The Organic-Functionalized Silica Nanoparticles as Lipase Carriers for Biocatalytic Application: Future Perspective in Biodegradation
Abstract
1. Introduction
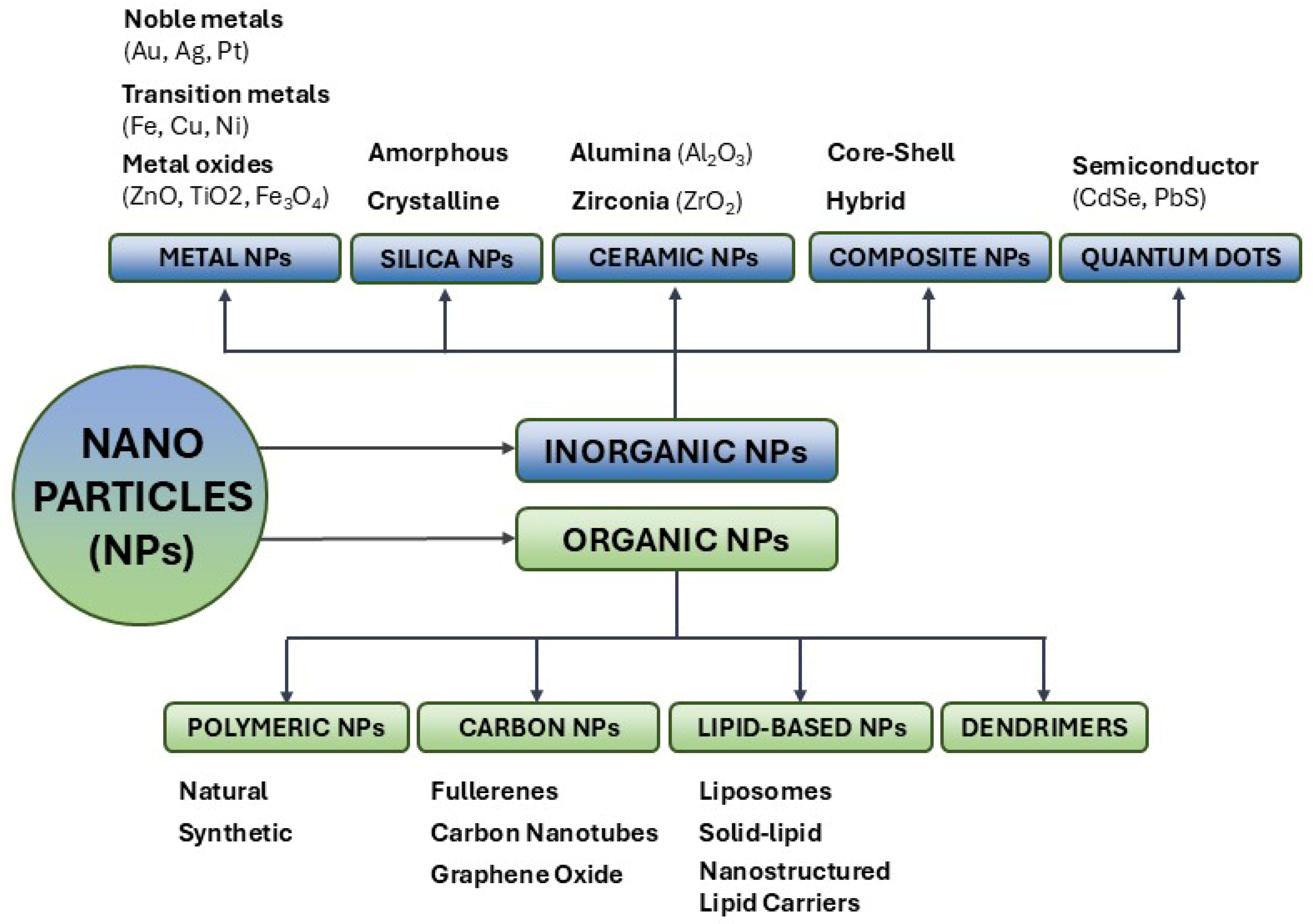
2. Nanoparticles as Support for Lipase Immobilization
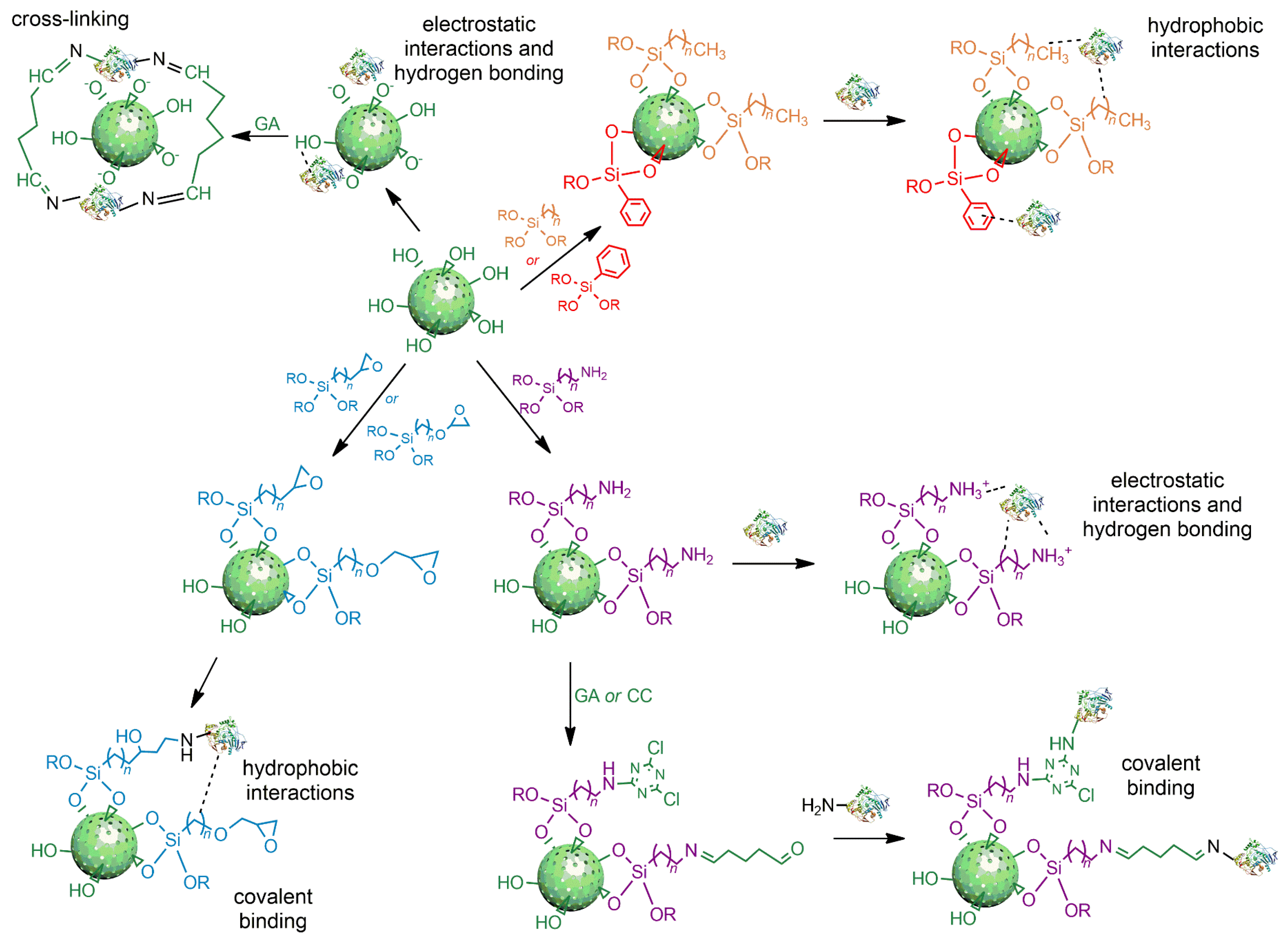
2.1. Silica Nanoparticles
2.2. Strategies for Lipase Immobilization on Silica Nanoparticles
| Support | Lipase Source | Modification | Immobilization Method | Application | Ref |
|---|---|---|---|---|---|
| f-SiNPs | CAL B | - | Adsorption | Geranyl acetate synthesis | [46] |
| f-SiNPs | CAL B | - | Adsorption | Geranyl acetate synthesis | [49] |
| w-SiNPs | CRL | - | Adsorption | Biodiesel | [50] |
| M-SiNPs | BCL | - | Adsorption | Biodiesel | [51] |
| SiNPs | CRL | - | Adsorption | Racemization of ibuprofen | [52] |
| N-SiNPs | CRL | →APTES; →APTES → CC | Adsorption + covalent | p-NPP hydrolysis | [56] |
| M-SiNPs | PPL | →APTES | Adsorption | Triacetin hydrolysis | [53] |
| SiNPs | TLL | →APTES; →OCTES | Adsorption | Cetyl octanoate, cetyl stearate, and cetyl oleate synthesis | [54] |
| M-SiNPs | RML | →APTMS → AA → CiC | covalent | Racemization of ibuprofen | [83] |
| M-SiN (flower) | CAL | →APTMS | Adsorption | Ethyl levulinate synthesis | [55] |
| M-SiNPs | TLL | →PEI | Adsorption | Ethyl valerate synthesis (apple flavor) | [57] |
| M-SiNPs | CRL | →APTES → GA | Cross-linking | Olive oil hydrolysis | [58] |
| SiNPs (virus like) | CAL B | →APTES → GA | Cross-linking | Lauryl levulinate synthesis | [84] |
| SiNPs | CRL | →APTES → GA | Cross-linking | Racemization of naproxen | [59] |
| Fe3O4@SiNPs | NS81006 from ANL | →APTES → GA; →MPTMS → GA | Cross-linking | Biodiesel | [60] |
| Fe3O4@SiNPs | CRL | →APTES → GA | Cross-linking | p-NPA hydrolysis | [61] |
| Fe3O4@SiNP/GO | CRL | →APTES → GA | Cross-linking | Ethyl valerate synthesis | [62] |
| Fe3O4@SiNPs | BCL | →GA | Cross-linking | Biodiesel | [63] |
| Fe3O4@SiNPs | PPL | →APTES → GA | Cross-linking | p-NPP hydrolysis | [64] |
| SiNPs | CAL B | →GA | Cross-linking | p-NPP hydrolysis | [85] |
| Fe3O4@SiNPs | ROL | →APTES → GA | Covalent | Biodiesel | [65] |
| SiNPs | RNL | →ClPTES | Adsorption | Quinizarin diester hydrolysis | [66] |
| h-SiNPs | CRL | →TMPS →TMPhS →TMOS →TMODS | Adsorption | Phytosterol ester synthesis | [67] |
| Fe3O4@SiNPs | BL sp. | →TPDACl | Adsorption | Biodiesel | [68] |
| w-SiNPs | CRL | →PDTES | Adsorption | Biodiesel | [69] |
| f-SiNPs | CRL | →GOPTMS | Covalent | Flavor ester synthesis | [76] |
| M-SiNPs | CALB; TLL; RML | →GOPTMS | Covalent | Biodiesel | [74] |
| SiNPs | TLL | →OTMS + GOPMDMS | Covalent | Biodiesel | [77] |
| SiNPs | CRL | → pGMA →pGMA → Cys(EDC+NHS) | Covalent | p-NPP hydrolysis | [78] |
| SiNPs | CRL | APTES→BIBB → p(GMA-co-SBMA) | Covalent and adsorption | p-NPP hydrolysis | [79] |
| SiNPs | RML | →GOPTMS | Covalent | Fish oil hydrolysis | [72] |
| Fe3O4@SiNPs | CAL B | →GOPTMS | Covalent | Biodiesel | [75] |
| M-SiNPs | CAL B RML | →GOPTMS | Covalent | Racemization of ibuprofen | [73] |
| Fe3O4@SiNPs | CRL | →GMA | Covalent | Olive oil hydrolysis | [80] |
| M-SiNPs | CAL B | →CPTES → H2OS4 | Covalent (EDC+NHS) | Tributyrin hydrolysis | [81] |
| Fe3O4@SiNPs | PPL | →APTES → SA | Covalent (EDC+NHS) | Inhibitor screening | [82] |
| SiNPs Fe3O4@SiNPs | FE-01 from TLL | →APTMS → GA | Covalent | Degradation of PCL | [24] |
| SiNPs | BCL | →GOPTMS; →APTMS → HDGE | Covalent | Degradation of PCL | [23] |
| M-SiNPs | PPL | →APTES → GA; →APTES → DTMS → GA; →DTMS → GA; →APTMS → HDGE, →GOPTMS; →GOPMTS → PEI → GA, | Covalent | Synthesis of biodegradable polymer | [86] |
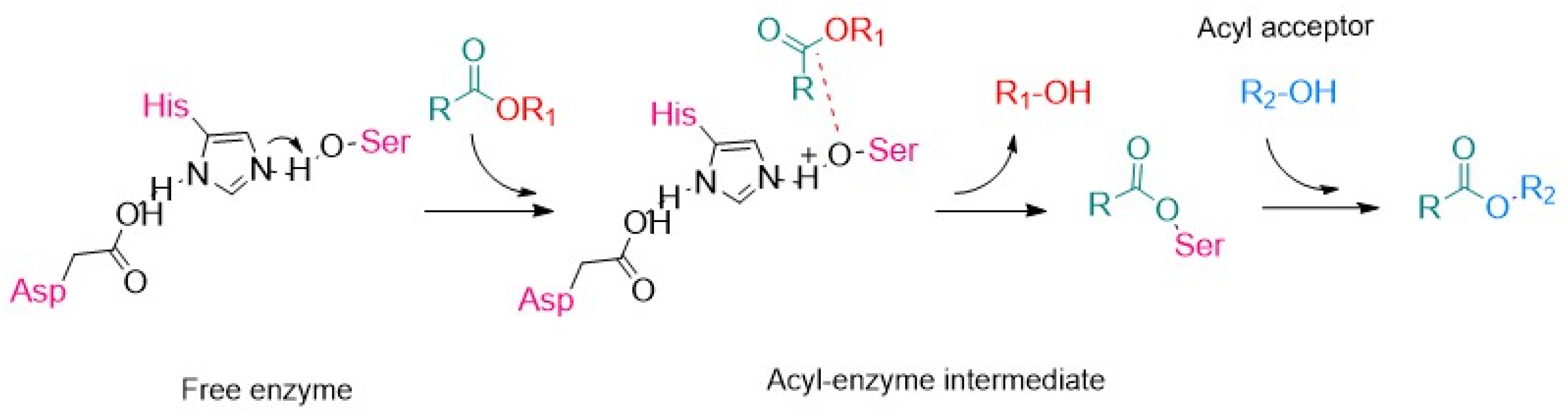
3. Application of Lipase SiNPs
3.1. Biodiesel Production
3.2. Flavor Esters Production
