrode in the Cu2+ precursor solution to allow ionic adsorption, (ii) briefly drying the surface in air, (iii) immersing it in the NaOH solution to trigger CuO formation, (iv) allowing another short air-drying period, (v) rinsing with warm deionized water to eliminate residual reactants, and (vi) drying again before initiating the next deposition cycle. This cycle was repeated to achieve uniform and strongly adherent CuO layers on the Ag electrode. To ensure consistent film quality, all working solutions including the cationic, anionic, and rinsing water were replaced with fresh ones after every few deposition cycles. Following the final cycle, the coated electrodes were air-dried and subsequently placed in an oven for several hours to ensure complete drying and stabilization of the CuO films.
Deposition experiments were conducted with 10, 15, 20, and 25 cycles to study CuO thin film growth. The experiment was repeated under identical conditions multiple times to assess the reproducibility. A schematic representation of the SILAR method for CuO thin film preparation is illustrated in Scheme 1 below.
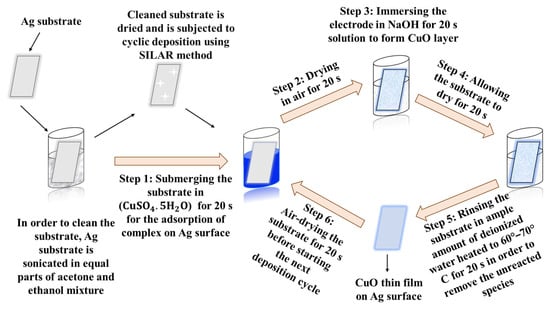
Scheme 1.
Illustration of a schematic representation of the modified SILAR method for CuO/Ag electrode preparation.
The modified SILAR approach used in this work offers distinct advantages over conventional low-cost deposition techniques. By optimizing the cationic and anionic immersion times, maintaining moderate bath temperatures, and introducing controlled rinsing between cycles, the modified SILAR process ensures uniform nucleation and gradual growth of CuO/Ag nanoparticles on the substrate. This modification minimizes random agglomeration, leading to smoother, well-adhered, and highly porous CuO films on the Ag electrode with improved interparticle connectivity [83]. Such tunability in film thickness and morphology is difficult to achieve through simple chemical bath or drop-casting methods, underscoring the suitability of the modified SILAR method for fabricating efficient catalytic electrodes.
The morphology and elemental analysis of CuO/Ag was examined using SEM and EDX from JEOL (Tokyo, Japan), XPS was applied to evaluate the oxidation states of the species on the modified electrode surface with a DLD spectrometer (Kratos Axis-Ultra; Kratos Analytical Ltd., Manchester, UK) with an Al Kα radiation source (1486.6 eV).
3.3. Electrochemical Experiments
All electrochemical experiments were conducted employing a conventional three-electrode system. A modified CuO/Ag electrode having a geometrical area of 0.3848 cm2 served as the working electrode. The counter and reference electrodes were Pt and Ag/AgCl (sat. KCl), respectively. Electrochemical measurements were conducted using several electrochemical workstations, including the PGSTAT 128N (Autolab, Utrecht, The Netherlands), CHI-660 potentiostat (CHI Instruments, Austin, TX, USA), and PINE Wave Driver 20 (PGSTATs, Grove City, PA, USA). All electrochemical measurements were conducted in 0.1 M NaOH solution under ambient laboratory temperature in alkaline medium.
To achieve inert and oxygenated environments, the electrolyte was purged with high-purity N2 and O2 gases, respectively, for 10 min at a flow rate of 1 L min−1 prior to measurements. This purging process was maintained during voltammetric scans and chronoamperometric experiments at a reduced flow rate of 0.5 L min−1.
All potentials were initially recorded as E/V against the Ag/AgCl (sat. KCl) reference electrode. The obtained data were later presented with iRs compensation and converted to the reversible hydrogen electrode (RHE) scale using Equation (6) [84].
