Synthesis of NiCu–Polymeric Membranes for Electro-Oxidizing Ethylene Glycol Molecules in Alkaline Medium
Abstract
1. Introduction
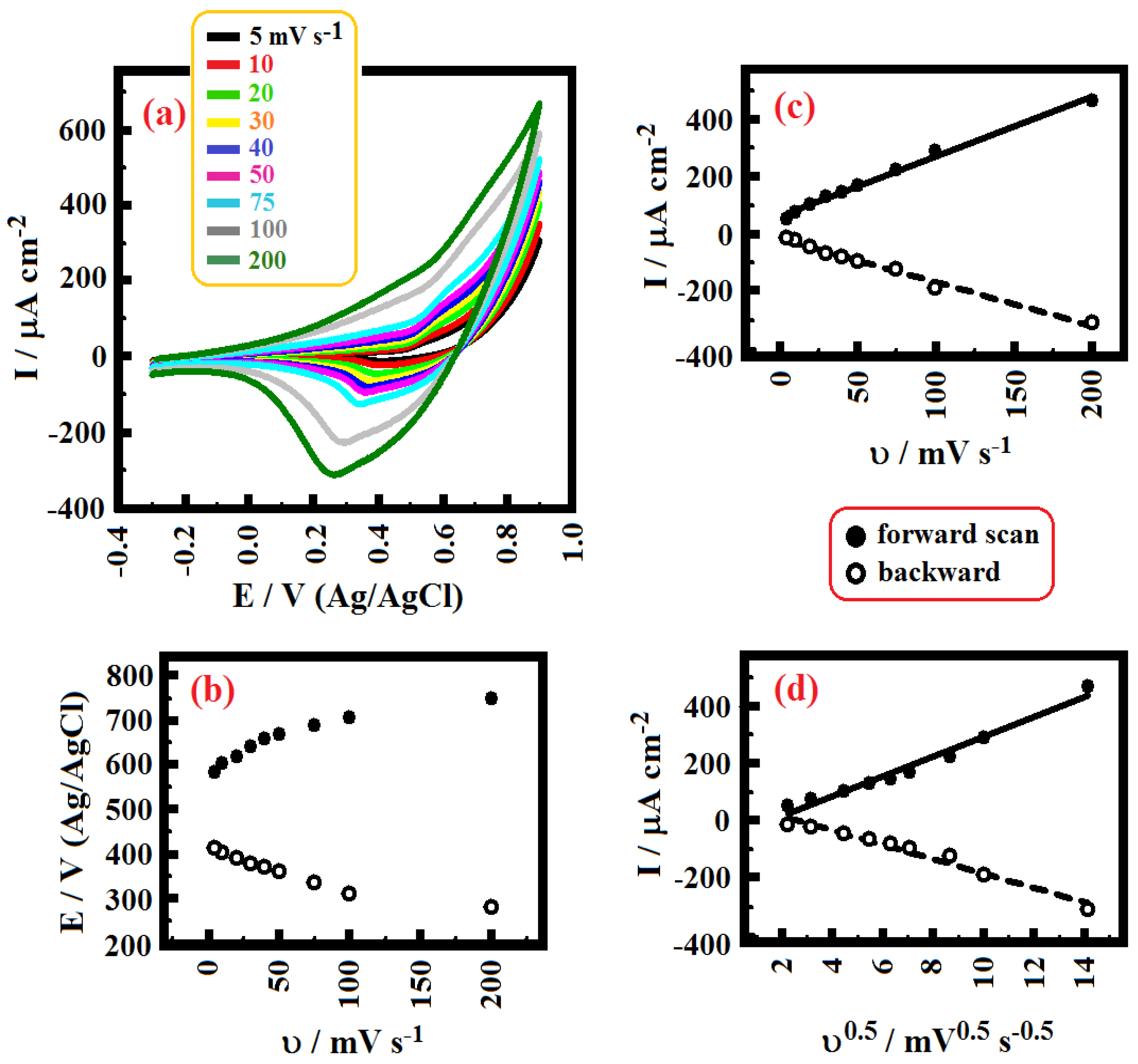
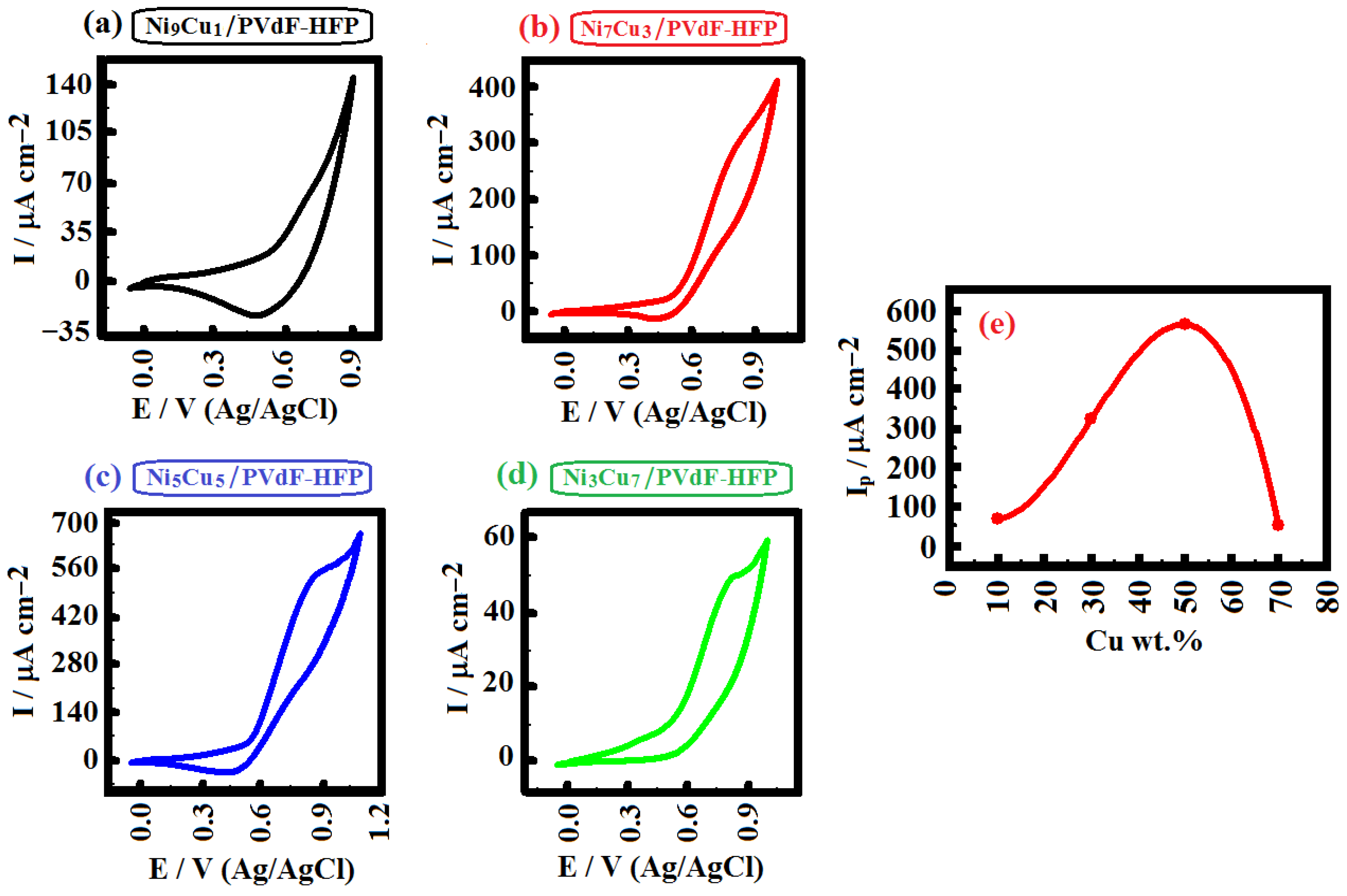
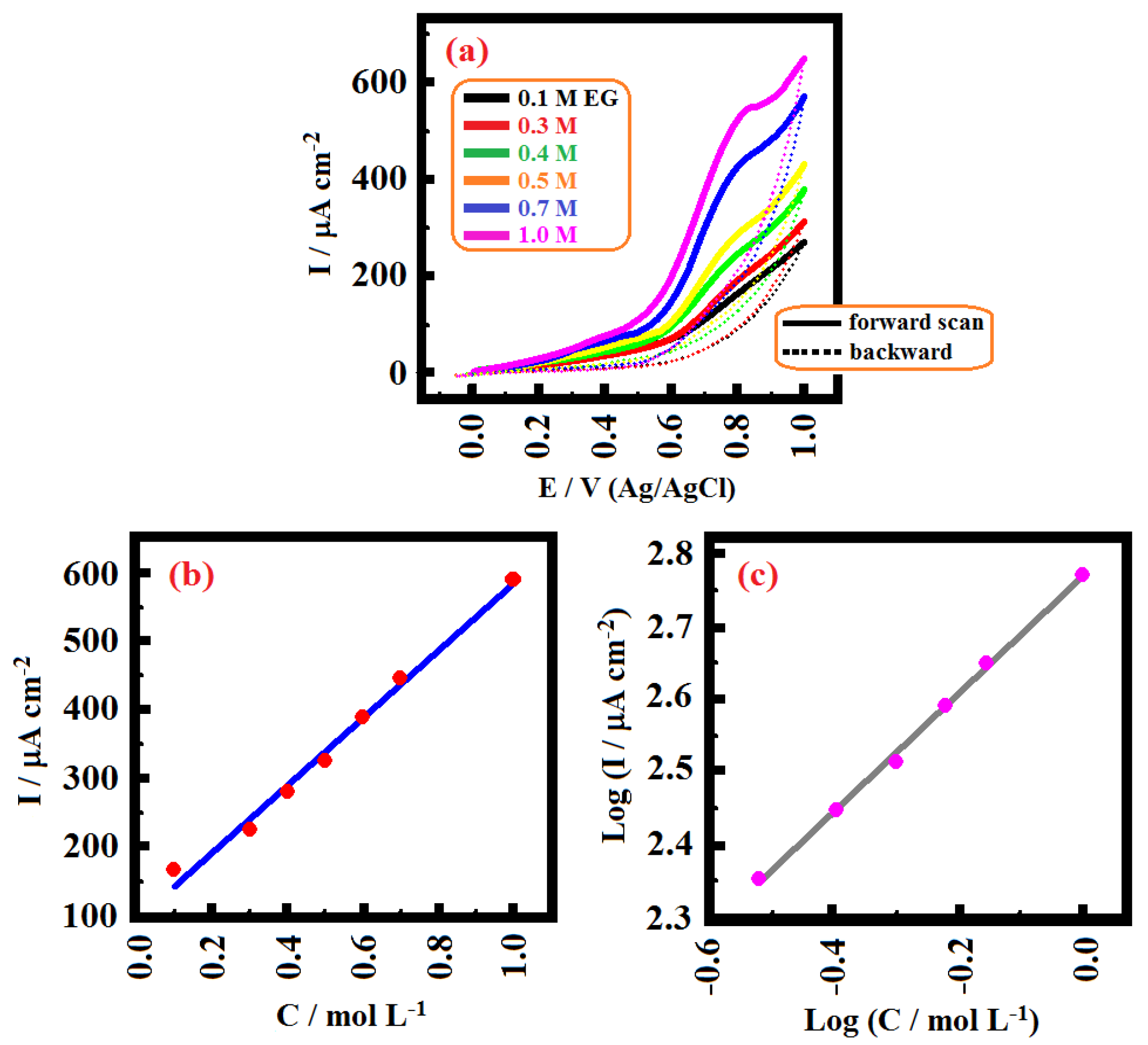
2. Results and Discussion
3. Experimental Section
3.1. Chemicals
3.2. The Formation of Metallic PVdF–HFP Membrane Surfaces in Altered Ni:Cu Proportions
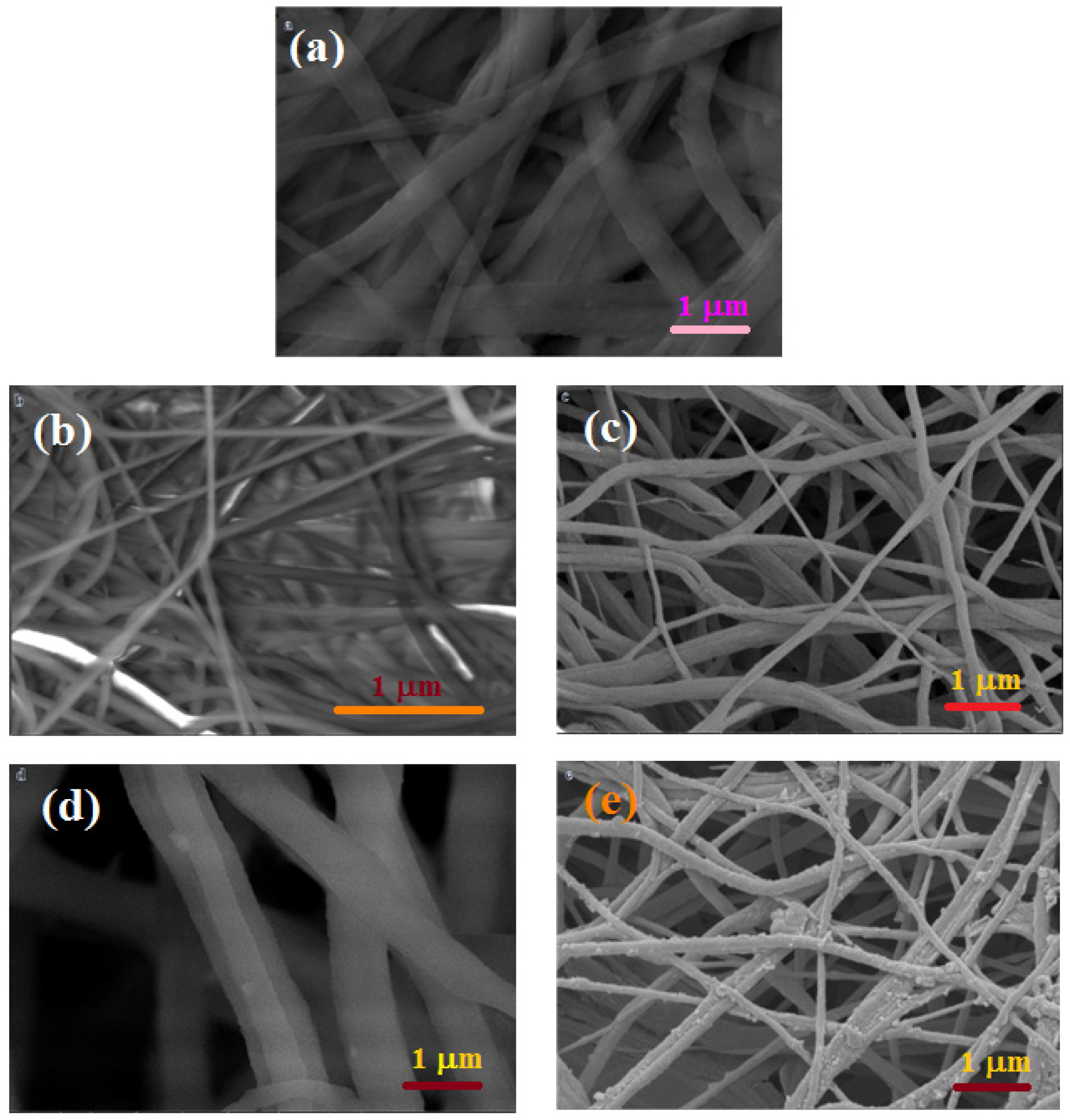
3.3. The Physical Characterization of NiCu/PVdF–HFP Membranes Using Convenient Analysis Tools
3.4. The Electrochemical Investigation of Varied NiCu/PVdF–HFP Membranes Activity for Electro-Oxidizing Ethylene Glycol Molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Govindarajan, D.; Murugadoss, G.; Kirubaharan, K.; Manavalan, R.K.; Manibalan, G.; Shaikh, J.; Etesami, M.; Kheawhom, S. Improved electrochemical supercapacitive properties of CuO-Ni(OH)2 nanocomposites by eco-friendly low-temperature synthesis. J. Alloys Compd. 2023, 942, 169130. [Google Scholar] [CrossRef]
- Meng, Z.-Y.; Li, Y.; Wang, S.-X.; Qiu, Z.-M.; Suen, N.-T.; Guo, X.-T.; Pi, Y.-C.; Pang, H. Boosting hydrogen oxidation performance of bimetallic telluride by electrochemical surface engineering. Rare Met. 2025, 44, 4669–4678. [Google Scholar] [CrossRef]
- Ren, X.; Qiu, L.; Li, M.; Tian, F.; He, L.; Guo, X.; Wu, F.; Liu, Y.; Sheng, J.; Yang, W.; et al. Hierarchically interfacial sulfide/phosphides achieve industry-level water electrolyzer in alkaline conditions at 3 A cm−2. Appl. Catal. B Environ. Energy 2025, 378, 125622. [Google Scholar] [CrossRef]
- Yan, X.; Yu, S.; Tang, Y.; Sun, D.; Xu, L.; Xue, C. Triangular AgAu/Pt core–shell nanoframes with a dendritic Pt shell and enhanced electrocatalytic performance toward the methanol oxidation reaction. Nanoscale 2018, 10, 2231–2235. [Google Scholar] [CrossRef]
- Qiu, X.; Li, T.; Deng, S.; Cen, K.; Xu, L.; Tang, Y. A general strategy for the synthesis of PtM (M=Fe, Co, Ni) decorated three-dimensional hollow graphene nanospheres for efficient methanol electrooxidation. Chem. A Eur. J. 2018, 24, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Cermenek, B.; Ranninger, J.; Feketeföldi, B.; Letofsky-Papst, I.; Kienzl, N.; Bitschnau, B.; Hacker, V. Novel highly active carbon supported ternary PdNiBi nanoparticles as anode catalyst for the alkaline direct ethanol fuel cell. Nano Res. 2019, 12, 683–693. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Lu, C.; Li, S.; Zhu, M. Molten salt technique for the synthesis of carbon-based materials for supercapacitors. Green Chem. 2023, 25, 10209–10234. [Google Scholar] [CrossRef]
- Zikhali, M.; Matthews, T.; Selepe, C.T.; Adegoke, K.A.; Mugadza, K.; Gwebu, S.S.; Maxakato, N.W. Electrocatalytic performance of Pd-based electro-catalysts supported on CNTs for isopropanol and ethanol electro-oxidation in alkaline medium. Mater. Today Chem. 2023, 30, 101484. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper(I) oxide catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- An, L.; Chen, R. Recent progress in alkaline direct ethylene glycol fuel cells for sustainable energy production. J. Power Sources 2016, 329, 484–501. [Google Scholar] [CrossRef]
- Ma, S.; Sadakiyo, M.; Luo, R.; Heima, M.; Yamauchi, M.; Kenis, P.J.A. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 2016, 301, 219–228. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Xiao, H.; Chen, W.; Zhao, M.; Jia, J. Shape-control of super-branched Pd–Cu alloys with enhanced electrocatalytic performance for ethylene glycol oxidation. Chem. Commun. 2018, 54, 13363–13366. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, G.; Zhao, Y.; Wu, L.; Wang, Y.; Song, Y.; Tian, P.; Wang, X. Controllable morphology of Pd-loaded potassium tantalates with high catalytic performance for ethylene glycol electrooxidation. Electrochim. Acta 2021, 376, 137978. [Google Scholar] [CrossRef]
