Abstract
The catalytic activity and selectivity of Co-based catalysts supported on home-made nanoporous carbon was studied as a function of the type of alkali promoter (Ca and Mg). The catalysts were characterized by N2 adsorption/desorption isotherms, temperature-programmed reduction, CO chemisorption, and X-ray diffraction patterns. The catalysts were compared against carbon-supported alkali-promoted Ni-based catalysts and Re-containing catalysts. The catalytic activity of the Co-based catalyst was clearly enhanced in the presence of Ca and Mg, and it was higher than the Ni-based catalysts and comparable to that obtained using an ReC catalyst. The initial activity of the Mg-promoted catalyst increased by a factor of up to 2.5 times higher compared to the non-promoted catalyst. Moreover, this catalyst showed a turnover frequency of up to 5 times higher than equivalent carbon-supported Re-based catalysts. Significant changes were not observed in the selectivity of products after the incorporation of alkali, with cyclohexane being the main product. However, it was demonstrated that the presence of alkali led to a faster and higher production of cyclohexane from the demethoxylation of phenol and the dehydrogenation of cyclohexanol. The present results suggest that Co-based catalysts are an economical alternative for the catalytic conversion of representative target molecules from bio-oil feed.
1. Introduction
It is well known that metal incorporation into porous carbons leads to an enhancement of the catalytic hydrodeoxygenation (HDO) conversion of phenolic molecules from bio-oil feed into upgraded chemicals and fuels [1,2]. The HDO of guaiacol has become an interesting target molecule that is commonly used to study the activity of catalysts [1,2,3,4,5,6,7]. For instance, Re [5,6] and ReMo [7] have been reported in the HDO of guaiacol, highlighting the formation of an active rhenium carbide phase [5,6] and a synergistic effect with Mo [6], promoting the demethylation of guaiacol to catechol. Thus, the formation of active and stable metal carbide phases is one of the advantages of using nanoporous carbons as support. However, alternative catalysts to Re, Mo, and expensive noble metals are required for the HDO of phenolic compounds. Several works [1,2,4] have reported high conversion rates of Ni-based catalysts in the HDO of guaiacol. In situ, the formation of nickel carbide phases has also been reported by our group during the hydrogenation of ethylene [8,9] and dry methane reforming [10,11,12]. In addition, Ni-based catalysts have shown representative activity in several hydrotreatment processes, including hydrodesulfurization [13,14,15] and hydrodenitrogenation [16,17,18]. In a preliminary study, our group reported [19] the promoter effect of alkali metals (Ca and Mg) on the catalytic activity and selectivity of Ni-based activated carbon-supported catalysts. Contrary to the trends observed in the initial rates of reaction, the turnover frequencies showed higher values when the alkali content was increased from 1 wt.% to 5 wt.%, suggesting the formation of a mixture of NiO-CaO and NiO-MgO oxides with boosted reactivity. In addition, our group reported [19] remarkable changes in the selectivity of products as a function of the type of alkali promotor. For instance, Mg led to the formation of cyclohexane, while Ca promoted representative benzene yields.
However, the control of the selectivity of products is still a major challenge. For example, the deoxygenation of guaiacol to high-value aromatic molecules such as benzene, toluene, and xylene (BTX) is of great interest, but the highly active hydrogenation of Ni favors a different mechanism. In this sense, because cobalt-based mineral stocks are lower than those of nickel, Co-based catalysts are more expensive than Ni-based catalysts. Recent works have shown that Co-based materials can be used as an alternative catalyst for the HDO of guaiacol [20,21,22,23,24]. These works reported on the optimization of reaction parameters, the influence of feedstock conditions, the optimization of catalyst composition, and the influence of the catalytic supports, among others. In addition, the more acidic chemical nature of Co-based catalysts compared to Ni-based catalysts could drive higher reactivities for processes involving thermolysis of C-O bonds, resulting in major deoxygenation activity. As reported elsewhere [19], the selective conversion of guaiacol is of major importance for the development of biorefinery industries because this molecule may suffer from parallel reactions that affect the efficiency of the process. For instance, one pathway is the demethoxylation (DMO) of guaiacol to form phenol, which can be hydrogenated (HYD) to form cyclohexanol. However, at the same time, phenol can enter a direct deoxygenation (DDO) pathway to produce benzene, which can be hydrogenated to cyclohexane. A second pathway is primary hydrogenation (HYD) to produce cyclohexanol, which can be dehydrogenated (DHY) to cyclohexane. Finally, a third pathway could be the formation of anisole through the direct deoxygenation (DDO) of guaiacol, but this product is not common. Though the influence of different catalysts has been clearly reported [24], the influence of promoters on the catalytic activity and selectivity of Co-based catalysts in HDO reactions still produces controversial results. It has been reported that changes in the mechanisms of reactions have been found during guaiacol conversion when Mo [1,7] and W [1] are used as promotors of Ni- and Re-based catalysts, respectively. It has been reported that the activity of Ni-based catalysts in dry methane reforming [10,11] and CO2 methanation [25], as well as iron-carbide-based catalysts in the HDO of phenols [26], decreases in the presence of alkali promoters. However, alkali permitted the control of product selectivity in these works. Keeping these results in mind, it should be noted that to the best of our knowledge, the effects of alkali promotors on carbon-supported Co-based catalysts have not been reported in the HDO of guaiacol. The present work aims to study the HDO of guaiacol on a nanoporous carbon-supported Co-based catalyst. The influence of Ca and Mg on the catalytic activity and selectivity was verified and compared against Ni-based catalysts.
