Abstract
This paper presents the results of a study on the effect of lipase on the methane fermentation of sewage sludge. The process was conducted at 37 °C for 20 days for five sludge mixtures. Excess sludge inoculated with digested sludge constituted the control sample. The other four samples are the aforementioned mixtures with the addition of lipase in amounts representing 0, 1, 2, 3, and 4% (w/w) with respect to sludge dry weight. The organic matter decomposition rate was 27.1% in the control sludge and from 33.5 to 46.7% in the disintegrated sludge. During the digestion of the control sludge, the total amount of biogas was 5802 mL·L−1. In sewage sludge enzymatically disintegrated by lipase, there was an increase in biogas from 15 to 26%. In the disintegrated sludge, an almost complete (95–100%) reduction in E. coli and Salmonella spp. was achieved. Therefore, enzymatic disintegration can be an effective alternative to physical and chemical disintegration methods.
1. Introduction
Sewage sludge is a dispersive system with a diverse composition, which causes difficulties in processing. The most common methods of sewage sludge disposal include biological or chemical stabilization, drying, and incineration. In the treatment process, there is a decomposition of organic compounds and a reduction in the volume of sludge and its preparation for further processing, use, or management. In large wastewater treatment plants, the most commonly used sludge disposal process is anaerobic biological stabilization [1,2,3,4].
The mineralization of biodegradable organic substrates leads to an improved sludge dewatering capacity and a reduction in pathogenic organisms. The fermentation process is considered to have been properly carried out when organic compounds in the digested sludge account for 45 to 55%. The amount and composition of biogas produced by fermentation depend on the properties of the fermented substrate, including protein, carbohydrate, and fat content. The methane content of biogas can range from 50 to 84%. During the process, mineralized biomass with a high content of biogenic compounds is obtained [5,6,7,8,9,10].
Improving the anaerobic stabilization process can be achieved by subjecting wastewater sludge to disintegration. The application of sludge disintegration can influence the following: accelerating the hydrolysis of organic compounds, reducing the weight of sludge, increasing the efficiency of mineralization of organic matter, increasing biogas production, and inactivating pathogenic microorganisms. Sewage sludge disintegration methods, depending on the disintegrating agent, can be divided into: mechanical, thermal, chemical, biochemical, and hybrid, which are a combination of different methods [11,12,13,14,15,16].
Enzymatic disintegration is one of the modern methods of treating sewage sludge. The process involves the use of hydrolytic enzymes such as proteases, lipases, amylases, or cellulases, which catalyze the breakdown of complex organic compounds into simpler, more easily biodegradable forms. The biochemical method is based on the enzymatic breakdown of long-chain organic compounds, such as proteins, carbohydrates, and fats, into simpler, smaller molecules. The introduction of enzymes into sewage sludge leads to the breakdown of cellular structures and the release of organic compounds from inside the cells of microorganisms and increases the availability of substrates for living heterotrophic biomass [17,18,19,20].
Lipases (EC 3.1.1.3) are among the most versatile enzymes, finding applications in many industries such as pharmaceuticals, dairy, cleaning products, cosmetics, and many others. They belong to a group of hydrolases that cause the cutting of chemical bonds in the process of hydrolysis. They are water-soluble enzymes that hydrolyze water-insoluble substrates into more polar lipolytic products. They are commonly found in living organisms, both in plants, animals and microorganisms, playing a key role in metabolic processes related to digestion and fat metabolism. Lipases are distinguished from other enzymes by their ability to act on water-insoluble substrates. Crucial to their activity is carrying out reactions at the interface between the insoluble substrate and the aqueous phase in which the enzyme is dissolved. The speed of the reaction depends on the size of the contact area between the enzyme and the substrate. Lipase is active in an alkaline environment (pH 7.0–9.0). In an aqueous environment, lipases catalyze the hydrolysis reaction of triacylglycerols, releasing diacylglycerol, monoacylglycerol, glycerol, and fatty acids into the substrate [21,22,23].
