Synergistic Radical and Non-Radical Pathways in Phenol Degradation: Electron Transfer Mechanism Dominated by N-Doped Carbon/Peroxymonosulfate System
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Nitrogen-Doped HA-Based Carbon Materials
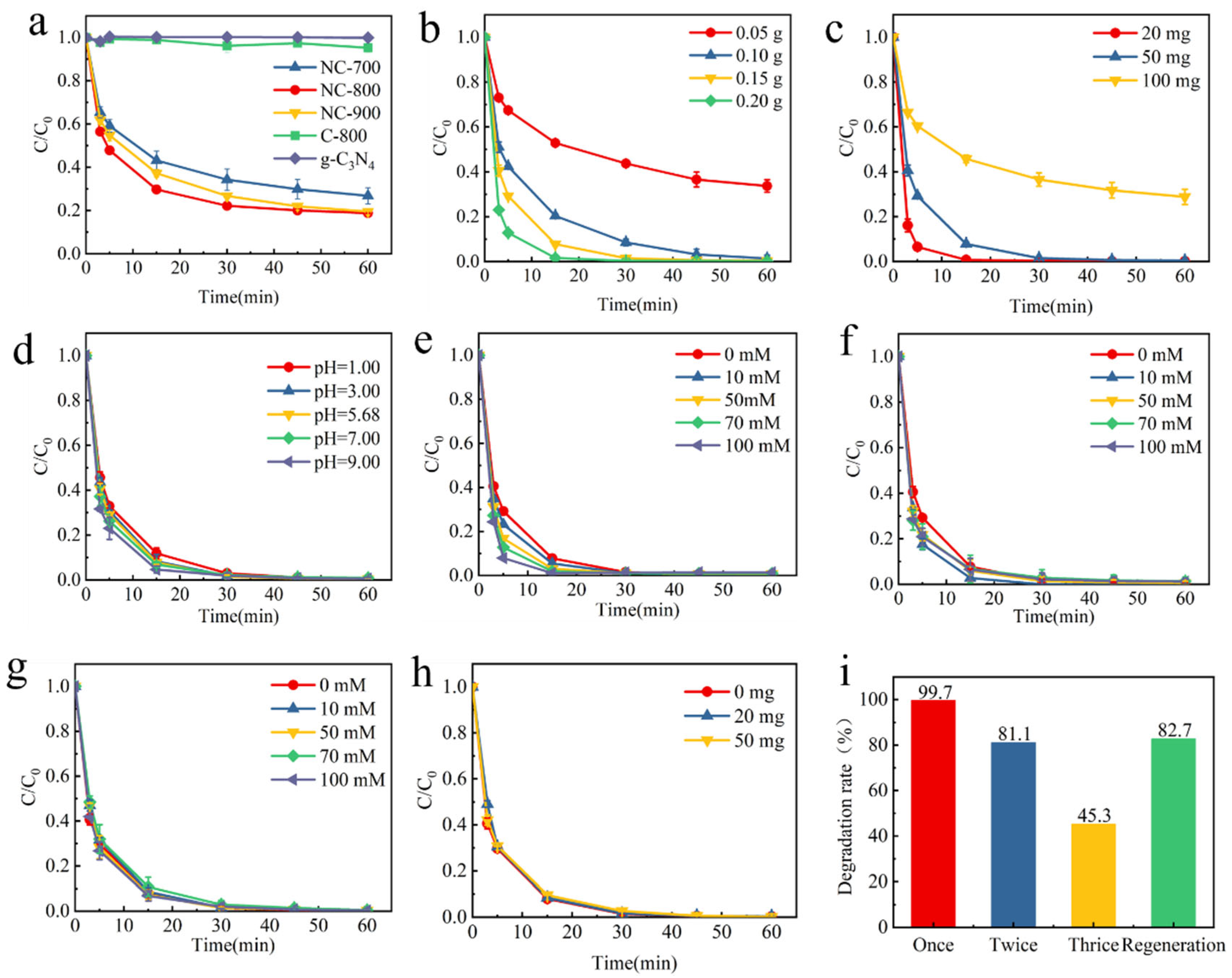
2.2. Performance Evaluation of Nitrogen-Doped HA-Based Carbon Materials Activated PMS
2.3. The Mechanism of NC-800 Activation of PMS
2.3.1. Detection of Active Substances
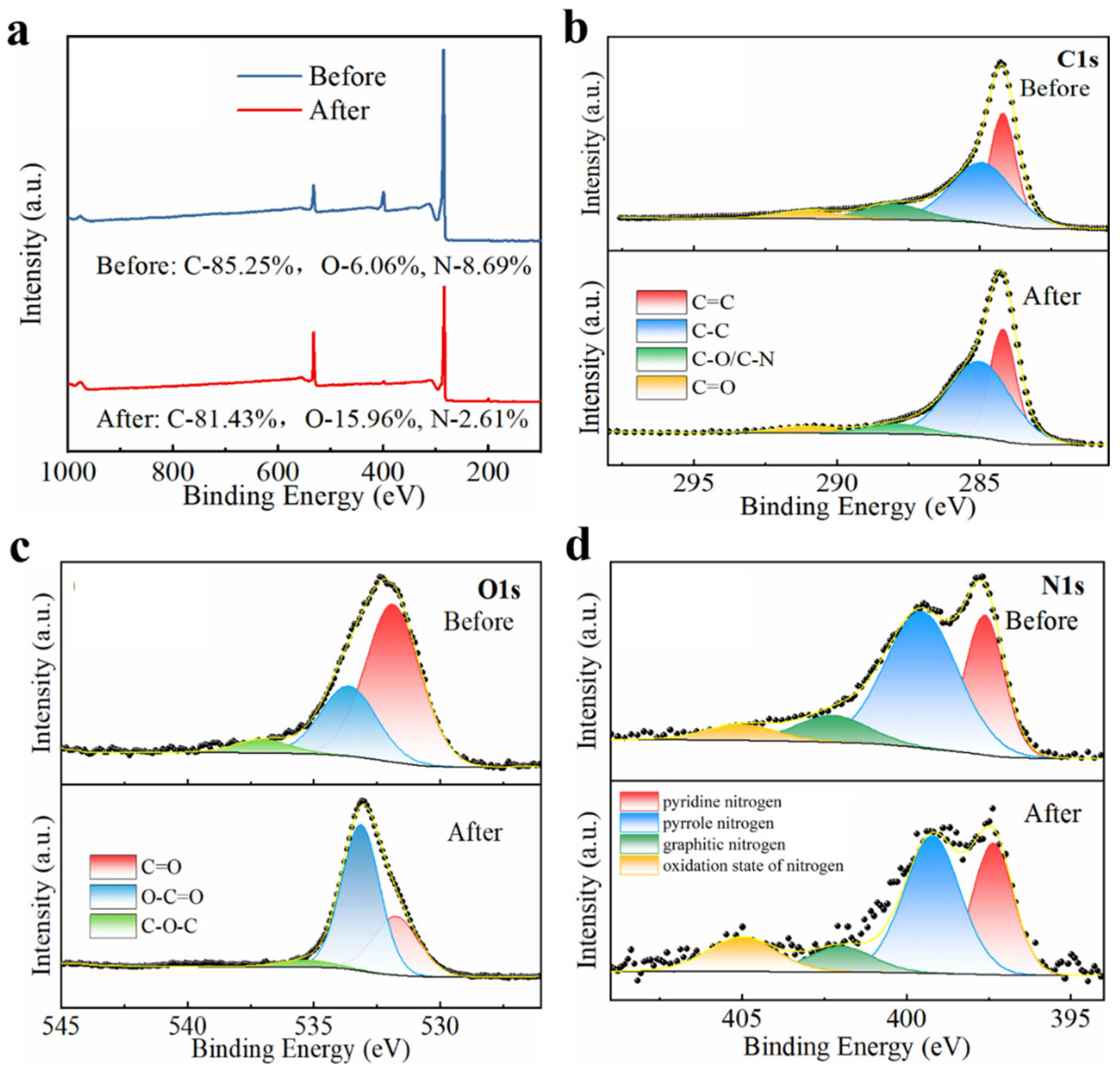
2.3.2. The Changes in the Surface Structure of NC-800 Before and After the Reaction
2.3.3. The Potential Activation Mechanisms of the NC-800/PMS System
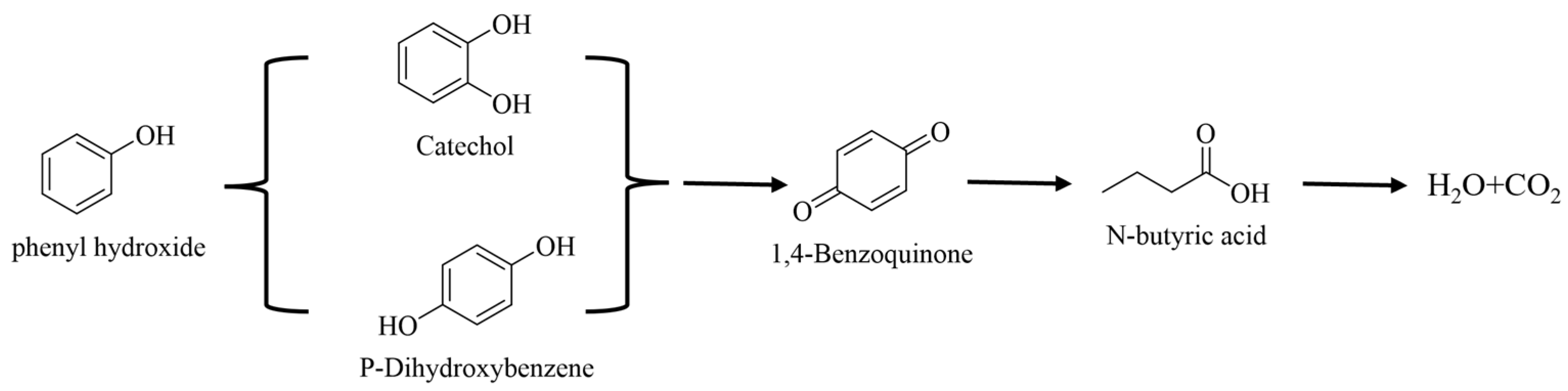
2.4. The Possible Pathways for Phenol Degradation
2.5. Material Comparison
3. Experimental Sections
3.1. Materials and Reagents
3.2. Preparation and Structural Characterization of Nitrogen-Doped HA-Based Carbon Materials
3.3. Material Performance Evaluation and Methods
3.4. Materials Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, J.X.; Wan, N.; Li, L.C.; Li, Z.X.; Han, H.J. Review on treatment technologies of coal gasification wastewater in China. J. Clean. Prod. 2022, 333, 130166. [Google Scholar] [CrossRef]
- Wang, W.; Han, H.J. Recovery strategies for tackling the impact of phenolic compounds in a UASB reactor treating coal gasification wastewater. Bioresour. Technol. 2012, 103, 95–100. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Zhang, A.; Liu, Z.; Yang, Z.; Li, X.; Li, Z. Construction of aldehyde-based, ester-based hyper-cross-linked polar resin and its selective adsorption mechanism for phenol in coal chemical wastewater. Environ. Res. 2024, 246, 118140. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.G.; Sun, H.Q.; Wang, S.B. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.P.; Yin, Z.L.; Liu, Z.F.; Liu, Y.L.; Yang, Z.; Yang, W.B. Adsorption and catalysis of peroxymonosulfate on carbocatalysts for phenol degradation: The role of pyrrolic-nitrogen. Appl. Catal. B Environ. 2022, 319, 121891. [Google Scholar] [CrossRef]