2. Results and Discussion
2.1. Characterization of Catalysts
Figure 1 shows that all N2 adsorption/desorption isotherms are type I (b), indicating that the pore structure is mainly composed of micropores. The samples show a H4 hysteresis loop [27] in the desorption branch, in agreement with the slit-shaped pores that are characteristic of nanoporous carbons. Table 1 summarizes the textural properties of the AC and Co-based catalysts. The AC is characterized by a high SBET of ca. 966 m2 g−1 and a VTot of ca. 0.60 cm3 g−1. The ratio between the micropore volume and the total pore volume (Vmicro/Vtotal) yields 72%, indicating that the nanoporous carbon is characterized by a pore framework lower than 2 nm. The Wp of the AC is ca. 2.48 nm, which is 3.5 times higher than the molecular dynamic diameter of guaiacol (0.71 nm). Accordingly, the diffusion of guaiacol molecules from the bulk of the solution to the carbon pores is not limited by the pore framework, especially considering that the carbon supports are characterized by slit-type pores.
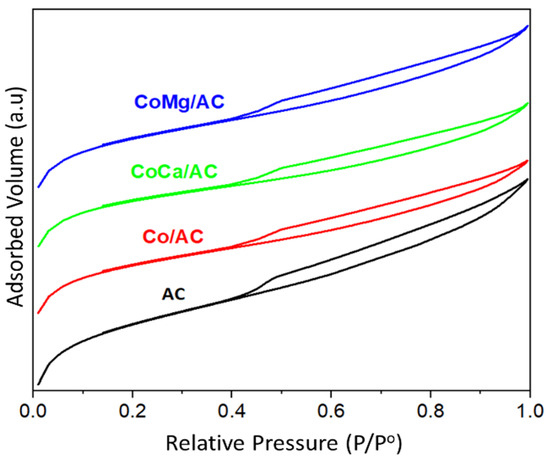
Figure 1.
N2 adsorption/desorption isotherms at −196 °C with AC and Co-based catalysts.

Table 1.
Summary of textural and structural properties of Co-based catalysts.
The experimental conditions used in the present study have been previously optimized [5,6,7], and it can be confirmed that the reaction is not controlled by diffusional phenomena. The representative contribution of mesopores (28%) supports this inference. Accordingly, it is reasonable to use the Gurvich rule [28] to estimate the mean pore width (Wp) of the AC and Co-based catalysts using Equation (1) and the data from Table 1.
Wp = 4·(VTot/SBET)
As can be seen from Table 1, the mean pore width (Wp) of AC is lightly affected by the impregnation of Co and the alkali-promoted Co-based catalysts. The Wp decreased from 2.48 nm for the non-impregnated AC to 2.42 nm, 2.31 nm, and 2.42 nm for Co/AC, CoCa/AC, and CoMg/AC, respectively. The impregnation of Co decreases the SBET and VTot values from 966 m2·g−1 and 0.60 cm3·g−1 to 792 m2·g−1 and 0.48 cm3·g−1, respectively, suggesting that most of the cobalt is in the micropore framework. The successive impregnation of Co/AC with Ca or with Mg did not affect the SBET and VTot of the Co/AC catalysts, but the proportion of the Vmicro/Vtotal ratio increased from 69% for Co/AC up to 74% and 73% for CoCa/AC and CoMg/AC, respectively. This result suggests that the alkali-promotors are preferentially located in the mesopore region, specifically in the small mesopores (2–3 nm). Interestingly, after Ca impregnation, the mean pore size of the Co/AC decreased to 2.31 nm, while the impregnation of Mg did not affect the mean pore size of the Co/AC catalyst. This result agrees with a higher ionic radius for Ca2+ (ca. 1 Å) compared to that for Mg2+ (ca. 0.7 Å), suggesting that CaO is preferentially located in the small mesopores, while MgO is in the large micropores.