Lipases can affect the microflora composition in wastewater treatment and sludge treatment processes. Microorganisms capable of decomposing fats may become the dominant group, affecting the entire structure of the microbial ecosystem. Lipases can produce negative effects in some microorganisms. Some pathogens may be sensitive to free fatty acids formed by lipases, which can alter the composition of microbial cell membranes, leading to their inactivation [24,25,26].
The aim of the study was to determine changes in the physical and chemical properties of sludge disintegrated with the lipase enzyme subjected to methane fermentation and the effect of the enzyme on the abundance of Escherichia coli, Salmonella sp., and Legionella sp.
2. Results
2.1. Physical, Chemical, and Microbiological Properties of Sewage Sludge
The results of physical and chemical analyses of sludge and supernatant liquids performed before the fermentation process and after 10 and 20 days of incubation are shown in Table 1. The inspection of parameters such as pH, volatile fatty acid content, and alkalinity makes it possible to assess the correctness of the fermentation process. During fermentation, there is an increase in volatile fatty acids and a decrease in alkalinity. To evaluate the relationship between these parameters, their quotient is used as an indicator. Disruption of the balance of the fermentation process manifests itself in changes in the VFA/alkalinity ratio and a drop in pH. The process is disrupted when the pH value decreases and the VFA/alkalinity ratio increases. With proper fermentation, the ratio of VFA to alkalinity should not exceed 0.3, and the lower it is, the more favorable. Well-digested sludge has a pH in the range of 7.2–7.8 and an alkalinity of 3000 to 5500 mgCaCO3·L−1. On the other hand, the optimal level of VFA after fermentation is in the range of 100–500 mgCH3COOH·L−1 [27,28,29]. In the control sewage sludge (0%) after the fermentation process, the alkalinity was 3215 mgCaCO3·L−1, and the concentration of volatile fatty acids did not exceed 250 mgCH3COOH·L−1. Similar final values were observed in test sewage sludge into which lipase was introduced. Alkalinity in the disintegrated sewage sludge after digestion was in the range of 3000 to 3200 mgCaCO3·L−1, and the concentration of volatile fatty acids was in the range of 145 to 224 mgCH3COOH·L−1. The quotient of volatile fatty acids to alkalinity, in both control and test sludge, remained below the 0.3 value.

Table 1.
Changes in physico-chemical properties of sludge and supernatant during the fermentation process.
Before the start of the digestion process, the COD value in the filtrate supernatants separated from the mixture of excess and digested sewage sludge was 570 mgO2·L−1. The introduction of the enzyme into the sludge was followed by the decomposition of macromolecular organic compounds into simpler products, as evidenced by the increase in COD values.
The dry matter content of the sewage sludge before the process was 23.3 g·L−1, and the proportion of organic matter and mineral matter were 61.5 and 38.5%, respectively. After the process, the dry matter content was 18.4 g·L−1 in the control sludge (0%), of which 43.2% was roasting residue and 56.8% was roasting loss. The degree of organic matter decomposition was 27.1%. In the sewage sludge to which lipase was introduced at a rate of 1% by weight (w/w) with respect to the dry weight content of the sludge, there was a 27.8% loss of dry weight, and the degree of organic matter decomposition was higher than in the control sludge, exceeding 33.5%. In sewage sludge enzymatically disintegrated with lipase at 2%, the dry weight after 20 days was 15.85 g·L−1. The degree of organic matter decomposition was 38.6%. The dry residue in the sludge with 3% enzyme addition after 20 days of incubation decreased to 14.32 g·L−1, while the 4% addition decreased to 13.52 g·L−1. The degree of organic matter decomposition was 43.9 and 46.7% for an enzyme dose of 3 and 4%, respectively.