- Bi, P.; Hong, W.; Shang, C.; Wang, J.; Wang, E. Bimetallic PdRu nanosponges with a tunable composition for ethylene glycol oxidation. RSC Adv. 2016, 6, 12486–12490. [Google Scholar] [CrossRef]
- Fu, X.; Wan, C.; Huang, Y.; Duan, X. Noble metal based electrocatalysts for alcohol oxidation reactions in alkaline media. Adv. Funct. Mater. 2022, 32, 2106401. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Saadawi, A.; Madih, K.; Hefny, R. Multiphase nickel-based carbon nanofibers for enhanced electrooxidation of urea and glycerol. Next Mater. 2025, 9, 101036. [Google Scholar] [CrossRef]
- Chowdhury, M.A.S.; Islam, M.M.; Jamal, M. Green synthesis of nickel oxide nanoparticles using Allium cepa stalks and investigation of their antibacterial activity. Results Chem. 2025, 16, 102328. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Wabaidur, S.M.; Islam, M.A.; Dhanusuraman, R.; Ponnusamy, V.K. Facile electrochemical fabrication of nickel-coated polydiphenylamine (Ni/PDPA) nanocomposite material as efficient anode catalyst for direct alcohol fuel cell application. Fuel 2022, 324 Part B, 124424. [Google Scholar] [CrossRef]
- Khatun, E.; Ramona, G.; Sobota, L.; Bondue, C.J.; Tschulik, K. Au-Ni rotating ring-disk electrode (RRDE) study: A new and rapid screening technique for quantitative detecting aldehyde during electrochemical alcohol oxidation reaction. Electrochim. Acta 2025, 519, 145803. [Google Scholar] [CrossRef]
- Guo, C.; Ren, X.; Guo, C.; Akram, N.; Mansha, M.S.; Ahmad, A.; Khawaja, S.; Wang, J. Fabrication of CdS/Ni-MOF bifunctional catalyst for the photocatalytic hydrogen evolution coupled with benzyl alcohol’s oxidation. J. Photochem. Photobiol. A Chem. 2025, 462, 116181. [Google Scholar] [CrossRef]
- Ma, M.; Yang, H.; Li, Q.; Ma, J.; Wu, W.; Zhang, Y.; Cao, X.; Huang, Y. Graphite encapsulated high active nickel-molybdenum/nickel oxide porous nanosheet composites as enhanced oxygen evolution reaction electrocatalysts. J. Alloys Compd. 2025, 1017, 179073. [Google Scholar] [CrossRef]
- Bera, A.; Mondal, A.; Maity, P.; Das, A.; Hati, G.; Khatua, B.B. The construction of a flexible solid-state asymmetric supercapacitor device using nickel-copper oxide nanoplates and carbon nanosphere serving as positive and negative electrode, respectively with excellent electrochemical capabilities. Mater. Sci. Eng. B 2024, 305, 117404. [Google Scholar] [CrossRef]
- Subedi, B.; Khatoon, N.; Gaire, M.; Majed, A.; He, J.; Zhang, X.; Chrisey, D.B. Rapid fabrication of binder free nickel cobalt oxide electrodes with dendritic nanostructure for electrochemical energy storage applications. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134251. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Muniratnam, K.; Shim, J. Nickel ferrite as an efficient electrocatalyst for alcohol oxidation reactions. Mater. Lett. 2025, 381, 137752. [Google Scholar] [CrossRef]
- Wan, J.; Mu, X.; Jin, Y.; Zhu, J.; Xiong, Y.; Li, T.; Li, R. Nitrogen-doped nickel-molybdenum oxide as a highly efficient electrocatalyst for benzyl alcohol oxidation. Green Chem. 2022, 24, 4870–4876. [Google Scholar] [CrossRef]
- Hedayatinasab, F.; Ghaffarinejad, A. Enhanced ethanol oxidation using nickel-tungsten alloy: Durable catalysts for direct ethanol fuel cells. J. Environ. Chem. Eng. 2025, 13, 115228. [Google Scholar] [CrossRef]
