Pore Structure Modification of the Mixed Metal Oxides Derived from Co-Al Layered Double Hydroxides and Catalytic Performance Enhancement for Aerobic Oxidation of Benzyl Alcohol
Abstract
1. Introduction
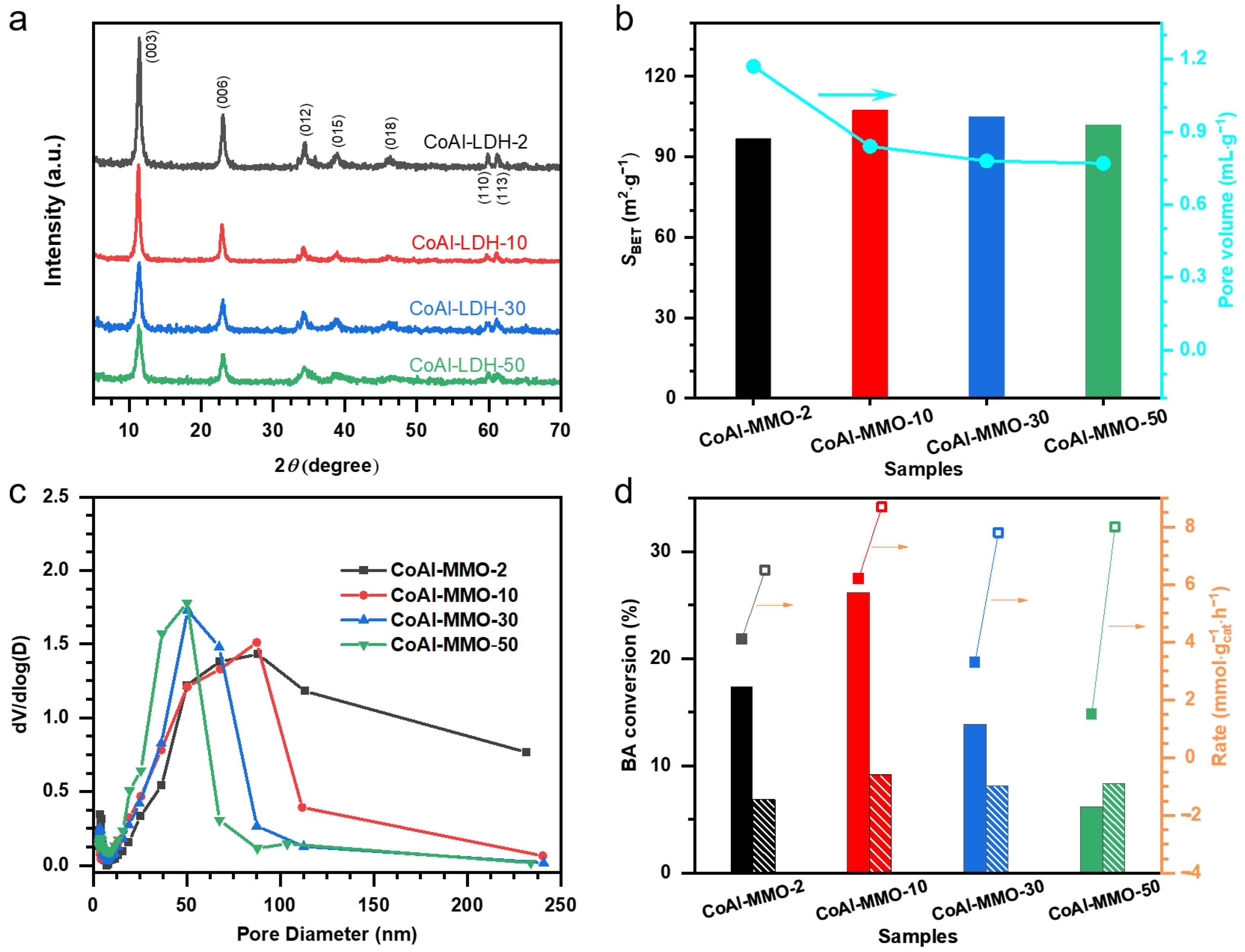
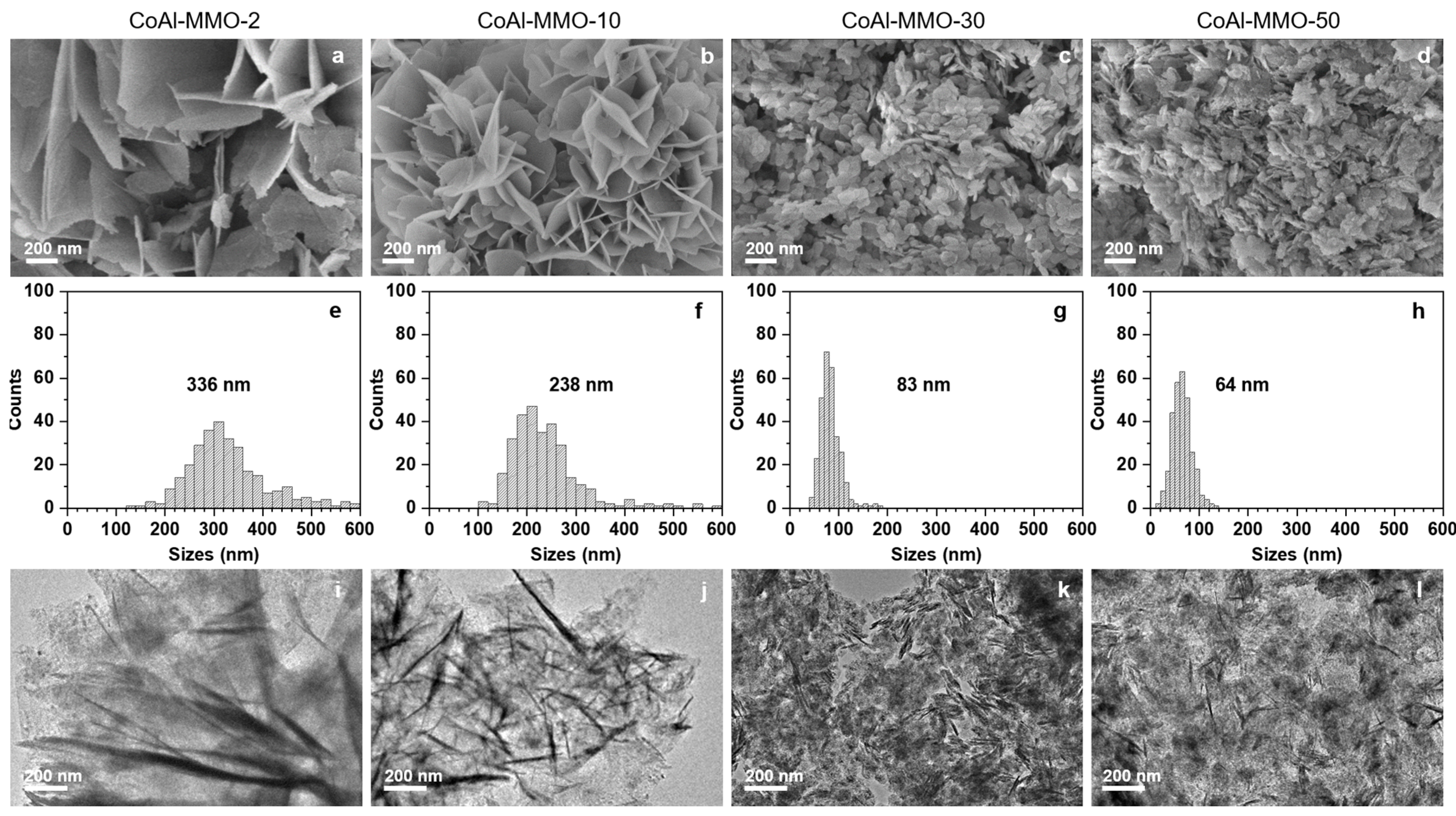
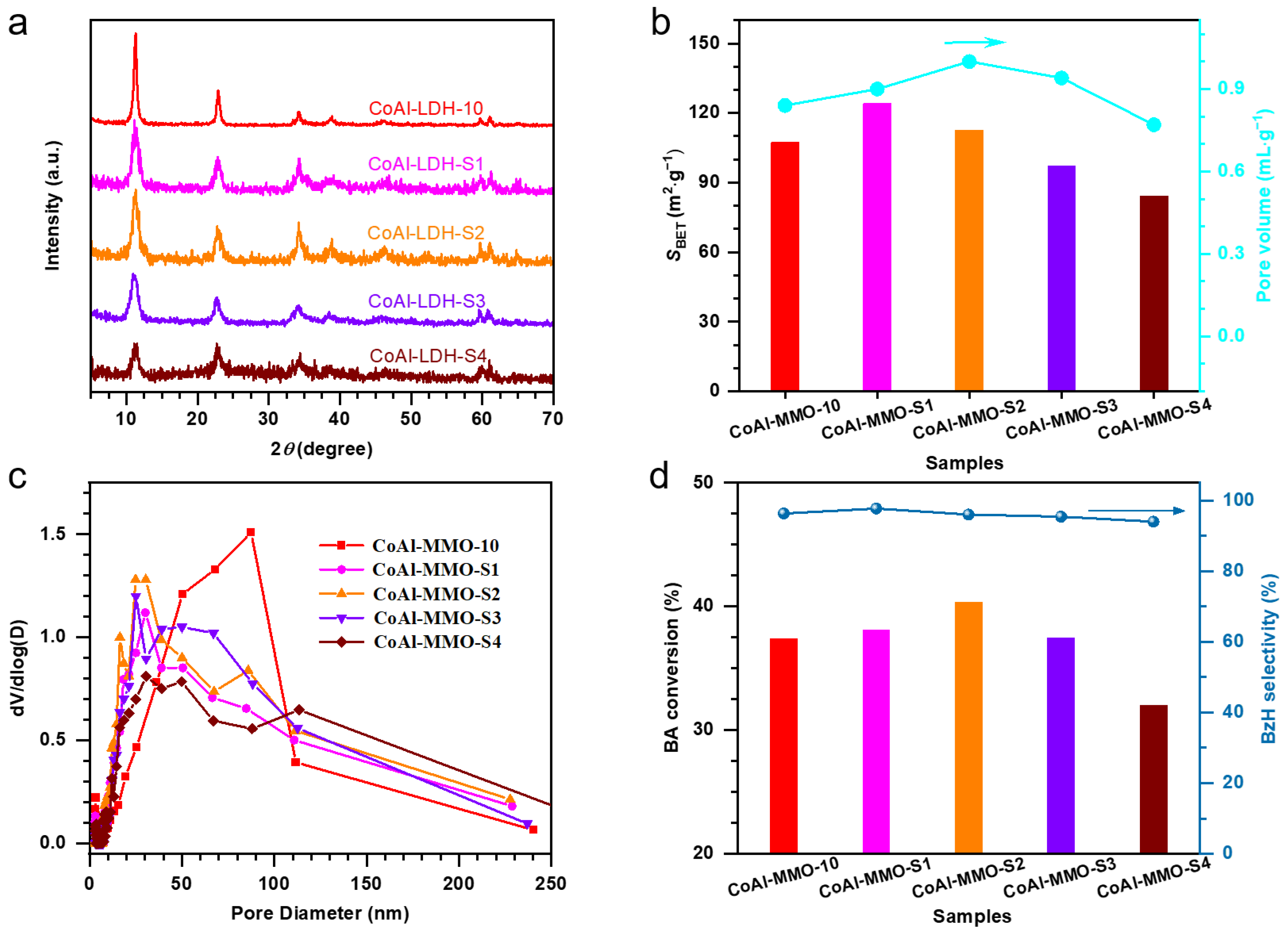
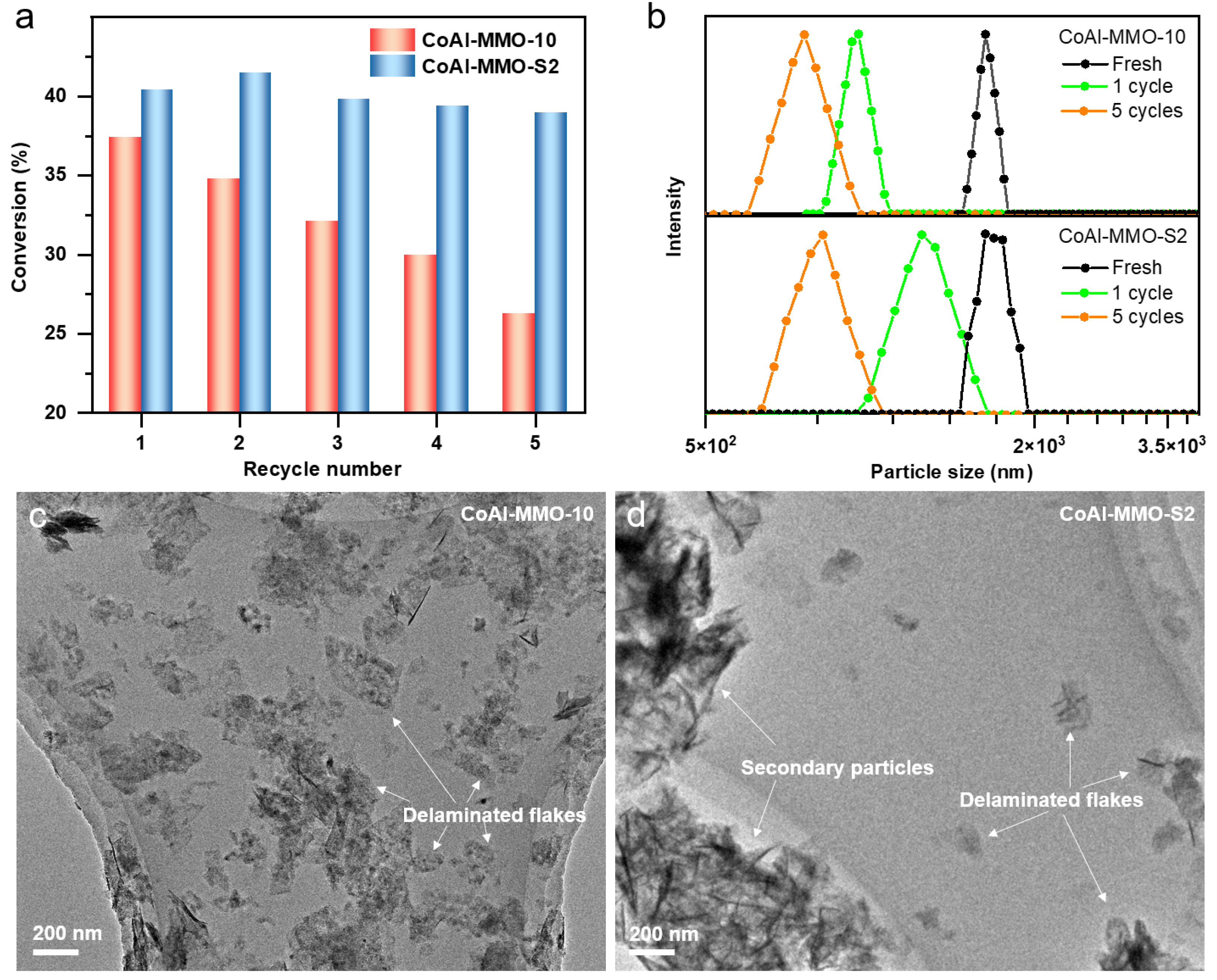
2. Results and Discussion
3. Experimental Section
3.1. Preparation of Samples
3.2. Catalyst Characterization
3.3. Catalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Zhang, M.; Xu, C.; Liu, G.; Zhang, K.; Zhang, Z.; Peng, H.-Q.; Liu, B.; Zhang, W. Recent Developments in Nickel-Based Layered Double Hydroxides for Photo(-/)electrocatalytic Water Oxidation. ACS Nano 2024, 18, 16413–16449. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Wu, X.; Chen, Y. Recent advance on VOCs oxidation over layered double hydroxides derived mixed metal oxides. Chin. J. Catal. 2020, 41, 550–560. [Google Scholar] [CrossRef]
- Pelalak, R.; Hassani, A.; Heidari, Z.; Zhou, M. State-of-the-art recent applications of layered double hydroxides (LDHs) material in Fenton-based oxidation processes for water and wastewater treatment. Chem. Eng. J. 2023, 474, 145511. [Google Scholar] [CrossRef]
- Liang, R.; Du, Y.; Xiao, P.; Cheng, J.; Yuan, S.; Chen, Y.; Yuan, J.; Chen, J. Transition Metal Oxide Electrode Materials for Supercapacitors: A Review of Recent Developments. Nanomaterials 2021, 11, 1248. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.-F.; Sun, X.; Duan, X. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: Identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef]