- Sun, H.Q.; Liu, S.Z.; Zhou, G.L.; Ang, H.M.; Tadé, M.O.; Wang, S.B. Reduced Graphene Oxide for Catalytic Oxidation of Aqueous Organic Pollutants. ACS Appl. Mater. Inter. 2012, 4, 5466–5471. [Google Scholar] [CrossRef]
- Zhong, S.; Zhou, H.Y.; Zhu, Z.S.; Ren, S.Y.; Vongsvivut, J.; Zhou, P.; Duan, X.G.; Wang, S.B. Overlooked Impacts of Alcohols in Electro-H2O2 and Fenton Chemistry. Environ. Sci. Technol. 2024, 58, 14585–14593. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Wang, S.B. Comment on “Activation of Persulfate by Graphitized Nanodiamonds for Removal of Organic Compounds”. Environ. Sci. Technol. 2017, 51, 5351–5352. [Google Scholar] [CrossRef]
- Indrawirawan, S.; Sun, H.Q.; Duan, X.G.; Wang, S.B. Nanocarbons in different structural dimensions (0-3D) for phenol adsorption and metal-free catalytic oxidation. Appl. Catal. B Environ. 2015, 179, 352–362. [Google Scholar] [CrossRef]
- Yang, Y.-j.; Wang, B.; Guo, X.-j.; Zou, C.-w.; Tan, X.-d. Investigating adsorption performance of heavy metals onto humic acid from sludge using Fourier-transform infrared combined with two-dimensional correlation spectroscopy. Environ. Sci. Pollut. Res. 2019, 26, 9842–9850. [Google Scholar] [CrossRef]
- Li, Y.; Jia, X.; Li, X.; Liu, P.; Zhang, X.; Guo, M. Study on the potential of sludge-derived humic acid as energy storage material. Waste Manag. 2023, 162, 55–62. [Google Scholar] [CrossRef]
- Wang, C.Q.; Jin, R.Y.; He, Z.D.; Qiao, Y.N.; Wang, Y.; Wang, K.; Lu, Y.R.; Wang, X.J.; Liu, D.D. A new water treatment technology for degradation of B[a]A by hydrodynamic cavitation and chlorine dioxide oxidation. Ultrason. Sonochem 2020, 61, 104834. [Google Scholar] [CrossRef]
