The Key Role of Carbon Materials in the Biological and Photocatalytic Reduction of Nitrates for the Sustainable Management of Wastewaters
Abstract
1. Introduction
2. Results and Discussion
2.1. Biological Treatment
2.1.1. Materials Characterization
2.1.2. Performance of Adhered Biofilms
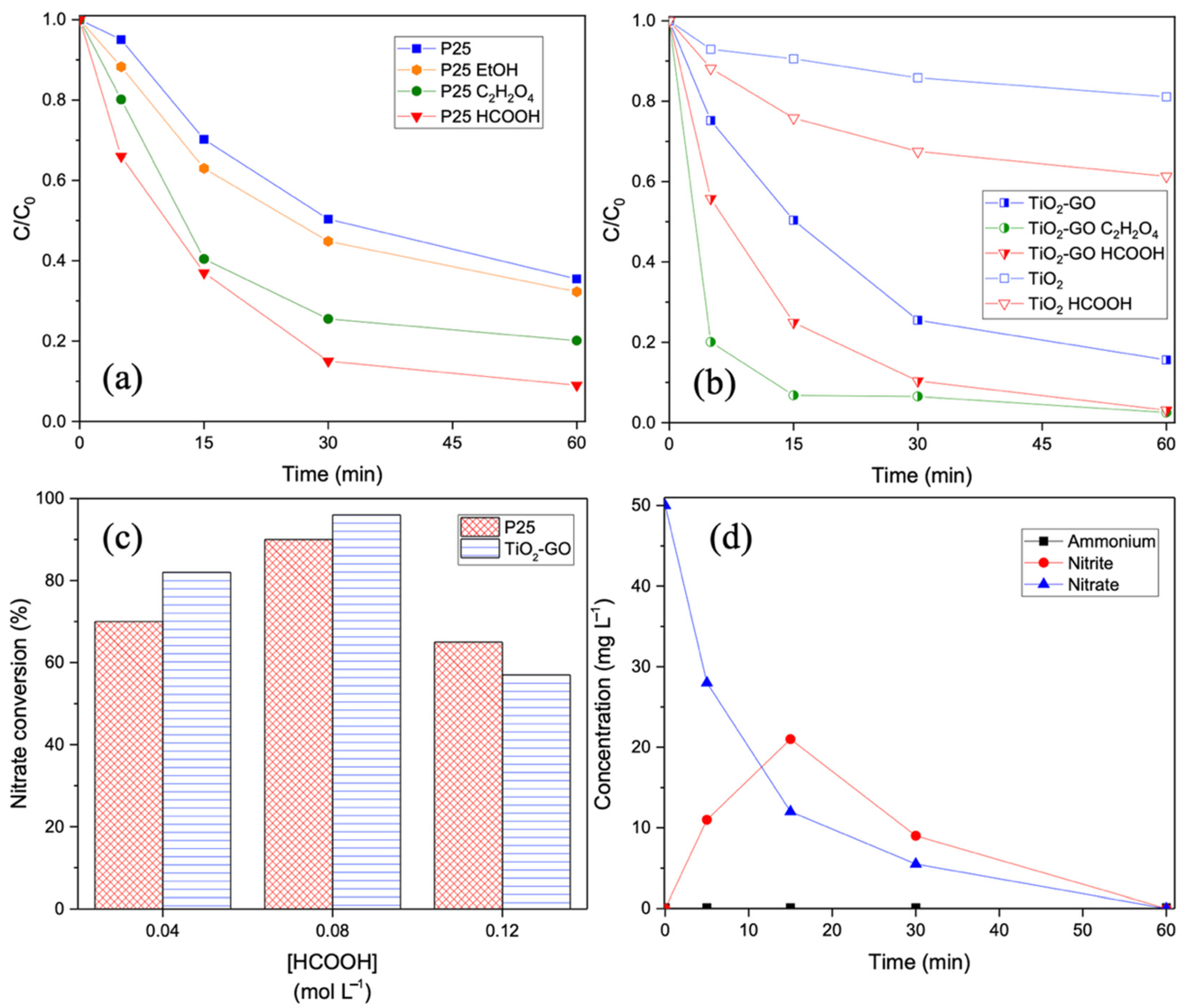
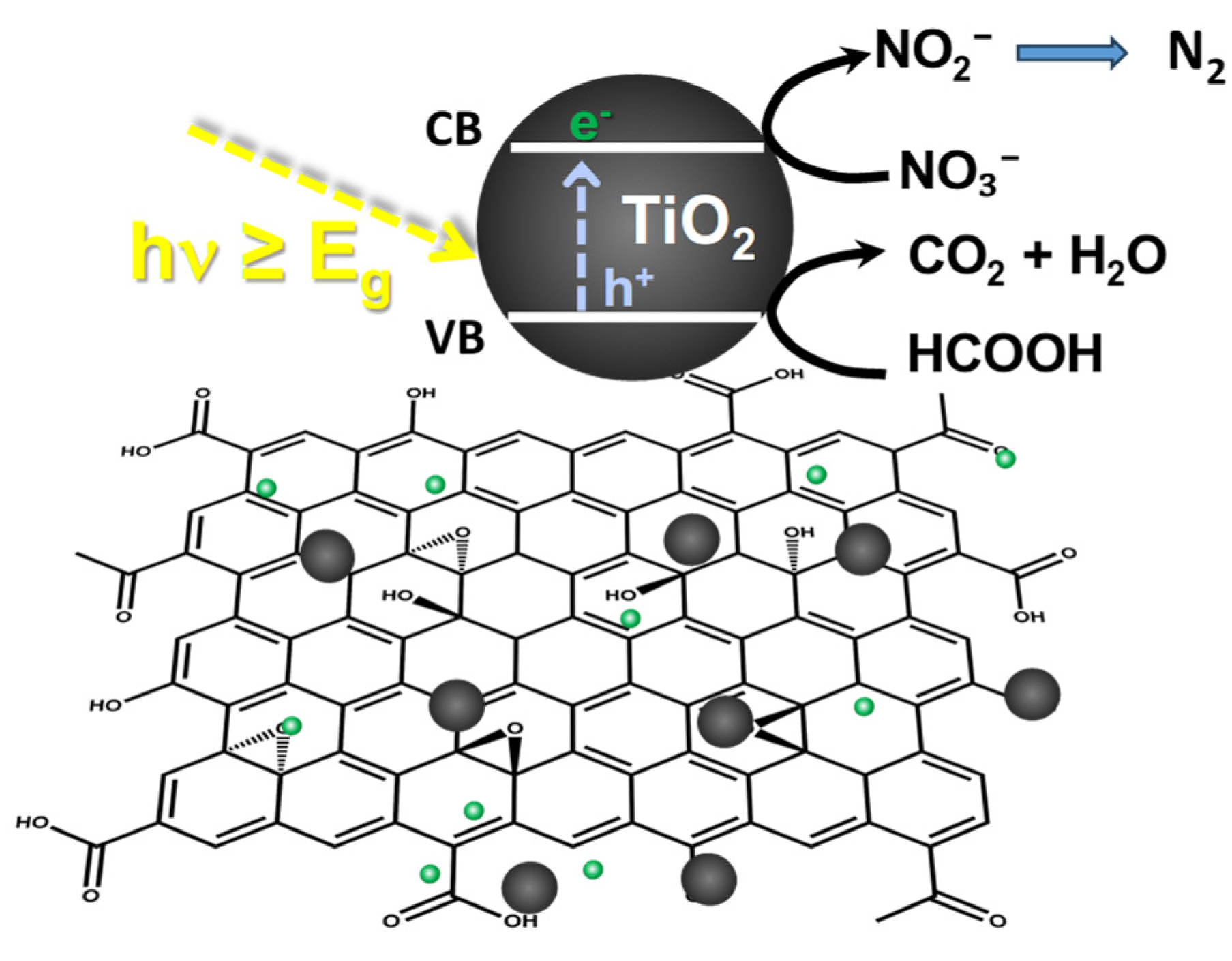
2.2. Photocatalytic Reduction of Nitrates
2.2.1. Catalyst Characterization
2.2.2. Photocatalytic Performance of the Samples
2.2.3. Influence of pH
2.2.4. Effect of Hole Scavengers
3. Materials and Methods
3.1. Synthesis of Carbon Materials and TiO2–GO Composites
3.2. Characterization Techniques
3.3. Nitrate Removal
3.3.1. Biological Experiments
3.3.2. Photocatalytic Reduction of Nitrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Jakobsen, F.; Ferrera, I.; Yebra, L.; Mercado, J.M. Two Decades of Satellite Surface Chlorophyll a Concentration (1998–2019) in the Spanish Mediterranean Marine Waters (Western Mediterranean Sea): Trends, Phenology and Eutrophication Assessment. Remote Sens. Appl. Soc. Environ. 2022, 28, 100855. [Google Scholar] [CrossRef]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 27 August 2025).