On the other hand, Figure 2a shows the XRD patterns of AC and carbon-supported Co-based catalysts. The AC is characterized by two broad peaks at 2θ = 24.0° and 43.3°, respectively, corresponding to the (002) and (101) planes of amorphous carbon, as reported elsewhere [29], with the hexagonal phase (JCPDS, 41-1487). It can be highlighted that the Co-based catalysts were not calcined to preserve the carbonaceous matrix, but the samples were activated in situ and passivated before structural characterization, as described in the Section 3. Figure 2a shows that all of the Co catalysts presented two diffraction planes. The first is in the shape of a hump at 2θ = 43.8°, overlapping with the diffraction plane of carbon, with a peak at 2θ = 51.8° ascribed to elemental Co0 [30] (JCPDS 15-0806). In addition, the Co/AC catalyst shows an intense and broad diffraction plane at 2θ = 30.2° and another broad diffraction plane of low intensity at 2θ = 31.4° attributed to the Co3O4 species (JCPDS 76-1802) [30]. As expected, diffraction peaks for CaO and MgO were not detected in the XRD patterns of CoCa(1%)/AC and CoMg(1%)/AC because their composition in the catalyst is only ca. 1 wt.%. Therefore, these peaks are diluted by the AC support. However, the CoMg/AC catalyst (1%) presented a broad and highly intense plane at 2θ = 13°, which can be assigned to MgO [31]. This result suggests a low degree of interaction between the Co-Mg species, leading to the formation of MgO agglomerations on the carbon surface. Furthermore, the incorporation of 1% of CaO and MgO would favor the reduction of Co3O4 to elemental Co [32].
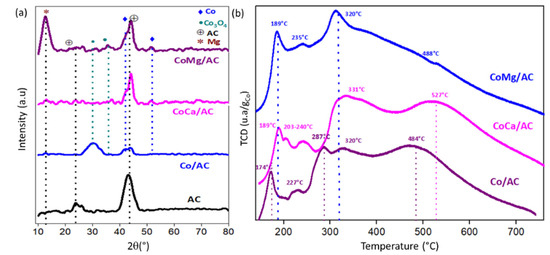
Figure 2.
(a): X-ray diffraction patterns (XRD). (b): Temperature-programmed reduction (TPR) profiles in terms of TCD response.
Table 1 also shows the average particle sizes for the Co crystallites obtained using the Scherrer equation based on the diffraction plane at 2θ = 43.8°. The average particle size decreases from 23 nm for the Co/AC catalyst to 11 nm and 12 nm with the incorporation of 1 wt.% CaO and MgO, respectively. This result suggests that the basic chemical nature of CaO and MgO promotes the dispersion of Co crystallites on the surface of the activated carbon. In other words, the presence of alkali promotors inhibits the interaction between Co particles and the carbon matrix. However, the formation of cobalt carbide phases along the reductive pretreatment of catalysts is not discarded [8,9] due to the presence of the carbon support.
Figure 2b shows the temperature-programmed reduction (TPR) profiles in terms of the TCD response of the AC-supported Co-based catalysts. The TPR results are summarized in Table 2. The two peaks observed below 200 °C and around 287–331 °C can be attributed to the two-stage reduction of the Co3O4 to metallic cobalt [33,34]. This indicates the reduction of Co3O4 to CoO and the reduction of CoO to Co.

Table 2.
Summary of reduction peaks obtained from TPR analysis.
The reduction peaks of the cobalt species shift to higher temperatures due to the presence of Ca and Mg, in a very similar way to that observed on Ni catalysts [19]. This result agrees with a decrease in the mean particle size observed using XRD. Accordingly, it can be suggested that both alkali promoters influence the interaction between Co particles and the carbon surface [10,11]. The high temperature peak observed around 484–527 °C can be attributed to the methanation of the support, as reported elsewhere [8].
CO chemisorption tests were also performed for the present catalysts. Table 3 shows a summary of the CO chemisorption results. The catalysts showed lower CO chemisorption than the CoCa/AC catalyst, which also showed the lowest particle size (11 nm), similar to that obtained from the XRD analysis (Table 1).

Table 3.
Summary of CO chemisorption analysis compared with mean particle size obtained based on XRD patterns.
However, CO uptake on CoMg/AC and particle size were similar to that observed for the non-promoted catalysts. These results suggest that CaO crystallites promote a higher metallic dispersion of Co crystallites, while the more basic MgO did not affect the active phase dispersion. In other words, it seems that MgO decorates Co particles, promoting a decrease in the chemisorption of CO. This trend is opposite when CaO is used as an alkali promotor. Accordingly, changes in the catalytic activity for guaiacol conversion as a function of the type of alkali are expected.
2.2. Catalytic Activity
Figure 3 shows the catalytic activity obtained on the alkali-promoted Co-based catalysts supported on nanoporous carbon, while Table 4 shows a summary of the kinetic parameters.
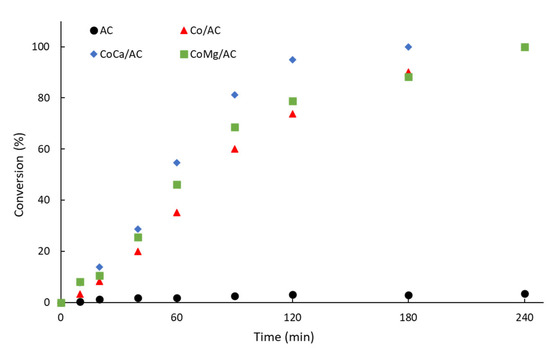
Figure 3.