- Yu, H.J.; Shang, L.; Bian, T.; Shi, R.; Waterhouse, G.I.N.; Zhao, Y.F.; Zhou, C.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. Nitrogen-Doped Porous Carbon Nanosheets Templated from g-C3N4 as Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. Adv. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Wang, Y.X.; Kang, J.; Wang, S.B. N-Doping-Induced Nonradical Reaction on Single-Walled Carbon Nanotubes for Catalytic Phenol Oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Wang, X.B.; Qin, Y.L.; Zhu, L.H.; Tang, H.Q. Nitrogen-Doped Reduced Graphene Oxide as a Bifunctional Material for Removing Bisphenols: Synergistic Effect between Adsorption and Catalysis. Environ. Sci. Technol. 2015, 49, 6855–6864. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Pan, B. Fe(III)-Doped g-C3N4 Mediated Peroxymonosulfate Activation for Selective Degradation of Phenolic Compounds via High-Valent Iron-Oxo Species. Environ. Sci. Technol. 2018, 52, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Ren, X.; Ding, S.; Fu, N.; Wei, Y.; Yang, Z.; Yao, X. Effective degradation of phenol by activating PMS with bimetallic Mo and Ni Co-doped g-C3N4 composite catalyst: A Fenton-like degradation process promoted by non-free radical 1O2. Environ. Res. 2023, 243, 117848. [Google Scholar] [CrossRef] [PubMed]
- Martha, S.; Nashim, A.; Parida, K.M. Facile synthesis of highly active g-C3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816–7824. [Google Scholar] [CrossRef]
- Zhu, S.S.; Huang, X.C.; Ma, F.; Wang, L.; Duan, X.G.; Wang, S.B. Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.L.; Nie, G.; Zhang, H.; Duan, X.G.; Wang, S.B. Insights into the Electron-Transfer Regime of Peroxydisulfate Activation on Carbon Nanotubes: The Role of Oxygen Functional Groups. Environ. Sci. Technol. 2020, 54, 1267–1275. [Google Scholar] [CrossRef]
