Effects of Sodium-to-OSDA Ratio in the Synthesis Gel on SSZ-39 Formation and Material Properties
Abstract
1. Introduction
2. Results
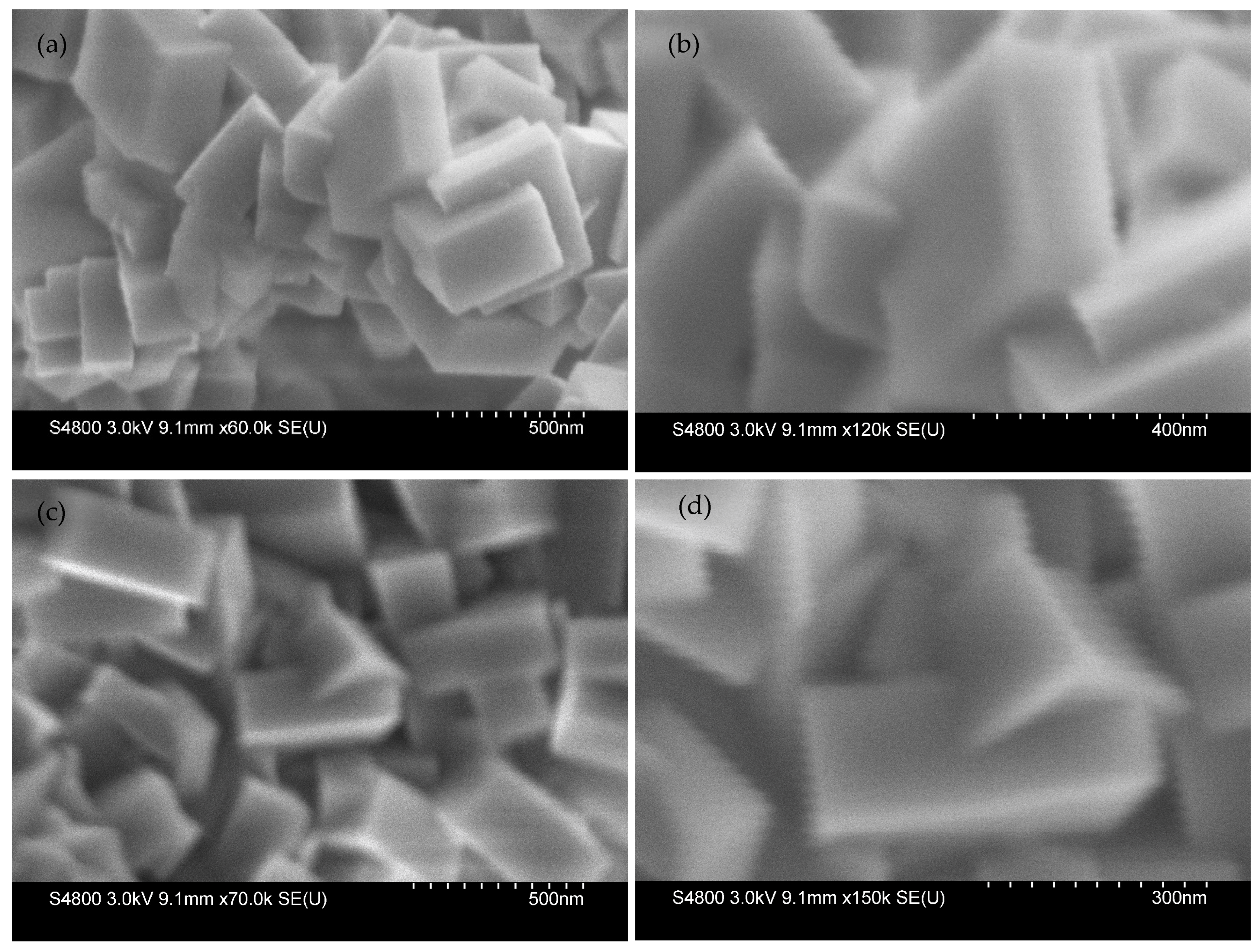
2.1. Phase Selectivity as a Function of Gel Composition
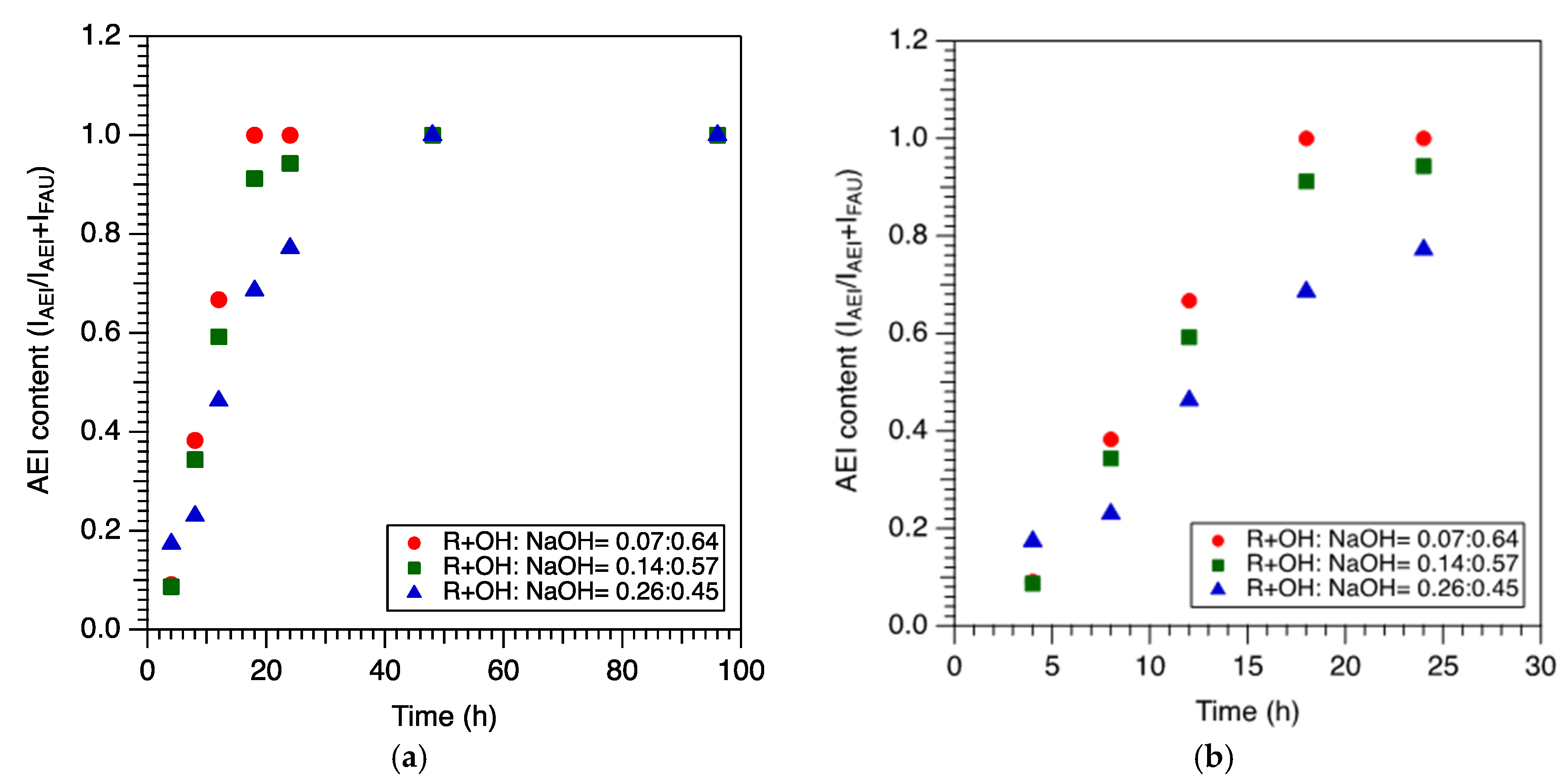
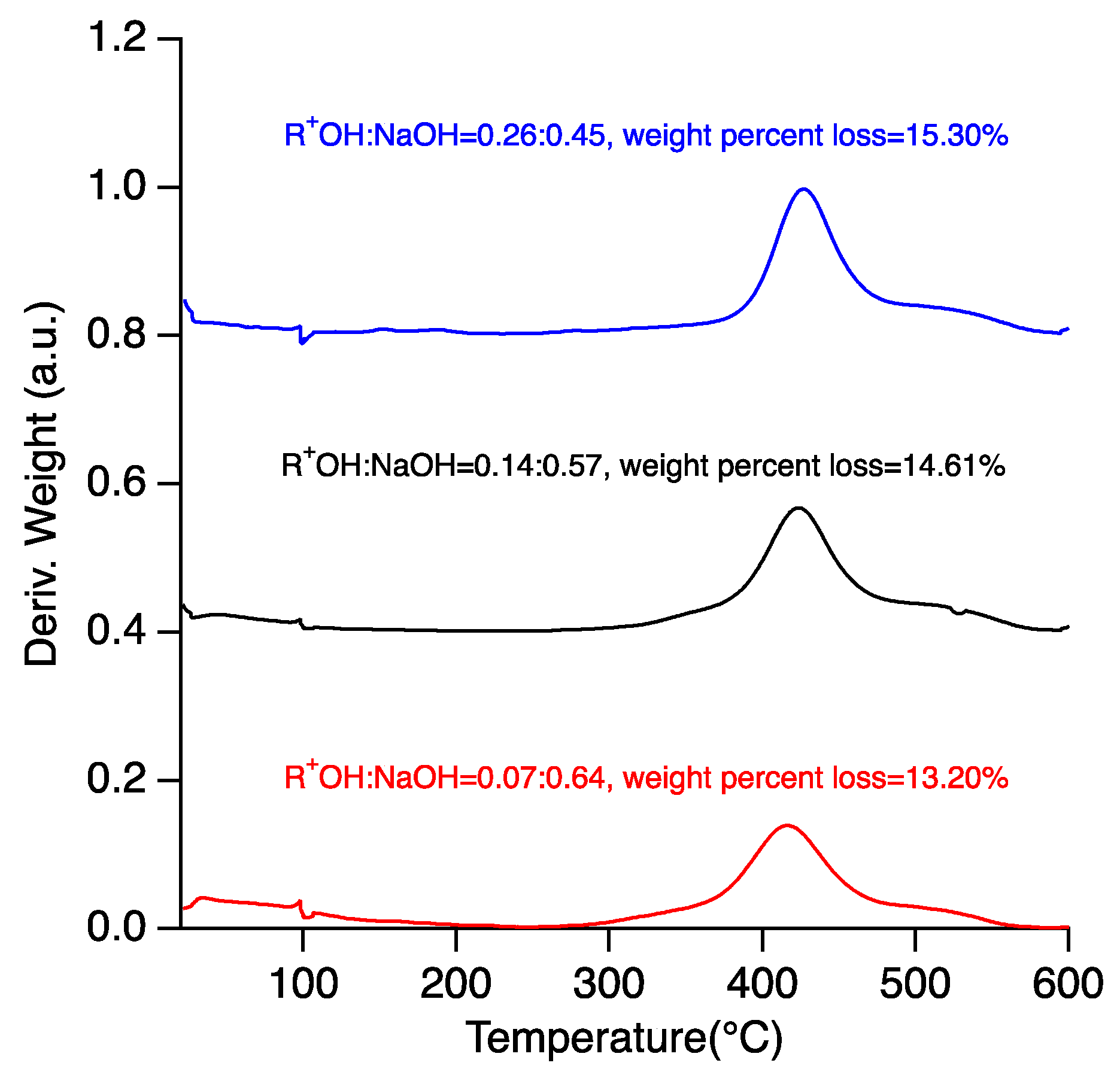
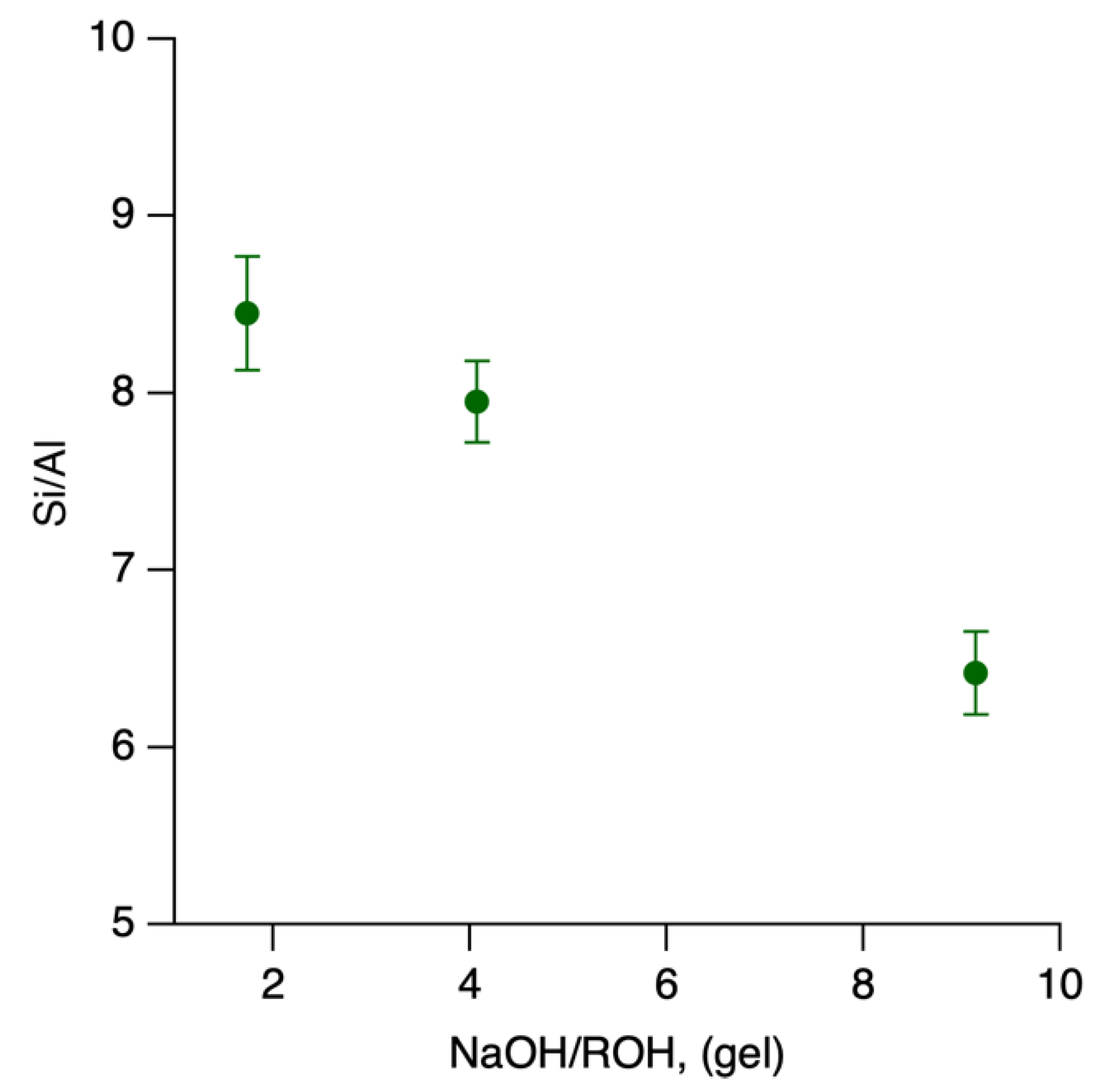
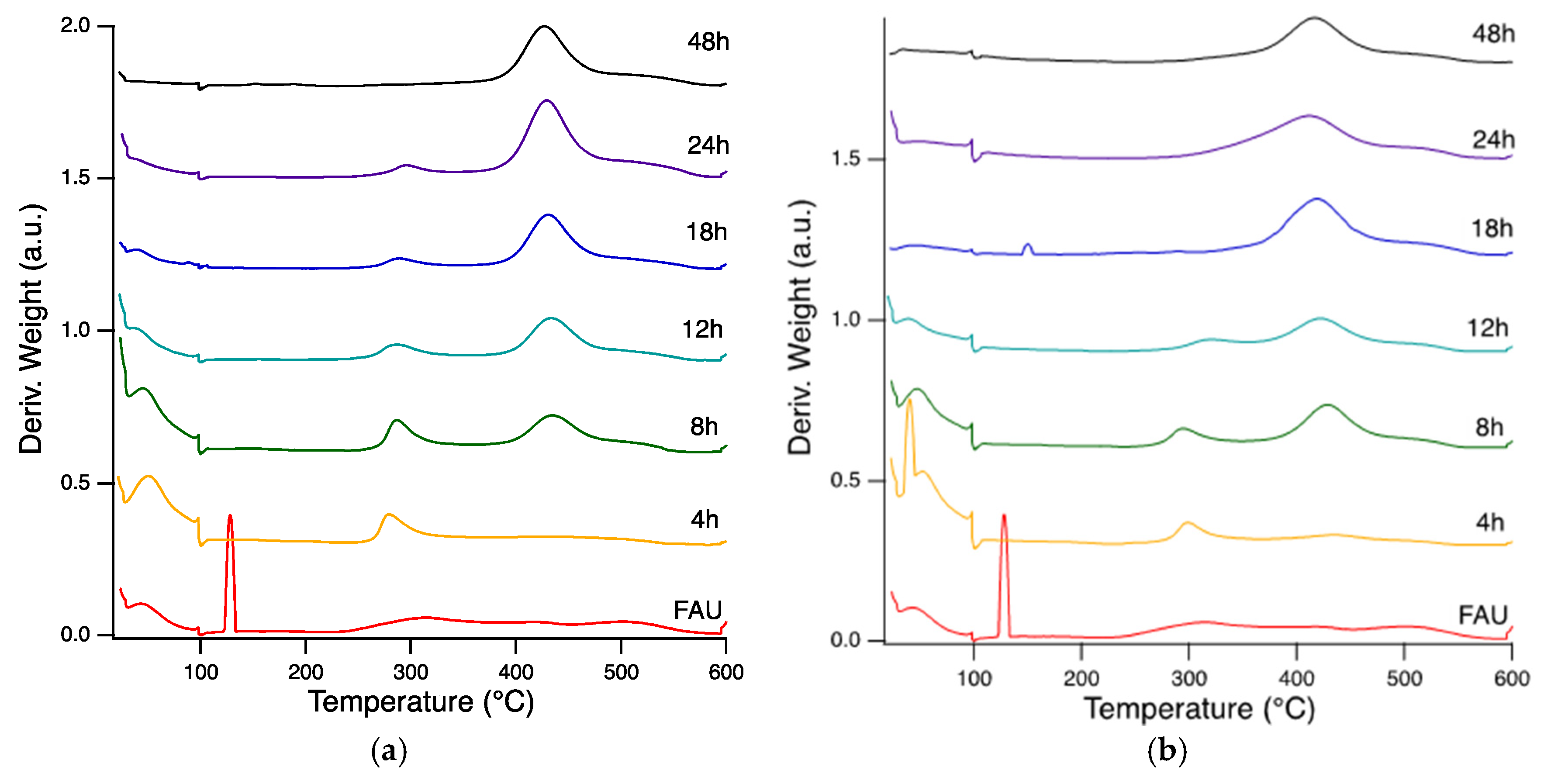
2.2. Effect of NaOH/ROH on Crystallization Kinetics and Framework Composition of SSZ-39
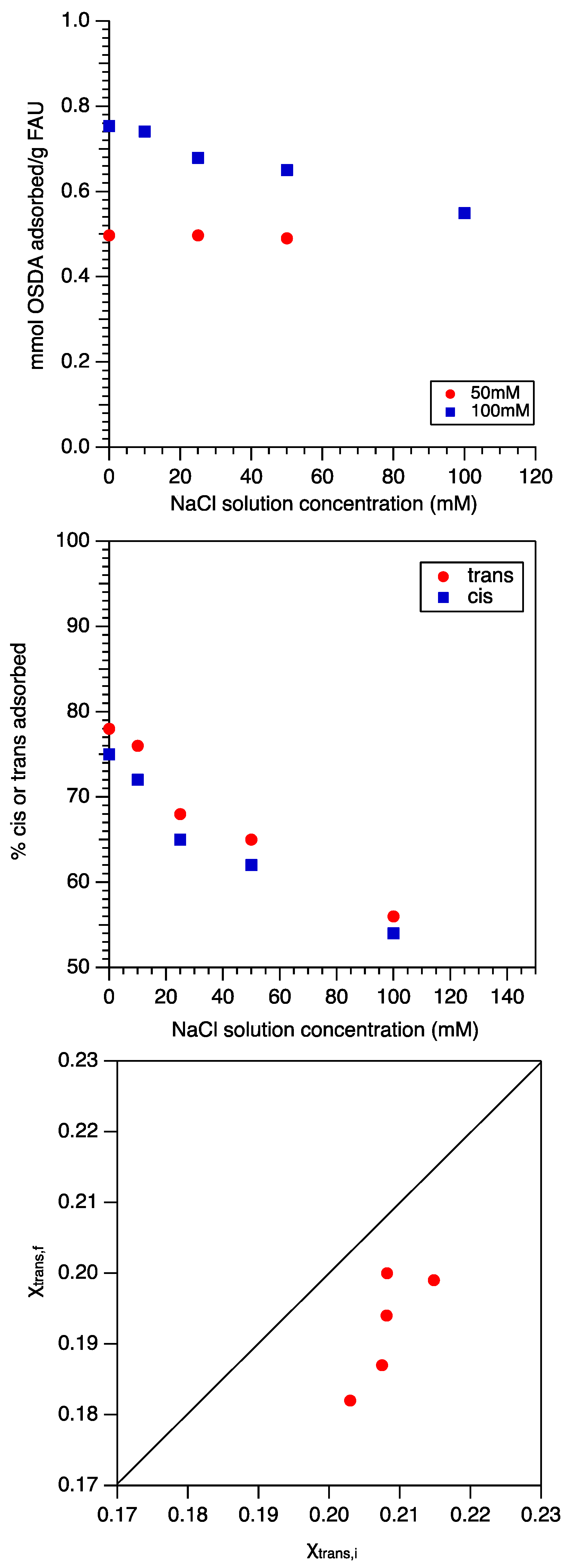
2.3. OSDA Binding Studies
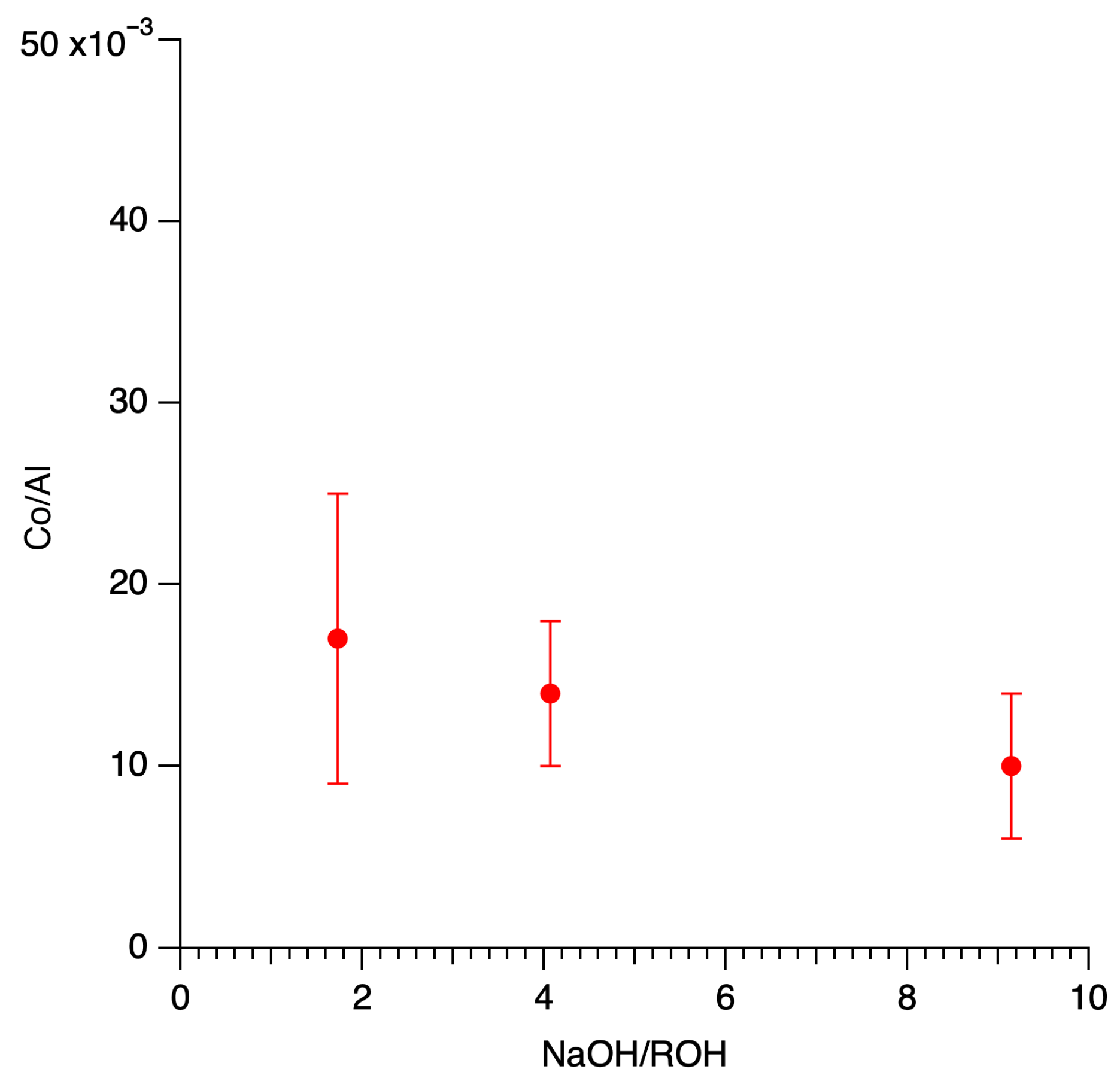
2.4. Local Ordering of Aluminum
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. SSZ-39 Synthesis
4.3. OSDA Adsorption Studies
4.4. Cobalt Titration
4.5. Analytical
4.6. DFT Calculations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, A.W.; Lee, G.S.; Zones, S.I. Phase Selectivity in the Syntheses of Cage-Based Zeolite Structures: An Investigation of Thermodynamic Interactions between Zeolite Hosts and Structure Directing Agents by Molecular Modeling. Microporous Mesoporous Mater. 2006, 90, 129–144. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: Precursors, Intermediates and Reaction Mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Dusselier, M.; Davis, M.E. Small-Pore Zeolites: Synthesis and Catalysis. Chem. Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of New Zeolite Structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, F.-S. Green Routes for Synthesis of Zeolites. Chem. Rev. 2014, 114, 1521–1543. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; Deem, M.W.; Davis, M.E. Synthesis of a Specified, Silica Molecular Sieve by Using Computationally Predicted Organic Structure-Directing Agents. Angew. Chem. Int. Ed. 2014, 53, 8372–8374. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, J.R.; Gounder, R. Controlling the Isolation and Pairing of Aluminum in Chabazite Zeolites Using Mixtures of Organic and Inorganic Structure-Directing Agents. Chem. Mater. 2016, 28, 2236–2247. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dong, M.; Fan, S.; Zhao, T.; Wang, J.; Fan, W. Strategies to Control Zeolite Particle Morphology. Chem. Soc. Rev. 2019, 48, 885–907. [Google Scholar] [CrossRef]
- Moliner, M.; Martínez, C.; Corma, A. Multipore Zeolites: Synthesis and Catalytic Applications. Angew. Chem. Int. Ed. 2015, 54, 3560–3579. [Google Scholar] [CrossRef]
- Wagner, P.; Nakagawa, Y.; Lee, G.S.; Davis, M.E.; Elomari, S.; Medrud, R.C.; Zones, S.I. Guest/Host Relationships in the Synthesis of the Novel Cage-Based Zeolites SSZ-35, SSZ-36, and SSZ-39. J. Am. Chem. Soc. 2000, 122, 263–273. [Google Scholar] [CrossRef]
- Zones, S.I.; Burton, A.W.; Lee, G.S.; Olmstead, M.M. A Study of Piperidinium Structure-Directing Agents in the Synthesis of Silica Molecular Sieves under Fluoride-Based Conditions. J. Am. Chem. Soc. 2007, 129, 9066–9079. [Google Scholar] [CrossRef]
- Gábová, V.; Dědeček, J.; Čejka, J. Control of Al Distribution in ZSM-5 by Conditions of Zeolite Synthesis. Chem. Commun. 2003, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Borry, R.W.; Kim, Y.H.; Huffsmith, A.; Reimer, J.A.; Iglesia, E. Structure and Density of Mo and Acid Sites in Mo-Exchanged H-ZSM5 Catalysts for Nonoxidative Methane Conversion. J. Phys. Chem. B 1999, 103, 5787–5796. [Google Scholar] [CrossRef]
- Bortnovsky, O.; Sobalík, Z.; Wichterlová, B. Exchange of Co(II) Ions in H-BEA Zeolites: Identification of Aluminum Pairs in the Zeolite Framework. Microporous Mesoporous Mater. 2001, 46, 265–275. [Google Scholar] [CrossRef]