Catalytic activity of alkali-promoted Co-based catalysts supported on nanoporous carbon during guaiacol conversion.

Table 4.
Summary of kinetic results for guaicol conversion on alkali-promoted carbon-supported Co-based catalysts.
It can be seen that the catalytic activity of the activated carbon is negligible (Figure 3); between 0.2 and 3.5% guaiacol converted after 10 min and 240 min reactions. The non-promoted Co/AC catalysts showed an initial conversion (after 10 min) of guaiacol of ca. 3.3%, while the alkali-promoted catalysts showed higher values of ca. 7.7% and 8.1%, respectively, compared to the CoCa/AC and CoMg/AC catalysts. However, the Coca/AC catalyst showed a faster trend than the CoMg/AC catalysts, achieving 100% conversion after 180 min and 240 min, respectively. As reported elsewhere [19], despite the Mg-promoted catalysts being slightly more active under initial conditions, the CoCa/AC became more active, suggesting the formation of an active species—likely a mixture of CoO-CaO—along the reaction, in agreement with the differences and changes observed in the textural parameters, the XRD patterns, the TPR profiles, and CO consumption.
According to the initial guaiacol conversion, the initial reaction rate (ro) of the Ca and Mg-promoted catalysts in Table 4 shows a clear increase in the catalytic activity compared to the Co/AC catalyst; up to ca. 2.3 and ca. 2.5 higher for CoCa/AC and CoMg/AC, respectively. However, the analysis of the first 90 min of the reaction in Figure 3 shows that the CoCa/AC catalyst has a greater slope than CoMg/AC. Therefore, it can be suggested that the CoCa/AC catalyst develops a better dispersion along the reaction. In other words, CoMg has less activity because it has less dispersion, but its initial reaction sites are more powerful; or, it shows that Mg is favoring the activity of Co.
It has been widely reported [1,2,6,8,9,10,11,12] that the activity of carbon-supported catalysts can be strongly conditioned by the ratio between the micropore volume, the total volume of the pores (Vmic/Vtot), and the mean pore width of the porous carbons. This influence can be of major importance during the initial steps of the reaction. However, as discussed above, the Wp of the AC is ca. 2.48 nm, which is ca. 3.5 times higher than the molecular dynamic diameter of guaiacol (0.71 nm). Accordingly, no limitation in the diffusion of guaiacol molecules from the bulk of solution through the porous framework is expected in the Co/AC catalysts. As noted above, the impregnation of Co/AC with Ca or with Mg did not affect the SBET and VTot of Co/AC catalysts, but the Vmic/Vtot ratio increased from 69% for Co/AC up to 74% and 73% for CoCa/AC and CoMg/AC, respectively, suggesting that the alkali-based crystallites are preferentially located in the small mesopore region (2–3 nm).
The turnover frequencies (TOF) estimated using Equation (2) based on the initial reaction rate (ro) and CO consumption showed higher values for CoMg/AC than for CoCa/AC: 0.72 s−1 vs 0.41 s−1, respectively. This result seems to be a contradiction, since CoCa/AC achieved 100% guaiacol conversion with lower reaction times than the CoMg/AC catalyst. However, it should be kept in mind that TOF is not a kinetic term. If the active phase were the same in the presence of both alkali promoters, then the TOF should be the same for all catalysts. However, the higher TOF value observed in the CoMg/AC catalyst suggests that Co is being enhanced by MgO in some way, making the active site more active along the reaction than the other two catalysts. It should be remarked that the presence of CaO also enhances the activity of Co, but Mg promotes a higher catalytic activity in terms of the initial rates and TOF. Thus, the results suggest that the active sites probed by CO-chemisorption are not the only active sites present over the promoted catalysts.
2.3. Selectivity of Products
Figure 4a–c show the kinetics of the appearance and disappearance of the main products detected during the conversion of guaiacol for the Co/AC, CoCa/AC, and CoMg/AC catalysts, respectively. Figure 4d shows the yields of the main products after 240 min. The selectivity trends observed in Figure 4 suggest that guaiacol conversion seems to follow parallel reactions.
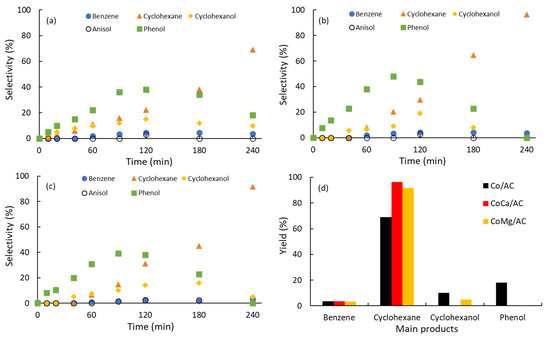
Figure 4.
Main products detected during the conversion of guaiacol. (a) Co/AC; (b) CoCa/AC; (c) CoMg/AC; (d) yields of products obtained after 240 min.
