BioTemplated Fe3+-Doped g-C3N4 Heterojunction Micromotors for the Degradation of Tetracycline through the Photo-Fenton Reaction
Abstract
1. Introduction
2. Results
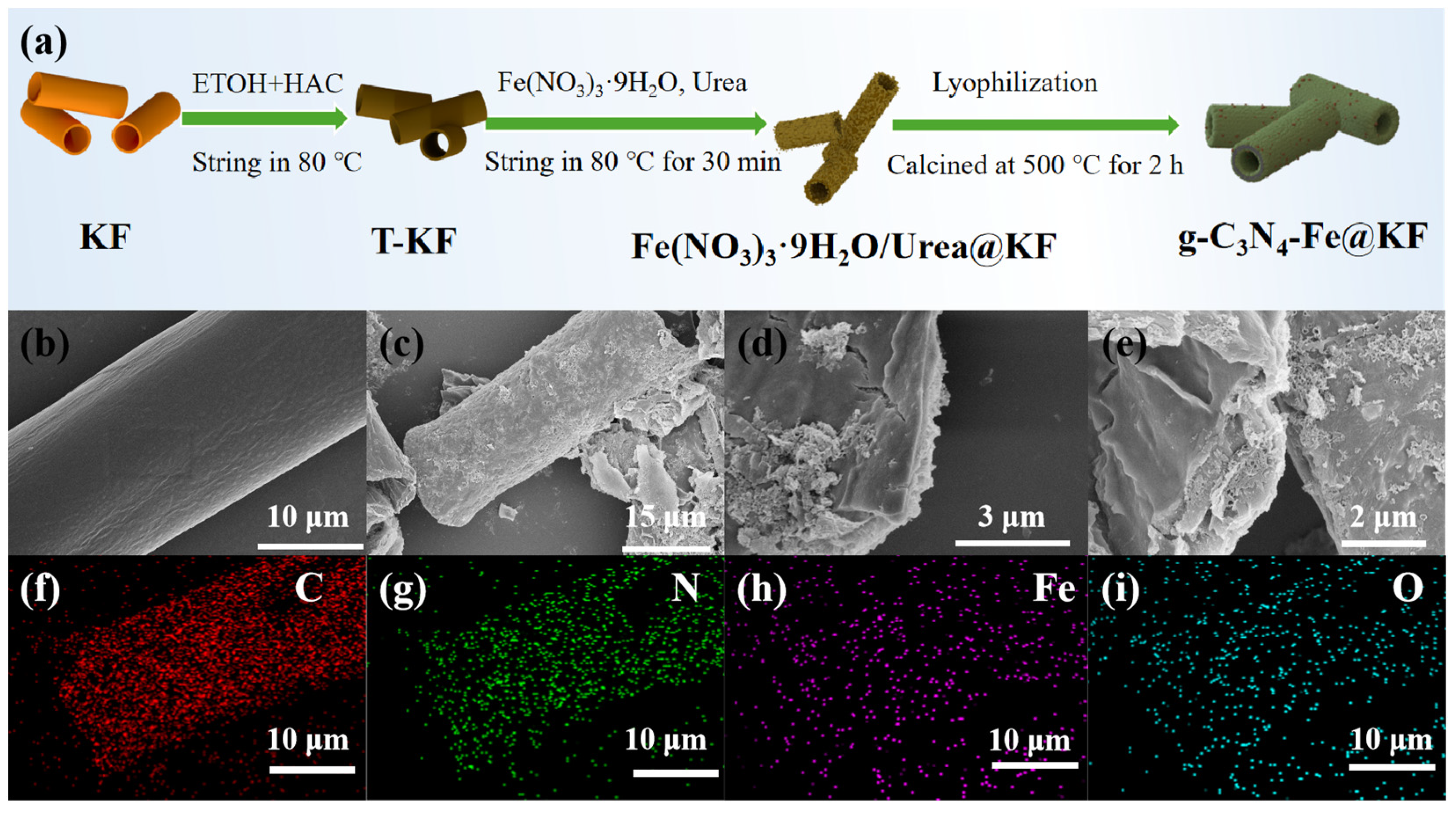
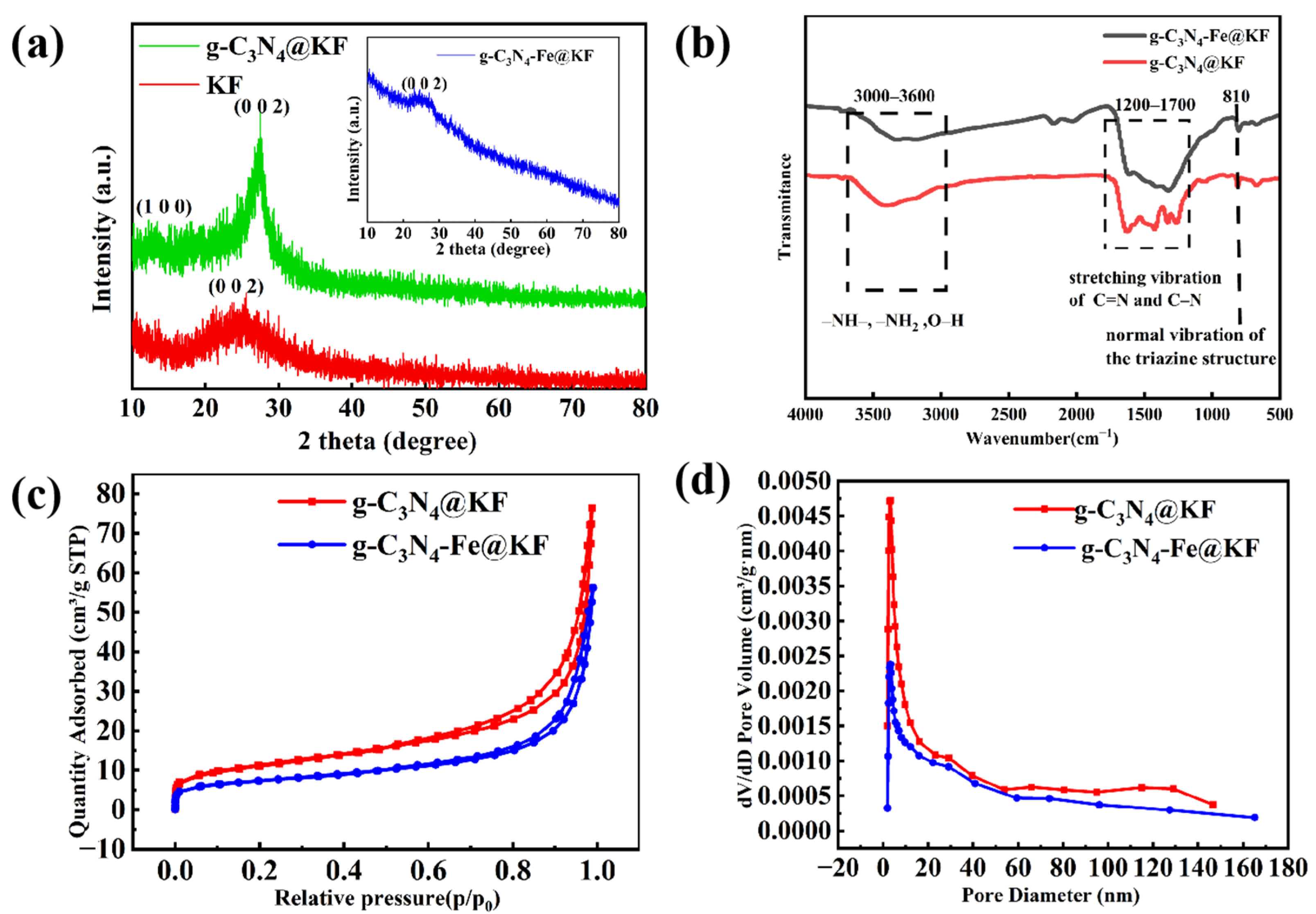
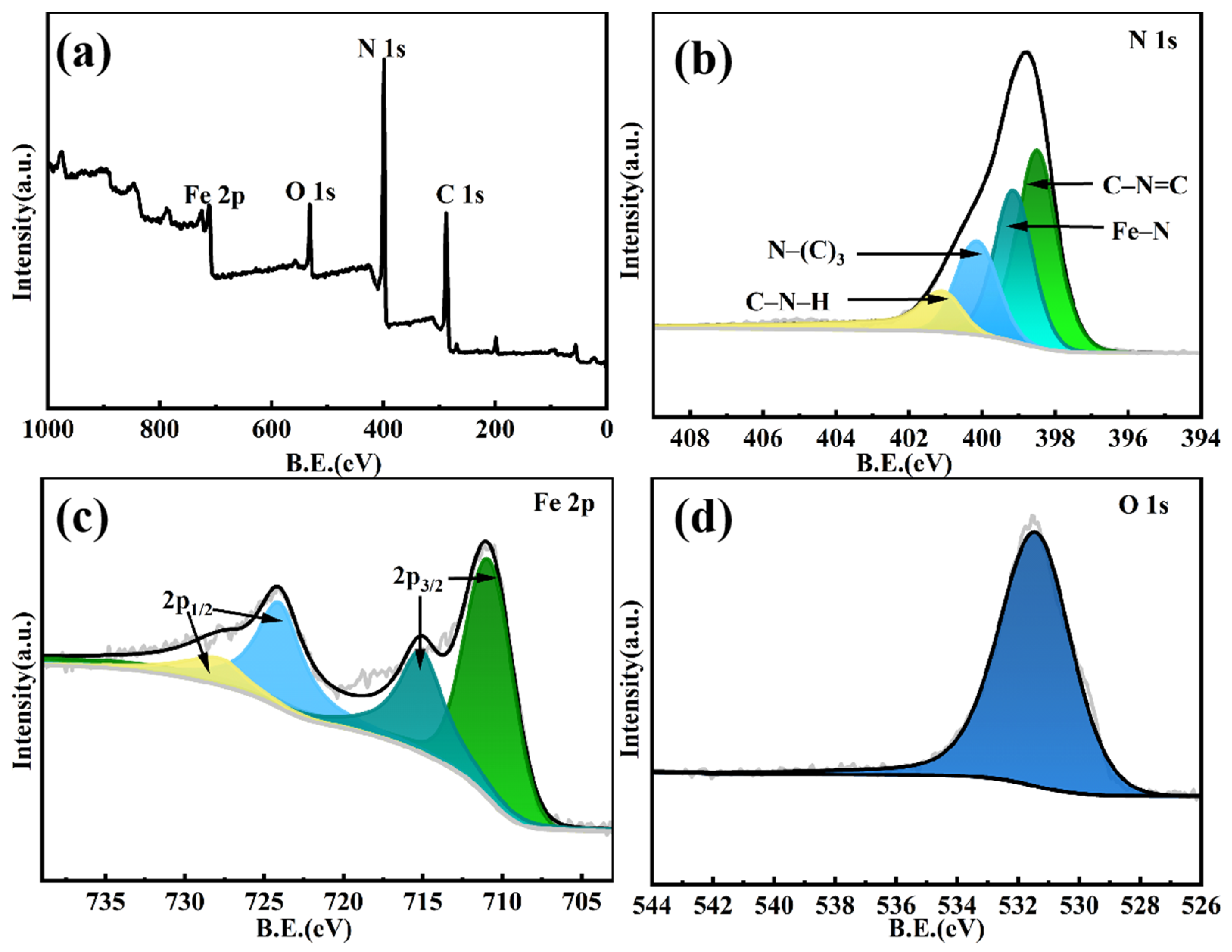
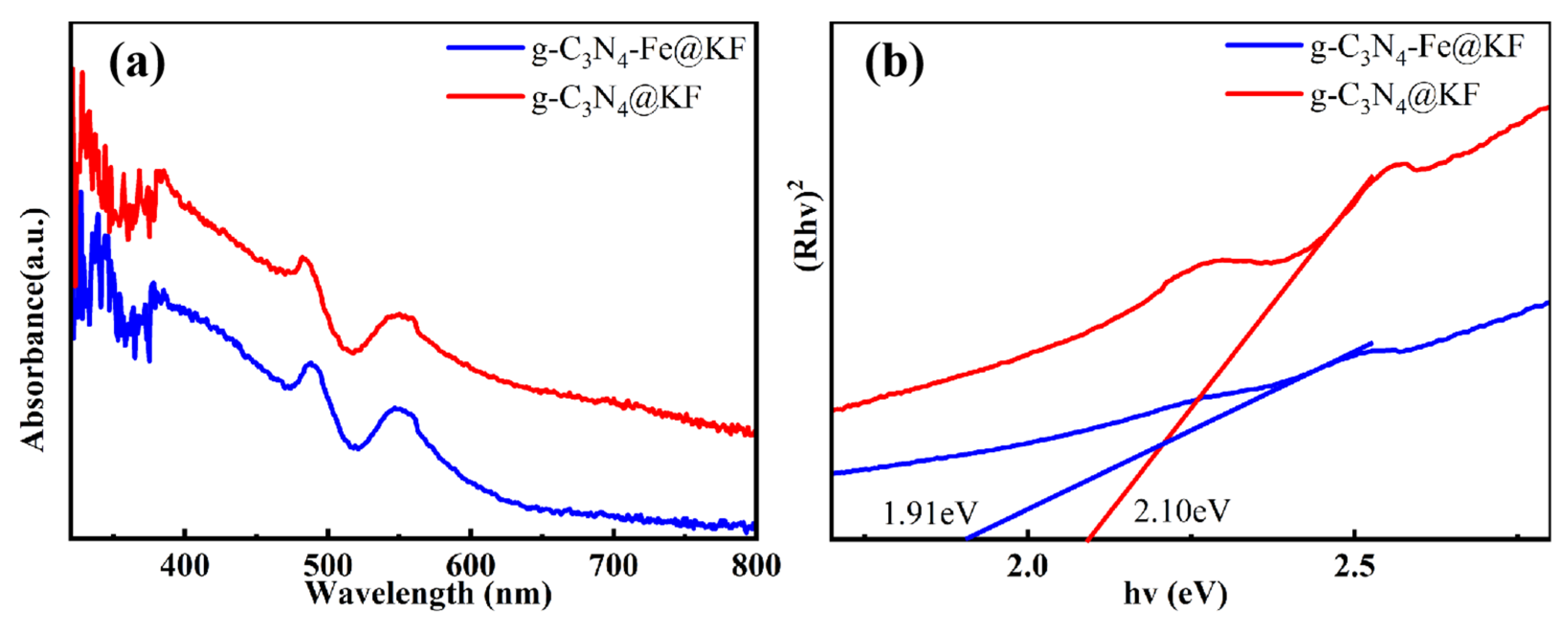
2.1. Fabrication and Characterization of g-C3N4-Fe@KF Micromotors
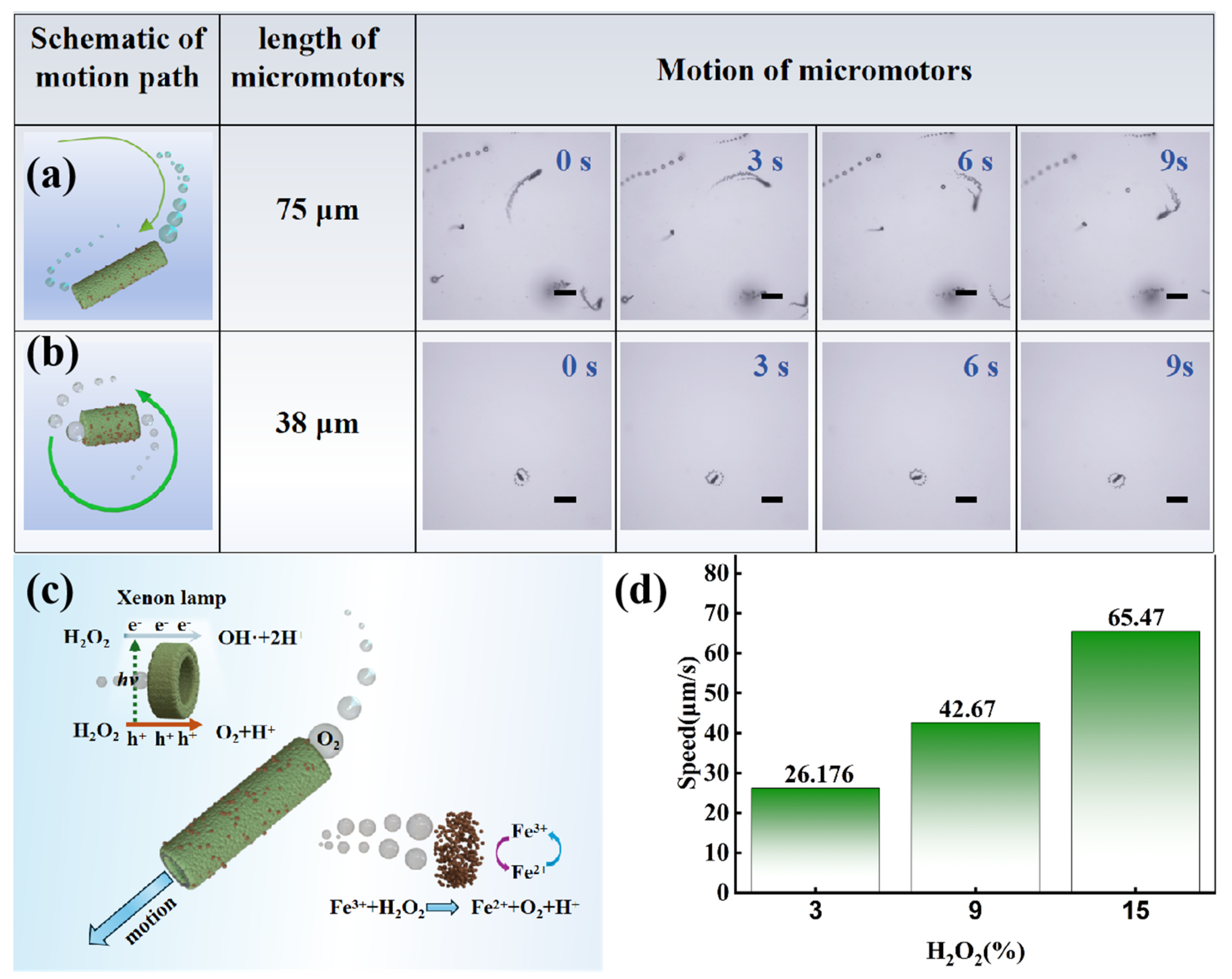
2.2. Motion Behavior of g-C3N4-Fe@KF Micromotors
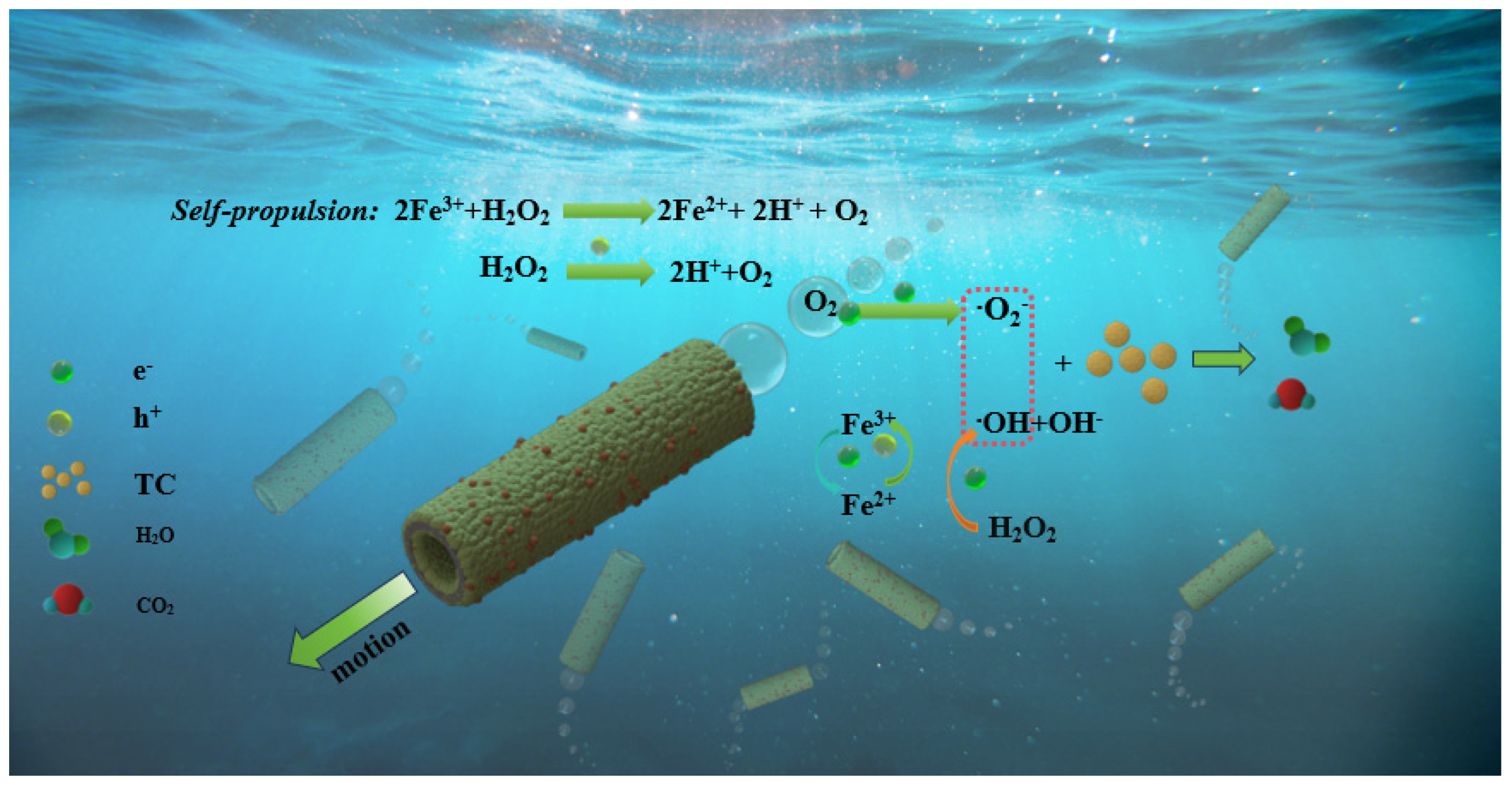
2.3. Photocatalytic Activity of g-C3N4-Fe@KF Micromotors
3. Materials and Methods
3.1. Materials
3.2. Preparation of g-C3N4-Fe@KF Micromotors
3.3. Characterization of g-C3N4-Fe@KF Micromotors
3.4. Motion Characterization of g-C3N4-Fe@KF Micromotors
3.5. Degradation of TC
3.6. Reusability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Jan, S.; Mishra, A.K.; Bhat, M.A.; Bhat, M.A.; Jan, A.T. Pollutants in aquatic system: A frontier perspective of emerging threat and strategies to solve the crisis for safe drinking water. Environ. Sci. Pollut. Res. 2023, 30, 113242–113279. [Google Scholar] [CrossRef]
- Okoye, C.O.; Nyaruaba, R.; Ita, R.E.; Okon, S.U.; Addey, C.I.; Ebido, C.C.; Opabunmi, A.O.; Okeke, E.S.; Chukwudozie, K.I. Antibiotic resistance in the aquatic environment: Analytical techniques and interactive impact of emerging contaminants. Environ. Toxicol. Pharmacol. 2022, 96, 103995. [Google Scholar] [CrossRef] [PubMed]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Mangla, D.; Annu; Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, M.F.; Sapawe, N. A review on the current techniques and technologies of organic pollutants removal from water/wastewater. Mater. Today Proc. 2020, 31, A158–A165. [Google Scholar] [CrossRef]
- Kanaujiya, D.K.; Paul, T.; Sinharoy, A.; Pakshirajan, K. Biological treatment processes for the removal of organic micropollutants from wastewater: A review. Curr. Pollut. Rep. 2019, 5, 112–128. [Google Scholar] [CrossRef]