The values of selected parameters of the digestion process are shown in Table 2. In the control sewage sludge (0%), it was calculated that 0.46 L·g−1 of biogas and 0.27 L·g−1 of methane were obtained from 1 g of dry organic matter of sewage sludge fed into the reactor. For the disintegrated sludge, the amount of biogas ranged from 0.54 to 0.62 L·g−1, and methane from 0.32 to 0.40 L·g−1, and increased with the amount of enzyme added. According to the literature, biogas production during mesophilic fermentation is in the range of 0.3–0.6 L·g−1. The load of organic pollutants in the chambers was 0.46 g·L−1·d−1. After sludge digestion with lipase, a statistically significant increase in organic matter decomposition was observed at each dose (Table 3). The obtained results correlated with the author’s previous studies [30,31].

Table 2.
Parameters of the sewage sludge methane fermentation process.

Table 3.
The value of Student’s t-distribution (td = 4.303).
The concentrations of phosphate and ammonium nitrogen are shown in Figure 1 and Figure 2. The concentrations of phosphate and ammonium nitrogen before the process began were 89.4 mg PO43−·L−1 and 115.6 mg N-NH4+·L−1, respectively. After 10 days, an increase in both phosphate and ammonium nitrogen concentrations was observed in all mixtures tested. After this period, the phosphate and ammonium nitrogen concentrations continued to rise. This phenomenon is typical, as extending the duration of methane fermentation causes a further breakdown of organic compounds, which in turn leads to the release of phosphates into the supernatant [32]. During anaerobic digestion, approximately 70% of organic nitrogen is mineralized, converting into ammonium nitrogen (N-NH4+) and free ammonia nitrogen (N-NH3). The concentrations of these forms are influenced by pH and temperature. As the pH increases, NH4+ hydrolyzes to NH3 [33], which then escapes from the reactor along with the biogas, which is also confirmed by a decrease in the ammonia content of the biogas. During the fermentation process, organic nitrogen and phosphorus compounds undergo hydrolysis and degradation, releasing nutrients into the supernatants. The increase in ammonium nitrogen concentration resulted from the ammonification process. The addition of the enzyme enhanced the rate of nutrient release compared to the control sample, likely due to the heightened metabolic activity. Similar trends were observed in Montusiewicz’s study on the effect of bioaugmentation on nutrient release during the methane digestion of sewage sludge [34]. The introduction of the enzyme lipase, at the adopted doses, into excess sewage sludge after 20 days contributed to an increase in the phosphate release rate by 14.4–24.2%, and ammonium nitrogen by 68.3–70.8% with respect to the initial concentration.
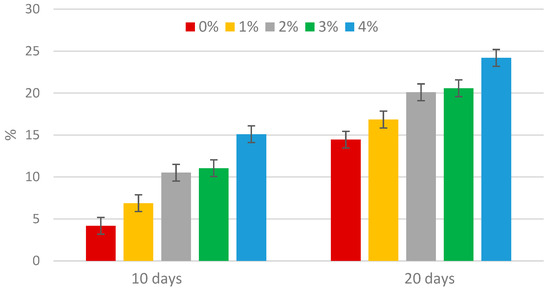
Figure 1.
Increase in phosphate during methane fermentation of sewage sludge.
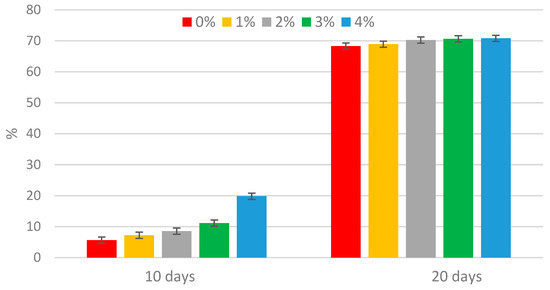
Figure 2.
Increase in ammonium nitrogen during methane fermentation of sewage sludge.