One reaction is the demethoxylation (DMO) of guaiacol, forming phenol that is the main product during a 120 min reaction time both in the absence and presence of alkali promoters. In other words, Ca promotes faster and higher production of phenol, while Mg did not affect phenol production. A second route is the hydrogenation (HYD) of guaiacol to produce cyclohexanol. The pathway is also increased by the promotion with Ca, while Mg did not affect the selectivity. For all the catalysts, the main product formed after the 120 min reaction was cyclohexane. The yields of cyclohexane were clearly higher in the presence of Ca and Mg after the 240 min reaction, suggesting that the dehydrogenation (DHY) of cyclohexanol to cyclohexane is the preferred pathway. In addition, it should also be mentioned that phenol can also undergo hydrogenation (HYD) to form cyclohexanol, but this product did not increase in concentration. On the contrary, cyclohexanol monotonically decreased after the 120 min reaction. Finally, it is also important to mention that a small proportion of phenol underwent direct deoxygenation (DDO), producing benzene, which eventually underwent hydrogenation to cyclohexane. An almost negligible production of anisole via the direct deoxygenation (DDO) of guaiacol was also found, but after the 240 min reaction, this product was not detected. CaO crystallites, a softer Lewis base than MgO, could be responsible for the demethoxylation (DMO) of guaiacol to phenol and the dehydrogenation (DHY) of cyclohexanol to cyclohexane. Accordingly, it can be suggested, as in the case of Ni-based catalysts [19], that Co crystallites are the active phase responsible for the hydrogenation (HYD) steps, mainly in the HYD of guaiacol to cyclohexanol and the HYD of benzene to cyclohexane. The above results confirm that both alkalis, mainly Ca, promote faster formation of saturated molecules.
2.4. General Discussion
Table 5 shows a comparison of the activity and selectivity results obtained for the present Co-based catalysts and Ni [19] and Re-based [5] catalysts supported by carbon supports. In the same experimental conditions of the reaction, in terms of the TOF, the Co-based catalysts were more active than the Ni-based catalysts supported by the same activated carbon [19], in agreement with the higher Lewis acidity of Co3O4 than NiO. It should be noted that the Co-based catalysts even developed higher activity than that reported for ReC catalysts [5].

Table 5.
Comparison of activity and selectivity results observed in the conversion of guaiacol for carbon-supported Co, Ni, and Re-based catalysts.
Furthermore, considering that the present Co-based catalysts only contained ca. 5%wt. of the active phase, it is interesting to highlight that these showed comparable catalytic activity to that observed for Ni-based catalysts supported on carbon nanotubes with 10%wt. Ni content [35]. However, Ni/CNT with 15%wt. Ni content is clearly more active than the present catalysts, but the cost of this catalyst is clearly higher than the present catalysts. Moreover, it should be highlighted that the TOF of CoMg/AC is two times higher than that reported for Ni(10%wt.)/CNT and is comparable to the value reported for Ni(15%wt.)/CNT. Despite the catalysts not being compared under isoconversion conditions, in terms of the selectivity of the products, the reaction mechanism did not seem to undergo remarkable changes for the Co- and Ni-based catalysts.
The present results suggest that Co-based catalysts are more selective to the formation of cyclohexane with shorter reaction times compared to Ni-based catalysts. It should be noted that both Co- and Ni-based carbon-supported catalysts led to the selectivity of a different pathway than that reported for ReC catalysts, which are highly selective to the formation of phenol and benzene. For the present catalysts, phenol was the major product after 120 min of reaction, but after this time, a remarkable increase in cyclohexanone was observed, while the Re-based catalysts led the conversion of phenol to benzene via a direct deoxygenation (DDO) pathway [19]. It would be interesting to evaluate the influence of different functional group surface chemistries and different pore size distributions of nanoporous carbons. Accordingly, studies of guaiacol conversion using bifunctional NiCo supported on different nanoporous carbons are being conducted in the absence and presence of both alkalis.
3. Materials and Methods
3.1. Synthesis of Activated Carbon and Catalysts
Porous carbon was prepared from apamate (T. Pentaphyla) sawdust via physical activation under CO2 flow (800 °C, 1 h) and labeled AC. The Co-based catalysts were prepared via wetness impregnation [8,9,10,11,12] of high-purity Co, Ca, and Mg nitrate solutions on the carbon support. The weights of the salts were adjusted to CoO (5 wt.%), CaO (1 wt.%), and MgO (1 wt.%). The Co-based catalyst is labelled Co/AC, and when the impregnation was followed by Ca or Mg, the catalysts are labeled CoCa/AC and CoMg/AC, respectively. The materials were activated in situ by purging catalysts [19] in a quartz reactor under an N2 flow (30 mL·min−1 at 20 °C for 10 min) and then reduced under H2 flow (60 mL·min−1, 450 °C for 4 h). The catalysts were passivated at 20 °C for 1 h with a 5% O2/N2 mixture (30 mL·min−1).