3.3. Racemizations
3.4. Synthesis of Antioxidants
3.5. Polymer Biodegradation
3.6. Polymerizations
3.7. Miscellaneous Applications
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kulkarni, S. Enzymes as Biocatalysts: Review on Investigations on Synthesis, Mechanism, Kinetics, Applications and Potential. Lett. Appl. NanoBioSci. 2022, 11, 3049–3064. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Prim. 2021, 1, 46. [Google Scholar] [CrossRef]
- Pyser, J.B.; Chakrabarty, S.; Romero, E.O.; Narayan, A.R.H. State-of-the-Art Biocatalysis. ACS Cent. Sci. 2021, 7, 1105–1116. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Wang, J.; Wilson, L.M.; Yan, Y. Protein Engineering for Improving and Diversifying Natural Products Biosynthesis. Trends Biotechnol. 2020, 38, 729–744. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, L.; Geng, Z.; Huo, Z.; Li, H.; Shen, X.; Peng, X.; Yan, R.; Cui, J.; Jia, S. Construction of Catalase@ hollow Silica Nanosphere: Catalase with Immobilized but Not Rigid State for Improving Catalytic Performances. Int. J. Biol. Macromol. 2024, 263, 130381. [Google Scholar] [CrossRef]
- Cao, L. Introduction: Immobilized Enzymes: Past, Present and Prospects; Wiley-VCH: Weinheim, Germany, 2005; ISBN 3527312323. [Google Scholar]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Spasojević, M.; Prodanović, O.; Pantić, N.; Popović, N.; Balaž, A.M.; Prodanović, R. The Enzyme Immobilization: Carriers and Immobilization Methods. J. Eng. Process. Manag. 2019, 11, 89–105. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.H. Lipase Immobilization with Support Materials, Preparation Techniques, and Applications: Present and Future Aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Mihailović, M.; Stojanović, M.; Banjanac, K.; Carević, M.; Prlainović, N.; Milosavić, N.; Bezbradica, D. Immobilization of Lipase on Epoxy-Activated Purolite® A109 and Its Post-Immobilization Stabilization. Process Biochem. 2014, 49, 637–646. [Google Scholar] [CrossRef]
- Prlainović, N.Ž.; Knežević-Jugović, Z.D.; Mijin, D.Ž.; Bezbradica, D.I. Immobilization of Lipase from Candida Rugosa on Sepabeads®: The Effect of Lipase Oxidation by Periodates. Bioprocess Biosyst. Eng. 2011, 34, 803–810. [Google Scholar] [CrossRef]
- Prlainović, N.; Milovanović, J.S.; Milašinović, N.Z.; Bezbradica, D.I.; Mijin, D. Multi-Walled Carbon Nanotubes as Lipase Carriers for Organic Synthesis: Current Trends and Recent Update. Hem. Ind. 2024, 78, 1–16. [Google Scholar] [CrossRef]
- Uddin, M.N.; Desai, F.; Asmatulu, E. Engineered Nanomaterials in the Environment: Bioaccumulation, Biomagnification and Biotransformation. Environ. Chem. Lett. 2020, 18, 1073–1083. [Google Scholar] [CrossRef]
- Ayub, J.; Saeed, M.U.; Hussain, N.; Zulfiqar, I.; Mehmood, T.; Iqbal, H.M.N.; Bilal, M. Designing Robust Nano-Biocatalysts Using Nanomaterials as Multifunctional Carriers—Expanding the Application Scope of Bio-Enzymes. Top. Cataysis 2023, 66, 625–648. [Google Scholar] [CrossRef]
- Bilal, M.; Fernandes, C.D.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Ferreira, L.F.R. Immobilized Lipases-Based Nano-Biocatalytic Systems—A Versatile Platform with Incredible Biotechnological Potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef]
- Bitar, A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Silica-Based Nanoparticles for Biomedical Applications. Drug Discov. Today 2012, 17, 1147–1154. [Google Scholar] [CrossRef]
- Prlainović Nevena, Ž.; Bezbradica Dejan, I.; Knežević-Jugović Zorica, D.; Kozlowska Roksana, T.; Mijin Dušan, Ž. A Kinetic Study of Candida Rugosa Lipase-Catalyzed Synthesis of 4,6-Dimethyl-3-Cyano-2-Pyridone. J. Braz. Chem. Soc. 2010, 21, 2285–2293. [Google Scholar] [CrossRef]
