The Bi-Modified (BiO)2CO3/TiO2 Heterojunction Enhances the Photocatalytic Degradation of Antibiotics
Abstract
1. Introduction
2. Results and Discussion
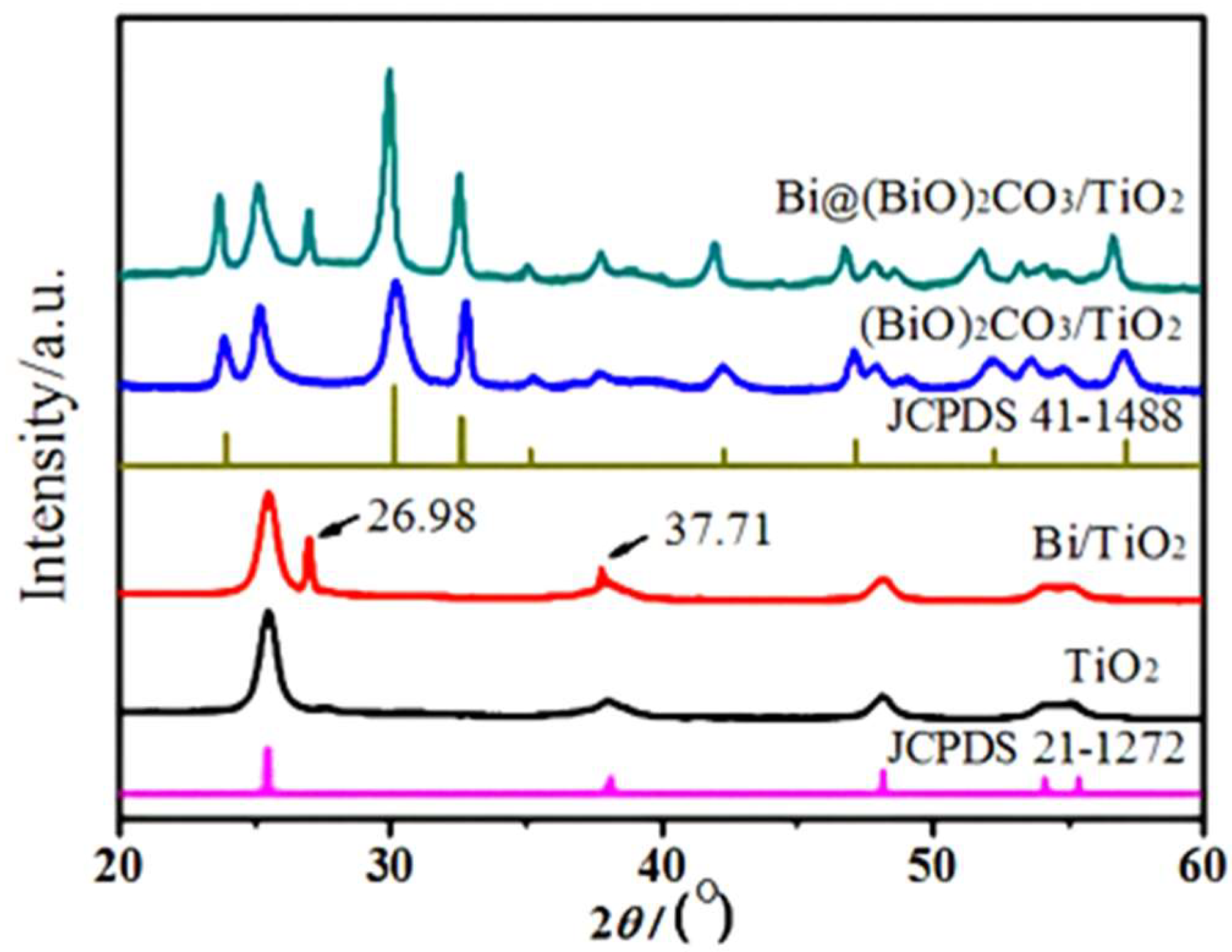
2.1. XRD Analysis
2.2. XPS Analysis
2.3. SEM and TEM Analysis
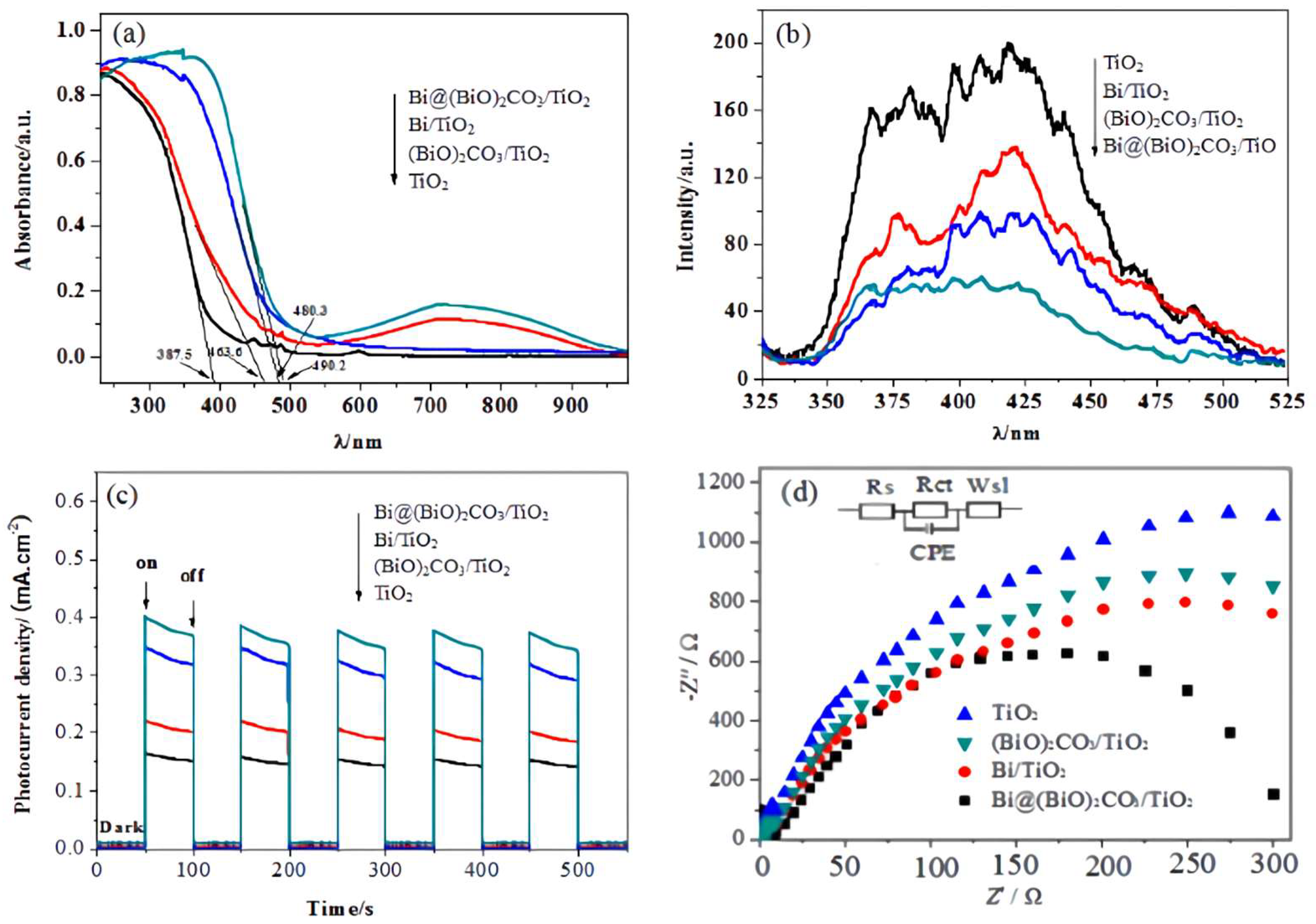
2.4. Photoelectric Performance Analysis
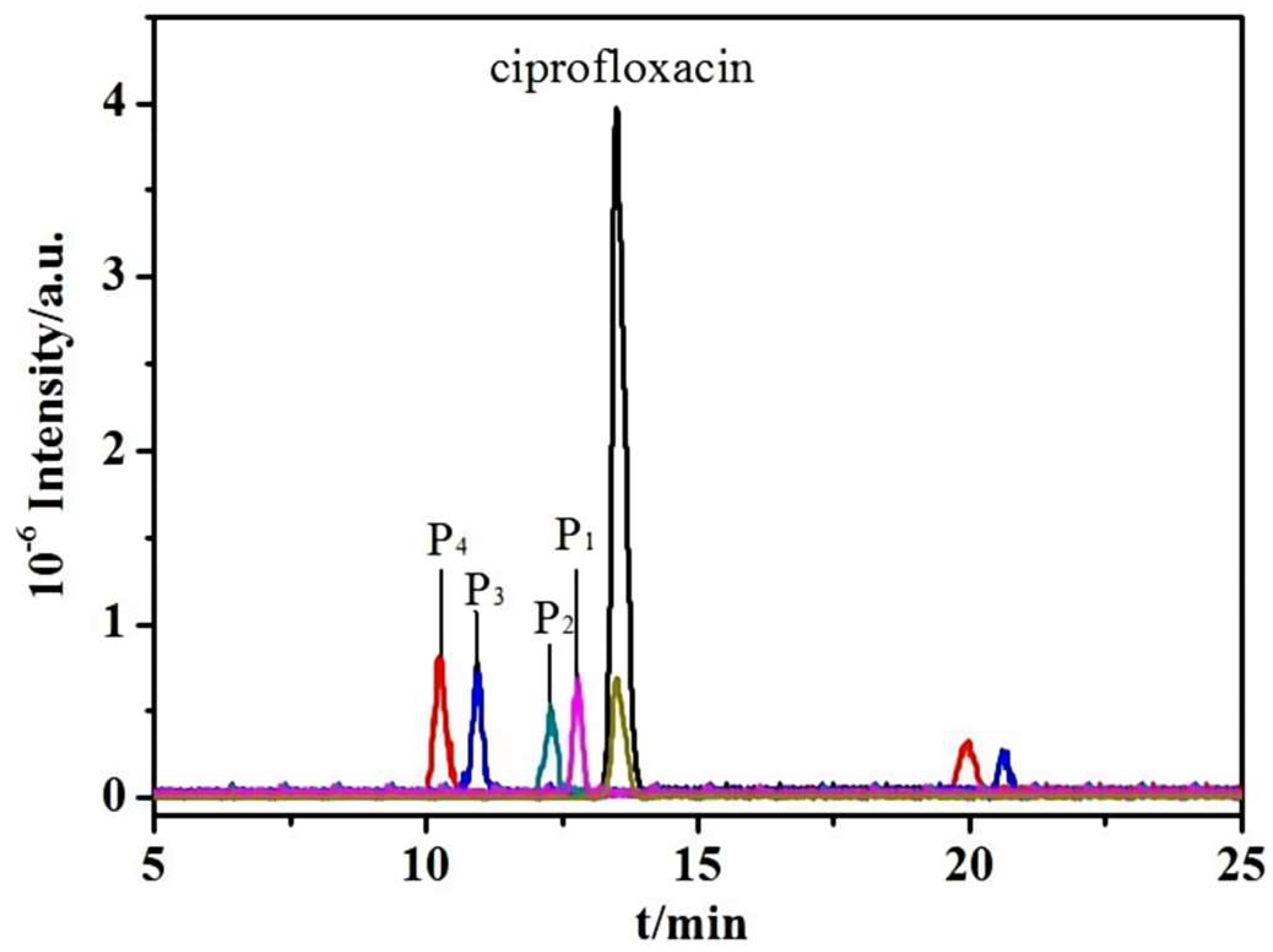
2.5. Photocatalytic Performance Analysis
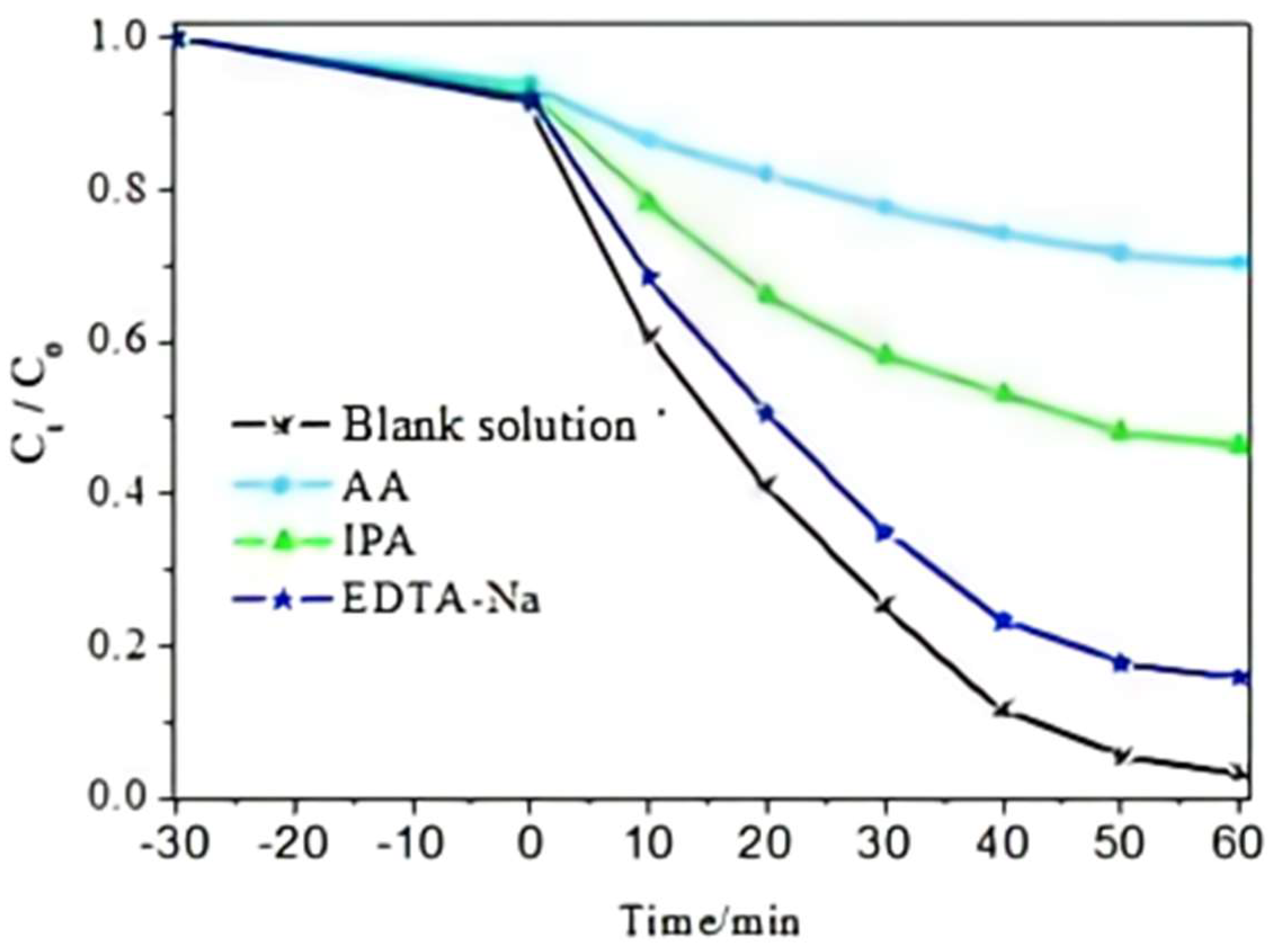
2.6. Analysis of Photocatalytic Mechanism
3. Experiment
3.1. Materials and Methods
3.2. Sample Preparation
3.3. Sample Characterization
3.4. Photocatalytic Degradation of Antibiotics
3.5. Photoelectric Performance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huyan, J.; Tian, Z.; Zhang, Y.; Zhang, H.; Shi, Y.; Gillings, M.R.; Yang, M. Dynamics of class 1 integrons in aerobic biofilm reactors spiked with antibiotics. Environ. Int. 2020, 140, 105816. [Google Scholar] [CrossRef] [PubMed]
- Ovis-Sánchez, J.O.; Perera-Pérez, V.D.; Buitrón, G.; Quintela-Baluja, M.; Graham, D.W.; Morales-Espinosa, R.; Carrillo-Reyes, J. Exploring resistomes and microbiomes in pilot-scale microalgae-bacteria wastewater treatment systems for use in low-resource settings. Sci. Total Environ. 2023, 882, 163545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Fang, C.; Zhang, Y.; Xu, Z.; Yan, Z.; Yao, K. Enhanced photocatalytic removal of antibiotics over graphitic carbon nitride induced by acetic acid post-treatment. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131165. [Google Scholar] [CrossRef]