Table 4 shows the changes in bacterial counts during fermentation in the supernatant liquids separated from sewage sludge. No Legionella spp. were detected in the supernatants either before or after the fermentation process. Before the process, in the supernatants separated from the mixture of excess and digested sewage sludge, the colony counts of E. coli bacteria were 5.0 ± 0.0 × 103 CFU·mL−1, and Salmonella spp. 1.7 ± 0.60 × 104 CFU·mL−1. After completion of the fermentation process, no Escherichia coli colonies were detected in the supernatant obtained from both the control sewage sludge and the sludge with the enzyme. The number of Salmonella spp. in the supernatants from mixed excess sludge with digested sewage sludge without lipase was 8.3 ± 0.03 × 103 CFU·mL−1. In contrast, the supernatants with a 2% (w/w) enzyme addition relative to the dry weight of the sewage sludge contained 6.6 ± 0.0 × 102 CFU·mL−1, while with a 3% addition, the count was 11.9 ± 0.07 × 102 CFU·mL−1.

Table 4.
Changes in colony counts of E. coli, Salmonella spp., and Legionella spp. (CFU·mL−1) in supernatant separated from sewage sludge.
The mechanism of lipase’s antibacterial action may be related to its ability to catalyze the hydrolysis of bacterial cell membrane lipids, leading to the destabilization of membrane structure and disruption of cellular functions. The products of this hydrolysis, such as free fatty acids and monoglycerides, exhibit additional antibacterial activity because they can damage the cell membranes of microorganisms. Studies have shown that lipases can reduce the abundance of pathogens such as Escherichia coli, Salmonella spp., and Legionella spp. in environments such as wastewater where lipid content is high. The introduction of lipases as a biotechnology agent into industrial or wastewater treatment systems can not only contribute to the degradation of fats, but also assist in reducing the abundance of unwanted microorganisms. The action of lipase on bacteria can lead to changes in cellular structure, enzymatic activity, and bacterial metabolism, and can affect their ability to survive in altered environmental conditions [24,25,26].
2.2. Production and Composition of Biogas
Figure 3 and Figure 4 illustrate the daily and cumulative biogas production throughout the digestion process, respectively. The highest biogas yield from both control and disintegrated sewage sludge was observed on the third day of digestion. The lowest biogas production occurred in the control sludge, while the highest was achieved with sludge treated with lipase at a concentration of 4% by weight (w/w) relative to the dry weight of the sludge.
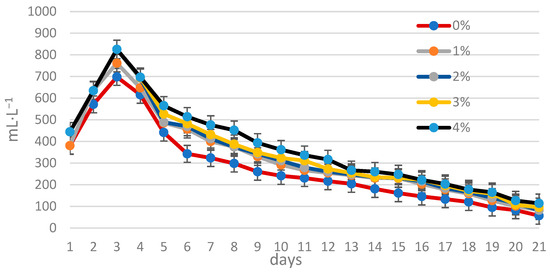
Figure 3.
Amount of biogas (mL−1·L−1 of sewage sludge) produced during fermentation.
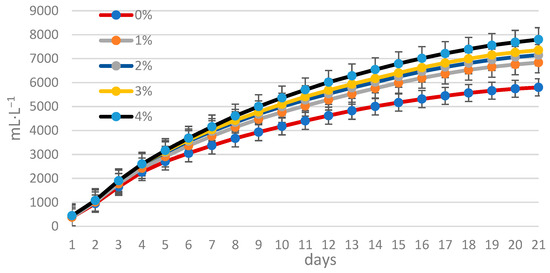
Figure 4.
The course of biogas production during sewage sludge digestion.
The highest amount of biogas per unit volume of sludge, at 7800 mL·L−1, was obtained during sludge digestion with the addition of lipase at 4%. For mixtures containing lipase at 1%, 2%, and 3% (w/w) relative to sludge dry weight, total biogas production was 6839, 7149, and 7356 mL·L−1, respectively. The lowest biogas production was recorded in the control sludge—5802 mL·L−1. These results indicate that increasing the amount of enzyme resulted in higher total biogas production. The introduction of lipase in amounts representing 1, 2, 3, and 4% by weight (w/w) with respect to sludge dry matter content was statistically significant on total biogas production (Table 3). Similar trends were obtained as in the authors’ previous studies [35,36].