- Zhang, T.; Xu, Q.; Liu, X.; Lei, Q.; Luo, J.; An, M.; Du, X.; Qiu, W.; Zhang, X.; Wang, F.; et al. Sources, Fate and Influencing Factors of Nitrate in Farmland Drainage Ditches of the Irrigation Area. J. Environ. Manag. 2024, 367, 122113. [Google Scholar] [CrossRef]
- Ateunkeng, J.G.; Boum, A.T.; Bitjoka, L. A Binary-Level Hybrid Intelligent Control Configuration for Sustainable Energy Consumption in an Activated Sludge Biological Wastewater Treatment Plant. J. Water Process Eng. 2024, 65, 105902. [Google Scholar] [CrossRef]
- Zhu, G.-C.; Lu, Y.-Z.; Xu, L.-R. Effects of the Carbon/Nitrogen (C/N) Ratio on a System Coupling Simultaneous Nitrification and Denitrification (SND) and Denitrifying Phosphorus Removal (DPR). Environ. Technol. 2021, 42, 3048–3054. [Google Scholar] [CrossRef]
- Lago, A.; Rocha, V.; Barros, O.; Silva, B.; Tavares, T. Bacterial Biofilm Attachment to Sustainable Carriers as a Clean-up Strategy for Wastewater Treatment: A Review. J. Water Process Eng. 2024, 63, 105368. [Google Scholar] [CrossRef]
- Ardiati, F.C.; Anita, S.H.; Nurhayat, O.D.; Chempaka, R.M.; Yanto, D.H.Y.; Watanabe, T.; Wilén, B.-M. Evaluation of Batch and Fed-Batch Rotating Drum Biological Contactor Using Immobilized Trametes Hirsuta EDN082 for Non-Sterile Real Textile Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 113241. [Google Scholar] [CrossRef]
- Barrabés, N.; Sá, J. Catalytic Nitrate Removal from Water, Past, Present and Future Perspectives. Appl. Catal. B Environ. 2011, 104, 1–5. [Google Scholar] [CrossRef]
- Cai, W.; Chen, C.; Bao, C.; Gu, J.; Li, K.; Jia, J. Nitrate Reduction to Nitrogen in Wastewater Using Mesoporous Carbon Encapsulated Pd–Cu Nanoparticles Combined with in-Situ Electrochemical Hydrogen Evolution. J. Environ. Manag. 2024, 362, 121346. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Domen, K.; Maruya, K.; Onishi, T. Photocatalytic Reduction of NO3− to Form NH3 over Pt–TiO2. Chem. Lett. 1987, 16, 1019–1022. [Google Scholar] [CrossRef]
- Pintar, A.; Šetinc, M.; Levec, J. Hardness and Salt Effects on Catalytic Hydrogenation of Aqueous Nitrate Solutions. J. Catal. 1998, 174, 72–87. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Faria, J.L.; Silva, C.G. Photocatalytic Nitrate Reduction over Pd–Cu/TiO2. Chem. Eng. J. 2014, 251, 123–130. [Google Scholar] [CrossRef]
- Bahadori, E.; Compagnoni, M.; Tripodi, A.; Freyria, F.; Armandi, M.; Bonelli, B.; Ramis, G.; Rossetti, I. Photoreduction of Nitrates from Waste and Drinking Water. Mater. Today: Proc. 2018, 5, 17404–17413. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Zhang, Z. TiO2-Based Catalysts for Photocatalytic Reduction of Aqueous Oxyanions: State-of-the-Art and Future Prospects. Environ. Int. 2020, 136, 105453. [Google Scholar] [CrossRef]