- Dědeček, J.; Kaucký, D.; Wichterlová, B. Co2+ Ion Siting in Pentasil-Containing Zeolites, Part 3. Microporous Mesoporous Mater. 2000, 35–36, 483–494. [Google Scholar] [CrossRef]
- Dědeček, J.; Kaucký, D.; Wichterlová, B. Al Distribution in ZSM-5 Zeolites: An Experimental Study. Chem. Commun. 2001, 970–971. [Google Scholar] [CrossRef]
- Dědeček, J.; Kaucký, D.; Wichterlová, B.; Gonsiorová, O. Co2+ Ions as Probes of Al Distribution in the Framework of Zeolites. ZSM-5 Study. Phys. Chem. Chem. Phys. 2002, 4, 5406–5413. [Google Scholar] [CrossRef]
- Dědeček, J.; Wichterlová, B. Co2+ Ion Siting in Pentasil-Containing Zeolites. I. Co2+ Ion Sites and Their Occupation in Mordenite. A Vis−NIR Diffuse Reflectance Spectroscopy Study. J. Phys. Chem. B 1999, 103, 1462–1476. [Google Scholar] [CrossRef]
- Bohinc, R.; Hoszowska, J.; Dousse, J.-C.; Błachucki, W.; Zeeshan, F.; Kayser, Y.; Nachtegaal, M.; Pinar, A.B.; van Bokhoven, J.A. Distribution of Aluminum over Different T-Sites in Ferrierite Zeolites Studied with Aluminum Valence to Core X-Ray Emission Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 29271–29277. [Google Scholar] [CrossRef]
- Chen, J.; Liang, T.; Li, J.; Wang, S.; Qin, Z.; Wang, P.; Huang, L.; Fan, W.; Wang, J. Regulation of Framework Aluminum Siting and Acid Distribution in H-MCM-22 by Boron Incorporation and Its Effect on the Catalytic Performance in Methanol to Hydrocarbons. ACS Catal. 2016, 6, 2299–2313. [Google Scholar] [CrossRef]
- Dedecek, J.; Balgová, V.; Pashkova, V.; Klein, P.; Wichterlová, B. Synthesis of ZSM-5 Zeolites with Defined Distribution of Al Atoms in the Framework and Multinuclear MAS NMR Analysis of the Control of Al Distribution. Chem. Mater. 2012, 24, 3231–3239. [Google Scholar] [CrossRef]