- Prlainović, N.Ž.; Bezbradica, D.I.; Knežević-Jugović, Z.D.; Marinković, A.D.; Mijin, D.Ž. Imobilizacija Enzima Na Ugljenične Nanocevi. Hem. Ind. 2011, 65, 423–430. [Google Scholar] [CrossRef]
- Prlainović, N.Ž.; Bezbradica, D.I.; Knežević-Jugović, Z.D.; Veličković, D.V.; Mijin, D.Ž. Enzymatic Synthesis of a Vitamin B6 Precursor. J. Serbian Chem. Soc. 2013, 78, 1491–1501. [Google Scholar] [CrossRef]
- Milašinović, N.; Jakovetić, S.; Knežević-Jugović, Z.; Milosavljević, N.; Lučić, M.; Filipović, J.; Kalagasidis Krušić, M. Catalyzed Ester Synthesis Using Candida Rugosa Lipase Entrapped by Poly(N-Isopropylacrylamide-Co-Itaconic Acid) Hydrogel. Sci. World J. 2014, 2014, 142123. [Google Scholar] [CrossRef]
- Milašinović, N.; Knežević-Jugović, Z.; Jakovljević, Ž.; Filipović, J.; Kalagasidis Krušić, M. Synthesis of N-Amyl Isobutyrate Catalyzed by Candida Rugosa Lipase Immobilized into Poly(N-Isopropylacrylamide-Co-Itaconic Acid) Hydrogels. Chem. Eng. J. 2012, 181–182, 614–623. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Lock, S.S.M.; Yap, P.S.; Cheah, K.W.; Chan, Y.H.; Yiin, C.L.; Ku, A.Z.E.; Loy, A.C.M.; Chin, B.L.F.; Chai, Y.H. Immobilized Enzyme/Microorganism Complexes for Degradation of Microplastics: A Review of Recent Advances, Feasibility and Future Prospects. Sci. Total Environ. 2022, 832, 154868. [Google Scholar] [CrossRef] [PubMed]
- Hegyesi, N.; Balogh-Weiser, D.; Pukánszky, B. Covalent Immobilization of an Enzyme on a Layered Silicate to Catalyze the Self-Degradation of PCL. Polym. Degrad. Stab. 2024, 229, 111003. [Google Scholar] [CrossRef]
- Krakor, E.; Gessner, I.; Wilhelm, M.; Brune, V.; Hohnsen, J.; Frenzen, L.; Mathur, S. Selective Degradation of Synthetic Polymers through Enzymes Immobilized on Nanocarriers. MRS Commun. 2021, 11, 363–371. [Google Scholar] [CrossRef]
- Gao, R.; Pan, H.; Kai, L.; Han, K. Microbial Degradation and Valorization of Poly(Ethylene Terephthalate) (PET) Monomers. World J. Microbiol. Biotechnol. 2022, 38, 89. [Google Scholar] [CrossRef]
- Dwivedee, B.P.; Soni, S.; Sharma, M.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Promiscuity of Lipase-Catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect 2018, 3, 2441–2466. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Yang, Y.; Yu, C. Silica-Based Nanoparticles for Enzyme Immobilization and Delivery. Chem. Asian J. 2022, 17, e202200573. [Google Scholar] [CrossRef]
- Thakur, K.; Attri, C.; Seth, A. Nanocarriers-Based Immobilization of Enzymes for Industrial Application. 3 Biotech 2021, 11, 427. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Hu, H.; Xu, J.; Wang, Z.; Du, Y.; Cui, J.; Jia, S. Enhanced Enzymatic Performance of Immobilized Lipase on Metal Organic Frameworks with Superhydrophobic Coating for Biodiesel Production. J. Colloid Interface Sci. 2021, 602, 426–436. [Google Scholar] [CrossRef]
- Shuai, W.; Kumar Das, R.; Naghdi, M.; Kaur Brar, S.; Verma, M. A Review on the Important Aspects of Lipase Immobilization on Nanomaterials. Biotechnol. Appl. Biochem. 2017, 64, 496–508. [Google Scholar] [CrossRef]
- Kuang, G.; Du, Y.; Lu, S.; Wang, Z.; Zhang, Z.; Fan, X.; Bilal, M.; Cui, J.; Jia, S. Silica@lipase Hybrid Biocatalysts with Superior Activity by Mimetic Biomineralization in Oil/Water Two-Phase System for Hydrolysis of Soybean Oil. LWT 2022, 160, 113333. [Google Scholar] [CrossRef]
- Cui, J.; Feng, Y.; Jia, S. Silica Encapsulated Catalase@metal-Organic Framework Composite: A Highly Stable and Recyclable Biocatalyst. Chem. Eng. J. 2018, 351, 506–514. [Google Scholar] [CrossRef]
- Zhong, L.; Jiao, X.; Hu, H.; Shen, X.; Zhao, J.; Feng, Y.; Li, C.; Du, Y.; Cui, J.; Jia, S. Activated Magnetic Lipase-Inorganic Hybrid Nanoflowers: A Highly Active and Recyclable Nanobiocatalyst for Biodiesel Production. Renew. Energy 2021, 171, 825–832. [Google Scholar] [CrossRef]