- Qin, J.; Liu, N.; Wei, Y.; Lu, Y.; Huang, Y.; Zhao, Q.; Ye, Z. The Mechanism of Efficient Photoreduction Nitrate over Anatase TiO2 in Simulated Sunlight. Chemosphere 2022, 307, 135921. [Google Scholar] [CrossRef]
- Shaban, Y.A.; El Maradny, A.A.; Al Farawati, R.K. Photocatalytic Reduction of Nitrate in Seawater Using C/TiO2 Nanoparticles. J. Photochem. Photobiol. A Chem. 2016, 328, 114–121. [Google Scholar] [CrossRef]
- Li, H.; Xue, S.; Cao, F.; Gao, C.; Wei, Q.; Li, R.; Zhou, A.; Wang, S.; Yue, X. Enhanced Nitrate Reduction by Metal Deposited g-C3N4/RGO/TiO2 Z-Schematic Photocatalysts: Performance and Mechanism Comparison of Pd-Cu and Ag. Chemosphere 2023, 325, 138336. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chen, R.; Xu, S.; Xia, X.; Zhao, F.; Ren, X.; Lu, Y.; Gao, L.; Bao, J.; Liu, A. Efficient Co and GO Co-Doped TiO2 Catalysts for the Electrochemical Reduction of Nitrate to Ammonia. Catal. Sci. Technol. 2025, 15, 1445–1455. [Google Scholar] [CrossRef]
- Venu Sreekala, S.; George, J.; Thoppil Ramakrishnan, R.; Puthenveedu Sadasivan Pillai, H. Novel Ternary Nanocomposite (TiO2@Fe3O4-Chitosan) System for Nitrate Removal from Water: An Adsorption Cum Photocatalytic Approach. Environ. Sci. Pollut. Res. 2024, 31, 50670–50685. [Google Scholar] [CrossRef]
- Shi, Y.; Xie, Y.; Xia, J.; Zhang, X.; Cheng, H.; Chen, J. Photo-Catalytic Reduction of Nitrate by Ag-TiO2/Formic Acid Under Visible Light: Selectivity of Nitrogen and Mechanism. Water 2025, 17, 155. [Google Scholar] [CrossRef]
- Bouteraa, M.; Panico, A.; Zamouche-Zerdazi, R.; Bencheikh-Lehocine, M.; Derbal, K.; Crispino, G.; Gisonni, C.; Ferraro, A.; Pirozzi, F. Moving Bed Biofilm Reactor Combined with an Activated Carbon Filter for Biological Nitrate Removal. Int. J. Chem. React. Eng. 2023, 21, 1241–1253. [Google Scholar] [CrossRef]
- Yang, H.; Tan, S.; Huang, Y.; Tang, X. Enhanced Nitrate Nitrogen Removal from Constructed Wetland via Fe3O4/Granular Activated Carbon Anode Microbial Electrolysis Cell under Low C/N Ratio. Water 2024, 16, 1377. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F. Textural and Mechanical Characteristics of Carbon Aerogels Synthesized by Polymerization of Resorcinol and Formaldehyde Using Alkali Carbonates as Basification Agents. Phys. Chem. Chem. Phys. 2010, 12, 10365. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Ritter, J.A. Effect of Synthesis PH on the Structure of Carbon Xerogels. Carbon 1997, 35, 1271–1278. [Google Scholar] [CrossRef]