The analysis of biogas composition revealed that the methane content ranged from 54% to 67%, carbon dioxide from 22% to 25%, and ammonia concentrations from 500 to 1130 ppm (Figure 5, Figure 6 and Figure 7). The addition of lipase at concentrations of 1, 2, 3, and 4% by weight (w/w) relative to the dry matter content of sewage sludge showed no statistically significant impact on the methane percentage in the biogas (Table 3).
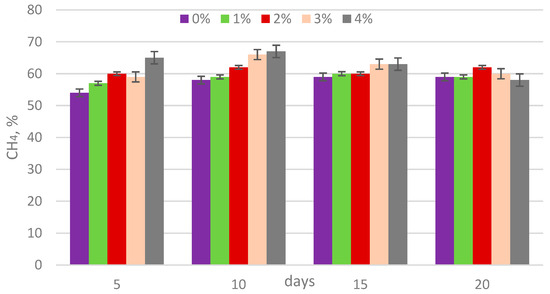
Figure 5.
CH4 content in biogas during digestion.
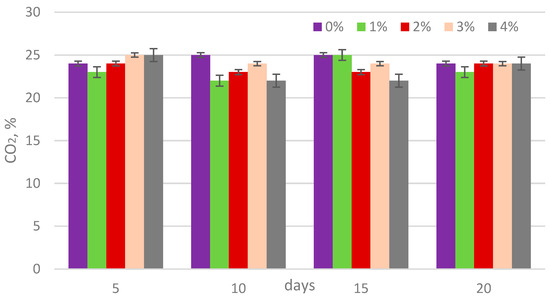
Figure 6.
CO2 content in biogas during digestion.
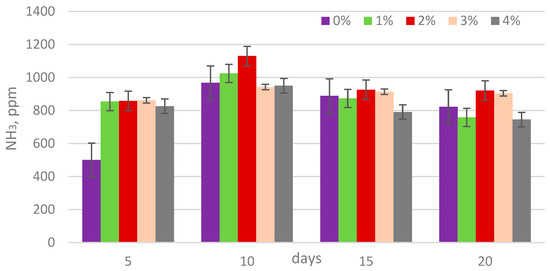
Figure 7.
NH3 content in biogas during digestion.
3. Materials and Methods
3.1. Materials and Course of Study
The research was conducted using sludge from a municipal wastewater treatment plant in which urban wastewater treatment is carried out through the processes of defosfatation, denitrification, and nitrification. Sewage sludge stabilization is carried out in separate digesters under anaerobic conditions. A mixture of thickened sludge is directed to the digesters. The mixture consists of raw sludge and excess sludge. Excess sewage sludge was taken upstream of the digester, while digested sewage sludge was taken from the sludge discharge pipeline from the enclosed separate fermentation chamber. The following samples were prepared for technological testing:
- −
- Excess and digested sewage sludge as an inoculum (in a volume ratio of 2:3)—control sample (0%)
- −
- Four mixtures of municipal sewage sludge of the composition as above into which lipase (Thermo Fisher Scientific, Waltham, MA, USA, >30,000 U/mg) was introduced in amounts representing 1, 2, 3, and 4% (w/w) by weight of the sludge dry matter content.
Anaerobic fermentation was carried out in glass batch bioreactors (single feed). The precipitates were incubated for 20 days at 37 °C without light. To ensure adequate contact between biomass and substrate and to remove biogas, the contents of the reactors were manually stirred once a day. To determine the course of the process, determinations of selected physico-chemical properties of the sewage sludge were made before the process and after 10 and 20 days of incubation. The following were determined in the sludge: dry residue and roasting residue (by weight method). The supernatants obtained from centrifuging the sludge were determined:
- −
- pH value—potentiometrically;
- −
- Alkalinity (ALK)—using the titration method against indicators;
- −
- Chemical oxygen demand (COD)—via Macherey–Nagel Nanocolor tube tests (Macherey–Nagel, Dueren, Germany) using a UV–Vis spectrophotometer (NANOCOLOR® UV/VIS II, Macherey–Nagel, Dueren, Germany);
- −
- Volatile fatty acids (VFA)—using the distillation method;
- −
- Phosphates (PO43−)—via the molybdenum method using a DR6000 spectrophotometer (Hach, Loveland, CO, USA);
- −
- Ammonium nitrogen (N-NH4+)—using Macherey–Nagel Nanocolor tube tests using a UV–Vis spectrophotometer (Macherey–Nagel, Dueren, Germany).