- Hajareh Haghighi, F.; Binaymotlagh, R.; Palocci, C.; Chronopoulou, L. Magnetic Iron Oxide Nanomaterials for Lipase Immobilization: Promising Industrial Catalysts for Biodiesel Production. Catalysts 2024, 14, 336. [Google Scholar] [CrossRef]
- Li, C.; Zhao, J.; Zhang, Z.; Jiang, Y.; Bilal, M.; Jiang, Y.; Jia, S.; Cui, J. Self-Assembly of Activated Lipase Hybrid Nanoflowers with Superior Activity and Enhanced Stability. Biochem. Eng. J. 2020, 158, 107582. [Google Scholar] [CrossRef]
- Maharramov, A.M.; Hasanova, U.A.; Suleymanova, I.A.; Osmanova, G.E.; Hajiyeva, N.E. The Engineered Nanoparticles in Food Chain: Potential Toxicity and Effects. SN Appl. Sci. 2019, 1, 1362. [Google Scholar] [CrossRef]
- Li, S.F.; Chen, J.P.; Wu, W.T. Electrospun Polyacrylonitrile Nanofibrous Membranes for Lipase Immobilization. J. Mol. Catal. B Enzym. 2007, 47, 117–124. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, J.; Salmon, S. Developing Enzyme Immobilization with Fibrous Membranes: Longevity and Characterization Considerations. Membranes 2023, 13, 532. [Google Scholar] [CrossRef]
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-Property Relationship for Different Mesoporous Silica Nanoparticles and Its Drug Delivery Applications: A Review. Front. Chem. 2022, 10, 823785. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Redhi, G.G. Green Synthesis of Metal Nanoparticles and Its Reaction Mechanisms. In Macabresque Humman Violation Hate Genocide, Mass Atrocity Enemy-Making; Oxford University Press: Oxford, UK, 2018; pp. 113–139. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.Y. Biosynthesis of Inorganic Nanomaterials Using Microbial Cells and Bacteriophages. Nat. Rev. Chem. 2020, 4, 638–656. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of Enzymes on Nanoinorganic Support Materials: An Update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Rogowska, M.; Dubis, A.; Szymański, K. Enzymes Immobilization on Fe3O4–Gold Nanoparticles. Appl. Surf. Sci. 2012, 258, 2783–2787. [Google Scholar] [CrossRef]
- Sonmez, M.; Georgescu, M.; Alexandrescu, L.; Gurau, D.; Ficai, A.; Ficai, D.; Andronescu, E. Synthesis and applications of Fe3O4/SiO2 core-shell materials. Curr. Pharm. Des. 2015, 21, 5324–5335. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Kleitz, F.; Linden, M.; Yu, C.; Popat, A. Silica Nanoparticles: A Review of Their Safety and Current Strategies to Overcome Biological Barriers. Adv. Drug Deliv. Rev. 2023, 203, 115115. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.C.; Pfromm, P.H.; Rezac, M.E. Immobilization of Candida Antarctica Lipase B on Fumed Silica. Process Biochem. 2009, 44, 62–69. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Li, T. Cheng Jianjun Nonporous Silica Nanoparticles for Nanomedicine Application. Nano Today 2013, 8, 290–312. [Google Scholar] [CrossRef]
- Cruz, J.C.; Würges, K.; Kramer, M.; Pfromm, P.H.; Rezac, M.E.; Czermak, P. Immobilization of Enzymes on Fumed Silica Nanoparticles for Applications in Nonaqueous Media. Methods Mol. Biol. 2011, 743, 147–160. [Google Scholar] [CrossRef]
- Pang, J.; Zhou, G.; Liu, R.; Li, T. Esterification of Oleic Acid with Methanol by Immobilized Lipase on Wrinkled Silica Nanoparticles with Highly Ordered, Radially Oriented Mesochannels. Mater. Sci. Eng. C 2016, 59, 35–42. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, W.; Zhou, L.; Ma, L.; He, Y.; Gao, J. Improved Performance of Lipase Immobilized on Tannic Acid-Templated Mesoporous Silica Nanoparticles. Appl. Biochem. Biotechnol. 2016, 179, 1155–1169. [Google Scholar] [CrossRef]