- Li, L.; Soyhan, I.; Warszawik, E.; van Rijn, P. Layered Double Hydroxides: Recent Progress and Promising Perspectives Toward Biomedical Applications. Adv. Sci. 2024, 11, 2306035. [Google Scholar] [CrossRef]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered double hydroxide-based nanomaterials for biomedical applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [Google Scholar] [CrossRef]
- Chen, B.-Q.; Xia, H.-Y.; Mende, L.K.; Lee, C.-H.; Wang, S.-B.; Chen, A.-Z.; Xu, Z.-P.; Kankala, R.K. Trends in Layered Double Hydroxides-Based Advanced Nanocomposites: Recent Progress and Latest Advancements. Adv. Mater. 2022, 9, 2200373. [Google Scholar] [CrossRef]
- Feng, J.; He, Y.; Liu, Y.; Du, Y.; Li, D. Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: General functionality and promising application prospects. Chem. Soc. Rev. 2015, 44, 5291–5319. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Liu, P.; Wang, H.; Geng, J.; Lei, H.; Zhuo, O. Interfaces and Oxygen Vacancies-Enriched Catalysts Derived from Cu-Mn-Al Hydrotalcite towards High-Efficient Water–Gas Shift Reaction. Molecules 2023, 28, 1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, X.; Wang, L.; Li, Q.; Xiong, R.; Zhang, Z.; Wu, B.; Yv, C.; Hu, X.; Li, S.; et al. Regulating crystal faces and pore size of layered double oxides for efficient phosphorus recovery from water. J. Water Process. Eng. 2025, 75, 108033. [Google Scholar] [CrossRef]