The bacterial counts of E. coli, Salmonella spp., and Legionella spp. were analyzed in the supernatants of centrifuged sewage sludge both before and after the process, following the methodology outlined in a previous study [36].
Atmospheric pressure and biogas pressure were monitored throughout the process using a manometer at 24 h intervals. The daily biogas volume was then calculated using the Boyle–Mariotte Equation (1):
where
- −
- p1—pressure in bioreactor, hPa;
- −
- V1—volume of free space in bioreactor, L;
- −
- p2—atmospheric pressure, hPa;
- −
- V2—calculated volume of biogas, L.
The composition of biogas (CH4, CO2, and NH3) was analyzed using a DP-28 BIO biogas analyzer (Nanosens, Wysogotowo, Poland).
3.2. Statistical Analysis
A Student’s t-test was performed to evaluate the statistical significance of changes in selected physical and chemical parameters. A confidence level of 0.95 was applied, with a degrees of freedom value of 2, corresponding to a theoretical t-Student distribution value (tdk) of 4.303. All calculations were conducted using SPSS Statistics software (SPSS Amos 29).
4. Conclusions
The study showed that the enzymatic disintegration of sewage sludge using lipase significantly increases the efficiency of the methane fermentation process. The introduction of lipase in amounts ranging from 1% to 4% (w/w) of sludge dry weight contributed to an increase in the degree of organic matter decomposition from 27.1% (control sludge) to a maximum of 46.7%. The amount of biogas produced increased by 15–26% compared to the control sample, with the highest biogas and methane values obtained in samples with 4% lipase. The process of enzymatic de-synthesis also contributed to the almost complete elimination of Escherichia coli and Salmonella spp. bacteria, indicating the potential of lipase in reducing pathogens. In addition, an increase in the release of phosphate and ammonium nitrogen was observed, suggesting an intensification of biochemical transformations.
The study demonstrated that adding lipase to the sludge prior to digestion significantly enhanced the digestion process. The research findings led to the following conclusions:
- −
- The addition of lipase to sewage sludge effectively improves the methane fermentation process. The introduction of the enzyme at a rate of 1 to 4% relative to the dry weight of the sludge contributed to an increase in the degree of organic matter decomposition from 27.1% (in the control sample) to a maximum of 46.7%.
- −
- Enzymatic disintegration of the sludge with lipase helped to increase biogas production by 15–26%. The amount of biogas increased to 0.62 L·g−1 of dry organic matter, compared to 0.46 L·g−1 in the control sample.
- −
- The use of lipase resulted in an almost complete reduction in the abundance of pathogenic Escherichia coli and Salmonella spp. bacteria (95–100%), highlighting the potential of this method in improving the sanitary safety of sludge.
- −
- The introduction of lipase increased the release of phosphate (by 14.4–24.2%) and ammonium nitrogen (by 68.3–70.8%) compared to the control sample, indicating an intensification of the mineralization processes of organic compounds.
- −
- The results confirm that the enzymatic disintegration of sludge using lipase is an effective alternative to physical and chemical methods, offering increased biogas production efficiency and pathogen elimination.
- −
- The use of enzymes in the disintegration process can be a competitive alternative to physical and chemical methods, providing high efficiency while reducing negative environmental impacts.