3.2. Characterization
The adsorption/desorption isotherms of N2 at −196 °C were measured using 3Flex equipment from Micromeritics (Norcross, GA, USA). The catalysts were previously degassed at 300 °C under a vacuum for 4 h using a SmartVacPrep instrument (Micromeritics). The specific surface area (SBET) was estimated using the Brunauer–Emmett–Teller model (BET). The total pore volume (Vtotal) was estimated at a relative pressure (P/Po) of ca. 0.97, while the micropore volume (Vmicro) was calculated using the t-plot equation.
Temperature-programmed reduction combined with mass spectrometry (TPR-MS) was performed using 3Flex equipment (Micromeritics) combined with Cirrus 2 (MKS Spectra Product, Andover, MA, USA). The samples were heated at a rate of 10 °C min−1 to 1050 °C with a mixture of 5% H2-Ar (100 mL·min−1). CO chemisorption was performed at 110 °C for 30 min and then reduced to 350 °C for 60 min. CO uptake was determined using the Sinfelt model. The dispersion and the crystallite size were calculated assuming a stoichiometry factor of 1.5 for CO chemisorption to Co for cubic particles.
Structural characterization was performed using X-ray diffraction (XRD) over an angular range of 10° ≤ 2θ ≤ 80° with a scanning step of 0.02°. The measurements were carried out using a Bruker diffractometer (D8 model, Billerica, MA, USA) with Cu-Kα radiation (λ = 1.5406 Å).
3.3. Catalytic Tests
The activity of the catalysts was evaluated using a stirred-batch reactor (Parr Model 4590, Moline, IL, USA) with 100 mL for 4 h. In a typical test, 150 mg of catalyst, 2.3 g of guaiacol (18,500 μmol), and 60 g of dodecane, (high purity, > 95%, Merck, Darmstadt, Germany) were mixed by stirring (ca. 650 rpm). Prior to heating to 300 °C, the reactor was purged with N2. The reactor was then charged with 5 MPa H2. Aliquots of ca. 0.1 mL were collected and analyzed using gas chromatography (Nexis GC2030, Shimadzu) equipped with an Elite-1 column (Perkin Elmer, Waltham, MA, USA). The product selectivity was estimated based on the fractional conversion of guaiacol (Xt). The initial reaction rate (ro, μmol·g−1·s−1) was estimated [5,19] using Equation (2), where b corresponds to the initial slope obtained by plotting conversion vs. time (s), n°GUA is the initial guaiacol mol in the reactor, and m is the mass of the catalyst (g).
ro = b · n°GUA/m
For the calculation of ro, only the data in the low-conversion region (≤30%) were considered. The turnover frequency (TOF, s−1) was estimated by considering that CO uptake (μmol·g−1) is chemisorbed on the active sites of catalysts according to Equation (3).
TOF = ro/COuptake
4. Conclusions
The results obtained in the present work provide a foundation for the future in HDO processes within the framework of biorefinery. For instance, Co-based catalysts supported on nanoporous carbons showed a representative catalytic activity for the guaiacol reaction, which was more active than the Ni-based catalysts, but more importantly, these catalysts developed higher activity than that reported for more expensive Re/AC catalysts. This boosted activity can be attributed to a combination of parameters. First, Co-based catalysts lead the selectivity to cyclohexane and phenol through pathways involving demethoxylation of phenol and dehydrogenation of cyclohexanol. These pathways are even favored by the presence of alkali promotors, mainly CaO crystallites. In other words, the present catalysts promote the cleavage of oxygenated groups such as methoxy and hydroxy without missing the hydrogenation function, mainly ascribed to the Co phases. The characterization of catalysts, together with the initial rate of guaiacol conversion and TOF estimations, suggest that porous carbon promotes the formation of novel active phases along the reaction, leading to an enhancement of activity for the case of the CoCa/AC catalyst. As a general conclusion, the present nanoporous carbon-supported alkali-promoted Co-based catalysts can be used as an alternative to more expensive catalysts based on noble metals.
Author Contributions
D.S.-G. contributed to the catalytic tests and characterization. E.B. contributed to the characterization and review. N.E. contributed to the conceptualization, writing, interpretation of data, and review. P.S.P. contributed to the synthesis of catalysts, review, and curation. J.M. contributed to the synthesis of catalysts, conceptualization, writing of the original draft, interpretation of data, and review. All authors have read and agreed to the published version of the manuscript.
Funding
N. Escalona thanks the Millennium Science Initiative Program NCN2021_090, ANID-FONDECYT 1220763, and Fondequip N° EQM160070. J. Matos thanks ANID-ANILLO ATE220014 and ANID-FONDECYT 1220228. P.S. Poon thanks ANID-FONDEF ID23I10085 and ANID-FONDECYT 1240641. The authors thank Patricio Baeza (PUCV) for the XRD analyses.