- Kim, C.W.; Heo, N.H.; Seff, K. Framework Sites Preferred by Aluminum in Zeolite ZSM-5. Structure of a Fully Dehydrated, Fully Cs + -Exchanged ZSM-5 Crystal (MFI, Si/Al = 24). J. Phys. Chem. C 2011, 115, 24823–24838. [Google Scholar] [CrossRef]
- Pashkova, V.; Klein, P.; Dedecek, J.; Tokarová, V.; Wichterlová, B. Incorporation of Al at ZSM-5 Hydrothermal Synthesis. Tuning of Al Pairs in the Framework. Microporous Mesoporous Mater. 2015, 202, 138–146. [Google Scholar] [CrossRef]
- Pashkova, V.; Sklenak, S.; Klein, P.; Urbanova, M.; Dědeček, J. Location of Framework Al Atoms in the Channels of ZSM-5: Effect of the (Hydrothermal) Synthesis. Chem.-Eur. J. 2016, 22, 3937–3941. [Google Scholar] [CrossRef]
- Pinar, A.B.; Gómez-Hortigüela, L.; McCusker, L.B.; Pérez-Pariente, J. Controlling the Aluminum Distribution in the Zeolite Ferrierite via the Organic Structure Directing Agent. Chem. Mater. 2013, 25, 3654–3661. [Google Scholar] [CrossRef]
- Song, C.; Chu, Y.; Wang, M.; Shi, H.; Zhao, L.; Guo, X.; Yang, W.; Shen, J.; Xue, N.; Peng, L.; et al. Cooperativity of Adjacent Brønsted Acid Sites in MFI Zeolite Channel Leads to Enhanced Polarization and Cracking of Alkanes. J. Catal. 2017, 349, 163–174. [Google Scholar] [CrossRef]
- Vjunov, A.; Fulton, J.L.; Huthwelker, T.; Pin, S.; Mei, D.; Schenter, G.K.; Govind, N.; Camaioni, D.M.; Hu, J.Z.; Lercher, J.A. Quantitatively Probing the Al Distribution in Zeolites. J. Am. Chem. Soc. 2014, 136, 8296–8306. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Biligetu, T.; Wang, Y.; Tatsumi, T. Unique Al Distribution in the MFI Framework and Its Impact on Catalytic Properties. Chem. Lett. 2017, 46, 798–800. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Control of the Al Distribution in the Framework of ZSM-5 Zeolite and Its Evaluation by Solid-State NMR Technique and Catalytic Properties. J. Phys. Chem. C 2015, 119, 15303–15315. [Google Scholar] [CrossRef]