- Karanasios, K.A.; Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. Hydrogenotrophic Denitrification of Potable Water: A Review. J. Hazard. Mater. 2010, 180, 20–37. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular Electron Transfer via Microbial Nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- van Loosdrecht, M.C.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J. Electrophoretic Mobility and Hydrophobicity as a Measured to Predict the Initial Steps of Bacterial Adhesion. Appl. Environ. Microbiol. 1987, 53, 1898–1901. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Physicochemical Regulation of Biofilm Formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef]
- Bautista-Toledo, M.I.; Maldonado-Hódar, F.J.; Morales-Torres, S.; Pastrana-Martínez, L.M. Supported Biofilms on Carbon–Oxide Composites for Nitrate Reduction in Agricultural Waste Water. Molecules 2021, 26, 2987. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J. Advances in the Development of Nanostructured Catalysts Based on Carbon Gels. Catal. Today 2013, 218–219, 43–50. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J.; Morales-Torres, S.; Ribeiro, F.; Silva, E.R.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Oliveira, F.A.C. Development of Carbon Coatings for Cordierite Foams: An Alternative to Cordierite Honeycombs. Langmuir 2008, 24, 3267–3273. [Google Scholar] [CrossRef]
- Paritosh, K.; Kesharwani, N. Biochar Mediated High-Rate Anaerobic Bioreactors: A Critical Review on High-Strength Wastewater Treatment and Management. J. Environ. Manag. 2024, 355, 120348. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Su, J.; Wang, J.; Ali, A.; Qian, K.; Wu, X. Modified Biochar Improved Simultaneous Nitrate Removal and Soluble Microbial Products Regulation in Low Carbon Wastewater: Insights from the Biocarrier and Community Function. J. Water Process Eng. 2024, 65, 105762. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M.T. Advanced Nanostructured Photocatalysts Based on Reduced Graphene Oxide–TiO2 Composites for Degradation of Diphenhydramine Pharmaceutical and Methyl Orange Dye. Appl. Catal. B Environ. 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Silva, C.G.; Faria, J.L. Photocatalytic Oxidation of Benzene Derivatives in Aqueous Suspensions: Synergic Effect Induced by the Introduction of Carbon Nanotubes in a TiO2 Matrix. Appl. Catal. B Environ. 2010, 101, 81–89. [Google Scholar] [CrossRef]
- Pérez-Poyatos, L.T.; Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hódar, F.J. Novel Strategies to Develop Efficient Carbon/TiO2 Photocatalysts for the Total Mineralization of VOCs in Air Flows: Improved Synergism between Phases by Mobile N-, O- and S-Functional Groups. Chem. Eng. J. 2025, 508, 160986. [Google Scholar] [CrossRef]