- Xu, Z.; Jia, Y.; Huang, B.; Zhao, D.; Long, X.; Hu, S.; Li, C.; Dao, G.; Chen, B.; Pan, X. Spatial distribution, pollution characteristics, and health risks of antibiotic resistance genes in China: A review. Environ. Chem. Lett. 2023, 21, 2285–2309. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, H. Insights into the pathways, intermediates, influence factors and toxicological properties in the degradation of tetracycline by TiO2-based photocatalysts. J. Environ. Chem. Eng. 2023, 11, 110587. [Google Scholar] [CrossRef]
- Bian, C.; Zhou, B.; Mo, F.; Liu, X.; Sun, P.; Dong, X. Post-synthetically covalent modification of g-C3N4 to regulate electronic structure and investigation of photocatalytic activity for eliminating antibiotics. Sep. Purif. Technol. 2023, 325, 124556. [Google Scholar] [CrossRef]
- Kakhki, S.; Khiadani, M.; Naderi, K.; Forough, M.; Kholghi, V.; Yektay, S. Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: A systematic review. Sci. Total Environ. 2022, 811, 151404. [Google Scholar] [CrossRef]
- Molaei, M.J. Recent advances in hydrogen production through photocatalytic water splitting: A review. Fuel 2024, 365, 131159. [Google Scholar] [CrossRef]