- Hou, T.; Yu, S.; Zhou, M.; Wu, M.; Liu, J.; Zheng, X.; Li, J.; Wang, J.; Wang, X. Effective removal of inorganic and organic heavy metal pollutants with poly (amino acid)-based micromotors. Nanoscale 2020, 12, 5227–5232. [Google Scholar] [CrossRef] [PubMed]
- Orozco, J.; Mercante, L.A.; Pol, R.; Merkoçi, A. Graphene-based Janus micromotors for the dynamic removal of pollutants. J. Mater. Chem. A 2016, 4, 3371–3378. [Google Scholar] [CrossRef]
- Wu, J.; Yu, H.; Liu, W.; Dong, C.; Wu, M.; Zhang, C. Enhanced degradation of organic pollutant by bimetallic catalysts decorated micromotor in advanced oxidation processes. J. Environ. Chem. Eng. 2022, 10, 107034. [Google Scholar] [CrossRef]
- Shang, Y.; Cai, L.; Liu, R.; Zhang, D.; Zhao, Y.; Sun, L. Self-Propelled Structural Color Cylindrical Micromotors for Heavy Metal Ions Adsorption and Screening. Small 2022, 18, 2204479. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, H.; Liu, J.; Zhou, M.; Pan, J. Crescent-shaped micromotor sorbents with sulfonic acid functionalized convex surface: The synthesis by A Janus emulsion strategy and adsorption for Li+. J. Hazard. Mater. 2022, 422, 126870. [Google Scholar] [CrossRef] [PubMed]
- Ussia, M.; Urso, M.; Dolezelikova, K.; Michalkova, H.; Adam, V.; Pumera, M. Active Light-Powered Antibiofilm ZnO Micromotors with Chemically Programmable Properties. Adv. Funct. Mater. 2021, 31, 2101178. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S.; Wang, Q.; Chen, W.; Liu, B.; Zhao, Y.-D. Light-controlled microbots gathering as a sterilization platform for highly efficient capturing, concentrating and killing targeted bacteria. Chem. Eng. J. 2022, 435, 135067. [Google Scholar] [CrossRef]
- Liu, W.; Ge, H.; Ding, X.; Lu, X.; Zhang, Y.; Gu, Z. Cubic nano-silver-decorated manganese dioxide micromotors: Enhanced propulsion and antibacterial performance. Nanoscale 2020, 12, 19655–19664. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, P.; Liu, Q.; Ran, P.; Xie, S.; Wei, J.; Li, X. Pyroelectric Janus nanomotors for synergistic electrodynamic-photothermal-antibiotic therapies of bacterial infections. Acta Biomater. 2023, 162, 20–31. [Google Scholar] [CrossRef]