ERHE = EAg/AgCl + 0.197 + 0.059 pH
Rotating ring–disc electrode (RRDE) experiments were conducted in an O2-saturated 1.0 M NaOH solution at a rotation speed of 600 rpm and a scan rate of 0.05 V s−1. The platinum ring was maintained at a constant potential of 1.3 V vs. RHE to quantify the formation of H2O2, as reported in previous studies [85]. The geometric surface areas of the glassy carbon disc and platinum ring were 0.2376 cm2 and 0.2356 cm2, respectively. The electron transfer number (n) and peroxide yield (% H2O2) during the ORR were determined using Equations (9) and (10) [86,87].
where Id is the disc current, Ir refers to the ring current, and N is the collection efficiency of the RRDE (0.38) provided by the manufacturer (Pine Research Instrumentation, Inc., Durham, NC, USA).
4. Conclusions
In the quest for efficient and cost-effective electrocatalysts for the ORR in alkaline medium, we successfully developed a CuO thin film-modified Ag electrode. The CuO/Ag electrode prepared through 15 SILAR cycles demonstrated remarkable and effective ORR activity. The modified CuO/Ag electrode enhanced catalytic performance by lowering overpotential and decreasing Tafel slope by 32 mV dec−1. Furthermore, the electrode showed excellent selectivity toward the four-electron pathway, producing less than a 6% H2O2 yield, indicating nearly complete conversion of O2 to H2O. Although the proposed electrode might have some limitations regarding the electrolyte concentration under the alkaline condition, the electrode is an eco-friendly and cost-effective tool that can replace noble metals in the case of ORR processes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15111012/s1, Figure S1: (A) Comparative CVs of the SC15CuO/Ag electrode and Pt/C electrode recorded under identical experimental conditions of O2 saturated 0.1 M NaOH solution. (B) LSVs obtained under hydrodynamic conditions at 600 rpm with a scan rate of 0.050 V s−1 for both the electrodes; Table S1: Comparison of ORR performance between Pt/C and SILAR modified SC15CuO/Ag electrodes; Figure S2: (A) LSVs obtained under hydrodynamic conditions at various rotation rates (100–1000 rpm) using a scan rate of 0.050 V s−1. (B) Koutecky–Levich plots at different potentials during ORR; Figure S3: SEM cross-sectional images of CuO film thickness on Ag substrates at scales of 300 μm: (A) SC10 CuO/Ag with a thickness of 62.5 μm, (B) SC15 CuO/Ag with a thickness of 81.2 μm (before-reaction), (C) SC15 CuO/Ag with a thickness of 70.3 μm (after-reaction), (D) SC20 CuO/Ag with a thickness of 98.9 μm, and (E) SC25 CuO/Ag with a thickness of 117.3 μm; Figure S4: (A) Variation of electrochemically active surface area (ECSA) and (B) kinetic current density (jk) as a function of CuO layer thickness for SC10–C25CuO/Ag electrodes synthesized by varying the number of SILAR cycles. Both ECSA and jk exhibit a maximum at ~82.1 µm (SC15CuO/Ag), indicating the optimal balance between catalytic surface area and charge-transfer efficiency.
Author Contributions
Formal analysis, investigation, data plotting, visualization, and original draft writing, R.L.; conceptualization and editing, M.I.H.; review and editing, N.R.S. and M.K.; funding acquisition, resources, and editing, M.R.; review and editing, J.U.; supervision, project administration, review and editing, funding acquisition, conceptualization, M.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Ongoing Research Funding Program, (ORFFT-2025-105-1), King Saud University, Riyadh, Saudi Arabia for financial support.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to the fact that the project is still in its early stage and will be further developed in future studies. Public deposition of the raw datasets at this point may compromise ownership and the integrity of ongoing work.