- Ren, C.; Li, Q.; Ling, C.; Wang, J. Mechaniam-Guided Design of Photocatalysts for CO2 Reduction toward Multiarbon Products. J. Am. Chem. Soc. 2023, 145, 28276. [Google Scholar] [CrossRef]
- Si, S.; Shou, H.; Mao, Y.; Bao, X.; Zhai, G.; Song, K.; Wang, Z.; Wang, P.; Liu, Y.; Zheng, Z.; et al. Low-Coordination Single Au Atoms on Ultrathin ZnIn2S4 Nanosheets for Selective Photocatalytic CO2 Reduction towards CH4. Angew. Chem. Int. Ed. 2022, 61, e202209446. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Sun, Y.; Zhao, Z.; Zhou, Y.; Feng, X.; Wu, Z. A semimetal bismuth element as a direct plasmonic photocatalyst. Chem. Commun. 2014, 50, 10386–10389. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xiong, T.; Yan, S.; Wang, H.; Sun, Y.; Zhang, Y.; Huang, H.; Wu, Z. Facets and defects cooperatively promote visible light plasmonic photocatalysis with Bi nanowires@BiOCl nanosheets. J. Catal. 2016, 344, 401–410. [Google Scholar] [CrossRef]
- Qu, L.L.; Luo, Z.J.; Tang, C. One Step Synthesis of Bi@Bi2O3@Carboxylate-rich Carbon Spheres with Enhanced Photocatalytic Performance. Mater. Res. Bull. 2013, 48, 4601–4605. [Google Scholar] [CrossRef]
- Li, L.; Gao, H.; Yi, Z.; Wang, S.; Wu, X.; Li, R.; Yang, H. Comparative investigation on synthesis, morphological tailoring and photocatalytic activities of Bi2O2CO3 nanostructures. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128758. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Huang, B.; Yang, K.; Zhang, X.; Qin, X.; Dai, Y. Preparation, electronic structure, and photocatalytic properties of Bi2O2CO3 nanosheet. Appl. Surf. Sci. 2010, 257, 172–175. [Google Scholar] [CrossRef]