Data Availability Statement
The data and materials presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cordero-Lanzac, T.; Palos, R.; Arandes, J.M.; Castaño, P.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J. Stability of an acid activated carbon based bifunctional catalyst for the raw bio-oil hydrodeoxygenation. Appl. Catal. B Environ. 2017, 203, 389–399. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Hita, I.; García-Mateos, F.J.; Castaño, P.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J. Adaptable kinetic model for the transient and pseudo-steady states in the hydrodeoxygenation of raw bio-oil. Chem. Engin. J. 2020, 400, 124679. [Google Scholar] [CrossRef]
- Fan, X.-D.; Wu, Y.-J.; Tu, R.; Sun, Y.; Jiang, E.-C.; Xu, X.-W. Hydrodeoxygenation of guaiacol via rice husk char supported Ni based catalysts: The influence of char supports. Renew. Energy 2020, 157, 1035–1045. [Google Scholar] [CrossRef]
- Wu, X.; Ge, Q.; Zhu, X. Vapor phase hydrodeoxygenation of phenolic compounds on group 10 metal-based catalysts: Reaction mechanism and product selectivity control. Catal. Today 2021, 365, 143–161. [Google Scholar] [CrossRef]
- Blanco, E.; Dongil, A.B.; García-Fierro, J.L.; Escalona, N. Insights in supported rhenium carbide catalysts for hydroconversion of lignin-derived compounds. Appl. Catal. A Gen. 2020, 599, 117600. [Google Scholar] [CrossRef]
- Blanco, E.; Cabeza, P.; Naharro Ovejero, V.; Contreras, C.; Dongil, A.B.; Ghampson, I.T.; Escalona, N. Effect of carbon support and functionalization on the synthesis of rhenium carbide and its use on HDO of guaiacol. Catal. Today 2023, 420, 114031. [Google Scholar] [CrossRef]
- Blanco, E.; Díaz de León, J.N.; García-Fierro, J.L.; Escalona, N. Study of supported bimetallic MoRe carbides catalysts for guaiacol conversion. Catal. Today 2021, 367, 290–296. [Google Scholar] [CrossRef]
- Matos, J.; Brito, J.; Laine, J. Activated carbon supported Ni-Mo: Effects of pretreatments and composition on catalyst reducibility and on ethylene conversion. Appl. Catal. A Gen. 1997, 152, 27–42. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J. Ethylene conversion on activated carbon supported NiMo catalysts: Effect of the Support. Appl. Catal. A Gen. 2003, 241, 25–38. [Google Scholar] [CrossRef]
- Matos, J.; Díaz, K.; García, V.; Cordero, T.C.; Brito, J.L. Methane transformation in presence of carbon dioxide on activated carbon supported nickel-calcium catalysts. Catal. Lett. 2006, 109, 163–169. [Google Scholar] [CrossRef]
- Díaz, K.; García, V.; Matos, J. Activated carbon supported Ni-Ca: Influence of reaction parameters on activity and stability of catalyst on methane reformation. Fuel 2007, 86, 1337–1344. [Google Scholar] [CrossRef]
- Goscianska, J.; Pietzrak, R.; Matos, J. Catalytic performance of ordered mesoporous carbons modified with lanthanides in dry methane reforming. Catal. Today 2018, 301, 204–216. [Google Scholar] [CrossRef]
- Tian, F.; Wang, W.; Liu, B.; Pan, Y.; Dong, B.; Li, Y.; Guo, H.; Chai, Y.; Liu, C. Synergistic effect between CoSx and MoS2 at the micrometer scale: Considerable promotion of the hydrodesulfurization of DBT. Chem. Engin. J. 2024, 484, 149579. [Google Scholar] [CrossRef]
- Medina Cervantes, J.A.; Díaz de León, J.N.; Fuentes Moyado, S.; Alonso-Núñez, G. Influence of precursor compounds on the structural and catalytic properties of CoNiMo/SBA-15 catalysts used in the hydrodesulfurization of dibenzothiophene. Mol. Catal. 2023, 547, 113399. [Google Scholar] [CrossRef]
- Wang, E.; Yang, F.; Song, M.; Chen, G.; Zhang, Q.; Wang, F.; Bing, L.; Wang, G.; Han, D. Recent advances in the unsupported catalysts for the hydrodesulfurization of fuel. Fuel Proc. Technol. 2022, 235, 107386. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, W.; Xu, Z.; Xu, Z.; Wang, X.; Wei, Q.; Zhou, Y. In situ synthesis of Co modified SAPO-5 molecular sieves and the application in quinoline hydrodenitrogenation of their NiWS supported catalysts. Chem. Engin. Sci. 2023, 284, 119428. [Google Scholar] [CrossRef]