- Liu, W.; Ge, H.; Gu, Z.; Lu, X.; Li, J.; Wang, J. Electrochemical deposition tailors the catalytic performance of MnO2-based micromotors. Small 2018, 14, 1802771. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Wang, W.; Li, M.; Zhang, M.-J.; Tang, M.-J.; Su, Y.-Y.; Liu, Z.; Ju, X.-J.; Xie, R.; Chu, L.-Y. Facile fabrication of bubble-propelled micromotors carrying nanocatalysts for water remediation. Ind. Eng. Chem. Res. 2018, 57, 4562–4570. [Google Scholar] [CrossRef]
- Chen, C.; Karshalev, E.; Guan, J.; Wang, J. Magnesium-based micromotors: Water-powered propulsion, multifunctionality, and biomedical and environmental applications. Small 2018, 14, 1704252. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zheng, C.; Song, X.; Gan, Q.; Lin, J. High-efficiency removal of organic pollutants by visible-light-driven tubular heterogeneous micromotors through a photocatalytic Fenton process. J. Colloid. Interface. Sci. 2023, 630, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; He, J.; Chen, L.; Chen, K.; Li, Y.; Zhang, K.; Jin, Z.; Liu, J.; Wang, C.; Wang, X.; et al. A 2D-g-C3N4 nanosheet as an eco-friendly adsorbent for various environmental pollutants in water. Chemosphere 2017, 171, 192–201. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Oh, D.; Lee, C.S.; Kumari, N.; Lee, I.S.; Chang, Y.-S. Carbon-nitride-based micromotor driven by chromate-hydrogen peroxide redox system: Application for removal of sulfamethaxazole. J. Colloid Interface Sci. 2021, 597, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.; Joshua, K.K.; Bice, S.M.; Vincent, O.N. A review of the current status of graphitic carbon nitride. Crit. Rev. Solid State Mat. Sci. 2021, 46, 189–217. [Google Scholar]