- Cen, W.; Xiong, T.; Tang, C.; Yuan, S.; Dong, F. Effects of morphology and crystallinity on the photocatalytic activity of (BiO)2CO3 nano/microstructures. Ind. Eng. Chem. Res. 2014, 53, 15002–15011. [Google Scholar] [CrossRef]
- Nie, J.; Gao, J.; Shen, Q.; Zhang, W.; Rao, F.; Hojamberdiev, M.; Zhu, G.Q. Flower-like Bi0/CeO2−δ plasmonic photocatalysts with enhanced visible-light-induced photocatalytic activity for NO removal. Sci. China Mater. 2020, 63, 2272–2280. [Google Scholar] [CrossRef]
- Li, Y.; Cao, T.; Mei, Z.; Li, X.; Sun, D. Separating type I heterojunction of NaBi(MoO4)2/Bi2MoO6 by TiO2 nanofibers for enhanced visible-photocatalysis. Chem. Phys. 2020, 533, 110696. [Google Scholar] [CrossRef]
- Qin, F.; Li, G.; Xiao, H.; Lu, Z.; Sun, H.; Chen, R. Large-scale synthesis of bismuth hollow nanospheres for highly efficient Cr(VI) removal. Dalton Trans. 2012, 41, 11263–11266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duang, F.; Chen, M.Q.; Xie, Y. Synthetic Bi2O2CO3 nanostructures: Novel photocatalyst with controlled special surface exposed. J. Mol. Catal. A Chem. 2010, 317, 34–40. [Google Scholar] [CrossRef]