Acknowledgments
The authors would like to thank Ongoing Research Funding Program, (ORFFT-2025-105-1), King Saud University, Riyadh, Saudi Arabia for financial support. The authors also would like to thank the research centre of Shahjalal University of Science and Technology, Bangladesh is acknowledged for supporting a research grant (PS/2025/03).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maddukuri, S.; Malka, D.; Chae, M.S.; Elias, Y.; Luski, S.; Aurbach, D. On the Challenge of Large Energy Storage by Electrochemical Devices. Electrochim. Acta 2020, 354, 136771. [Google Scholar] [CrossRef]
- Steinhauser, G.; Brandl, A.; Johnson, T.E. Comparison of the Chernobyl and Fukushima Nuclear Accidents: A Review of the Environmental Impacts. Sci. Total Environ. 2014, 470, 800–817. [Google Scholar] [CrossRef]
- Amponsah, N.Y.; Troldborg, M.; Kington, B.; Aalders, I.; Hough, R.L. Greenhouse Gas Emissions from Renewable Energy Sources: A Review of Lifecycle Considerations. Renew. Sustain. Energy Rev. 2014, 39, 461–475. [Google Scholar] [CrossRef]
- Bogdanov, D.; Farfan, J.; Sadovskaia, K.; Aghahosseini, A.; Child, M.; Gulagi, A.; Oyewo, A.S.; de Souza Noel Simas Barbosa, L.; Breyer, C. Radical Transformation Pathway towards Sustainable Electricity via Evolutionary Steps. Nat. Commun. 2019, 10, 1077. [Google Scholar] [CrossRef]
- Zhang, J.; Xuan, Y. Performance Improvement of a Photovoltaic—Thermoelectric Hybrid System Subjecting to Fluctuant Solar Radiation. Renew. Energy 2017, 113, 1551–1558. [Google Scholar] [CrossRef]
- Taljan, G.; Fowler, M.; Cañizares, C.; Verbič, G. Hydrogen Storage for Mixed Wind-Nuclear Power Plants in the Context of a Hydrogen Economy. Int. J. Hydrogen Energy 2008, 33, 4463–4475. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Ustolin, F.; Taccani, R. Fuel Cells for Airborne Usage: Energy Storage Comparison. Int. J. Hydrogen Energy 2018, 43, 11853–11861. [Google Scholar] [CrossRef]
- Lin, L.; Miao, N.; Wallace, G.G.; Chen, J.; Allwood, D.A. Engineering Carbon Materials for Electrochemical Oxygen Reduction Reactions. Adv. Energy Mater. 2021, 11, 2100695. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.X.; Liu, J.N.; Ren, D.; Li, B.Q.; Huang, J.Q.; Zhang, Q. Quantitative Kinetic Analysis on Oxygen Reduction Reaction: A Perspective. Nano Mater. Sci. 2021, 3, 313–318. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition Metal Oxide Nanocatalysts for Oxygen Reduction Reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.; Möller, T.; Strasser, P. Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO2RR). Adv. Mater. 2019, 31, 1805617. [Google Scholar] [CrossRef] [PubMed]
- Siddika, M.; Hosen, N.; Althomali, R.H.; Al-Humaidi, J.Y.; Rahman, M.M.; Hasnat, M.A. Kinetics of Electrocatalytic Oxygen Reduction Reaction over an Activated Glassy Carbon Electrode in an Alkaline Medium. Catalysts 2024, 14, 164. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, J.; Chen, Y.; Shen, Q.; Ding, C.; Zhang, S.; Zhang, J. Advanced Porous Platinum Group Metal Nano-Structural Electrocatalysts for Water Electrolysis, Fuel Cells and Metal-Air Batteries. Coord. Chem. Rev. 2025, 543, 216958. [Google Scholar] [CrossRef]