- Schmithorst, M.B.; Prasad, S.; Moini, A.; Chmelka, B.F. Direct Detection of Paired Aluminum Heteroatoms in Chabazite Zeolite Catalysts and Their Significance for Methanol Dehydration Reactivity. J. Am. Chem. Soc. 2023, 145, 18215–18220. [Google Scholar] [CrossRef]
- Di Iorio, J.R.; Li, S.; Jones, C.B.; Nimlos, C.T.; Wang, Y.; Kunkes, E.; Vattipalli, V.; Prasad, S.; Moini, A.; Schneider, W.F.; et al. Cooperative and Competitive Occlusion of Organic and Inorganic Structure-Directing Agents within Chabazite Zeolites Influences Their Aluminum Arrangement. J. Am. Chem. Soc. 2020, 142, 4807–4819. [Google Scholar] [CrossRef]
- Di Iorio, J.R.; Nimlos, C.T.; Gounder, R. Introducing Catalytic Diversity into Single-Site Chabazite Zeolites of Fixed Composition via Synthetic Control of Active Site Proximity. ACS Catal. 2017, 7, 6663–6674. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Moini, A.; Gounder, R.; Maginn, E.J.; Schneider, W.F. Influence of an N, N, N-Trimethyl-1-Adamantyl Ammonium (TMAda+) Structure Directing Agent on Al Distributions and Pair Features in Chabazite Zeolite. Chem. Mater. 2022, 34, 10811–10822. [Google Scholar] [CrossRef]
- Lee, S.; Nimlos, C.T.; Kipp, E.R.; Wang, Y.; Gao, X.; Schneider, W.F.; Lusardi, M.; Vattipalli, V.; Prasad, S.; Moini, A.; et al. Evolution of Framework Al Arrangements in CHA Zeolites during Crystallization in the Presence of Organic and Inorganic Structure-Directing Agents. Cryst. Growth Des. 2022, 22, 6275–6295. [Google Scholar] [CrossRef]
- Krishna, S.H.; Goswami, A.; Wang, Y.; Jones, C.B.; Dean, D.P.; Miller, J.T.; Schneider, W.F.; Gounder, R. Influence of Framework Al Density in Chabazite Zeolites on Copper Ion Mobility and Reactivity during NOx Selective Catalytic Reduction with NH3. Nat. Catal. 2023, 6, 276–285. [Google Scholar] [CrossRef]