- Lv, X.M.; Zhang, Y.F.; Wang, Y.; Zhang, G.J.; Liu, J.; Lu, Y. Formation of coal-based carbon nanotubes by Fe-K catalyst. J. Anal. Appl. Pyrolysis 2022, 161, 105400. [Google Scholar] [CrossRef]
- Ahmed, S.; An, J.; Zubair, M.; Sun, H.-J.; Shim, J.; Park, G. Relationship between Morphology and Performance of Manganese-Derived Catalysts for Rechargeable Zinc-Air Batteries. J. Phys. Chem. Lett. 2025, 16, 6667–6673. [Google Scholar] [CrossRef]
- Hong, Y.C.; Peng, J.L.; Zhao, X.G.; Yan, Y.; Lai, B.; Yao, G. Efficient degradation of atrazine by CoMgAl layered double oxides catalyzed peroxymonosulfate: Optimization, degradation pathways and mechanism. Chem. Eng. J. 2019, 370, 354–363. [Google Scholar] [CrossRef]
- Dikdim, J.M.D.; Gong, Y.; Noumi, G.B.; Sieliechi, J.M.; Zhao, X.; Ma, N.; Yang, M.; Tchatchueng, J.B. Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation. Chemosphere 2019, 217, 833–842. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Rodriguez, V.; Montero, E.; Romero, A.; Santos, A. Methanol-enhanced degradation of carbon tetrachloride by alkaline activation of persulfate: Kinetic model. Sci. Total Environ. 2019, 666, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, H.G.; Zhang, Y.L.; Tang, W.H.; Cheng, X.; Liu, H.W. Activation of peroxymonosulfate by BiVO4 under visible light for degradation of Rhodamine B. Chem. Phys. Lett. 2016, 653, 101–107. [Google Scholar] [CrossRef]
- de Andrade, J.R.; Vieira, M.G.A.; Carlos da Silva, M.G.; Wang, S. Oxidative degradation of pharmaceutical losartan potassium with N-doped hierarchical porous carbon and peroxymonosulfate. Chem. Eng. J. 2020, 382, 122971. [Google Scholar] [CrossRef]