- Bhuvanendran, N.; Ravichandran, S.; Lee, S.; Sanij, F.D.; Kandasamy, S.; Pandey, P.; Su, H.; Lee, S.Y. Recent Progress in Pt-Based Electrocatalysts: A Comprehensive Review of Supported and Support-Free Systems for Oxygen Reduction. Coord. Chem. Rev. 2024, 521, 216191. [Google Scholar] [CrossRef]
- Yang, L.; Vukmirovic, M.B.; Su, D.; Sasaki, K.; Herron, J.A.; Mavrikakis, M.; Liao, S.; Adzic, R.R. Tuning the Catalytic Activity of Ru@Pt Core-Shell Nanoparticles for the Oxygen Reduction Reaction by Varying the Shell Thickness. J. Phys. Chem. C 2013, 117, 1748–1753. [Google Scholar] [CrossRef]
- Diesen, E.; Dudzinski, A.M.; Reuter, K.; Bukas, V.J. Origin of Electrocatalytic Selectivity during the Oxygen Reduction Reaction on Au(111). ACS Catal. 2025, 15, 5403–5411. [Google Scholar] [CrossRef]
- Pu, Y.; Chen, J.L.; Zhao, J.W.; Feng, L.; Zhu, J.; Jiang, X.; Li, W.X.; Liu, J.X. Nature of the Active Center for the Oxygen Reduction on Ag-Based Single-Atom Alloy Clusters. JACS Au 2024, 4, 2886–2895. [Google Scholar] [CrossRef]
- Neyerlin, K.C.; Srivastava, R.; Yu, C.; Strasser, P. Electrochemical Activity and Stability of Dealloyed Pt-Cu and Pt-Cu-Co Electrocatalysts for the Oxygen Reduction Reaction (ORR). J. Power Sources 2009, 186, 261–267. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Kokkinidis, G.; Stoychev, D.; Lazarov, V.; Papoutsis, A.; Milchev, A. Electroless Deposition of Pt on Ti: Part II. Catalytic Activity for Oxygen Reduction. J. Electroanal. Chem. 2001, 511, 20–30. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, Y.; Sun, J.; Zhang, S.; Zhang, J. Perceptions of Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction Reaction. Mater. Sci. Eng. R Rep. 2025, 165, 101027. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Electrocatalytic Oxygen Reduction Reaction. In PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer: London, UK, 2008; pp. 89–134. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Wang, J.; Gao, Y.; Shi, P. In Situ Deposition of Highly Dispersed Pt Nanoparticles on Carbon Black Electrode for Oxygen Reduction. J. Electrochem. Soc. 2006, 153, A1261. [Google Scholar] [CrossRef]
- Okada, T.; Yoshida, M.; Hirose, T.; Kasuga, K.; Yu, T.; Yuasa, M.; Sekine, I. Oxygen Reduction Characteristics of Graphite Electrodes Modified with Cobalt Di-Quinolyldiamine Derivatives. Electrochim. Acta 2000, 45, 4419–4429. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent Progress of Electrochemical Production of Hydrogen Peroxide by Two-Electron Oxygen Reduction Reaction. Adv. Sci. 2021, 8, 2100076. [Google Scholar] [CrossRef]
- Shahid, M.M.; Zhan, Y.; Alizadeh, M.; Sagadevan, S.; Paiman, S.; Oh, W.C. A Glassy Carbon Electrode Modified with Tailored Nanostructures of Cobalt Oxide for Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2020, 45, 18850–18858. [Google Scholar] [CrossRef]
- Lu, X.F.; Xia, B.Y.; Zang, S.Q.; Lou, X.W. Metal–Organic Frameworks Based Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem.—Int. Ed. 2020, 132, 4634–4650. [Google Scholar] [CrossRef]