- Lu, P.; Li, W.; Yang, S.; Wei, Y.; Zhang, Z.; Li, Y. Layered double hydroxides (LDHs) as novel macropore-templates: The importance of porous structures for forward osmosis desalination. J. Membr. Sci. 2019, 585, 175–183. [Google Scholar] [CrossRef]
- Pahalagedara, M.N.; Pahalagedara, L.R.; Kuo, C.-H.; Dharmarathna, S.; Suib, S.L. Ordered Mesoporous Mixed Metal Oxides: Remarkable Effect of Pore Size on Catalytic Activity. Langmuir 2014, 30, 8228–8237. [Google Scholar] [CrossRef]
- Rabe, A.; Jaugstetter, M.; Hiege, F.; Cosanne, N.; Ortega, K.F.; Linnemann, J.; Tschulik, K.; Behrens, M. Tailoring Pore Size and Catalytic Activity in Cobalt Iron Layered Double Hydroxides and Spinels by Microemulsion-Assisted pH-Controlled Co-Precipitation. ChemSusChem 2023, 16, e202202015. [Google Scholar] [CrossRef]
- Wang, A.; Ma, Y.; Zhao, D. Pore engineering of Porous Materials: Effects and Applications. ACS Nano 2024, 18, 22829–22854. [Google Scholar] [CrossRef]
- Wu, F.; Bai, J.; Feng, J.; Xiong, S. Porous mixed metal oxides: Design, formation mechanism, and application in lithium-ion batteries. Nanoscale 2015, 7, 17211–17230. [Google Scholar] [CrossRef]
- Duan, M.; Liu, S.; Jiang, Q.; Guo, X.; Zhang, J.; Xiong, S. Recent progress on preparation and applications of layered double hydroxides. Chin. Chem. Lett. 2022, 33, 4428–4436. [Google Scholar] [CrossRef]
- Prevot, V.; Tokudome, Y. 3D hierarchical and porous layered double hydroxide structures: An overview of synthesis methods and applications. J. Mater. Sci. 2017, 52, 11229–11250. [Google Scholar] [CrossRef]