- Paz Carmona, H.; Tišler, Z.; Svobodová, E.; Akhmetzyanova, U. Co-processing of atmospheric gas oil with rapeseed oil over sulfur-free supported and phosphorus-modified Co-Mo and Ni-Mo carbide catalysts. Catal. Lett. 2022, 152, 3814–3824. [Google Scholar] [CrossRef]
- Klimov, O.V.; Vatutina, Y.V.; Nadeina, K.A.; Kazakov, M.O.; Gerasimov, E.Y.; Prosvirin, I.P.; Larina, T.V.; Noskov, A.S. CoMoB/Al2O3 catalysts for hydrotreating of diesel fuel. The effect of the way of the boron addition to a support or an impregnating solution. Catal. Today 2018, 305, 192–202. [Google Scholar] [CrossRef]
- Matos, J.; Samudio-González, D.; Blanco, E.; Poon, P.S.; Escalona, N. Alkali-driven selectivity of products on carbon-supported Ni-based catalysts during the HDO of guaiacol. Fuel 2024, 374, 132442. [Google Scholar] [CrossRef]
- Jiang, D.; Lin, M.; Yan, Y.; Zhan, L.; Li, R.; Wu, Y. Highly selective hydrogenation of guaiacol to cyclohexanol over carbon-encapsulated highly dispersed cobalt catalyst. Chem. Eng. Sci. 2024, 290, 119779. [Google Scholar] [CrossRef]
- Kim, H.; Lim, Y.H.; Park, J.H.; Ha, J.-M.; Kim, D.H. Hydrodeoxygenation of guaiacol over physically mixed Co/TiO2 and WO3/TiO2 catalysts. Green Chem. 2024, 26, 2692–2704. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Z.; Qin, J.; Chen, M.; Dong, L.; Mao, W.; Zhou, X.; Long, Y.; Ma, J. Highly efficient and selective hydrodeoxygenation of guaiacol to cyclohexanol over a rod-like CoNi-C catalyst. Fuel 2023, 353, 129216. [Google Scholar] [CrossRef]
- Hongkailers, S.; Pattiya, A.; Hinchiranan, N. Hydrodeoxygenation of oxygenates derived from biomass pyrolysis using titanium dioxide-supported cobalt catalysts. Molecules 2023, 28, 7468. [Google Scholar] [CrossRef]
- Wu, L.; Wei, J.; Zhang, Y.; He, Y.; Wang, X.; Guo, H.; Tang, Y.; Tan, L. The selective hydrodeoxygenation of guaiacol to cyclohexanol over cobalt-modified TS-1 catalysts. Micro. Mesoporous Mater. 2023, 348, 112347. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, G.; Sun, W.; Bai, Y.; Zheng, L.; Han, X.; Wu, L. Influence of Mg-promoted Ni-based catalyst supported on coconut shell carbon for CO2 methanation. Chem. Sel. 2019, 4, 838–845. [Google Scholar] [CrossRef]
- Zhang, J.; Sudduth, B.; Sun, J.; Kovarik, L.; Engelhard, M.H.; Wang, Y. Elucidating the active site and the role of alkali metals in selective hydrodeoxygenation of phenols over iron-carbide-based catalyst. ChemSusChem 2021, 14, 4546–4555. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Helmich, M.; Luckas, M.; Pasel, C.; Bathen, D. Characterization of microporous activated carbons using molecular probe method. Carbon 2014, 74, 22–31. [Google Scholar]
- Shao, M.; Wang, D.; Yu, G.; Hu, B.; Yu, W.; Qian, Y. The synthesis of carbon nanotubes at low temperature via carbon suboxide disproportionation. Carbon 2004, 42, 183–185. [Google Scholar] [CrossRef]
- Ghods, B.; Meshkani, F.; Rezaei, M. Effects of alkaline earth promoters on the catalytic performance of the nickel catalysts supported on high surface area mesoporous magnesium silicate in dry reforming reaction. Inter. J. Hydrog. Energy 2016, 41, 22913–22921. [Google Scholar] [CrossRef]
- Chiou, J.Y.Z.; Liu, S.W.; Ho, K.F.; Huang, H.H.; Tang, C.W.; Wang, C.B. Ca-modified Co/SBA-15 catalysts for hydrogen production through ethanol steam reforming. Inter. Lett. Chem. Phys. Astron. 2013, 24, 1–16. [Google Scholar]
- He, K.; Dong, Y.M.; Li, Z.; Yin, L.; Zhang, A.M.; Zheng, Y.C. Catalytic ozonation of phenol in water with natural brucite and magnesia. J. Hazardous Mater. 2008, 159, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Comparison of ethanol steam reforming using Co and Ni catalysts supported on SBA-15 modified by Ca and Mg. Fuel Process. Technol. 2016, 146, 99–109. [Google Scholar] [CrossRef]
- Du, H.; Zhu, H.; Chen, X.; Dong, W.; Lu, W.; Luo, W.; Ding, Y. Study on CaO-promoted Co/AC catalysts for synthesis of higher alcohols from syngas. Fuel 2016, 182, 42–49. [Google Scholar] [CrossRef]
- Dongil, A.B.; Ghampson, I.T.; García, R.; Fierro, J.L.G.; Escalona, N. Hydrodeoxygenation of guaiacol over Ni/carbon catalysts: Effect of the support and Ni loading. RSC Adv. 2016, 6, 2611. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).