Funding
This study was funded by the scientific subvention of Cardinal Stefan Wyszynski University in Warsaw. The funder of this research was the Ministry of Science and High Education.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Hu, H.; Ye, Z.; Tan, S.; Yin, K.; Chen, Z.; Guo, D.; Rong, H.; Wang, J.; Pan, Z.; et al. A review on turning sewage sludge to value-added energy and materials via thermochemical conversion towards carbon neutrality. J. Clean. Prod. 2022, 379, 134657. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, S.; Xu, Y.; Qian, G. Effects of phosphorus and iron on the composition and property of Portland cement clinker utilized incinerated sewage sludge ash. Constr. Build. Mater. 2022, 341, 127754. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, A.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Brix, H. Sludge dewatering and mineralization in sludge treatment reed beds. Water 2017, 9, 160. [Google Scholar] [CrossRef]
- Samara, E.; Matsi, T.; Zdragas, A.; Barbayiannis, N. Use of clay minerals for sewage sludge stabilization and a preliminary assessment of the treated sludge’s fertilization capacity. Environ. Sci. Pollut. Res. 2019, 26, 35387–35398. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to energy: A focus on the impact of substrate type in biogas production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Schnürer, A.; Jarvis, Å. Microbiology of the Biogas Process; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018. [Google Scholar]
- Grübel, K.; Wacławek, S.; Machnicka, A.; Nowicka, E. Synergetic disintegration of waste activated sludge: Improvement of the anaerobic digestion and hygienization of sludge. J. Environ. Sci. Health A 2018, 53, 1067–1074. [Google Scholar] [CrossRef]
- Zubrowska-Sudol, M.; Podedworna, J.; Sytek-Szmeichel, K.; Bisak, A.; Krawczyk, P.; Garlicka, A. The effects of mechanical sludge disintegration to enhance full-scale anaerobic digestion of municipal sludge. Therm. Sci. Eng. Prog. 2018, 5, 289–295. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Eskicioglu, C. Recent developments on thermal municipal sludge pretreatment technologies for enhanced anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 110, 423–443. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Krzemieniewski, M.; Rusanowska, P.; Zielińska, M.; Cydzik-Kwiatkowska, A.; Głowacka-Gil, A. Application of an innovative ultrasound disintegrator for sewage sludge conditioning before methane fermentation. J. Ecol. Eng. 2018, 19, 240–247. [Google Scholar] [CrossRef]
- Wang, X.; Gao, C.; Qi, X.; Zhang, Y.; Chen, T.; Xie, Y.; Zhang, A.; Gao, J. Enhancing sludge fermentation and anaerobic digestion by mechanical cutting pretreatment. Water Process Eng. 2021, 40, 101812. [Google Scholar] [CrossRef]
- Penghe, Z.; Yuling, L.; Chuanchuan, D.; Pengliang, W. Study on dissolution characteristics of excess sludge by low-temperature thermal hydrolysis and acid production by fermentation. ACS Omega 2020, 5, 26101–26109. [Google Scholar] [CrossRef] [PubMed]
- Barjenbruch, M.; Kopplow, O. Enzymatic, mechanical and thermal pre-treatment of surplus sludge. Adv. Environ. Res. 2003, 7, 715–720. [Google Scholar] [CrossRef]
- Roman, H.J.; Burgess, J.E.; Pletschke, B.I. Enzyme treatment to decrease solids and improve digestion of primary sewage sludge. Afr. J. Biotechnol. 2006, 5, 963–967. [Google Scholar]
- Burgess, J.E.; Pletschke, B.I. Hydrolytic enzymes in sewage sludge treatment: A mini-review. Water Sa 2008, 34, 343–350. [Google Scholar] [CrossRef]
- Myszograj, S.; Płuciennik-Koropczuk, E. Thermal disintegration of sewage sludge as a method of improving the biogas potential. Energies 2023, 16, 559. [Google Scholar] [CrossRef]