- Shang, E.; Li, Y.; Niu, J.; Zhou, Y.; Wang, T.; Crittenden, J.C. Relative importance of humic and fulvic acid on ROS generation, dissolution, and toxicity of sulfide nanoparticles. Water Res. 2017, 124, 595–604. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Liu, P.; Bai, X.; Du, X.; Jin, P.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Govindan, K.; Kim, D.-G.; Ko, S.-O. Role of N-Doping and O-Groups in Unzipped N-Doped CNT Carbocatalyst for Peroxomonosulfate Activation: Quantitative Structure–Activity Relationship. Catalysts 2022, 12, 845. [Google Scholar] [CrossRef]
- Li, N.; Li, R.; Duan, X.; Yan, B.; Liu, W.; Cheng, Z.; Chen, G.; Hou, L.a.; Wang, S. Correlation of Active Sites to Generated Reactive Species and Degradation Routes of Organics in Peroxymonosulfate Activation by Co-Loaded Carbon. Environ. Sci. Technol. 2021, 55, 16163–16174. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.G.; Wang, S.B. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Hu, W.; Xie, Y.; Lu, S.; Li, P.; Xie, T.; Zhang, Y.; Wang, Y. One-step synthesis of nitrogen-doped sludge carbon as a bifunctional material for the adsorption and catalytic oxidation of organic pollutants. Sci. Total Environ. 2019, 680, 51–60. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Lv, P.; She, L.; Zhong, Y.; Chen, Q.; Ye, Q.; Zeng, D.; Wang, J.; Yang, J.; Li, J.; Wu, G.; et al. Regulation mechanism of biochar on cobalt-doped copper oxide/peroxymonosulfate system: Key roles of superoxide radicals. Sep. Purif. Technol. 2025, 375, 133857. [Google Scholar] [CrossRef]
- Hou, J.F.; Yang, S.S.; Wan, H.Q.; Fu, H.Y.; Qu, X.L.; Xu, Z.Y.; Zheng, S.R. Highly effective catalytic peroxymonosulfate activation on N-doped mesoporous carbon for o-phenylphenol degradation. Chemosphere 2018, 197, 485–493. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Hu, Z.-T.; Sun, Y.-M.; Webster, R.D.; Li, S.-Z.; Lim, T.-T. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl. Catal. B Environ. 2018, 225, 243–257. [Google Scholar] [CrossRef]