- Theis, J.R.; Ura, J.; Getsoian, A.B.; Prikhodko, V.Y.; Thomas, C.R.; Pihl, J.A.; Lardinois, T.M.; Gounder, R.; Wei, X.; Ji, Y.; et al. Effect of Framework Al Pairing on NO Storage Properties of Pd-CHA Passive NOx Adsorbers. Appl. Catal. B 2023, 322, 122074. [Google Scholar] [CrossRef]
- Umhey, C.E.; Guo, J.; Cui, Z.; Shantz, D.F.; Kulkarni, A.; McEwen, J.-S. Elucidating the Impact of Cis–Trans Organic Structure Directing Agent Isomer Ratios on the Aluminum Distribution Within SSZ-39. Chem. Mater. 2024, 36, 11852–11862. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Yamasaki, Y.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. One-Pot Synthesis of Phosphorus-Modified AEI Zeolites Derived by the Dual-Template Method as a Durable Catalyst with Enhanced Thermal/Hydrothermal Stability for Selective Catalytic Reduction of NOx by NH3. Chem. Lett. 2016, 45, 122–124. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, L.; Wu, Q.; Meng, X.; Xiao, F.-S. Advances in the Synthesis and Application of the SSZ-39 Zeolite. Inorg. Chem. Front. 2022, 9, 1047–1057. [Google Scholar] [CrossRef]
- Sada, Y.; Chokkalingam, A.; Iyoki, K.; Yoshioka, M.; Ishikawa, T.; Naraki, Y.; Yanaba, Y.; Yamada, H.; Ohara, K.; Sano, T.; et al. Tracking the Crystallization Behavior of High-Silica FAU during AEI-Type Zeolite Synthesis Using Acid Treated FAU-Type Zeolite. RSC Adv. 2021, 11, 23082–23089. [Google Scholar] [CrossRef]
- Tsunoji, N.; Shimono, D.; Tsuchiya, K.; Sadakane, M.; Sano, T. Formation Pathway of AEI Zeolites as a Basis for a Streamlined Synthesis. Chem. Mater. 2020, 32, 60–74. [Google Scholar] [CrossRef]
- Guo, A.; Liu, H.; Li, Y.; Luo, Y.; Ye, D.; Jiang, J.; Chen, P. Recent Progress in Novel Zeolite Catalysts for Selective Catalytic Reduction of Nitrogen Oxides. Catal. Today 2023, 422, 114212. [Google Scholar] [CrossRef]