- Huang, H.; Tian, N.; Jin, S.; Zhang, Y.; Wang, S. Syntheses, characterization and nonlinear optical properties of a bismuth subcarbonate Bi2O2CO3. Solid State Sci. 2014, 30, 1–5. [Google Scholar] [CrossRef]
- Pang, B.; Miao, J.; Wang, H.; Wu, C.; Wu, L.; Yuan, G.; Wang, X. Construction of fast charge-transferred 0D/2D BiOBr/Bi2WO6 S-scheme heterojunction with enhanced photocatalytic performance. Appl. Surf. Sci. 2024, 649, 159104. [Google Scholar] [CrossRef]
- Song, D.; Li, M.; Yang, F.; Yu, M.; Li, Z.; Chen, J.; Zhang, X.; Zhou, W. Plasmon Bi in-situ anchored on BiOCl nanosheets assembled microspheres towards optimized photothermal-photocatalytic performance. Chin. Chem. Lett. 2024, 35, 108591. [Google Scholar] [CrossRef]
- Giovannini, T.; Bonatti, L.; Lafiosca, P.; Nicoli, L.; Castagnola, M.; Illobre, P.G.; Corni, S.; Cappelli, C. Do we really need quantum mechanics to describe plasmonic properties of metal nanostructures? ACS Photonics 2022, 9, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Q.; Chen, C.; Jiang, H.; Su, F.; Hu, Q.; Guo, Z. Microwave-hydrothermal synthesis of beta-bismuth (III) oxide nanopowders and their enhanced photocatalytic properties. Powder Technol. 2020, 370, 226–236. [Google Scholar] [CrossRef]
- Dong, F.; Li, Q.; Sun, Y.; Ho, W.K. Noble metal-like behavior of plasmonic Bi particles as a cocatalyst deposited on (BiO)2CO3 microspheres for efficient visible light photocatalysis. ACS Catal. 2014, 4, 4341–4350. [Google Scholar] [CrossRef]