- Feng, J.; Cai, R.; Magliocca, E.; Luo, H.; Higgins, L.; Romario, G.L.F.; Liang, X.; Pedersen, A.; Xu, Z.; Guo, Z.; et al. Iron, Nitrogen Co-Doped Carbon Spheres as Low Cost, Scalable Electrocatalysts for the Oxygen Reduction Reaction. Adv. Funct. Mater. 2021, 31, 2102974. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Nia, P.M.; Etesami, M.; Abdullah, E.C.; Abdullah, L.C. Electrocatalytic Activity of Starch/Fe3O4/Zeolite Bionanocomposite for Oxygen Reduction Reaction. Arab. J. Chem. 2020, 13, 1297–1308. [Google Scholar] [CrossRef]
- Qiang, Z.; Chang, J.H.; Huang, C.P. Electrochemical Generation of Hydrogen Peroxide from Dissolved Oxygen in Acidic Solutions. Water Res. 2002, 36, 85–94. [Google Scholar] [CrossRef]
- Nzilu, D.M.; Madivoli, E.S.; Makhanu, D.S.; Wanakai, S.I.; Kiprono, G.K.; Kareru, P.G. Green Synthesis of Copper Oxide Nanoparticles and Its Efficiency in Degradation of Rifampicin Antibiotic. Sci. Rep. 2023, 13, 14030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Ni, X.; Song, J.; Zheng, H. Synthesis and Electrochemical Properties of Different Sizes of the CuO Particles. J. Nanopart. Res. 2008, 10, 839–844. [Google Scholar] [CrossRef]
- Vukmirovic, M.B.; Vasiljevic, N.; Dimitrov, N.; Sieradzki, K. Diffusion-Limited Current Density of Oxygen Reduction on Copper. J. Electrochem. Soc. 2003, 150, B10. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Z.; Liu, X.; Zhang, Y.; Hu, J. Room-Temperature Ferromagnetism in CuO Sol-Gel Powders and Films. J. Magn. Magn. Mater. 2010, 322, 1994–1998. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Electrodeposition of Copper: The Nucleation Mechanisms. Electrochim. Acta 2002, 47, 2901–2912. [Google Scholar] [CrossRef]
- Vila, M.; Díaz-Guerra, C.; Piqueras, J. Optical and Magnetic Properties of CuO Nanowires Grown by Thermal Oxidation. J. Phys. D Appl. Phys. 2010, 43, 135403. [Google Scholar] [CrossRef]
- Feng, J.K.; Xia, H.; Lai, M.O.; Lu, L. Electrochemical Performance of CuO Nanocrystal Film Fabricated by Room Temperature Sputtering. Mater. Res. Bull. 2011, 46, 424–427. [Google Scholar] [CrossRef]
- Singh, I.; Bedi, R.K. Studies and Correlation among the Structural, Electrical and Gas Response Properties of Aerosol Spray Deposited Self Assembled Nanocrystalline CuO. Appl. Surf. Sci. 2011, 257, 7592–7599. [Google Scholar] [CrossRef]
- Mageshwari, K.; Sathyamoorthy, R. Physical Properties of Nanocrystalline CuO Thin Films Prepared by the SILAR Method. Mater. Sci. Semicond. Process. 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Ratnayake, S.P.; Ren, J.; Colusso, E.; Guglielmi, M.; Martucci, A.; Della Gaspera, E. SILAR Deposition of Metal Oxide Nanostructured Films. Small 2021, 17, 2101666. [Google Scholar] [CrossRef]
- Patil, A.S.; Lohar, G.M.; Fulari, V.J. Structural, Morphological, Optical and Photoelectrochemical Cell Properties of Copper Oxide Using Modified SILAR Method. J. Mater. Sci. Mater. Electron. 2016, 27, 9550–9557. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Tammeveski, K. Oxygen Reduction Reaction on Silver Catalysts in Alkaline Media: A Minireview. ChemElectroChem 2019, 6, 73–86. [Google Scholar] [CrossRef]
- Raimundo, R.A.; Santos, J.R.D.; Silva, T.R.; Vieira, P.S.; Pereira, T.O.; Araújo, A.J.M.; Loureiro, F.J.A.; Macedo, D.A.; Fagg, D.P. Enhancing Oxygen Evolution Reaction through Tailored Copper Oxide/Nickel Foam Interfaces. Mater. Sci. Eng. B 2026, 323, 118826. [Google Scholar] [CrossRef]