- Massaneiro, J.; Valério, T.L.; Pellosi, D.S.; da Silva, B.J.G.; Vidotti, M. Electrocatalytic oxidation of glycerol performed by nickel/cobalt alloys: Adding value to a common subproduct of chemical industry. Electrochim. Acta 2024, 506, 145013. [Google Scholar] [CrossRef]
- ElSayed, N.G.; Farghali, A.A.; El Rouby, W.M.A.; Hmamm, M.F.M. Silver decorated nickel oxide nanoflake/carbon nanotube nanocomposite as an efficient electrocatalyst for ethanol oxidation. Nanoscale Adv. 2024, 6, 5133–5144. [Google Scholar] [CrossRef] [PubMed]
- Abdel Hameed, R.M.; Abutaleb, A.; Zouli, N.; Yousef, A. Facile synthesis of electrospun transition metallic nanofibrous mats with outstanding activity for ethylene glycol electro-oxidation in alkaline solution. Mol. Catal. 2022, 522, 112186. [Google Scholar] [CrossRef]
- Suárez-Barajas, A.; Ramos-Castillo, C.M.; Olivas, A.; Guerra-Balcázar, M.; Álvarez-Contreras, L.; Arjona, N. Oxygen vacancy-enriched NiCo2O4 spinels/N-doped carbon nanotubes-graphene composites for the ethylene glycol electro-oxidation. Fuel 2024, 360, 130371. [Google Scholar] [CrossRef]
- Su, X.; Ren, J.; Meng, X.; Ren, X.; Tang, F. A novel platform for enhanced biosensing based on the synergy effects of electrospun polymer nanofibers and graphene oxides. Analyst 2013, 138, 1459–1466. [Google Scholar] [CrossRef]
- Kumar, G.G.; Kim, A.R.; Nahm, K.S.; Yoo, D.J. High proton conductivity and low fuel crossover of polyvinylidene fluoride–hexafluoro propylene–silica sulfuric acid composite membranes for direct methanol fuel cells. Curr. Appl. Phys. 2011, 11, 896–902. [Google Scholar] [CrossRef]
- Ali Shah, A.U.H.; Yasmeen, N.; Rahman, G.; Bilal, S. High electrocatalytic behavior of Ni impregnated conducting polymer coated platinum and graphite electrodes for electrooxidation of methanol. Electrochim. Acta 2017, 224, 468–474. [Google Scholar] [CrossRef]
- Kazim, H.; Sabri, M.; Al-Othman, A.; Tawalbeh, M. Functionalized conducting polymers in photocatalysis and opportunities for artificial intelligence applications. Nano-Struct. Nano-Objects 2024, 40, 101371. [Google Scholar] [CrossRef]
- Bharatish, A.; Kumar, A.; Siddhanth, K.S.; Manikant, V.; Jagdish, P.; Sharma, A.; Solaiachari, S. On optimizing wettability, surface roughness and swelling behaviour of laser-polished 3D-printed PETG polymer for bio-medical implants. Polymer 2025, 330, 128482. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.M.; Khalil, K.A.; Yousef, A.; Jeon, K.-S.; Kim, H.Y. Encapsulation of CoS nanoparticles in PAN electrospun nanofibers: Effective and reusable catalyst for ammonia borane hydrolysis and dyes photodegradation. Ceram. Int. 2013, 39, 1469–1476. [Google Scholar] [CrossRef]
- Mandal, B.P.; Vasundhara, K.; Abdelhamid, E.; Lawes, G.; Salunke, H.G.; Tyagi, A.K. Improvement of magnetodielectric coupling by surface functionalization of nickel nanoparticles in Ni and polyvinylidene fluoride nanohybrids. J. Phys. Chem. C 2014, 118, 20819–20825. [Google Scholar] [CrossRef]
- Kumar, R.V.; Mastai, Y.; Diamant, Y.; Gedanken, A. Sonochemical synthesis of amorphous Cu and nanocrystalline Cu2O embedded in a polyaniline matrix. J. Mater. Chem. 2001, 11, 1209–1213. [Google Scholar] [CrossRef]
- Nagashree, K.L.; Ahmed, M.F. Electrocatalytic oxidation of methanol on Ni modified polyaniline electrode in alkaline medium. J. Solid State Electrochem. 2010, 14, 2307–2320. [Google Scholar] [CrossRef]
- Salman, M.; Aslam, M.; Mustafa, Z.; Ahmad, M.; Shehzad, A. Flexible polypyrrole/polyvinyl alcohol/titanium dioxide/graphene quantum dots nanocomposite films for energy storage devices. Diam. Relat. Mater. 2025, 152, 111996. [Google Scholar] [CrossRef]