- Moliner, M.; Franch, C.; Palomares, E.; Grill, M.; Corma, A. Cu–SSZ-39, an Active and Hydrothermally Stable Catalyst for the Selective Catalytic Reduction of NOx. Chem. Commun. 2012, 48, 8264. [Google Scholar] [CrossRef]
- Dusselier, M.; Deimund, M.A.; Schmidt, J.E.; Davis, M.E. Methanol-to-Olefins Catalysis with Hydrothermally Treated Zeolite SSZ-39. ACS Catal. 2015, 5, 6078–6085. [Google Scholar] [CrossRef]
- Wulfers, M.J.; Teketel, S.; Ipek, B.; Lobo, R.F. Conversion of Methane to Methanol on Copper-Containing Small-Pore Zeolites and Zeotypes. Chem. Commun. 2015, 51, 4447–4450. [Google Scholar] [CrossRef]
- Ransom, R.; Coote, J.; Moulton, R.; Gao, F.; Shantz, D.F. Synthesis and Growth Kinetics of Zeolite SSZ-39. Ind. Eng. Chem. Res. 2017, 56, 4350–4356. [Google Scholar] [CrossRef]
- Ransom, R.; Moulton, R.; Shantz, D.F. The Structure Directing Agent Isomer Used in SSZ-39 Synthesis Impacts the Zeolite Activity towards Selective Catalytic Reduction of Nitric Oxides. J. Catal. 2020, 382, 339–346. [Google Scholar] [CrossRef]
- Pokhrel, J.; Shantz, D.F. Continuous Partial Oxidation of Methane to Methanol over Cu-SSZ-39 Catalysts. J. Catal. 2023, 421, 300–308. [Google Scholar] [CrossRef]
- Dusselier, M.; Schmidt, J.E.; Moulton, R.; Haymore, B.; Hellums, M.; Davis, M.E. Influence of Organic Structure Directing Agent Isomer Distribution on the Synthesis of SSZ-39. Chem. Mater. 2015, 27, 2695–2702. [Google Scholar] [CrossRef]