- Madakannu, I.; Patil, I.; Kakade, B.; Datta, K.K.R. Electrocatalytic Oxygen Reduction Activity of AgCoCu Oxides on Reduced Graphene Oxide in Alkaline Media. Beilstein J. Nanotechnol. 2022, 13, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, F.; Jin, Y.; Liu, Z. Ag-Cu Nanoalloyed Film as a High-Performance Cathode Electrocatalytic Material for Zinc-Air Battery. Nanoscale Res. Lett. 2015, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Hasan, M.M.; Shabik, M.F.; Islam, F.; Nagao, Y.; Hasnat, M.A. Electroless Deposition of Gold Nanoparticles on a Glassy Carbon Surface to Attain Methylene Blue Degradation via Oxygen Reduction Reactions. Electrochim. Acta 2020, 360, 136966. [Google Scholar] [CrossRef]
- Scholz, F. Electroanalytical Methods: Guide to Experiments and Applications. In Electroanalytical Methods: Guide to Experiments and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–359. [Google Scholar] [CrossRef]
- Park, S.-M.; Yoo, J.-S. Peer Reviewed: Electrochemical Impedance Spectroscopy for Better Electrochemical Measurements. Anal. Chem. 2003, 75, 455 A–461 A. [Google Scholar] [CrossRef]
- Smith, D.E. Theory of the Faradaic Impedance: Relationship between Faradaic Impedances for Various Small Amplitude Alternating Current Techniques. Anal. Chem. 1964, 36, 962–970. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy─A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Schott, C.M.; Schneider, P.M.; Song, K.T.; Yu, H.; Götz, R.; Haimerl, F.; Gubanova, E.; Zhou, J.; Schmidt, T.O.; Zhang, Q.; et al. How to Assess and Predict Electrical Double Layer Properties. Implications for Electrocatalysis. Chem. Rev. 2024, 124, 12391–12462. [Google Scholar] [CrossRef]
- Martínez-Hincapié, R.; Wegner, J.; Anwar, M.U.; Raza-Khan, A.; Franzka, S.; Kleszczynski, S.; Čolić, V. The Determination of the Electrochemically Active Surface Area and Its Effects on the Electrocatalytic Properties of Structured Nickel Electrodes Produced by Additive Manufacturing. Electrochim. Acta 2024, 476, 143663. [Google Scholar] [CrossRef]
- Watt-Smith, M.J.; Friedrich, J.M.; Rigby, S.P.; Ralph, T.R.; Walsh, F.C. Determination of the Electrochemically Active Surface Area of Pt/C PEM Fuel Cell Electrodes Using Different Adsorbates. J. Phys. D Appl. Phys. 2008, 41, 174004. [Google Scholar] [CrossRef]
- Connor, P.; Schuch, J.; Kaiser, B.; Jaegermann, W. The Determination of Electrochemical Active Surface Area and Specific Capacity Revisited for the System MnOx as an Oxygen Evolution Catalyst. Z. Phys. Chem. 2020, 234, 979–994. [Google Scholar] [CrossRef]
- Hossain, M.I.; Monim, S.A.; Moushumy, Z.M.; Khansur, N.H.; Mahmud, I.; Rahaman, M.; Aldalbahi, A.; Firoz, S.H.; Hasnat, M.A. Synthesis of La2NiMnO6 Double Perovskite as a Highly Selective Electrocatalyst for Oxygen Reduction to Hydrogen Peroxide in Electrochemical Energy Conversion. ACS Appl. Energy Mater. 2025, 8, 949–963. [Google Scholar] [CrossRef]
- Nizam Uddin, S.M.; Yasir Abir, A.; Imran Hossain, M.; Akter, N.; Bin Islam, M.; Aoki, K.; Nagao, Y.; Maktedar, S.S.; Rahaman, M.; Abul Hasnat, M. Rhodium-Modified Glassy Carbon Electrode as a Promising Electrocatalyst for Oxygen Reduction Reaction in Phosphoric Acid Electrolytes. ChemNanoMat 2025, 11, e202400468. [Google Scholar] [CrossRef]