- Pérez-Molina, Á.; Morales-Torres, S.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Functionalization of Graphitic Carbon Nitride/ZnO Heterojunctions with Zinc Cyanamide Groups: A Powerful Approach for Photocatalytic Degradation of Anticancer Drugs. Sep. Purif. Technol. 2025, 364, 132306. [Google Scholar] [CrossRef]
- Regadera-Macías, A.M.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Maldonado-Hódar, F.J. Ethylene Removal by Adsorption and Photocatalytic Oxidation Using Biocarbon –TiO2 Nanocomposites. Catal. Today 2023, 413–415, 113932. [Google Scholar] [CrossRef]
- Mirikaram, N.; Pérez-Molina, Á.; Morales-Torres, S.; Salemi, A.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Photocatalytic Perfomance of ZnO-Graphene Oxide Composites towards the Degradation of Vanillic Acid under Solar Radiation and Visible-LED. Nanomaterials 2021, 11, 1576. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Falaras, P.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Role of Oxygen Functionalities on the Synthesis of Photocatalytically Active Graphene–TiO2 Composites. Appl. Catal. B Environ. 2014, 158–159, 329–340. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Design of Graphene-Based TiO2 Photocatalysts—a Review. Environ. Sci. Pollut. Res. 2012, 19, 3676–3687. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and Reduced Graphene Oxide as Efficient Photocatalysts for Hydrogen Evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- González, D.T.; Baeza, J.A.; Alemany-Molina, G.; Calvo, L.; Gilarranz, M.A.; Morallón, E.; Cazorla-Amorós, D. Catalytic Nitrate Reduction Using a Pd-Cu Catalysts Supported on Carbon Materials with Different Porous Structure. J. Environ. Chem. Eng. 2025, 13, 115979. [Google Scholar] [CrossRef]
- Sanchis, I.; Rodriguez, J.J.; Mohedano, A.F.; Diaz, E. N-Doped Activated Carbon as Support of Pd-Sn Bimetallic Catalysts for Nitrate Catalytic Reduction. Catal. Today 2023, 423, 114011. [Google Scholar] [CrossRef]
- Zhou, L.; Richard, C.; Ferronato, C.; Chovelon, J.-M.; Sleiman, M. Investigating the Performance of Biomass-Derived Biochars for the Removal of Gaseous Ozone, Adsorbed Nitrate and Aqueous Bisphenol A. Chem. Eng. J. 2018, 334, 2098–2104. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Asghar, B.H.M.; Muathen, H.A. Facile Synthesis of Mesoporous Bicrystallized TiO2(B)/Anatase (Rutile) Phases as Active Photocatalysts for Nitrate Reduction. Catal. Commun. 2012, 28, 58–63. [Google Scholar] [CrossRef]