- Lei, Z.-L.; Wan, L.; Lü, Q.-F. Carbon nanotubes modified V-Ti3C2Tx/poly(3,4-ethylenedioxythiophene) composite as a high-performance electrode for supercapacitor. Diam. Relat. Mater. 2024, 150, 111696. [Google Scholar] [CrossRef]
- Sundararajan, P.B.; Muthukrishnan, F.P.; Murugesan, A.; Chuang, H.-C.; Pandiyarajan, S. Nitrogen-sulphur dual doped reduced graphene oxide-polydiphenylamine supported PdSn nanocomposite: A bimetallic electrocatalyst for methanol electro-oxidation in alkaline solution. Mater. Sci. Eng. B 2024, 304, 117363. [Google Scholar] [CrossRef]
- Herranz, D.; Escudero-Cid, R.; Montiel, M.; Palacio, C.; Fatás, E.; Ocón, P. Poly(vinyl alcohol) and poly(benzimidazole) blend membranes for high performance alkaline direct ethanol fuel cells. Renew. Energy 2018, 127, 883–895. [Google Scholar] [CrossRef]
- Wring, S.A.; Hart, J.P. Chemically modified, carbon-based electrodes and their application as electrochemical sensors for the analysis of biologically important compounds. A review. Analyst 1992, 117, 1215–1229. [Google Scholar] [CrossRef]
- Nguyen, N.S.; Das, G.; Yoon, H.H. Nickel/cobalt-decorated 3D graphene nanocomposite electrode for enhanced electrochemical detection of urea. Biosens. Bioelectron. 2016, 77, 372–377. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Chen, G.; Yao, S.; Cong, B. Construction double electric field of sulphur vacancies as medium ZnS/Bi2S3-PVDF self-supported recoverable piezoelectric film photocatalyst for enhanced photocatalytic performance. Appl. Catal. B Environ. 2022, 301, 120792. [Google Scholar] [CrossRef]
- Su, P.; Zhang, D.; Zhu, M.; Liang, T.; Yang, N.; Zhao, H.; Zhang, D.; Liu, J.; Cai, P.; Pu, X. Re-usable Cd0.9Zn0.1S-ZnO-C/PVDF piezo-photocatalytic film with exceptional hydrogen evolution capability triggered by the synergetic advantages of piezoelectricity and S-Scheme heterojunction. J. Energy Chem. 2024, 96, 164–176. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, F.; Xiao, F.; Zhang, G.; Hou, H.; Bi, J.; Yan, S.; Hao, H. Construction of PVDF-HKUST-1/BiVO4 hybrid electrospun flexible fiber membrane for enhanced piezo-photocatalytic reduction performance of Cr(VI). Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 134046. [Google Scholar] [CrossRef]
- Kumar, G.G. Irradiated PVdF-HFP-montmorillonite composite membranes for the application of direct ethanol fuel cells. J. Mater. Chem. 2011, 21, 17382–17391. [Google Scholar] [CrossRef]
- Kumar, G.G.; Nahm, K.S.; Elizabeth, R.N. Electro chemical properties of porous PVdF-HFP membranes prepared with different nonsolvents. J. Membr. Sci. 2008, 325, 117–124. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Babu, K.J.; Kumar, G.G.; Kim, A.R.; Yoo, D.J. Flexible electrospun PVdF-HFP/Ni/Co membranes for efficient and highly selective enzyme free glucose detection. Ind. Eng. Chem. Res. 2014, 53, 10347–10357. [Google Scholar] [CrossRef]
- Kumar, T.R.; Babu, K.J.; Yoo, D.J.; Kim, A.R.; Kumar, G.G. Binder free and free-standing electrospun membrane architecture for sensitive and selective non-enzymatic glucose sensors. RSC Adv. 2015, 5, 41457–41467. [Google Scholar] [CrossRef]
- Ren, H.; Labidi, A.; Sial, A.; Miao, Z.; Zhao, Y.; Feng, X.; Luo, J.; Wang, C. Fluid-induced piezoelectric field enhanced photocatalytic antibiotic removal over nitrogen-doped carbon quantum dots/Bi2WO6-polyvinylidene fluoride-co-hexafluoropropylene membrane in aqueous environments. J. Colloid Interface Sci. 2025, 691, 137412. [Google Scholar] [CrossRef]