- Ghaly, H.A.; El-Kalliny, A.S.; Gad-Allah, T.A.; El-Sattar, N.E.A.A. Photodegradation of Naproxen Using Ag/AgCl–PANI Composite under Solar Light: Transformation Product and Reaction Kinetics. Kinet. Catal. 2021, 62, 367–374. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Poormohammadi, A.; Zamani, F.; Tahmasebi Birgani, Y.; Jorfi, S.; Gholizadeh, S.; Mohammadi, M.J.; Almasi, H. Activated persulfate by chelating agent Fe/complex for in situ degradation of phenol: Intermediate identification and optimization study. Res. Chem. Intermed. 2018, 44, 5501–5519. [Google Scholar] [CrossRef]
- Sanchez, I.; Stüber, F.; Font, J.; Fortuny, A.; Fabregat, A.; Bengoa, C. Elimination of phenol and aromatic compounds by zero valent iron and EDTA at low temperature and atmospheric pressure. Chemosphere 2007, 68, 338–344. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Huang, F.; Liu, Y.; Feng, L.; Jiang, J.; Zhang, L.; Qi, F.; Liu, C. Carbonized polyaniline activated peroxymonosulfate (PMS) for phenol degradation: Role of PMS adsorption and singlet oxygen generation. Appl. Catal. B Environ. 2021, 286, 119921. [Google Scholar] [CrossRef]
- Kou, L.D.; Fan, Q.F.; Yang, Y.H.; Duan, X.Y.; Jiang, K.; Wang, J. Polyaniline@g-C3N4 derived N-rich porous carbon for selective degradation of phenolic pollutants via peroxymonosulfate activation: An electron transfer mechanism. Chemosphere 2023, 311, 137022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, H.; Li, H.; Zhen, J.; Lv, W. Efficient activation of peroxymonosulfate by MoS2 intercalated MgCuFe layered double hydroxide for phenol pollutant control. J. Environ. Chem. Eng. 2023, 11, 109502. [Google Scholar] [CrossRef]
- Ding, S.; Ren, X.; Chen, R.; Yang, Z. Efficient degradation of Phenol by 1 T/2H-MoS2/CuFe2O4 activated peroxymonosulfate and mechanism research. Appl. Surf. Sci. 2023, 612, 155931. [Google Scholar] [CrossRef]