- Sá, J.; Agüera, C.A.; Gross, S.; Anderson, J.A. Photocatalytic Nitrate Reduction over Metal Modified TiO2. Appl. Catal. B Environ. 2009, 85, 192–200. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Silva, C.G.; Silva, A.M.T.; Pastrana-Martínez, L.M.; Han, C.; Morales-Torres, S.; Figueiredo, J.L.; Dionysiou, D.D.; Faria, J.L. Carbon-Based TiO2 Materials for the Degradation of Microcystin-LA. Appl. Catal. B Environ. 2015, 170–171, 74–82. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Liu, F.; Shao, Y.; Wang, J.; Wan, H.; Zheng, S. Photocatalytic Nitrate Reduction over Pt–Cu/TiO2 Catalysts with Benzene as Hole Scavenger. J. Photochem. Photobiol. A: Chem. 2010, 212, 113–121. [Google Scholar] [CrossRef]
- Stanbury, D.M. Reduction Potentials Involving Inorganic Free Radicals in Aqueous Solution. Adv. Inorg. Chem. 1989, 33, 69–138. [Google Scholar] [CrossRef]
- Shi, H.; Li, C.; Wang, L.; Wang, W.; Bian, J.; Meng, X. Photocatalytic Reduction of Nitrate Pollutants by Novel Z-Scheme ZnSe/BiVO4 Heterostructures with High N2 Selectivity. Sep. Purif. Technol. 2022, 300, 121854. [Google Scholar] [CrossRef]
- Li, Y.; Wasgestian, F. Photocatalytic Reduction of Nitrate Ions on TiO2 by Oxalic Acid. J. Photochem. Photobiol. A Chem. 1998, 112, 255–259. [Google Scholar] [CrossRef]
- Sowmya, A.; Meenakshi, S. Photocatalytic Reduction of Nitrate over Ag–TiO2 in the Presence of Oxalic Acid. J. Water Process Eng. 2015, 8, e23–e30. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Bansal, R.C.; Donnet, J.-B.; Stoeckli, F. Active Carbon; Marcel Dekker: New York, NY, USA, 1988; ISBN 0–8247–7842–1. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Leon y Leon, C.A.; Solar, J.M.; Calemma, V.; Radovic, L.R. Evidence for the Protonation of Basal Plane Sites on Carbon. Carbon 1992, 30, 797–811. [Google Scholar] [CrossRef]
- Kesserű, P.; Kiss, I.; Bihari, Z.; Polyák, B. Investigation of the Denitrification Activity of Immobilized Pseudomonas Butanovora Cells in the Presence of Different Organic Substrates. Water Res. 2002, 36, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
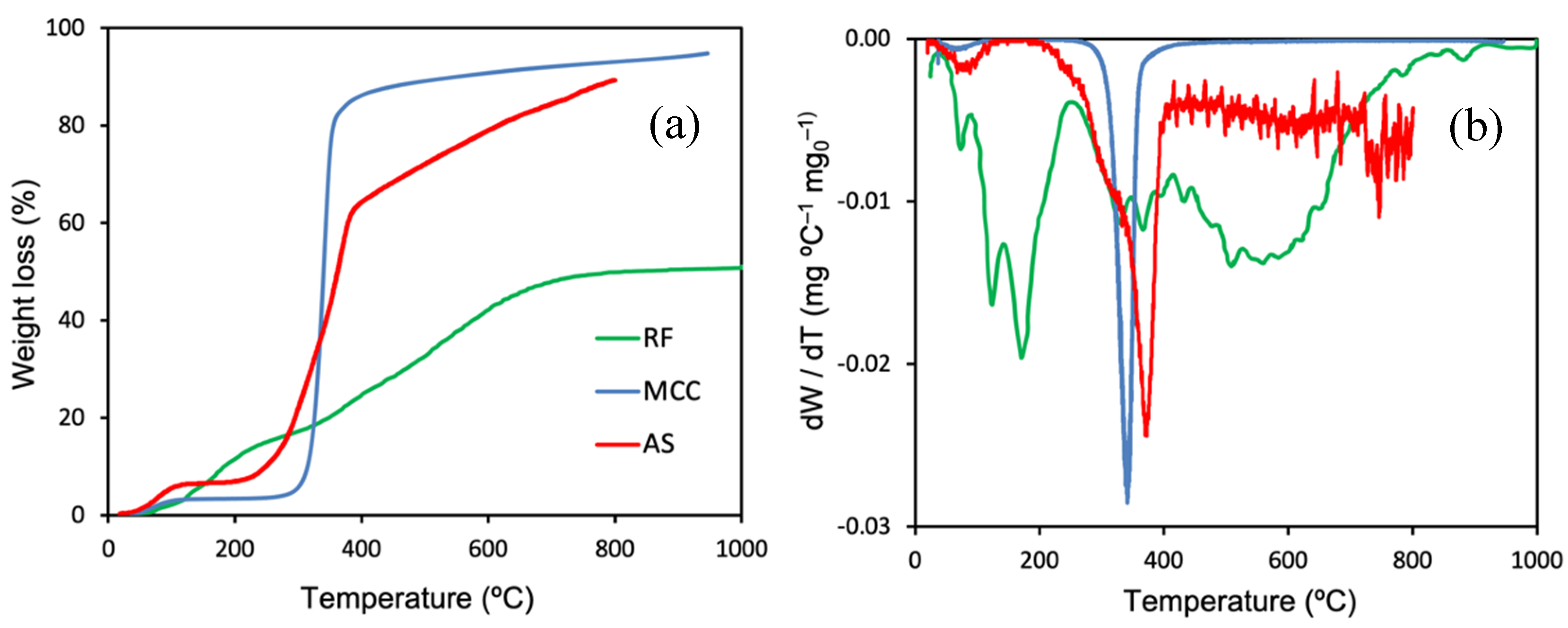

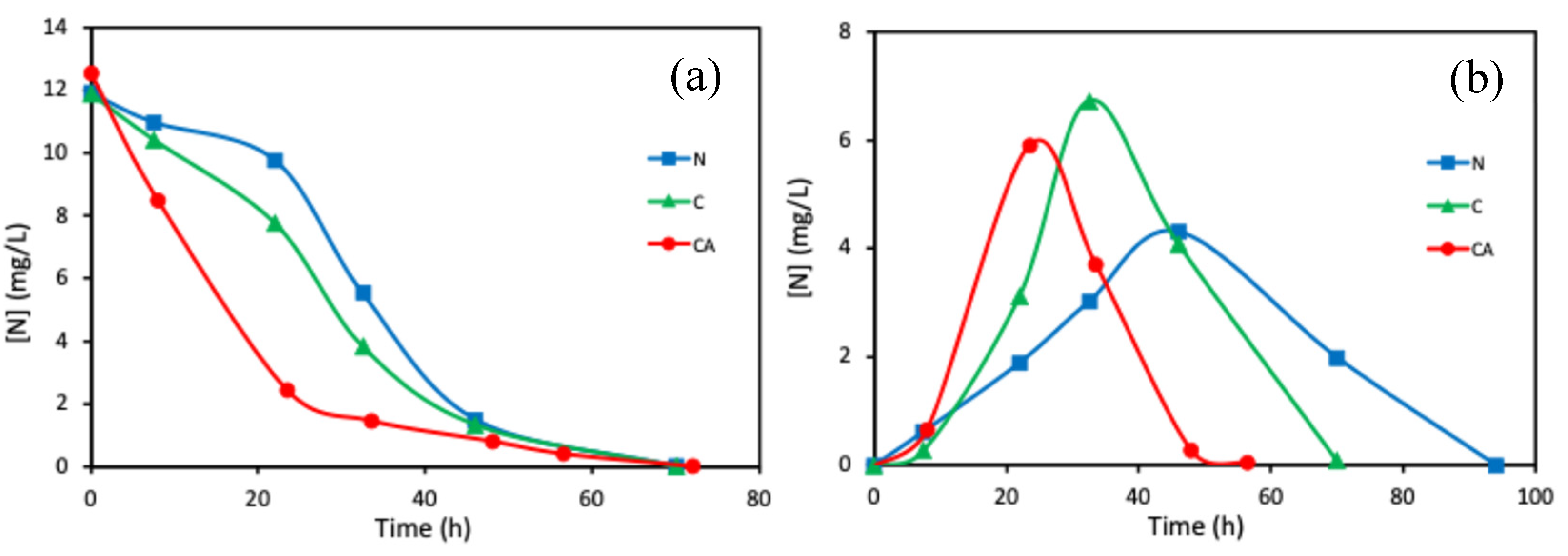
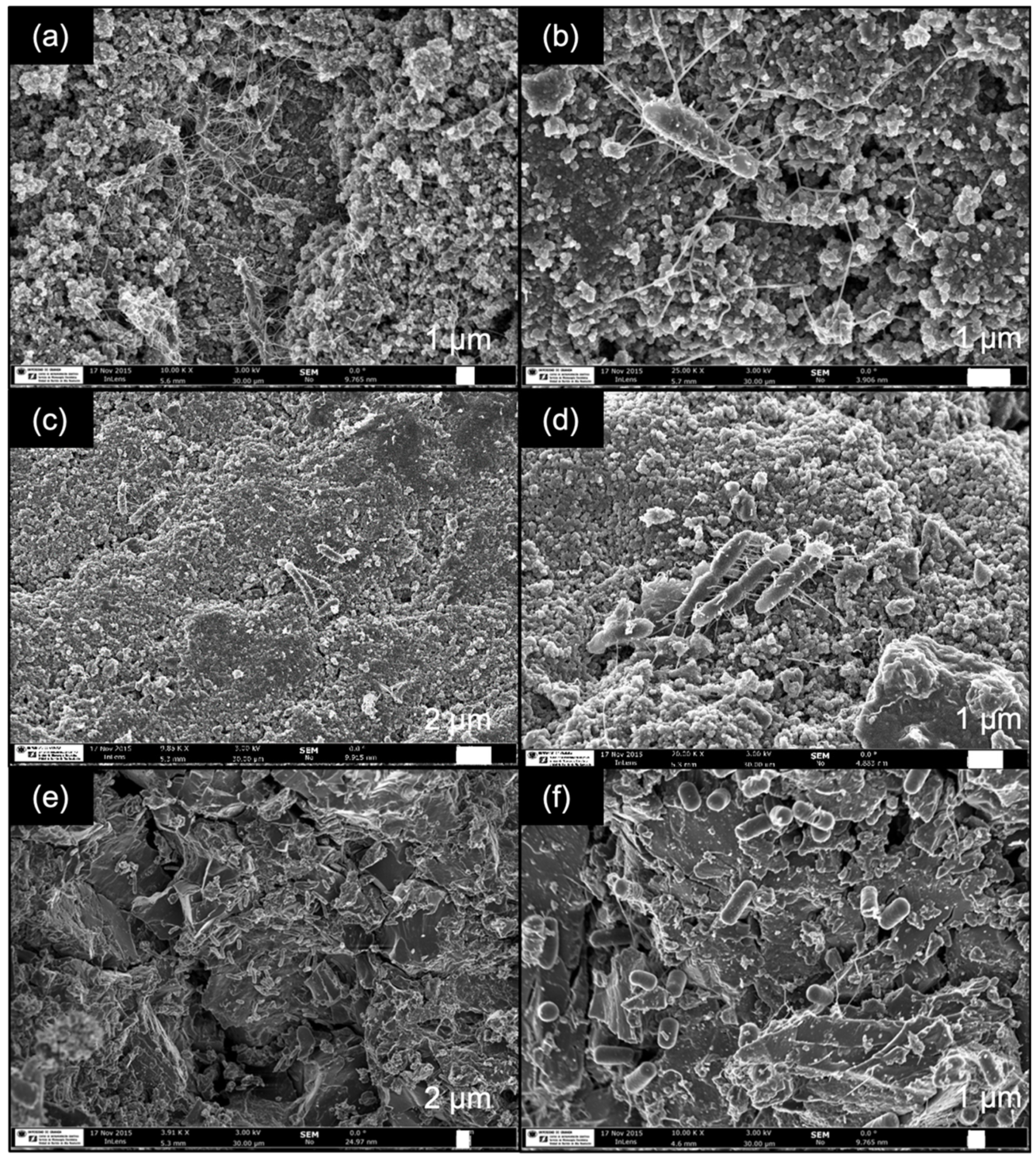

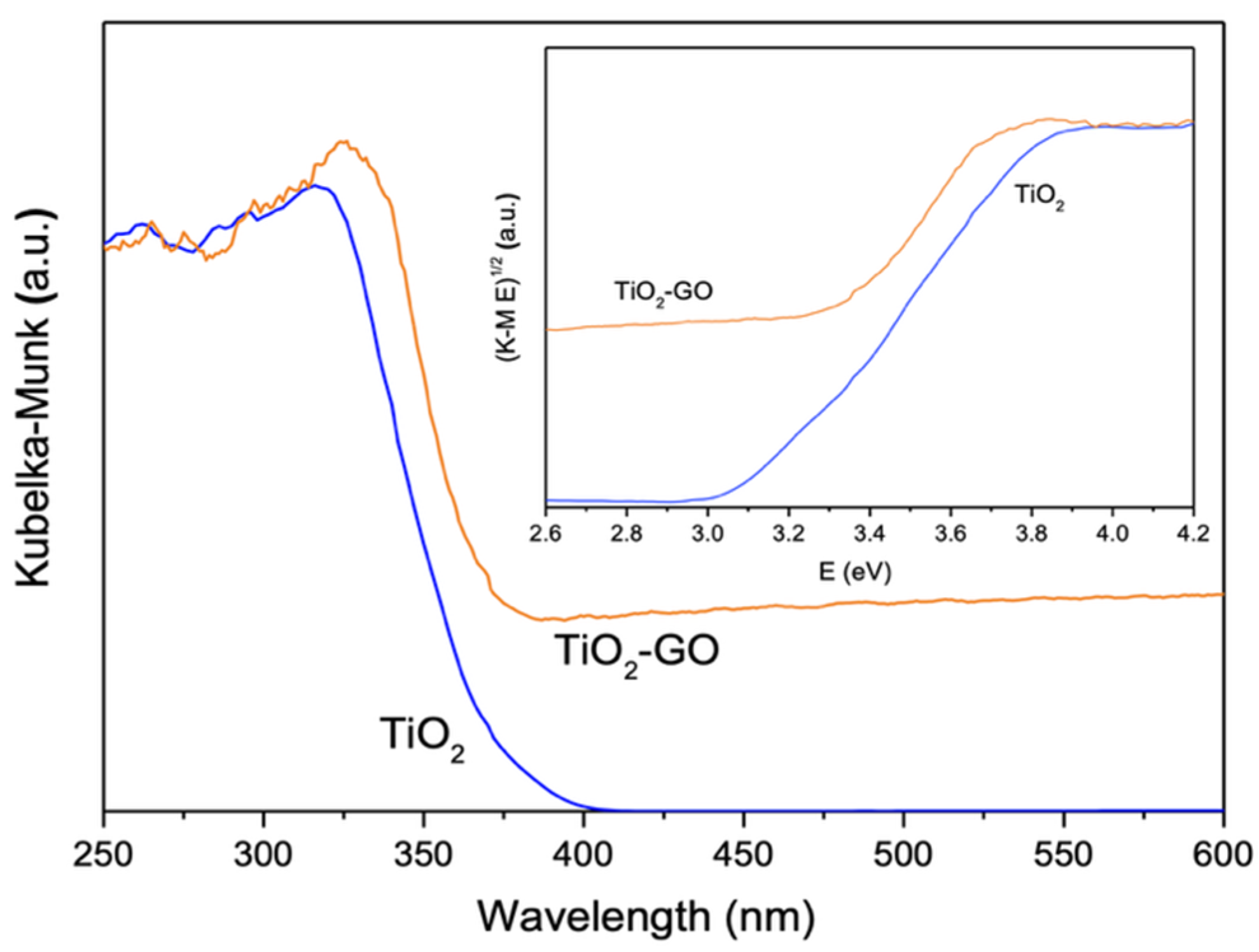


| Support | Ash (wt%) | pHPZC | SBET (m2 g−1) | VT (cm3 g−1) | W0 (cm3 g−1) | L0 (nm) | Vmeso (cm3 g−1) |
|---|---|---|---|---|---|---|---|
| CA | null | 6.3 | 594 | 0.69 | 0.21 | 2.5 | 0.48 |
| A | 0.3 | 10.6 | 913 | 0.47 | 0.32 | 1.4 | 0.15 |
| N | 4.8 | 11.0 | 1233 | 0.60 | 0.56 | 1.5 | 0.04 |
| Catalyst | SBET (m2 g−1) | VT (cm3 g−1) | pHPZC | Crystalline Phase (%) | Crystallite Size (nm) | Eg (eV) |
|---|---|---|---|---|---|---|