- Li, J.; Du, L.; Guo, S.; Chang, J.; Wu, D.; Jiang, K.; Gao, Z. Molybdenum iron carbide-copper hybrid as efficient electrooxidation catalyst for oxygen evolution reaction and synthesis of cinnamaldehyde/benzalacetone. J. Colloid Interface Sci. 2024, 673, 616–627. [Google Scholar] [CrossRef]
- Castro, A.S.; de Azevedo, L.S.; Bruni, A.T.; de Menezes, M.M.T.; Dockal, E.R.; de Oliveira, O.V.; Ferreira, B.; de Oliveira, M.F. Cocaine electrooxidation behavior, mechanism, and kinetics on a carbon paste electrode chemically modified with a cobalt or copper Schiff base complex. Forensic Chem. 2021, 26, 100363. [Google Scholar] [CrossRef]
- Joshi, K.K.; Chauhan, S.V.; Pataniya, P.M.; Sumesh, C.K. Efficient hybrid water splitting and direct electrooxidation of organic dye from wastewater using copper cobalt sulphide nanosheets. Int. J. Hydrogen Energy 2024, 58, 1562–1575. [Google Scholar] [CrossRef]
- Chen, C.; Jin, L.; Shang, H.; Song, T.; Gao, F.; Zhang, Y.; Wang, C.; Wang, C.; Du, Y. Monodispersed bimetallic platinum-copper alloy nanospheres as efficient catalysts for ethylene glycol electrooxidation. J. Colloid Interface Sci. 2019, 551, 81–88. [Google Scholar] [CrossRef]
- Meng, K.; Fan, Z.; Wen, H.; Hu, Y.; Zhang, W.; Chen, Z. Construction of a Cu3+–OH–Pt interface for enhancing glycerol electrooxidation coupled with hydrogen evolution. Green Chem. 2025, 27, 5376–5387. [Google Scholar] [CrossRef]
- Sumbal, S.; Łuczak, J.; Hassan, A.; Lieder, M. Role of Cu and Ni in dual sites layered electrocatalysts for ammonia electrooxidation. Appl. Catal. A Gen. 2025, 696, 120181. [Google Scholar] [CrossRef]
- Han, M.; Wang, N.; Zhang, B.; Xia, Y.; Li, J.; Han, J.; Yao, K.; Gao, C.; He, C.; Liu, Y.; et al. High-valent nickel promoted by atomically embedded copper for efficient water oxidation. ACS Catal. 2020, 10, 9725–9734. [Google Scholar] [CrossRef]
- He, Q.; Yao, K.; Wang, X.; Xia, X.; Leng, S.; Li, F. Room-temperature and solution-processable Cu-doped nickel oxide nanoparticles for efficient hole-transport layers of flexible large-area perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 41887–41897. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Cao, H.; Meng, W.; Wang, Q.; Bi, Y.; Liang, X.; Yang, H.; Zhang, L.; Lang, M.-F.; Sun, J. Nickel-copper oxide nanoflowers for highly efficient glucose electrooxidation. Int. J. Hydrogen Energy 2021, 46, 28527–28536. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Q.; Tong, Y.; Zhou, G.; Lin, C.; Zhao, Y.; Chen, P. Nickel-copper alloying arrays realizing efficient co-electrosynthesis of adipic acid and hydrogen. J. Energy Chem. 2025, 101, 7–15. [Google Scholar] [CrossRef]
- Chemchoub, S.; El Attar, A.; Oularbi, L.; Younssi, S.A.; Bentiss, F.; Jama, C.; El Rhazi, M. Electrosynthesis of eco-friendly electrocatalyst based nickel-copper bimetallic nanoparticles supported on poly-phenylenediamine with highest current density and early ethanol oxidation onset potential. Int. J. Hydrogen Energy 2022, 47, 39081–39096. [Google Scholar] [CrossRef]
- Gibson, P.; Schreuder-Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 469–481. [Google Scholar] [CrossRef]
- Kumar, G.G.; Shin, J.; Nho, Y.-C.; Hwang, I.S.; Fei, G.; Kim, A.R.; Nahm, K.S. Irradiated PVdF-HFP–tin oxide composite membranes for the applications of direct methanol fuel cells. J. Membr. Sci. 2010, 350, 92–100. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Wang, Y. Preparation of hollow poly(vinylidene fluoride) capsules containing nickel catalyst for hydrogen storage and production. Int. J. Energy Res. 2015, 39, 634–642. [Google Scholar] [CrossRef]