- Ren, T.; Zhao, L.K.; Zhang, X.; Gao, X.W.; Chen, H.; Liu, Z.; Yang, D.; Luo, W. Bin. Optimizing LaNiO3 Surface Structure for an Efficient Oxygen Reduction Reaction. J. Mater. Chem. A Mater. 2025, 13, 14737–14742. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, R.; Shi, S.; He, Y. Efficient Four-Electron Transfer Platinum-Based Oxygen Reduction Catalysts: A Mini Review. Int. J. Hydrogen Energy 2023, 48, 30391–30406. [Google Scholar] [CrossRef]
- Je, H.; Chow, K.F.; Chang, B.Y. Voltammetry of Constant Phase Elements: Analyzing Scan Rate Effects. J. Electrochem. Sci. Technol. 2024, 15, 427–435. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Allen J. Bard and Larry R. Faulkner, Electrochemical Methods: Fundamentals and Applications, New York: Wiley, 2001, 2nd Ed. Russ. J. Electrochem. 2002, 38, 1364–1365. [Google Scholar] [CrossRef]
- Mumtarin, Z.; Rahman, M.M.; Marwani, H.M.; Hasnat, M.A. Electro-Kinetics of Conversion of NO3− into NO2− and Sensing of Nitrate Ions via Reduction Reactions at Copper Immobilized Platinum Surface in the Neutral Medium. Electrochim. Acta 2020, 346, 135994. [Google Scholar] [CrossRef]
- Li, S.; Shi, L.; Guo, Y.; Wang, J.; Liu, D.; Zhao, S. Selective Oxygen Reduction Reaction: Mechanism Understanding, Catalyst Design and Practical Application. Chem. Sci. 2024, 15, 11188–11228. [Google Scholar] [CrossRef]
- Shabik, M.F.; Hasan, M.M.; Alamry, K.A.; Rahman, M.M.; Nagao, Y.; Hasnat, M.A. Electrocatalytic Oxidation of Ammonia in the Neutral Medium Using Cu2O.CuO Film Immobilized on Glassy Carbon Surface. J. Electroanal. Chem. 2021, 897, 115592. [Google Scholar] [CrossRef]
- Agbo, P.; Danilovic, N. An Algorithm for the Extraction of Tafel Slopes. J. Phys. Chem. C 2019, 123, 30252–30264. [Google Scholar] [CrossRef]
- Kirowa-Eisner, E.; Schwarz, M.; Gileadi, E. The Temperature Dependence of the Tafel Slope-I. Instrumentation, Calibration and a Study of the Reduction of Hydroxylamine on the Dme. Electrochim. Acta 1989, 34, 1103–1111. [Google Scholar] [CrossRef]
- Antipin, D.; Risch, M. Calculation of the Tafel Slope and Reaction Order of the Oxygen Evolution Reaction between PH 12 and PH 14 for the Adsorbate Mechanism. Electrochem. Sci. Adv. 2023, 3, e2100213. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. How Properly Are We Interpreting the Tafel Lines in Energy Conversion Electrocatalysis? Mater. Today Energy 2022, 29, 101123. [Google Scholar] [CrossRef]
- Wang, H.; Jerigova, M.; Hou, J.; Tarakina, N.V.; Delacroix, S.; López-Salas, N.; Strauss, V. Modulating between 2e− and 4e− Pathways in the Oxygen Reduction Reaction with Laser-Synthesized Iron Oxide-Grafted Nitrogen-Doped Carbon. J. Mater. Chem. A Mater. 2022, 10, 24156–24166. [Google Scholar] [CrossRef]
- Ahsan, M.; Dutta, A.; Akermi, M.; Alam, M.M.; Nizam Uddin, S.M.; Khatun, N.; Hasnat, M.A. Sulfur Adlayer on Gold Surface for Attaining H2O2 Reduction in Alkaline Medium: Catalysis, Kinetics, and Sensing Activities. J. Electroanal. Chem. 2023, 934, 117281. [Google Scholar] [CrossRef]