- Ghofrani, S.; Allameh, A.; Yaghmaei, P.; Norouzian, D. Immobilization of Candida Rugosa Lipase for Resolution of Racimic Ibuprofen. DARU J. Pharm. Sci. 2021, 29, 117–123. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, G.; Xu, Y.; Chen, J. Porcine pancreatic Lipase Immobilized in Amino-Functionalized Short Rod-Shaped Mesoporous Silica Prepared Using Poly(Ethylene Glycol) and Triblock Copolymer as Templates. J. Phys. Chem. C 2011, 115, 22191–22199. [Google Scholar] [CrossRef]
- Machado, N.B.; Miguez, J.P.; Bolina, I.C.A.; Salviano, A.B.; Gomes, R.A.B.; Tavano, O.L.; Luiz, J.H.H.; Tardioli, P.W.; Cren, É.C.; Mendes, A.A. Preparation, Functionalization and Characterization of Rice Husk Silica for Lipase Immobilization via Adsorption. Enzyme Microb. Technol. 2019, 128, 9–21. [Google Scholar] [CrossRef]
- Jia, B.; Liu, C.; Qi, X. Selective Production of Ethyl Levulinate from Levulinic Acid by Lipase-Immobilized Mesoporous Silica Nanoflowers Composite. Fuel Process. Technol. 2020, 210, 106578. [Google Scholar] [CrossRef]
- Banjanac, K.; Mihailović, M.; Prlainović, N.; Stojanović, M.; Carević, M.; Marinković, A.; Bezbradica, D. Cyanuric Chloride Functionalized Silica Nanoparticles for Covalent Immobilization of Lipase. J. Chem. Technol. Biotechnol. 2016, 91, 439–448. [Google Scholar] [CrossRef]
- Sadighi, A.; Motevalizadeh, S.F.; Hosseini, M.; Ramazani, A.; Gorgannezhad, L.; Nadri, H.; Deiham, B.; Ganjali, M.R.; Shafiee, A.; Faramarzi, M.A.; et al. Metal-Chelate Immobilization of Lipase onto Polyethylenimine Coated MCM-41 for Apple Flavor Synthesis. Appl. Biochem. Biotechnol. 2017, 182, 1371–1389. [Google Scholar] [CrossRef]
- Ali, Z.; Tian, L.; Zhao, P.; Zhang, B.; Ali, N.; Khan, M.; Zhang, Q. Immobilization of Lipase on Mesoporous Silica Nanoparticles with Hierarchical Fibrous Pore. J. Mol. Catal. B Enzym. 2016, 134, 129–135. [Google Scholar] [CrossRef]
- Song, Y.S.; Lee, H.U.; Lee, J.H.; Park, C.; Kim, S.W. Enzyme-Catalyzed Resolution of Racemate Using Enzyme Functionalized Silica Nanoparticles in the Presence of Surfactants. Process Biochem. 2011, 46, 817–820. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Effect of Silica Coating on Fe3O4 Magnetic Nanoparticles for Lipase Immobilization and Their Application for Biodiesel Production. Arab. J. Chem. 2019, 12, 4694–4706. [Google Scholar] [CrossRef]
- Nikolić, M.P.; Pavlović, K.V.; Stanojević-Nikolić, S.; Maričić, A.; Srdić, V.V. Synthesis and Characterization of Silica Core/Multilayered Cobalt Ferrite-Silica Shell Particles for Lipase Immobilization. Mater. Res. 2021, 24, e20210130. [Google Scholar] [CrossRef]
- Jacob, A.G.; Wahab, R.A.; Mahat, N.A. Ternary Biogenic Silica/Magnetite/Graphene Oxide Composite for the Hyperactivation of Candida Rugosa Lipase in the Esterification Production of Ethyl Valerate. Enzyme Microb. Technol. 2021, 148, 109807. [Google Scholar] [CrossRef]
- Karimi, M. Immobilization of Lipase onto Mesoporous Magnetic Nanoparticles for Enzymatic Synthesis of Biodiesel. Biocatal. Agric. Biotechnol. 2016, 8, 182–188. [Google Scholar] [CrossRef]
- Ranjbakhsh, E.; Bordbar, A.K.; Abbasi, M.; Khosropour, A.R.; Shams, E. Enhancement of Stability and Catalytic Activity of Immobilized Lipase on Silica-Coated Modified Magnetite Nanoparticles. Chem. Eng. J. 2012, 179, 272–276. [Google Scholar] [CrossRef]
- Esmi, F.; Nematian, T.; Salehi, Z.; Khodadadi, A.A.; Dalai, A.K. Amine and Aldehyde Functionalized Mesoporous Silica on Magnetic Nanoparticles for Enhanced Lipase Immobilization, Biodiesel Production, and Facile Separation. Fuel 2021, 291, 120126. [Google Scholar] [CrossRef]