| P25 | 52 | 6.5 | 85 anatase | 22 | 3.20 | |
| TiO2 | 118 | 0.11 | 3.5 | 100 anatase | 8 | 3.12 |
| TiO2–GO | 120 | 0.17 | 3.0 | 100 anatase | 4 | 2.95 |
| Approach | Catalyst/System | Conditions | Nitrate Removal | Selectivity | Ref. |
|---|---|---|---|---|---|
| Photocatalysis | Ag–TiO2/Formic acid | Visible light, pH 3 | High N2 selectivity, fast kinetics | N2 (no NH4+) | [20] |
| Photocatalysis | g-C3N4/Pd–Cu/rGO/TiO2 hybrid | Visible light | 58% | N2 | [17] |
| Photocatalysis | TiO2@Fe3O4–Chitosan composite | UV light, adsorption + photocatalysis | 70–80%/1 h | N2 | [19] |
| Photocatalysis | Carbon/TiO2 nanoparticles | pH 3 and 0.04 M of formic acid | 100%/60 min | N2 | [16] |
| Photocatalysis | Z-scheme ZnSe/BiVO4 | Hg lamp, formic acid | 89%/50 min | 91% N2 | [51] |
| Photocatalysis | TiO2 (P25) | pH 2.5, solar light, no scavenger | 60%/1 h | N2 (no NH4+) | This work |
| Photocatalysis | TiO2–GO | pH 2.5, solar light, and oxalic acid (0.08 M) | 100%/15 min | N2 (no NH4+) | This work |
| Biological | Moving bed biofilm reactor + AC filter | Synthetic groundwater, acetate C-source | High efficiency | N2 | [21] |
| Biological | Wetland + microbial electrolysis (Fe3O4/GAC anode) | Electrobiotic integration | 88.9% | NO2− or NH4+ in the effluent | [22] |
| Biological | E. coli biofilm on carbon xerogel (CA) | pH 7, anaerobic, and ethanol as C-source | 100% NO3− + NO2− removal | N2 | This work |
| Biological | E. coli biofilm on activated carbon (A, N) | pH 7, anaerobic | pH 7, anaerobic | N2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hódar, F.J. The Key Role of Carbon Materials in the Biological and Photocatalytic Reduction of Nitrates for the Sustainable Management of Wastewaters. Catalysts 2025, 15, 958. https://doi.org/10.3390/catal15100958
Pastrana-Martínez LM, Morales-Torres S, Maldonado-Hódar FJ. The Key Role of Carbon Materials in the Biological and Photocatalytic Reduction of Nitrates for the Sustainable Management of Wastewaters. Catalysts. 2025; 15(10):958. https://doi.org/10.3390/catal15100958
Chicago/Turabian StylePastrana-Martínez, Luisa M., Sergio Morales-Torres, and Francisco J. Maldonado-Hódar. 2025. "The Key Role of Carbon Materials in the Biological and Photocatalytic Reduction of Nitrates for the Sustainable Management of Wastewaters" Catalysts 15, no. 10: 958. https://doi.org/10.3390/catal15100958
APA StylePastrana-Martínez, L. M., Morales-Torres, S., & Maldonado-Hódar, F. J. (2025). The Key Role of Carbon Materials in the Biological and Photocatalytic Reduction of Nitrates for the Sustainable Management of Wastewaters. Catalysts, 15(10), 958. https://doi.org/10.3390/catal15100958