- Afzal, S.; Mehmood, A.; Jin, L.; Pan, K.; Ahmad, M.; Duan, D.; Wei, Y.; Raza, W.; Hao, T.; Sharif, H.M.A.; et al. Facile fabrication of cobalt doped manganese oxide with hierarchical porosity for peroxymonosulfate activation: Performance investigation, mechanistic insight to degrade organic pollutants in water and DFT study. J. Hazard. Mater. Adv. 2023, 9, 100244. [Google Scholar] [CrossRef]
- Yu, Y.L.; Yang, J.; Fan, X.F.; Liu, Y.M. Enhanced persulfate activation by nitrogen-doped mesoporous carbon for efficiently degrading organic matters. Environ. Sci. Pollut. Res. 2023, 30, 33795–33807. [Google Scholar] [CrossRef] [PubMed]
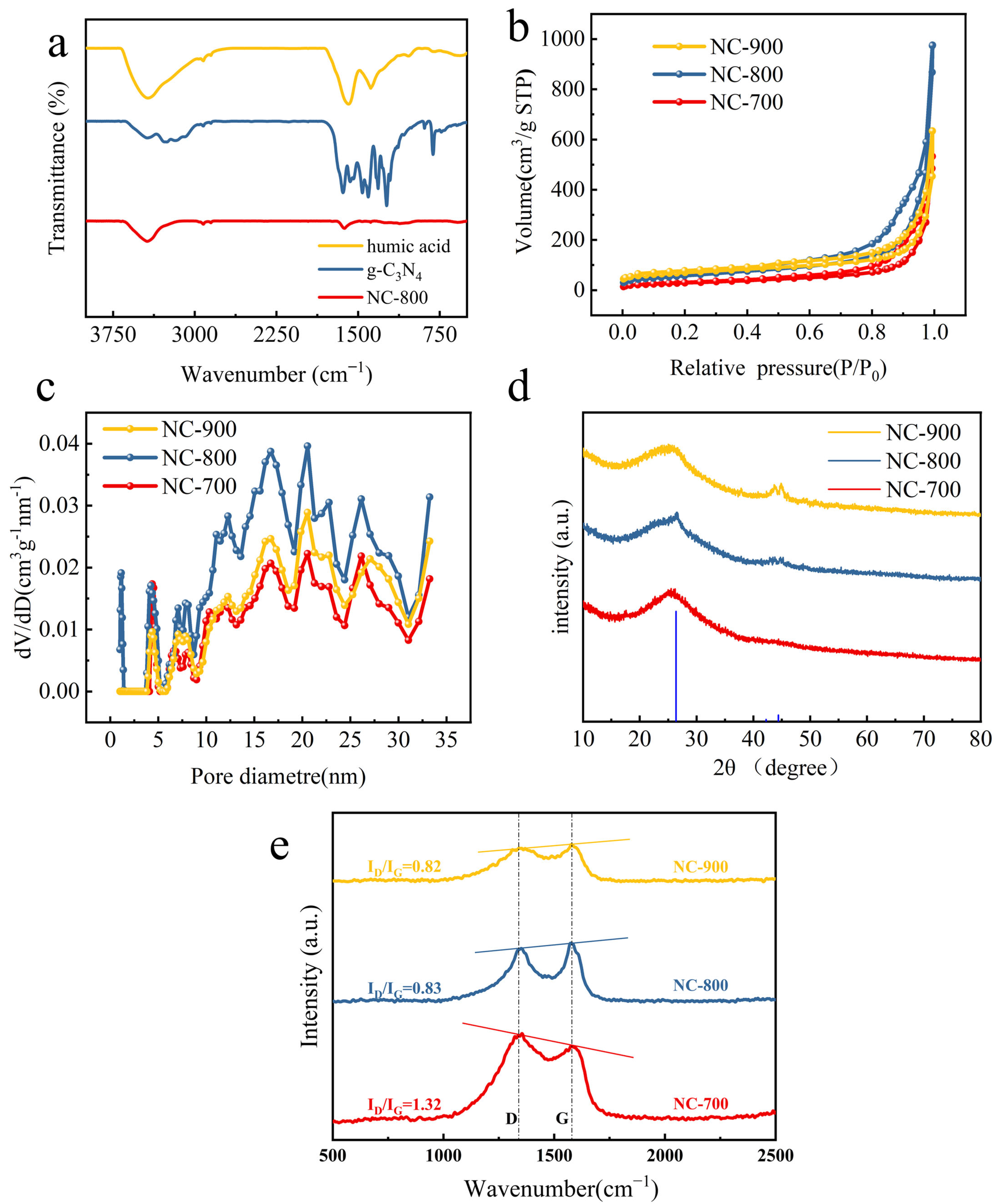
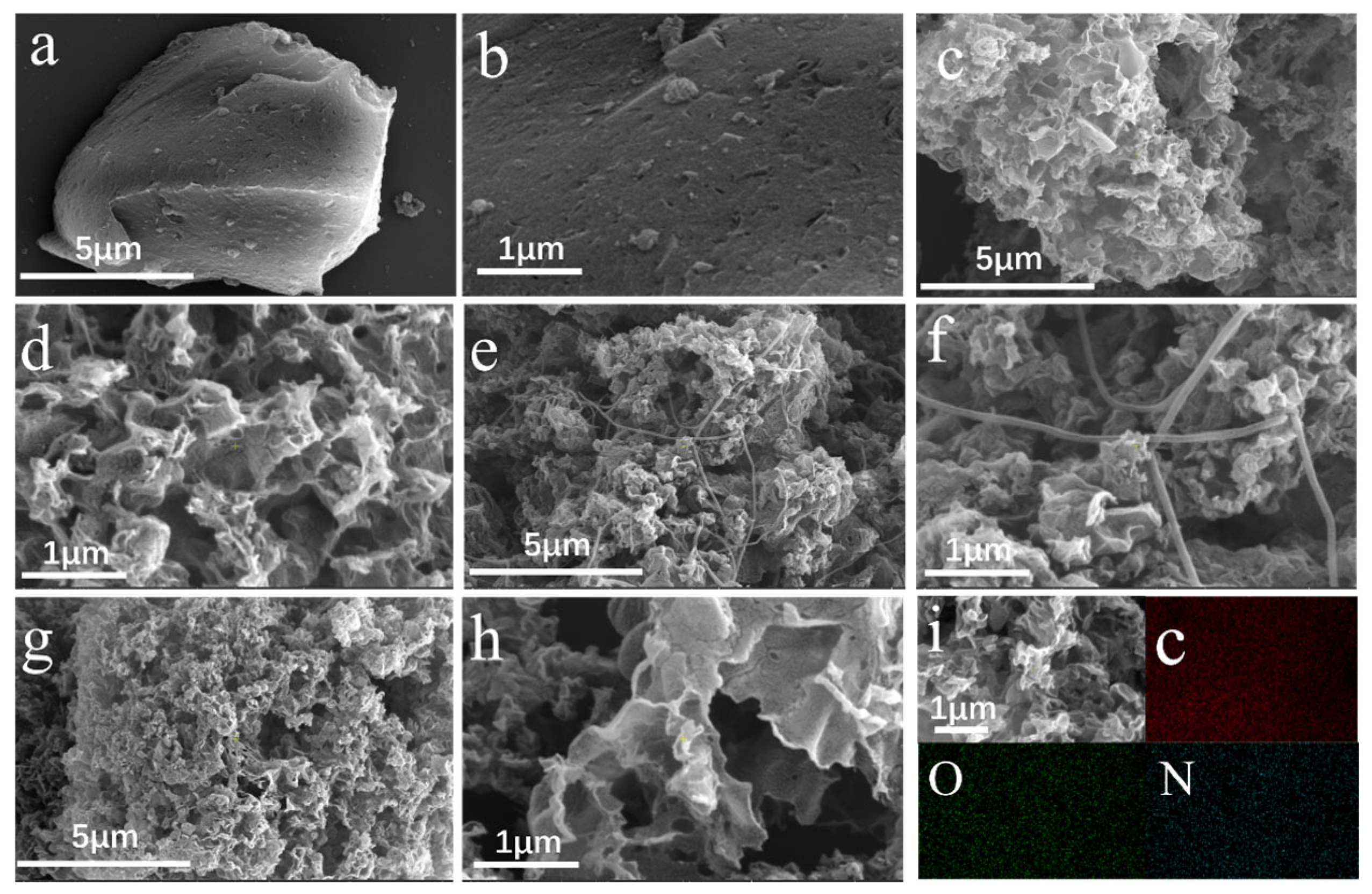
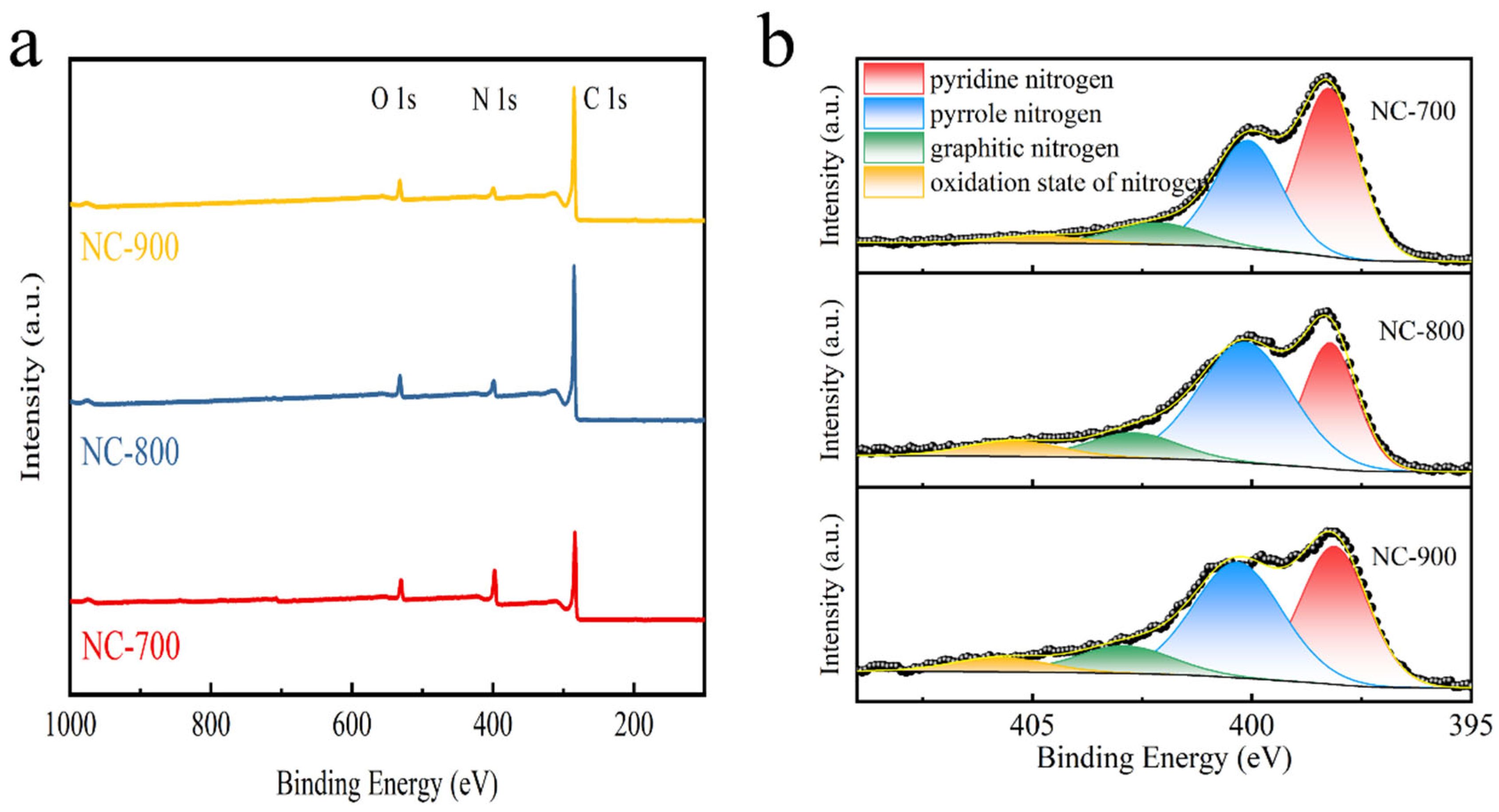

| Parameters | NC-700 | NC-800 | NC-900 |
|---|---|---|---|
| SBET (m2/g) | 104.17 | 209.11 | 130.29 |
| Pore volume (cm3/g) | 0.38 | 0.68 | 0.46 |
| Samples | Elemental Analysis (wt. %) | XPS (at. %) | ||||
|---|---|---|---|---|---|---|
| Cad | Nad | Had | Cad | Nad | Oad | |
| NC-700 | 65.32 | 23.66 | 1.91 | 71.67 | 20.84 | 7.49 |
| NC-800 | 76.66 | 7.85 | 1.41 | 85.25 | 8.69 | 6.06 |
| NC-900 | 80.24 | 6.73 | 1.39 | 86.95 | 6.98 | 6.07 |
| Samples | Pyridinic N | Pyrrolic N | Graphitic N | Oxidized N |
|---|---|---|---|---|
| NC-700 | 50.10% | 36.61% | 9.71% | 3.58% |
| NC-800 | 30.81% | 52.75% | 10.43% | 6.01% |
| NC-900 | 39.19% | 43.64% | 11.27% | 5.90% |
| Materials | Reaction Conditions | Degradation Rate Constant | Degradation Effect | Reusability | Literature |
|---|---|---|---|---|---|
| Carbonized polyaniline | [PH] = 0.94 mg/L; [catalyst] = 25 mg/L; [PMS] = 0.5 mmol/L | 0.431 min−1 | 100% 30 min | 29% after fourth times | [43] |
| Polyaniline@g-C3N4 | [PH] = 9.4 mg/L; [catalyst] = 100 mg/L; [PMS] = 1 mM | 0.075 min−1 | 100% 180 min | 46% after three times | [44] |
| MoS2/MgCuFe-LDH | [PH] = 1.88 mg/L; [catalyst] = 100 mg/L; [PMS] = 1.0 mM | 1.24 min−1 | 98% 10 min | [45] | |