- Sabatini, C.A.; Gehlen, M.H. Enzymatic Hydrolysis of Quinizarin Diester by Lipase in Silica Nanoparticles Investigated by Fluorescence Microscopy. J. Nanopart. Res. 2014, 16, 2093. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, M.Y.; Shi, J.; Zheng, M.M.; Huang, F.H. Preparation of Immobilized Lipase Based on Hollow Mesoporous Silica Spheres and Its Application in Ester Synthesis. Molecules 2019, 24, 395. [Google Scholar] [CrossRef]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Immobilization of Burkholderia Sp. Lipase on a Ferric Silica Nanocomposite for Biodiesel Production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef]
- Pota, G.; Bifulco, A.; Parida, D.; Zhao, S.; Rentsch, D.; Amendola, E.; Califano, V.; Costantini, A. Tailoring the Hydrophobicity of Wrinkled Silica Nanoparticles and of the Adsorption Medium as a Strategy for Immobilizing Lipase: An Efficient Catalyst for Biofuel Production. Microporous Mesoporous Mater. 2021, 328, 111504. [Google Scholar] [CrossRef]
- Jin, Q.; Jia, G.; Zhang, Y.; Yang, Q.; Li, C. Hydrophobic Surface Induced Activation of Pseudomonas Cepacia Lipase Immobilized into Mesoporous Silica. Langmuir 2011, 27, 12016–12024. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernández-Lorente, G.; Pedroche, J.; Fernández-Lafuente, R.; Guisan, J.M.; Tam, A.; Daminati, M. Epoxy Sepabeads: A Novel Epoxy Support for Stabilization of Industrial Enzymes via Very Intense Multipoint Covalent Attachment. Biotechnol. Prog. 2002, 18, 629–634. [Google Scholar] [CrossRef]
- Mohammadi, M.; Habibi, Z.; Dezvarei, S.; Yousefi, M.; Samadi, S.; Ashjari, M. Improvement of the Stability and Selectivity of Rhizomucor Miehei Lipase Immobilized on Silica Nanoparticles: Selective Hydrolysis of Fish Oil Using Immobilized Preparations. Process Biochem. 2014, 49, 1314–1323. [Google Scholar] [CrossRef]
- Mohammadi, M.; Gandomkar, S.; Habibi, Z.; Yousefi, M. One Pot Three-Component Reaction for Covalent Immobilization of Enzymes: Application of Immobilized Lipases for Kinetic Resolution of: Rac -Ibuprofen. RSC Adv. 2016, 6, 52838–52849. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; Yousefi, P.; Mohammadi, J.; Brask, J. Enzymatic Production of Biodiesel Using Lipases Immobilized on Silica Nanoparticles as Highly Reusable Biocatalysts: Effect of Water, t-Butanol and Blue Silica Gel Contents. Renew. Energy 2016, 91, 196–206. [Google Scholar] [CrossRef]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent Immobilization of Candida Antarctica Lipase on Core-Shell Magnetic Nanoparticles for Production of Biodiesel from Waste Cooking Oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- Banjanac, K.; Mihailović, M.; Prlainović, N.; Ćorović, M.; Carević, M.; Marinković, A.; Bezbradica, D. Epoxy-Silanization—Tool for Improvement of Silica Nanoparticles as Support for Lipase Immobilization with Respect to Esterification Activity. J. Chem. Technol. Biotechnol. 2016, 91, 2654–2663. [Google Scholar] [CrossRef]
- Cazaban, D.; Illanes, A.; Wilson, L.; Betancor, L. Bio-Inspired Silica Lipase Nanobiocatalysts for the Synthesis of Fatty Acid Methyl Esters. Process Biochem. 2018, 74, 86–93. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, C.; Liu, Y.; Dong, X.; Sun, Y. Cysteine-Modified Poly(Glycidyl Methacrylate) Grafted onto Silica Nanoparticles: New Supports for Significantly Enhanced Performance of Immobilized Lipase. Biochem. Eng. J. 2019, 145, 137–144. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, C.; Dong, X.; Sun, Y. Fabrication and Characterization of Epoxylated Zwitterionic Copolymer-Grafted Silica Nanoparticle as a New Support for Lipase Immobilization. Chin. J. Chem. Eng. 2020, 28, 1129–1135. [Google Scholar] [CrossRef]
- Lei, L.; Liu, X.; Li, Y.; Cui, Y.; Yang, Y.; Qin, G. Study on Synthesis of Poly(GMA)-Grafted Fe3O4/ SiOX Magnetic Nanoparticles Using Atom Transfer Radical Polymerization and Their Application for Lipase Immobilization. Mater. Chem. Phys. 2011, 125, 866–871. [Google Scholar] [CrossRef]