- McMahon, J.M.; Schatz, G.C.; Gray, S.K. Plasmonics in the ultraviolet with the poor metals Al, Ga, In, Sn, Tl, Pb, and Bi. Phys. Chem. Chem. Phys. 2013, 15, 5415–5423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, C.; Huang, R.; Peng, H.; Tang, X. Investigation of optical and photocatalytic properties of bismuth nanospheres prepared by a facile thermolysis method. J. Phys. Chem. C 2014, 118, 1155–1160. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Cao, T.; Du, J.; Qi, X.; Yan, H.; Xu, X. The Bi-Modified (BiO)2CO3/TiO2 Heterojunction Enhances the Photocatalytic Degradation of Antibiotics. Catalysts 2025, 15, 56. https://doi.org/10.3390/catal15010056
Gao Y, Cao T, Du J, Qi X, Yan H, Xu X. The Bi-Modified (BiO)2CO3/TiO2 Heterojunction Enhances the Photocatalytic Degradation of Antibiotics. Catalysts. 2025; 15(1):56. https://doi.org/10.3390/catal15010056
Chicago/Turabian StyleGao, Yue, Tieping Cao, Jinfeng Du, Xuan Qi, Hao Yan, and Xuefeng Xu. 2025. "The Bi-Modified (BiO)2CO3/TiO2 Heterojunction Enhances the Photocatalytic Degradation of Antibiotics" Catalysts 15, no. 1: 56. https://doi.org/10.3390/catal15010056
APA StyleGao, Y., Cao, T., Du, J., Qi, X., Yan, H., & Xu, X. (2025). The Bi-Modified (BiO)2CO3/TiO2 Heterojunction Enhances the Photocatalytic Degradation of Antibiotics. Catalysts, 15(1), 56. https://doi.org/10.3390/catal15010056