- Babu, K.J.; Senthilkumar, N.; Kim, A.R.; Kumar, G.G. Freestanding and binder free PVdF-HFP/Ni-Co nanofiber membrane as a versatile platform for the electrocatalytic oxidation and non-enzymatic detection of urea. Sens. Actuators B Chem. 2017, 241, 541–551. [Google Scholar] [CrossRef]
- Nath, A.K.; Kumar, A. Swift heavy ion irradiation induced enhancement in electrochemical properties of ionic liquid based PVdF-HFP-layered silicate nanocomposite electrolyte membranes. J. Membr. Sci. 2014, 453, 192–201. [Google Scholar] [CrossRef]
- Ozay, O.; Aktas, N.; Inger, E.; Sahiner, N. Hydrogel assisted nickel nanoparticle synthesis and their use in hydrogen production from sodium boron hydride. Int. J. Hydrogen Energy 2011, 36, 1998–2006. [Google Scholar] [CrossRef]
- Abutaleb, A. CuNi alloy NPs anchored on electrospun PVDF-HFP NFs catalyst for H2 production from sodium borohydride. Polymers 2023, 15, 474. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lv, C.; Zeng, N.; Liu, A.; Liu, Y.; Hu, L.; Li, P.; Yao, Y.; Cai, J.; Tang, T. Recent advances in Ni-based electrocatalysts for hydrogen evolution reaction. Energy Technol. 2023, 11, 2201048. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Guo, H.; Cheng, X.; Huang, Y.; Liu, C.; Zang, J.; Dong, L. Ni-based electrocatalysts for urea oxidation reaction: Mechanistic insights and recent advancements. J. Alloys Compd. 2024, 1008, 176591. [Google Scholar] [CrossRef]
- Abbas, M.; Abdel Hameed, R.M.; Al-Enizi, A.M.; Thamer, B.M.; Yousef, A.; El-Newehy, M.H. Decorated carbon nanofibers with mixed nickel–manganese carbides for methanol electro-oxidation in alkaline solution. Int. J. Hydrogen Energy 2021, 46, 6494–6512. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Hua, X.; Zhang, R. Methanol electrooxidation on glassy carbon electrode modified with bimetallic Ni(II)Co(II) salen complexes encapsulated in mesoporous zeolite A. Electrochim. Acta 2015, 163, 48–56. [Google Scholar] [CrossRef]
- Rostami, T.; Jafarian, M.; Miandari, S.; Mahjani, M.G.; Gobal, F. Synergistic effect of cobalt and copper on a nickel-based modified graphite electrode during methanol electro-oxidation in NaOH solution. Chin. J. Catal. 2015, 36, 1867–1874. [Google Scholar] [CrossRef]
- Rahmani, K.; Habibi, B. NiCo alloy nanoparticles electrodeposited on an electrochemically reduced nitrogen-doped graphene oxide/carbon-ceramic electrode: A low cost electrocatalyst towards methanol and ethanol oxidation. RSC Adv. 2019, 9, 34050–34064. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Wala, M.; Blacha-Grzechnik, A.; Stolarczyk, A.; Maciej, A.; Simka, W. Electrocatalytic methanol oxidation using Ni–Co–graphene composite electrodes. Int. J. Hydrogen Energy 2021, 46, 33272–33286. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Zhang, J.; Tse, Y.-H.; Pietro, W.J.; Lever, A.B.P. Electrocatalytic activity of N, N′, N′′, N′′′-tetramethyl-tetra-3,4-pyridoporphyrazinocobalt(II) adsorbed on a graphite electrode towards the oxidation of hydrazine and hydroxylamine. J. Electroanal. Chem. 1996, 406, 203–211. [Google Scholar] [CrossRef]
- Dantas, L.M.F.; dos Reis, A.P.; Tanaka, S.M.C.N.; Zagal, J.H.; Chen, Y.-Y.; Tanaka, A.A. Electrocatalytic oxidation of hydrazine in alkaline media promoted by iron tetrapyridinoporphyrazine adsorbed on graphite surface. J. Braz. Chem. Soc. 2008, 19, 720–726. [Google Scholar] [CrossRef]
- Abdel Hameed, R.M.; Medany, S.S. Influence of support material on the electrocatalytic activity of nickel oxide nanoparticles for urea electro-oxidation reaction. J. Colloid Interface Sci. 2018, 513, 536–548. [Google Scholar] [CrossRef]