- Zhong, L.; He, C.; Xiao, C.; Yao, C.; Pyatt, I.H.; Lu, Y. Covalent Immobilization of Candida Antarctica Lipase B on Functionalized Hollow Mesoporous Silica Nanoparticles. ChemistrySelect 2021, 6, 3453–3460. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Ren, X.Y.; Liu, Y.M.; Wei, Y.; Qing, L.S.; Liao, X. Covalent Immobilization of Porcine Pancreatic Lipase on Carboxyl-Activated Magnetic Nanoparticles: Characterization and Application for Enzymatic Inhibition Assays. Mater. Sci. Eng. C 2014, 38, 278–285. [Google Scholar] [CrossRef]
- Mohammadi, M.; Habibi, Z.; Gandomkar, S.; Yousefi, M. A Novel Approach for Bioconjugation of Rhizomucor Miehei Lipase (RML) onto Amine-Functionalized Supports; Application for Enantioselective Resolution of Rac-Ibuprofen. Int. J. Biol. Macromol. 2018, 117, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Wang, L.; Zhou, L.; Huang, Z.; Ma, L.; He, Y.; Shi, L.; Gao, J. Virus-like Organosilica Nanoparticles for Lipase Immobilization: Characterization and Biocatalytic Applications. Biochem. Eng. J. 2019, 144, 125–134. [Google Scholar] [CrossRef]
- Qian, J.; Huang, A.; Zhu, H.; Ding, J.; Zhang, W.; Chen, Y. Immobilization of Lipase on Silica Nanoparticles by Adsorption Followed by Glutaraldehyde Cross-Linking. Bioprocess Biosyst. Eng. 2023, 46, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, F.; Zhuo, R. Immobilized Lipase on Porous Silica Particles: Preparation and Application for Biodegradable Polymer Syntheses in Ionic Liquid at Higher Temperature. J. Mol. Catal. B Enzym. 2013, 94, 129–135. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase Promiscuity and Its Biochemical Applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Ling, F.W.; Jun, L.S. Proposed Kinetic Mechanism of the Production of Biodiesel from Palm Oil Using Lipase. Process Biochem. 2007, 42, 951–960. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Derewenda, U.; Derewenda, Z.S.; Dodson, G.G.; Lawson, D.M.; Turkenburg, J.P.; Bjorkling, F.; Huge-Jensen, B.; Patkar, S.A.; Thim, L. A Model for Interfacial Activation in Lipases from the Structure of a Fungal Lipase-Inhibitor Complex. Nature 1991, 351, 491–494. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and Application of Lipases in Organic Media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Chang, J.-S. Biodiesel Production Using Immobilized Lipase: Feasibility and Challenges. Biofuels Bioprod. Biorefin. 2016, 10, 896–916. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and Use of Immobilized Lipases in/on Nanomaterials: A Review from the Waste to Biodiesel Production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Idris, A.; Bukhari, A. Immobilized Candida Antarctica Lipase B: Hydration, Stripping off and Application in Ring Opening Polyester Synthesis. Biotechnol. Adv. 2012, 30, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Keyhani, A.; Akram, A.; Rahman, M.; Jenkins, B.; Stroeve, P. Hybrid Response Surface Methodology-Genetic Algorithm Optimization of Ultrasound-Assisted Transesterification of Waste Oil Catalysed by Immobilized Lipase on Mesoporous Silica/Iron Oxide Magnetic Core-Shell Nanoparticles. Environ. Technol. 2013, 34, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, J. Enzymatic Production of Biodiesel from Soybean Oil by Using Immobilized Lipase on Fe3O4/Poly(Styrene-Methacrylic Acid) Magnetic Microsphere as a Biocatalyst. Energy Fuels 2014, 28, 2624–2631. [Google Scholar] [CrossRef]
- Bayout, I.; Bouzemi, N.; Guo, N.; Mao, X.; Serra, S.; Riva, S.; Secundo, F. Natural Flavor Ester Synthesis Catalyzed by Lipases. Flavour Fragr. J. 2020, 35, 209–218. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef]