- Kohay, H.; Gazit, O.M. Regulating adsorption-desorption through controlled modification of the 2D pores in layered double hydroxides. Surf. Interfaces 2024, 44, 103810. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Kim, B.-K.; Hirata, S.; Inada, M.; Oh, J.-M. Particle size effect of layered double hydroxide on the porosity of calcined metal oxide. Appl. Clay Sci. 2020, 195, 105701. [Google Scholar] [CrossRef]
- Oka, Y.; Kuroda, Y.; Matsuno, T.; Kamata, K.; Wada, H.; Shimojima, A.; Kuroda, K. Preparation of Mesoporous Basic Oxides through Assembly of Monodispersed Mg–Al Layered Double Hydroxide Nanoparticles. Chem. A Eur. J. 2017, 23, 9362–9368. [Google Scholar] [CrossRef]
- Guo, C.; Xu, Y.; Ni, C.; Pan, X.; Tijing, L.D.; Shon, H.K.; Deng, N.; Huang, X. Tailoring pore size to enhance dissolution of layered double oxides for efficient nitrogen and phosphorus recovery via crystallization of struvite from wastewater. J. Colloid Interface Sci. 2025, 692, 137546. [Google Scholar] [CrossRef]
- Kim, N.; Gu, T.-H.; Shin, D.; Jin, X.; Shin, H.; Kim, M.G.; Kim, H.; Hwang, S.-J. Lattice Engineering to Simultaneously Control the Defect/Stacking Structures of Layered Double Hydroxide Nanosheets to Optimize Their Energy Functionalities. ACS Nano 2021, 15, 8306–8318. [Google Scholar] [CrossRef]
- Veerabhadrappa, M.G.; Maroto-Valer, M.M.; Chen, Y.; Garcia, S. Layered Double Hydroxides-Based Mixed Metal Oxides: Development of Novel Structured Sorbents for CO2 Capture Applications. Acs Appl. Mater. Interfaces 2021, 13, 11805–11813. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Guan, S.; Zhang, X.; Wang, B.; Cao, X.; Yu, Z.; He, Y.; Evans, D.G.; Feng, J.; et al. Ultrathin and Vacancy-Rich CoAl-Layered Double Hydroxide/Graphite Oxide Catalysts: Promotional Effect of Cobalt Vacancies and Oxygen Vacancies in Alcohol Oxidation. ACS Catal. 