- Afify, D.G.; Abdel Hameed, R.M.; Mohamed, A.M.; Ghayad, I.M. Outstanding activity and durability of supported NiCo2O4 nanoparticles for direct ethanol fuel cells. Appl. Organomet. Chem. 2025, 39, e7802. [Google Scholar] [CrossRef]
- Rodriguez, J.R.; Fuentes-Moyado, S.; Zepeda, T.A.; Díaz de León, J.N.; Cruz-Reyes, J.; Oropeza-Guzman, M.T.; Berhault, G.; Alonso-Núňez, G. Methanol electro-oxidation with alloy nanoparticles of Pt10−x–Fex supported on CNTs. Fuel 2016, 182, 1–7. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, Y.; Yang, X.; Yuan, Q. Au enables PdAu aerogel efficient catalysts for oxygen reduction and C2 alcohol oxidation. Int. J. Hydrogen Energy 2024, 78, 443–451. [Google Scholar] [CrossRef]
- Lao, X.; Li, Z.; Yang, L.; Zhang, B.; Ye, W.; Fu, A.; Guo, P. Monodispersed ultrathin twisty PdBi alloys nanowires assemblies with tensile strain enhance C2+ alcohols electrooxidation. J. Energy Chem. 2023, 79, 279–290. [Google Scholar] [CrossRef]
- El-Shafei, A.A. Electrocatalytic oxidation of methanol at a nickel hydroxide/glassy carbon modified electrode in alkaline medium. J. Electroanal. Chem. 1999, 471, 89–95. [Google Scholar] [CrossRef]
- Sun, H.; Ye, Y.; Liu, J.; Tian, Z.; Cai, Y.; Li, P.; Liang, C. Pure Ni nanocrystallines anchored on rGO present ultrahigh electrocatalytic activity and stability in methanol oxidation. Chem. Commun. 2018, 54, 1563–1566. [Google Scholar] [CrossRef]
- Afify, D.G.; Abdel Hameed, R.M.; Ghayad, I.M. Binary NiCu oxide nanoparticles onto graphite as promoting nanocatalysts for ethanol electro-oxidation process. Appl. Organomet. Chem. 2024, 38, e7418. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Abdel Hameed, R.M.; Al-Enizi, A.M.; Karim, A.; Yousef, A. Free-standing and binder-free nickel polymeric nanofiber membrane for electrocatalytic oxidation of ethanol in alkaline solution. J. Mater. Res. Technol. 2022, 21, 4591–4606. [Google Scholar] [CrossRef]
- Maritan, A.; Toigo, F. On skewed ARC plots of impedance of electrodes with an irreversible electrode process. Electrochim. Acta 1990, 35, 141–145. [Google Scholar] [CrossRef]
- Mao, Y.-H.; Chen, C.-Y.; Fu, J.-X.; Lai, T.-Y.; Lu, F.-H.; Tsai, Y.-C. Electrodeposition of nickel-copper on titanium nitride for methanol electrooxidation. Surf. Coat. Technol. 2018, 350, 949–953. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Rezaee, S. Vertically standing Cu2O nanosheets promoted flower-like PtPd nanostructures supported on reduced graphene oxide for methanol electro-oxidation. Electrochim. Acta 2018, 259, 36–47. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P. Superior catalytic performance of NiCo2O4 nanorods loaded rGO towards methanol electro-oxidation and hydrogen evolution reaction. J. Mol. Liq. 2019, 291, 111306. [Google Scholar] [CrossRef]
- Sheikhi, S.; Jalali, F. Hierarchical NiCo2O4/CuO-C nanocomposite derived from copper-based metal organic framework and Ni/Co hydroxides: Excellent electrocatalytic activity towards methanol oxidation. J. Alloys Compd. 2022, 907, 164510. [Google Scholar] [CrossRef]




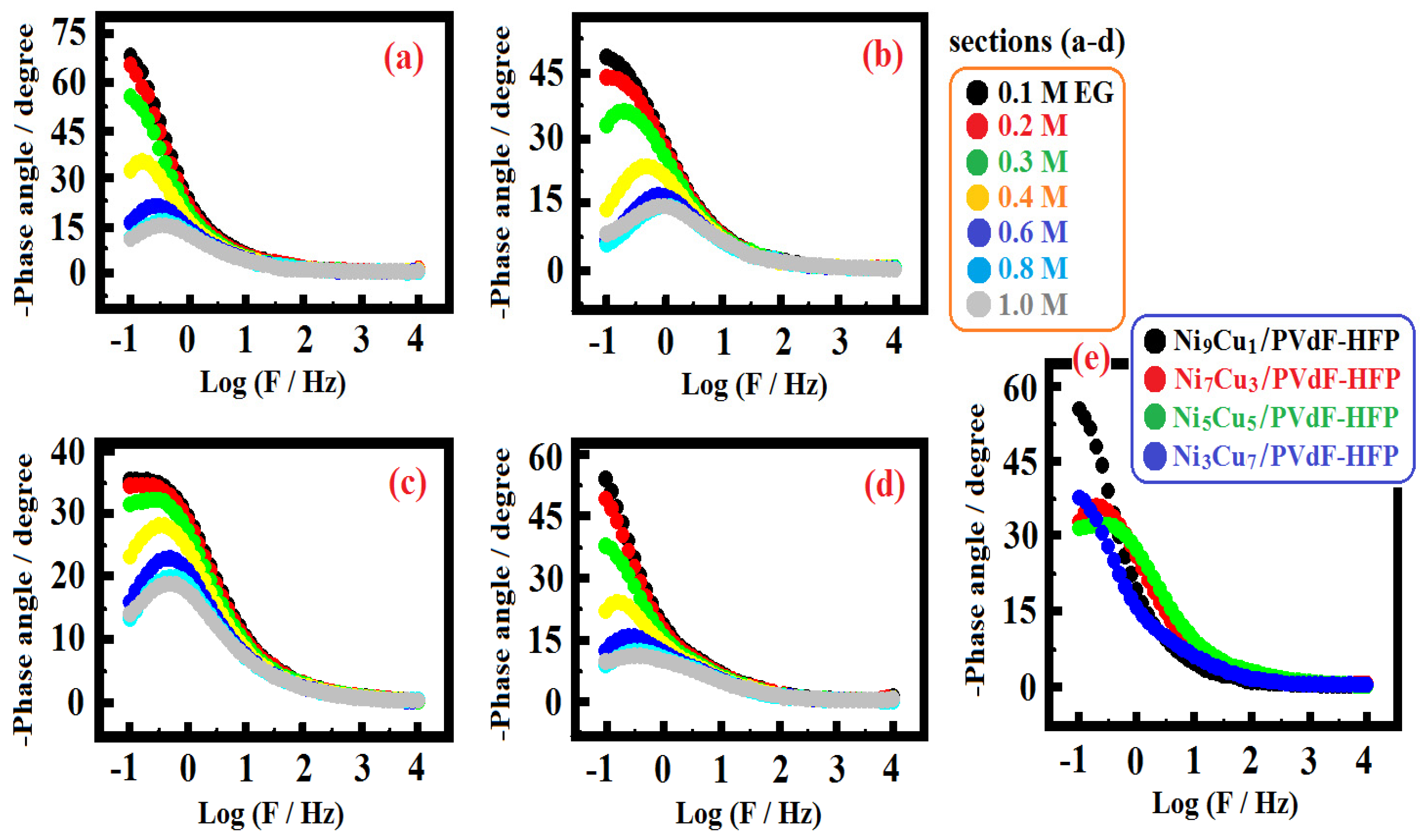

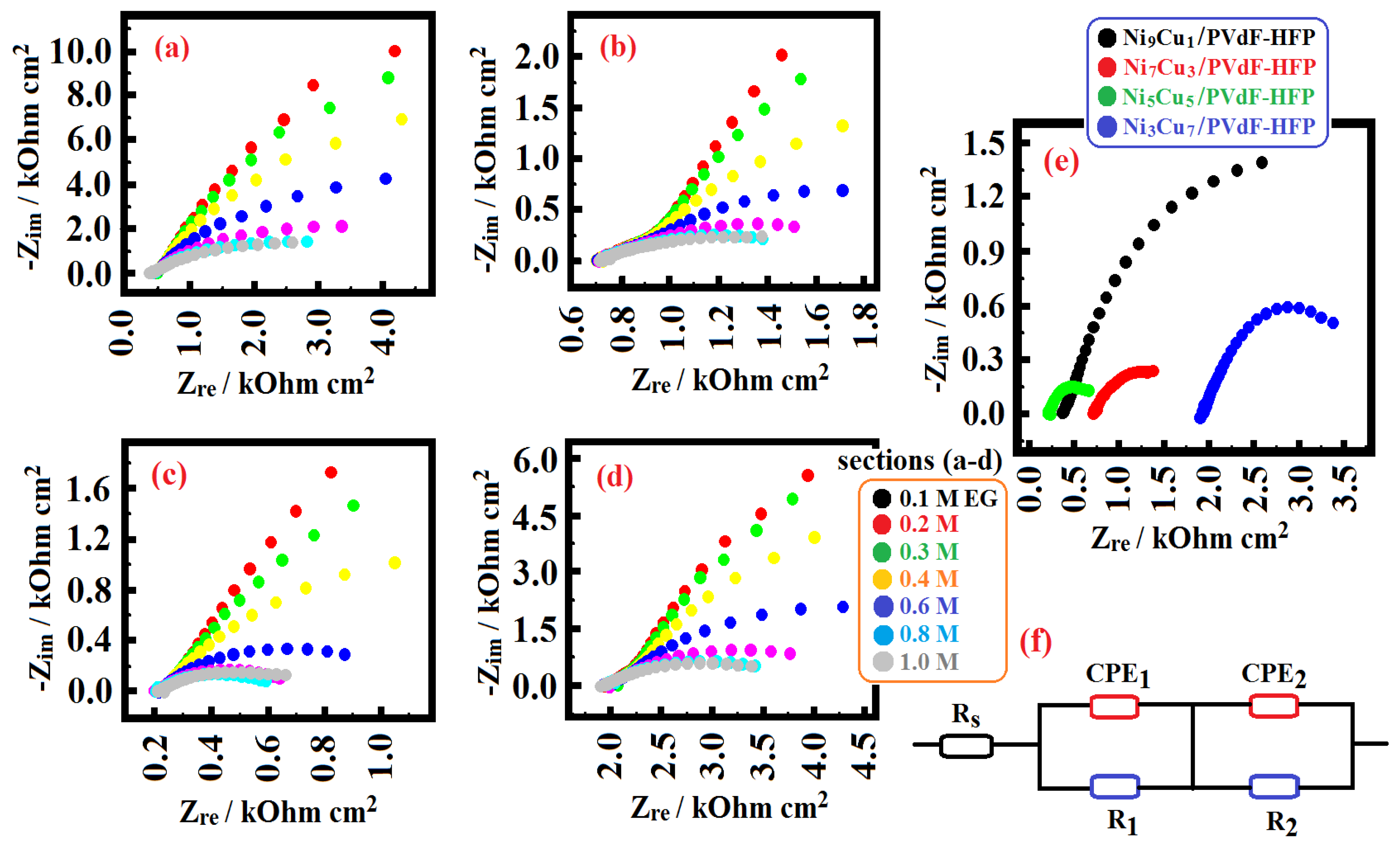

| Ethylene Glycol Concentration/M | Rs/ Ohm | Yo1/ Ohm−1 sa cm−2 × 10−3 | a1 | R1/ Ohm cm2 | Yo2/ Ohm−1 sa cm−2 × 10−4 | a2 | R2/ Ohm cm2 |
|---|---|---|---|---|---|---|---|
| (a) | |||||||
| 0.1 | 649.0 | 1.000 | 0.940 | 370.0 | 1.082 | 0.593 | 8478.0 |
| 0.2 | 597.0 | 1.519 | 0.992 | 384.4 | 1.106 | 0.579 | 8028.0 |
| 0.3 | 653.0 | 1.990 | 0.822 | 380.0 | 1.115 | 0.702 | 7661.0 |
| 0.4 | 624.8 | 2.553 | 0.820 | 374.6 | 1.125 | 0.701 | 7220.0 |
| 0.6 | 646.8 | 3.495 | 0.899 | 363.4 | 1.138 | 0.684 | 5774.0 |
| 0.8 | 623.1 | 4.584 | 0.947 | 366.2 | 1.160 | 0.692 | 4752.0 |