- Cui, Z.; Umhey, C.E.; Ukagha, O.C.; Ogunleye, M.; McEwen, J.-S.; Shantz, D.F. Quantifying How the Cis/Trans Ratio of N, N -Dimethyl-3,5-Dimethylpiperidinium Hydroxide Impacts the Growth Kinetics, Composition and Local Structure of SSZ-39. RSC Adv. 2025, 15, 7962–7972. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Lejaeghere, K.; Bihlmayer, G.; Björkman, T.; Blaha, P.; Blügel, S.; Blum, V.; Caliste, D.; Castelli, I.E.; Clark, S.J.; Dal Corso, A.; et al. Reproducibility in Density Functional Theory Calculations of Solids. Science 2016, 351, aad3000. [Google Scholar] [CrossRef] [PubMed]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Van Der Waals Density Functionals Applied to Solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef]

| NaOH/OSDA | Ratio Value | Phase-Pure SSZ-39 |
|---|---|---|
| 0.68/0.03 | 22.6 | No |
| 0.66/0.05 | 13.2 | No |
| 0.64/0.07 | 9.14 | Yes |
| 0.60/0.11 | 5.45 | Yes |
| 0.57/0.14 | 4.07 | Yes |
| 0.51/0.2 | 2.55 | Yes |
| 0.45/0.26 | 1.73 | Yes |
| 0.42/0.29 | 1.45 | No |
| 0.39/0.32 | 1.22 | No |
| 0.33/0.38 | 0.87 | No |
| 0.21/0.5 | 0.42 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.; Umhey, C.E.; Shantz, D.F.; McEwen, J.-S. Effects of Sodium-to-OSDA Ratio in the Synthesis Gel on SSZ-39 Formation and Material Properties. Catalysts 2025, 15, 989. https://doi.org/10.3390/catal15100989
Cui Z, Umhey CE, Shantz DF, McEwen J-S. Effects of Sodium-to-OSDA Ratio in the Synthesis Gel on SSZ-39 Formation and Material Properties. Catalysts. 2025; 15(10):989. https://doi.org/10.3390/catal15100989
Chicago/Turabian StyleCui, Zheng, Charles E. Umhey, Daniel F. Shantz, and Jean-Sabin McEwen. 2025. "Effects of Sodium-to-OSDA Ratio in the Synthesis Gel on SSZ-39 Formation and Material Properties" Catalysts 15, no. 10: 989. https://doi.org/10.3390/catal15100989
APA StyleCui, Z., Umhey, C. E., Shantz, D. F., & McEwen, J.-S. (2025). Effects of Sodium-to-OSDA Ratio in the Synthesis Gel on SSZ-39 Formation and Material Properties. Catalysts, 15(10), 989. https://doi.org/10.3390/catal15100989