| 1T/2H-MoS2/CuFe2O4 | [PH]= 20 mg/L; [catalyst]= 300 mg/L; [PMS] = 1.0 mM | 0.170 min−1 | 95.8% 40 min | More than 90% after 5 cycles | [46] |
| Co18-MnOx | [PH] = 20 mg/L; [catalyst] = 200 mg/L; [PMS] = 2.86 mM | 0.29 min−1 | 100% 18 min | 87.45% after three times | [47] |
| nitrogen-doped mesoporous carbon | [PH] = 70 mg/L; [catalyst] = 400 mg/L; [PMS] = 1.5 mM | 0.0192 min−1 | 95% 90 min | 45% after the third cycle | [48] |
| 8-g C3N4/Mo/Ni | [PH] = 20 mg/L; [catalyst] = 350 mg/L; [PMS] = 0.6 mM | 0.097 min−1 | 95% 20 min | 72% after the fifth cycle | [18] |
| NC-800 | [PH] = 50 mg/L; [catalyst] = 150 mg/L; [PMS] = 1.5 mM | 0.1214 min−1 | 99.62% 60 min | 45.3% after the third cycle | This thesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Wu, X.; Ma, P.; Wu, X.; Miao, Z. Synergistic Radical and Non-Radical Pathways in Phenol Degradation: Electron Transfer Mechanism Dominated by N-Doped Carbon/Peroxymonosulfate System. Catalysts 2025, 15, 968. https://doi.org/10.3390/catal15100968
He Q, Wu X, Ma P, Wu X, Miao Z. Synergistic Radical and Non-Radical Pathways in Phenol Degradation: Electron Transfer Mechanism Dominated by N-Doped Carbon/Peroxymonosulfate System. Catalysts. 2025; 15(10):968. https://doi.org/10.3390/catal15100968
Chicago/Turabian StyleHe, Qiongqiong, Xuewen Wu, Ping Ma, Xiaoqi Wu, and Zhenyong Miao. 2025. "Synergistic Radical and Non-Radical Pathways in Phenol Degradation: Electron Transfer Mechanism Dominated by N-Doped Carbon/Peroxymonosulfate System" Catalysts 15, no. 10: 968. https://doi.org/10.3390/catal15100968
APA StyleHe, Q., Wu, X., Ma, P., Wu, X., & Miao, Z. (2025). Synergistic Radical and Non-Radical Pathways in Phenol Degradation: Electron Transfer Mechanism Dominated by N-Doped Carbon/Peroxymonosulfate System. Catalysts, 15(10), 968. https://doi.org/10.3390/catal15100968