- Ismael, M. A review on graphitic nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A review. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Graphitic carbon nitride-based catalysts and their applications: A review. Nano-Struct. Nano-Objects 2020, 24, 100577. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Pan, B. Fe (III)-doped g-C3N4 mediated peroxymonosulfate activation for selective degradation of phenolic compounds via high-valent iron-oxo species. Environ. Sci. Technol. 2018, 52, 2197–2205. [Google Scholar] [CrossRef]
- Hanggara, S. Reducing agent-free formation of Cu (I) nanoclusters on gC3N4 for enhanced photocatalysis. J. Alloys Compd. 2017, 716, 119–127. [Google Scholar]
- Shi, J.; Bai, X.; Xu, L.; Jin, X.; Shi, X.; Jin, P. Facile preparation of Fe-C3N4 heterojunction for enhanced pollutant degradation in Fenton-like process. J. Water Process. Eng. 2022, 46, 102628. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, Y.; Lu, C.; Lin, X.; Fu, Z.; Shi, W.; Guo, F. Photocatalytic self-Fenton system of g-C3N4-based for degradation of emerging contaminants: A review of advances and prospects. Molecules 2023, 28, 5916. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shen, Q.; Zhaoa, B.; Xue, J.; Guan, R.; Liu, X.; Jia, H.; Xu, B. Facile synthesis of Fe-doped g-C3N4 for enhanced visible-light photocatalytic activity. Inorg. Chem. Commun. 2019, 107, 107451. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A facile one-step synthesis of Fe-doped g-C3N4 nanosheets and their improved visible-light photocatalytic performance. ChemCatChem 2017, 9, 1708–1715. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.; Chen, Z.; Zhang, Z.; Fang, X. Constructing a novel ternary Fe (III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B Environ. 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Li, Q.; Xu, H.; Zhou, G.; Cheng, F.; Wang, M.; Zhang, J.; Wang, Y.; Huang, X.; Wang, Q. Sulfite activation by Fe-doped g-C3N4 for metronidazole degradation. Sep. Purif. Technol. 2021, 272, 118928. [Google Scholar] [CrossRef]