- Zhou, R.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Determination of the Electron Transfer Number for the Oxygen Reduction Reaction: From Theory to Experiment. ACS Catal. 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, F.R.F.; Bard, A.J. Electrochemistry of Oxygen in Concentrated NaOH Solutions: Solubility, Diffusion Coefficients, and Superoxide Formation. J. Am. Chem. Soc. 2008, 131, 177–181. [Google Scholar] [CrossRef]
- Miah, M.R.; Masud, J.; Ohsaka, T. Kinetics of Oxygen Reduction Reaction at Electrochemically Fabricated Tin-Palladium Bimetallic Electrocatalyst in Acidic Media. Electrochim. Acta 2010, 56, 285–290. [Google Scholar] [CrossRef]
- John, A.S.; Gurumurthy, K. Synthesis and Characterization of CuO Nanoparticles from Bioleached Copper through Modified and Optimized Double Precipitation Method. ACS Omega 2025, 10, 10193–10198. [Google Scholar] [CrossRef]
- Xu, G.; Huang, J.; Li, X.; Chen, Q.; Xie, Y.; Liu, Z.; Kajiyoshi, K.; Wu, L.; Cao, L.; Feng, L. Heterostructured Cu/CuO Nanoparticles Embedded within N-Doped Carbon Nanosheets for Efficient Oxygen Reduction Reaction. Catalysts 2023, 13, 255. [Google Scholar] [CrossRef]
- Khater, D.Z.; Amin, R.S.; Mahmoud, M.; El-Khatib, K.M. Evaluation of Mixed Transition Metal (Co, Mn, and Cu) Oxide Electrocatalysts Anchored on Different Carbon Supports for Robust Oxygen Reduction Reaction in Neutral Media. RSC Adv. 2022, 12, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.L.; Graville, S.R.; Sermon, P.A.; Vong, M.S.W. Reduction of CuO-Containing Catalysts, CuO: II, XRD and XPS. React. Kinet. Catal. Lett. 1991, 44, 13–18. [Google Scholar] [CrossRef]
- Ando, F.; Gunji, T.; Tanabe, T.; Fukano, I.; Abruña, H.D.; Wu, J.; Ohsaka, T.; Matsumoto, F. Enhancement of the Oxygen Reduction Reaction Activity of Pt by Tuning Its D-Band Center via Transition Metal Oxide Support Interactions. ACS Catal. 2021, 11, 9317–9332. [Google Scholar] [CrossRef]
- Ali, K.; Sajid, M.; Abu Bakar, S.; Younus, A.; Ali, H.; Zahid Rashid, M. Synthesis of Copper Oxide (CuO) via Coprecipitation Method: Tailoring Structural and Optical Properties of CuO Nanoparticles for Optoelectronic Device Applications. Hybrid Adv. 2024, 6, 100250. [Google Scholar] [CrossRef]
- Patil, A.S.; Patil, M.D.; Lohar, G.M.; Jadhav, S.T.; Fulari, V.J. Supercapacitive Properties of CuO Thin Films Using Modified SILAR Method. Ionics 2017, 23, 1259–1266. [Google Scholar] [CrossRef]
- Patwary, M.A.M.; Hossain, M.A.; Ghos, B.C.; Chakrabarty, J.; Haque, S.R.; Rupa, S.A.; Uddin, J.; Tanaka, T. Copper Oxide Nanostructured Thin Films Processed by SILAR for Optoelectronic Applications. RSC Adv. 2022, 12, 32853–32884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wei, N.; Yao, Z.; Zhao, X.; Du, M.; Zhou, Q. Oxygen Vacancy-Based Ultrathin Co3O4 Nanosheets as a High-Efficiency Electrocatalyst for Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 5286–5295. [Google Scholar] [CrossRef]
- Gao, R.; Wang, J.; Huang, Z.F.; Zhang, R.; Wang, W.; Pan, L.; Zhang, J.; Zhu, W.; Zhang, X.; Shi, C.; et al. Pt/Fe2O3 with Pt–Fe Pair Sites as a Catalyst for Oxygen Reduction with Ultralow Pt Loading. Nat. Energy 2021, 6, 614–623. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The Path towards Sustainable Energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bashyam, R.; Zelenay, P. A Class of Non-Precious Metal Composite Catalysts for Fuel Cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).