2018, 8, 3104–3115. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Y.; Zhang, Q.; Tan, X.; Cui, H.; Li, F.; Lei, H.; Zhuo, O. Synthesis of Well-Crystallized Cu-Rich Layered Double Hydroxides and Improved Catalytic Performances for Water–Gas Shift Reaction. Catalysts 2025, 15, 546. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, J.-F.; Yang, J.-C.E.; Li, D.; Zhong, L.-B.; Zheng, Y.-M. Ultra-fast degradation of ciprofloxacin by the peroxymonosulfate activation using a Co/Al-LDH decorated magnetic hydrochar: Structural design, catalytic performance and synergistic effects. Chem. Eng. J. 2023, 477, 146961. [Google Scholar] [CrossRef]
- Tichit, D.; Layrac, G.; Alvarez, M.G.; Marcu, I.-C. Formation pathways of MII/MIII layered double hydroxides: A review. Appl. Clay Sci. 2024, 248, 107234. [Google Scholar] [CrossRef]
- Tathod, A.P.; Gazit, O.M. Fundamental Insights into the Nucleation and Growth of Mg–Al Layered Double Hydroxides Nanoparticles at Low Temperature. Cryst. Growth Des. 2016, 16, 6709–6713. [Google Scholar] [CrossRef]
- Martín, A.J.; Mitchell, S.; Mondelli, C.; Jaydev, S.; Pérez-Ramírez, J. Unifying views on catalyst deactivation. Nat. Catal. 2022, 5, 854–866. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Anand, N.; Raj, R.S.; Lubloy, E. Performance evaluation on engineering properties of sodium silicate binder as a precursor material for the development of cement-free concrete. Dev. Built Environ. 2022, 12, 100092. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Chen, H.; Wang, Y.Q.; Wang, G.; Li, L.H.; Zhong, M.; Bai, H. Effect of sodium silicate binder on the performance of ceramic coatings on copper prepared by the slurry method. Surf. Coat. Technol. 2022, 448, 128868. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Tan, X.; Hu, Y.; Cui, H.; Lin, X.; Li, F.; Lei, H.; Zhuo, O. Pore Structure Modification of the Mixed Metal Oxides Derived from Co-Al Layered Double Hydroxides and Catalytic Performance Enhancement for Aerobic Oxidation of Benzyl Alcohol. Catalysts 2025, 15, 1002. https://doi.org/10.3390/catal15111002
Zhang Q, Tan X, Hu Y, Cui H, Lin X, Li F, Lei H, Zhuo O. Pore Structure Modification of the Mixed Metal Oxides Derived from Co-Al Layered Double Hydroxides and Catalytic Performance Enhancement for Aerobic Oxidation of Benzyl Alcohol. Catalysts. 2025; 15(11):1002. https://doi.org/10.3390/catal15111002
Chicago/Turabian StyleZhang, Qian, Xia Tan, Yinjie Hu, Haonan Cui, Xiao Lin, Fei Li, Huibin Lei, and Ou Zhuo. 2025. "Pore Structure Modification of the Mixed Metal Oxides Derived from Co-Al Layered Double Hydroxides and Catalytic Performance Enhancement for Aerobic Oxidation of Benzyl Alcohol" Catalysts 15, no. 11: 1002. https://doi.org/10.3390/catal15111002
APA StyleZhang, Q., Tan, X., Hu, Y., Cui, H., Lin, X., Li, F., Lei, H., & Zhuo, O. (2025). Pore Structure Modification of the Mixed Metal Oxides Derived from Co-Al Layered Double Hydroxides and Catalytic Performance Enhancement for Aerobic Oxidation of Benzyl Alcohol. Catalysts, 15(11), 1002. https://doi.org/10.3390/catal15111002