- Muralidhar, R.; Marchant, R.; Nigam, P. Lipases in Racemic Resolutions. J. Chem. Technol. Biotechnol. 2001, 76, 3–8. [Google Scholar] [CrossRef]
- José, C.; Toledo, M.V.; Briand, L.E. Enzymatic Kinetic Resolution of Racemic Ibuprofen: Past, Present and Future. Crit. Rev. Biotechnol. 2016, 36, 891–903. [Google Scholar] [CrossRef]
- Torres, P.; Reyes-Duarte, D.; Ballesteros, A.; Plou, F. Lipase-Catalyzed Modification of Phenolic Antioxidants. Methods Mol. Biol. 2012, 861, 435–443. [Google Scholar]
- Qianchun, D.; Pin, Z.; Qingde, H.; Fenghong, H.; Fang, W.; Mingming, Z.; Xiao, Y.; Qi, Z.; Chang, Z. Chemical Synthesis of Phytosterol Esters of Polyunsaturated Fatty Acids with Ideal Oxidative Stability. Eur. J. Lipid Sci. Technol. 2011, 113, 441–449. [Google Scholar] [CrossRef]
- Šaraba, V.; Milovanovic, J.; Nikodinovic-Runic, J.; Budin, C.; de Boer, T.; Ciric, M. Brackish Groundwaters Contain Plastic- and Cellulose-Degrading Bacteria. Microb. Ecol. 2023, 86, 2747–2755. [Google Scholar] [CrossRef]
- Spasic, J.; Mandic, M.; Radivojevic, J.; Jeremic, S.; Vasiljevic, B.; Nikodinovic-Runic, J.; Djokic, L. Biocatalytic Potential of Streptomyces Spp. Isolates from Rhizosphere of Plants and Mycorrhizosphere of Fungi. Biotechnol. Appl. Biochem. 2018, 65, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D.N. Nanocomposites of Aliphatic Polyesters: An Overview of the Effect of Different Nanofillers on Enzymatic Hydrolysis and Biodegradation of Polyesters. Polym. Degrad. Stab. 2013, 98, 1908–1928. [Google Scholar] [CrossRef]
- Tang, C.; Wang, L.; Sun, J.; Chen, G.; Shen, J.; Wang, L.; Han, Y.; Luo, J.; Li, Z.; Zhang, P.; et al. Degradable Living Plastics Programmed by Engineered Spores. Nat. Chem. Biol. 2024. [Google Scholar] [CrossRef]
- Jeremic, S.; Milovanovic, J.; Mojicevic, M.; Bogojevic, S.S.; Nikodinovic-Runic, J. Understanding Bioplastic Materials—Current State and Trends. J. Serbian Chem. Soc. 2020, 85, 1507–1538. [Google Scholar] [CrossRef]
- Namekawa, S.; Suda, S.; Uyama, H.; Kobayashi, S. Lipase-Catalyzed Ring-Opening Polymerization of Lactones to Polyesters and Its Mechanistic Aspects. Int. J. Biol. Macromol. 1999, 25, 145–151. [Google Scholar] [CrossRef]
- He, F.; Wang, Y.; Feng, J.; Zhuo, R.; Wang, X. Synthesis of Poly[(5-Benzyloxy-Trimethylene Carbonate)-Co-(5,5-Dimethyl-Trimethylene Carbonate)] Catalyzed by Immobilized Lipase on Silica Particles with Different Size. Polymer 2003, 44, 3215–3219. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanović, J.; Banjanac, K.; Nikolić, J.; Nikodinović-Runić, J.; Prlainović, N.Ž. The Organic-Functionalized Silica Nanoparticles as Lipase Carriers for Biocatalytic Application: Future Perspective in Biodegradation. Catalysts 2025, 15, 54. https://doi.org/10.3390/catal15010054
Milovanović J, Banjanac K, Nikolić J, Nikodinović-Runić J, Prlainović NŽ. The Organic-Functionalized Silica Nanoparticles as Lipase Carriers for Biocatalytic Application: Future Perspective in Biodegradation. Catalysts. 2025; 15(1):54. https://doi.org/10.3390/catal15010054
Chicago/Turabian StyleMilovanović, Jelena, Katarina Banjanac, Jasmina Nikolić, Jasmina Nikodinović-Runić, and Nevena Ž. Prlainović. 2025. "The Organic-Functionalized Silica Nanoparticles as Lipase Carriers for Biocatalytic Application: Future Perspective in Biodegradation" Catalysts 15, no. 1: 54. https://doi.org/10.3390/catal15010054
APA StyleMilovanović, J., Banjanac, K., Nikolić, J., Nikodinović-Runić, J., & Prlainović, N. Ž. (2025). The Organic-Functionalized Silica Nanoparticles as Lipase Carriers for Biocatalytic Application: Future Perspective in Biodegradation. Catalysts, 15(1), 54. https://doi.org/10.3390/catal15010054