- Li, R.; Huang, J.; Cai, M.; Huang, J.; Xie, Z.; Zhang, Q.; Liu, Y.; Liu, H.; Lv, W.; Liu, G. Activation of peroxymonosulfate by Fe doped g-C3N4/graphene under visible light irradiation for Trimethoprim degradation. J. Hazard. Mater. 2020, 384, 121435. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Wang, Q.; Cheng, H. High-efficiency photo-Fenton Fe/g-C3N4/kaolinite catalyst for tetracycline hydrochloride degradation. Appl. Clay Sci. 2021, 212, 106213. [Google Scholar] [CrossRef]
- Bai, X.; Shi, J.; Xu, L.; Jin, X.; Shi, X.; Jin, P. Fe-g-C3N4/reduced graphene oxide lightless application for efficient peroxymonosulfate activation and pollutant mineralization: Comprehensive exploration of reactive sites. Sci. Total Environ. 2023, 855, 158799. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Dou, M.; Ji, J.; Wang, F. Fe–N x moiety-modified hierarchically porous carbons derived from porphyra for highly effective oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 1526–1532. [Google Scholar] [CrossRef]
- Miao, W.; Liu, Y.; Chen, X.; Zhao, Y.; Mao, S. Tuning layered Fe-doped g-C3N4 structure through pyrolysis for enhanced Fenton and photo-Fenton activities. Carbon 2020, 159, 461–470. [Google Scholar] [CrossRef]
- Huang, W.; Manjare, M.; Zhao, Y. Catalytic nanoshell micromotors. J. Phys. Chem. C 2013, 117, 21590–21596. [Google Scholar] [CrossRef]
- Mou, F.; Li, Y.; Chen, C.; Li, W.; Yin, Y.; Ma, H.; Guan, J. Single-Component TiO2 Tubular Microengines with Motion Controlled by Light-Induced Bubbles. Small 2015, 11, 2564–2570. [Google Scholar] [CrossRef]
- Ding, C.; Kang, S.; Li, W.; Gao, W.; Zhang, Z.; Zheng, L.; Cui, L. Mesoporous structure and amorphous Fe-N sites regulation in Fe-g-C3N4 for boosted visible-light-driven photo-Fenton reaction. J. Colloid Interface Sci. 2022, 608, 2515–2528. [Google Scholar] [CrossRef]
- Li, K.; Liang, Y.; Yang, H.; An, S.; Shi, H.; Song, C.; Guo, X. New insight into the mechanism of enhanced photo-Fenton reaction efficiency for Fe-doped semiconductors: A case study of Fe/g-C3N4. Catal. Today 2021, 371, 58–63. [Google Scholar] [CrossRef]