| 1.0 | 618.4 | 5.389 | 0.974 | 368.8 | 1.200 | 0.625 | 4230.0 |
| (b) | |||||||
| 0.1 | 1463.1 | 1.432 | 0.825 | 134.3 | 1.526 | 0.470 | 4487.0 |
| 0.2 | 1461.7 | 2.120 | 0.852 | 137.7 | 1.815 | 0.340 | 3490.0 |
| 0.3 | 1478.5 | 2.991 | 0.852 | 158.5 | 2.366 | 0.457 | 2890.0 |
| 0.4 | 1460.1 | 3.851 | 0.866 | 141.4 | 3.115 | 0.593 | 1926.0 |
| 0.6 | 1438.2 | 5.793 | 0.875 | 143.4 | 4.128 | 0.644 | 1206.0 |
| 0.8 | 1420.3 | 7.455 | 0.882 | 157.7 | 5.190 | 0.564 | 1062.0 |
| 1.0 | 1406.0 | 9.512 | 0.883 | 160.4 | 6.107 | 0.500 | 1056.0 |
| (c) | |||||||
| 0.1 | 412.7 | 3.794 | 0.940 | 79.0 | 2.594 | 0.485 | 1900.0 |
| 0.2 | 409.5 | 4.536 | 0.918 | 72.0 | 2.905 | 0.489 | 1543.0 |
| 0.3 | 407.1 | 5.146 | 0.915 | 67.4 | 3.282 | 0.520 | 1180.0 |
| 0.4 | 404.7 | 5.882 | 0.895 | 74.5 | 3.854 | 0.510 | 894.5 |
| 0.6 | 404.0 | 7.171 | 0.896 | 69.7 | 4.818 | 0.568 | 522.2 |
| 0.8 | 405.4 | 8.526 | 0.836 | 59.8 | 5.828 | 0.514 | 408.1 |
| 1.0 | 410.9 | 10.366 | 0.853 | 52.6 | 6.600 | 0.489 | 392.6 |
| (d) | |||||||
| 0.1 | 3906.0 | 1.208 | 0.918 | 400.7 | 1.309 | 0.672 | 5158.0 |
| 0.2 | 3912.0 | 1.758 | 0.946 | 373.5 | 1.338 | 0.544 | 4598.0 |
| 0.3 | 3960.0 | 2.410 | 0.937 | 357.4 | 1.381 | 0.633 | 4062.0 |
| 0.4 | 3960.0 | 2.998 | 0.999 | 337.6 | 1.486 | 0.685 | 3680.0 |
| 0.6 | 3916.0 | 4.468 | 0.999 | 384.8 | 1.538 | 0.621 | 2970.0 |
| 0.8 | 3872.0 | 5.543 | 1.000 | 331.6 | 1.725 | 0.589 | 2262.0 |
| 1.0 | 3878.0 | 6.834 | 1.000 | 364.2 | 1.875 | 0.665 | 1954.0 |
| Nanomaterial | Rs/ Ohm | Yo1/ Ohm−1 sa cm−2 × 10−3 | a1 | R1/ Ohm cm2 | Yo2/ Ohm−1 sa cm−2 × 10−4 | a2 | R2/ Ohm cm2 |
|---|---|---|---|---|---|---|---|
| Ni9Cu1/PVdF–HFP | 618.4 | 5.389 | 0.974 | 368.8 | 1.200 | 0.625 | 4230.0 |
| Ni7Cu3/PVdF–HFP | 1406.0 | 9.512 | 0.883 | 160.4 | 6.107 | 0.500 | 1056.0 |
| Ni5Cu5/PVdF–HFP | 410.9 | 10.366 | 0.853 | 52.6 | 6.600 | 0.489 | 392.6 |
| Ni3Cu7/PVdF–HFP | 3878.0 | 6.834 | 1.000 | 364.2 | 1.875 | 0.665 | 1954.0 |
| E/mV | Rs/ Ohm | Yo1/ Ohm−1 sa cm−2 × 10−3 | a1 | R1/ Ohm cm2 | Yo2/ Ohm−1 sa cm−2 × 10−4 | a2 | R2/ Ohm cm2 |
|---|---|---|---|---|---|---|---|
| 350 | 388.4 | 3.199 | 0.835 | 59.3 | 1.603 | 0.570 | 1976.0 |
| 400 | 377.0 | 4.396 | 0.879 | 71.1 | 2.054 | 0.508 | 1644.0 |
| 450 | 378.8 | 4.756 | 0.906 | 56.5 | 2.340 | 0.565 | 1536.0 |
| 500 | 379.6 | 5.002 | 0.932 | 58.0 | 2.654 | 0.534 | 1318.0 |
| 550 | 382.4 | 5.076 | 0.932 | 73.5 | 3.130 | 0.511 | 1249.0 |
| 600 | 418.4 | 5.277 | 0.937 | 58.3 | 3.438 | 0.574 | 1158.0 |
| 650 | 423.2 | 5.557 | 0.922 | 74.0 | 3.554 | 0.517 | 1088.0 |
| 700 | 424.2 | 5.664 | 0.924 | 60.8 | 3.627 | 0.533 | 1048.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, A.; Abdel Hameed, R.M.; Maafa, I.M.; Abutaleb, A. Synthesis of NiCu–Polymeric Membranes for Electro-Oxidizing Ethylene Glycol Molecules in Alkaline Medium. Catalysts 2025, 15, 959. https://doi.org/10.3390/catal15100959
Yousef A, Abdel Hameed RM, Maafa IM, Abutaleb A. Synthesis of NiCu–Polymeric Membranes for Electro-Oxidizing Ethylene Glycol Molecules in Alkaline Medium. Catalysts. 2025; 15(10):959. https://doi.org/10.3390/catal15100959
Chicago/Turabian StyleYousef, Ayman, R. M. Abdel Hameed, Ibrahim M. Maafa, and Ahmed Abutaleb. 2025. "Synthesis of NiCu–Polymeric Membranes for Electro-Oxidizing Ethylene Glycol Molecules in Alkaline Medium" Catalysts 15, no. 10: 959. https://doi.org/10.3390/catal15100959
APA StyleYousef, A., Abdel Hameed, R. M., Maafa, I. M., & Abutaleb, A. (2025). Synthesis of NiCu–Polymeric Membranes for Electro-Oxidizing Ethylene Glycol Molecules in Alkaline Medium. Catalysts, 15(10), 959. https://doi.org/10.3390/catal15100959