- Kocabaş, D.S.; Lyne, J.; Ustunol, Z. Hydrolytic enzymes in the dairy industry: Applications, market and future perspectives. Trends Food Sci. Tech. 2022, 119, 467–475. [Google Scholar] [CrossRef]
- Goswami, D.; Basu, J.K.; De, S. Lipase applications in oil hydrolysis with a case study on castor oil: A review. Crit. Rev. Biotechnol. 2013, 33, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Aloulou, A.; Rodriguez, J.A.; Fernandez, S.; van Oosterhout, D.; Puccinelli, D.; Carrière, F. Exploring the specific features of interfacial enzymology based on lipase studies. BBA Adv. 2006, 1761, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, H.K. Antibacterial activity of emulsions containing unsaturated fatty acid ergosterol esters synthesized by lipase-mediated transesterification. Enzym. Microb. Technol. 2020, 139, 109581. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. The antimicrobial properties of milkfat after partial hydrolysis by calf pregastric lipase. Chem. Biol. Interact. 2002, 140, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.Y.; Shi, Y.G.; Wu, Y.; Bian, L.Q.; Zhu, Y.J.; Huang, X.Y.; Pan, Y.; Zeng, L.Y.; Zhang, R.R. Lipase-catalyzed synthesis of sucrose monolaurate and its antibacterial property and mode of action against four pathogenic bacteria. Molecules 2018, 23, 1118. [Google Scholar] [CrossRef]
- Ma, S.; Hu, H.; Wang, J.; Liao, K.; Ma, H.; Ren, H. The characterization of dissolved organic matter in alkaline fermentation of sewage sludge with different pH for volatile fatty acids production. Water Res. 2019, 164, 114924. [Google Scholar] [CrossRef]
- Kardos, L.; Juhasz, A.; Palko, G.Y.; Olah, J.; Barkacs, K.; Zaray, G.Y. Comparing of mesophilic and thermophilic anaerobic fermented sewage sludge based on chemical and biochemical tests. Appl. Ecol. Environ. Res. 2011, 9, 293–302. [Google Scholar] [CrossRef]
- Junior, I.V.; de Almeida, R.; Cammarota, M.C. A review of sludge pretreatment methods and co-digestion to boost biogas production and energy self-sufficiency in wastewater treatment plants. J. Water Process Eng. 2021, 40, 101857. [Google Scholar] [CrossRef]
- Macherzyński, B.; Włodarczyk-Makuła, M.; Wszelaka-Rylik, M.; Wojewódka, D. Evaluation of the possibility of PAH degradation by a consortium of fermentation bacteria. Desalination Water Treat. 2020, 186, 325–333. [Google Scholar] [CrossRef]
- Macherzyński, B.; Włodarczyk-Makuła, M. Biochemical neutralization of coke excess sewage sludge during anaerobic digestion process. Chem. Biochem. Eng. Q. 2018, 32, 239–246. [Google Scholar] [CrossRef]
- Yu, B.; Xiao, X.; Wang, J.; Hong, M.; Deng, C.; Li, Y.Y.; Liu, J. Enhancing phosphorus recovery from sewage sludge using anaerobic-based processes: Current status and perspectives. Bioresour. Technol. 2021, 341, 125899. [Google Scholar] [CrossRef]
- Di Costanzo, N.; Cesaro, A.; Di Capua, F.; Esposito, G. Exploiting the nutrient potential of anaerobically digested sewage sludge: A review. Energies 2021, 14, 8149. [Google Scholar] [CrossRef]
- Montusiewicz, A. Impact bioaugmentation on nutrient release in anaerobic digestion of sewage sludge. Proc. ECOpole 2015, 9, 269–277. [Google Scholar] [CrossRef]
- Macherzyński, B.; Włodarczyk-Makuła, M. Evaluation of the possibility of disposal of coke sludge in the co-fermentation process. Annu. Set Environ. Prot. 2015, 17, 1142–1161. [Google Scholar]
- Macherzyński, B.; Popowska-Nowak, E.; Włodarczyk-Makuła, M.; Bień, B.; Wszelaka-Rylik, M. Intensification of Energy Production in the Anaerobic Digestion Process of Sewage Sludge Using Enzymatic Disintegration. Energies 2025, 18, 11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).