- Simmchen, J.; Magdanz, V.; Sanchez, S.; Chokmaviroj, S.; Ruiz-Molina, D.; Baeza, A.; Schmidt, O.G. Effect of surfactants on the performance of tubular and spherical micromotors–a comparative study. RSC Adv. 2014, 4, 20334–20340. [Google Scholar] [CrossRef]
- Qi, A.; Hang, Z.; Ning, L.; Shuai, W.; Shuo, C. Fe-doped g-C3N4 synthesized by supramolecular preorganization for enhanced photo-Fenton activity. Sep. Purif. Technol. 2023, 315, 123673. [Google Scholar]
- Lai, X.; Ning, X.-A.; Zhang, Y.; Li, Y.; Li, R.; Chen, J.; Wu, S. Treatment of simulated textile sludge using the Fenton/Cl− system: The roles of chlorine radicals and superoxide anions on PAHs removal. Environ. Res. 2021, 197, 110997. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, B.; Dekanovsky, L.; Wei, S.; Khezri, B.; Hartman, T.; Li, J.; Sofer, Z. Integration of BiOI nanosheets into bubble-propelled micromotors for efficient water purification. FlatChem 2021, 30, 100294. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, J.; Yan, X.; Wang, F.; Yang, W.; Ng, D.H.; Yang, J. Active magnetic Fe3+-doped BiOBr micromotors as efficient solar photo-fenton catalyst. J. Clean. Prod. 2020, 252, 119573. [Google Scholar] [CrossRef]
- Yang, W.; Xu, C.; Lyu, Y.; Lan, Z.; Li, J.; Ng, D.H.L. Hierarchical hollow α-Fe2O3/ZnFe2O4/Mn2O3 Janus micromotors as dynamic and efficient microcleaners for enhanced photo-Fenton elimination of organic pollutants. Chemosphere 2023, 338, 139530. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Y.; Chen, X.; Liu, W.; Li, J. Simultaneous Removal of Antibiotics and Heavy Metals with Poly (Aspartic Acid)-Based Fenton Micromotors. Chem.–Asian J. 2021, 16, 1930–1936. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Lyu, Y.; Yan, X.; Yang, P.; Zuo, M. Bioinspired 3D hierarchical BSA-NiCo2O4@ MnO2/C multifunctional micromotors for simultaneous spectrophotometric determination of enzyme activity and pollutant removal. J. Clean. Prod. 2021, 309, 127294. [Google Scholar] [CrossRef]
- Wang, S.; Long, J.; Jiang, T.; Shao, L.; Li, D.; Xie, X.; Xu, F. Magnetic Fe3O4/CeO2/g-C3N4 composites with a visible-light response as a high efficiency Fenton photocatalyst to synergistically degrade tetracycline. Sep. Purif. Technol. 2021, 278, 119609. [Google Scholar] [CrossRef]
- Mao, S.; Liu, C.; Wu, Y.; Xia, M.; Wang, F. Porous P, Fe-doped g-C3N4 nanostructure with enhanced photo-Fenton activity for removal of tetracycline hydrochloride: Mechanism insight, DFT calculation and degradation pathways. Chemosphere 2022, 291, 133039. [Google Scholar] [CrossRef]
- Guo, T.; Wang, K.; Zhang, G.; Wu, X. A novel α-Fe2O3@ g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance. Appl. Surf. Sci. 2019, 469, 331–339. [Google Scholar] [CrossRef]
- Bicalho, H.A.; Lopez, J.L.; Binatti, I.; Batista, P.F.; Ardisson, J.D.; Resende, R.R.; Lorençon, E.J. Facile synthesis of highly dispersed Fe (II)-doped g-C3N4 and its application in Fenton-like catalysis. Mol. Catal. 2017, 435, 156–165. [Google Scholar] [CrossRef]
- He, W.; Jia, H.; Li, Z.; Miao, C.Q.; Lu, R.; Zhang, S.; Zhang, Z. Magnetic recyclable g-C3N4/Fe3O4@ MIL-100 (Fe) ternary catalyst for photo-Fenton degradation of ciprofloxacin. J. Environ. Chem. Eng. 2022, 10, 108698. [Google Scholar] [CrossRef]

| Element | Element Proportion (wt%) |
|---|---|
| C | 49.8 |
| N | 34.1 |
| Fe | 9.4 |
| O | 6.7 |
| Material | Pollutant | Light Conditions | Degradation Time and Conditions | Degradation Rate | Reference |
|---|---|---|---|---|---|
| rGO/ZnO/BiOI/Co-Pi/Pt micromotors (40 mg/L) | R6G (10 mg/L) | Blue laser (435 nm) | 60 min, H2O2 | 94% | [50] |
| Fe0.11Bi0.89OBr/Fe3O4/Mn3O4 micromotor (10 mg/L) | MB (10 mg/L) | Sunlight | 50 min, H2O2 | 98% | [51] |
| α-Fe2O3/ZnFe2O/Mn2O3 micromotors (50 mg) | MB (5 mg/L, 50 mL) | Sunlight | 45 min, H2O2 | 95% | [52] |
| PASP/Fe2O3-MnO2 micromotor (160 mg/L) | TC (30 mg/L) | - | 50 min, H2O2 | 90% | [53] |
| BSA-NiCo2O4@MnO2/C micromotors (50 mg) | TCH (10 mg/L, 50 mL) | - | 180 min, H2O2 | 97.6% | [54] |
| Fe3O4/CeO2/g-C3N4 composites (50 mg) | TCH (50 mg/L) | Visible light | 120 min, H2O2 | 96.63% | [55] |
| Porous P, Fe-doped g-C3N4 nanostructure material (30 mg) | TCH (20 mg/L) | Visible light | 60 min, H2O2 | 98% | [56] |
| α-Fe2O3@ g-C3N4 catalyst (50 mg) | TC (40 mg/L, 100 mL) | Visible light | 100 min, H2O2 | 92% | [57]. |
| Fe(II)-doped g-C3N4 catalyst (50 mg) | MB (50 mg/L, 100 mL) | Visible light | 90 min, H2O2 | 90% (5%-Fe/CN) 100% (10%-Fe/CN) | [58] |
| g-C3N4/Fe3O4 @MIL-100(Fe) (0.67 g/L) | CIP (200 mg/L) | Visible light | 150 min, H2O2 | 94.7% | [59] |
| g-C3N4-Fe@KF micromotors (2 mg) | TC (50 mg/L, 50 mL) | Xenon lamp irradiation | 70 min, H2O2 | 96.79% | Current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Q.; Zhang, J.; Wang, J.; Wei, Y.; Chen, S.; Cai, S.; Xiao, X.; Zheng, C. BioTemplated Fe3+-Doped g-C3N4 Heterojunction Micromotors for the Degradation of Tetracycline through the Photo-Fenton Reaction. Catalysts 2024, 14, 579. https://doi.org/10.3390/catal14090579
Gan Q, Zhang J, Wang J, Wei Y, Chen S, Cai S, Xiao X, Zheng C. BioTemplated Fe3+-Doped g-C3N4 Heterojunction Micromotors for the Degradation of Tetracycline through the Photo-Fenton Reaction. Catalysts. 2024; 14(9):579. https://doi.org/10.3390/catal14090579
Chicago/Turabian StyleGan, Qingbao, Jianwei Zhang, Jinglin Wang, Yuntian Wei, Shikun Chen, Shuguang Cai, Xueqing Xiao, and Chan Zheng. 2024. "BioTemplated Fe3+-Doped g-C3N4 Heterojunction Micromotors for the Degradation of Tetracycline through the Photo-Fenton Reaction" Catalysts 14, no. 9: 579. https://doi.org/10.3390/catal14090579
APA StyleGan, Q., Zhang, J., Wang, J., Wei, Y., Chen, S., Cai, S., Xiao, X., & Zheng, C. (2024). BioTemplated Fe3+-Doped g-C3N4 Heterojunction Micromotors for the Degradation of Tetracycline through the Photo-Fenton Reaction. Catalysts, 14(9), 579. https://doi.org/10.3390/catal14090579






