A Critical Review of Clay Mineral-Based Photocatalysts for Wastewater Treatment
Abstract
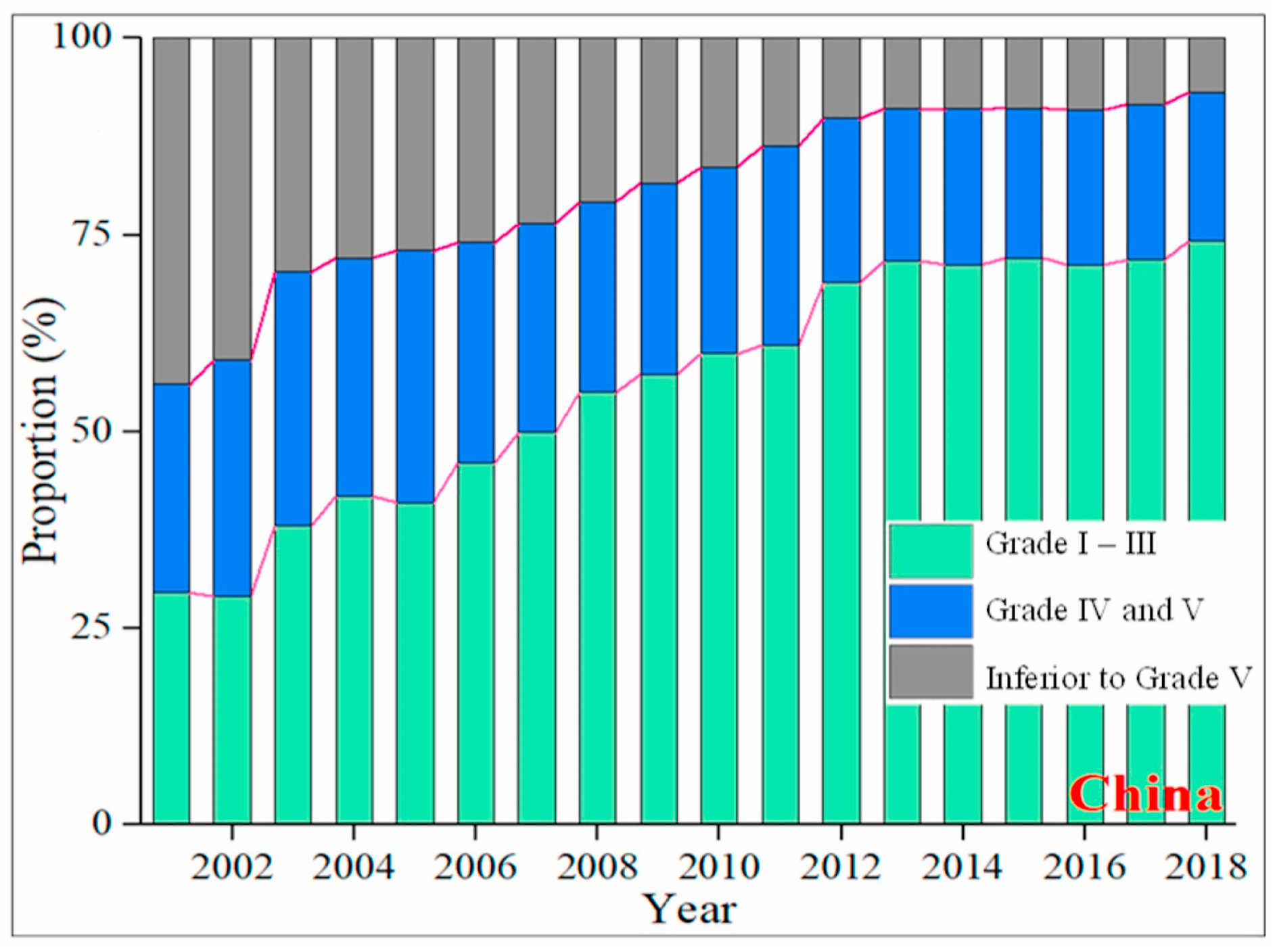
1. Introduction
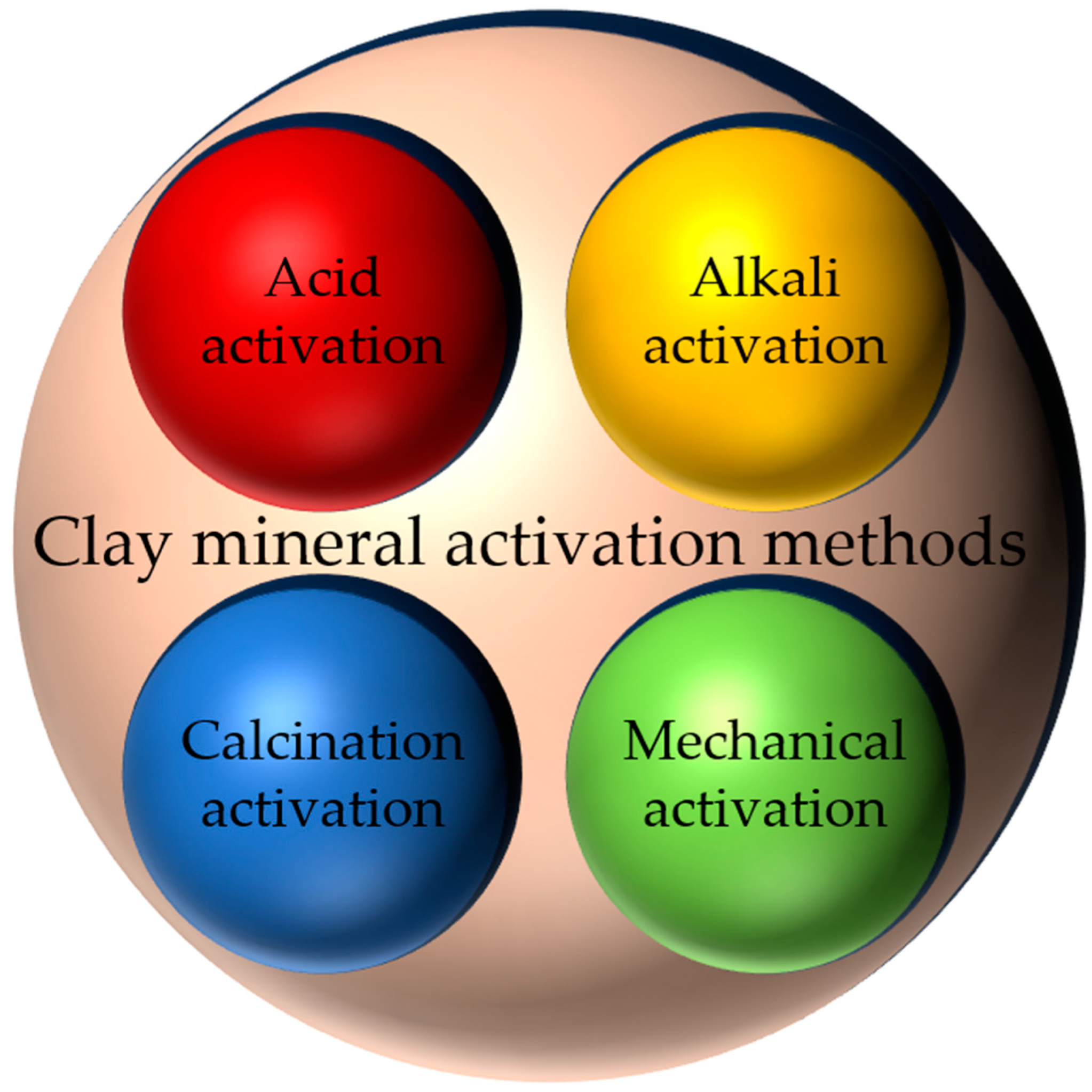
2. Clay Mineral Activation Methods
2.1. Acid Activation
2.2. Alkali Activation
2.3. Calcination Activation
2.4. Mechanical Activation
3. Photocatalyst Modification Strategies
3.1. Doping
3.1.1. Metal-Ion Doping
3.1.2. Nonmetallic-Ion Doping
3.2. Deposition
3.2.1. Metal Deposition
3.2.2. Noble Metal Deposition
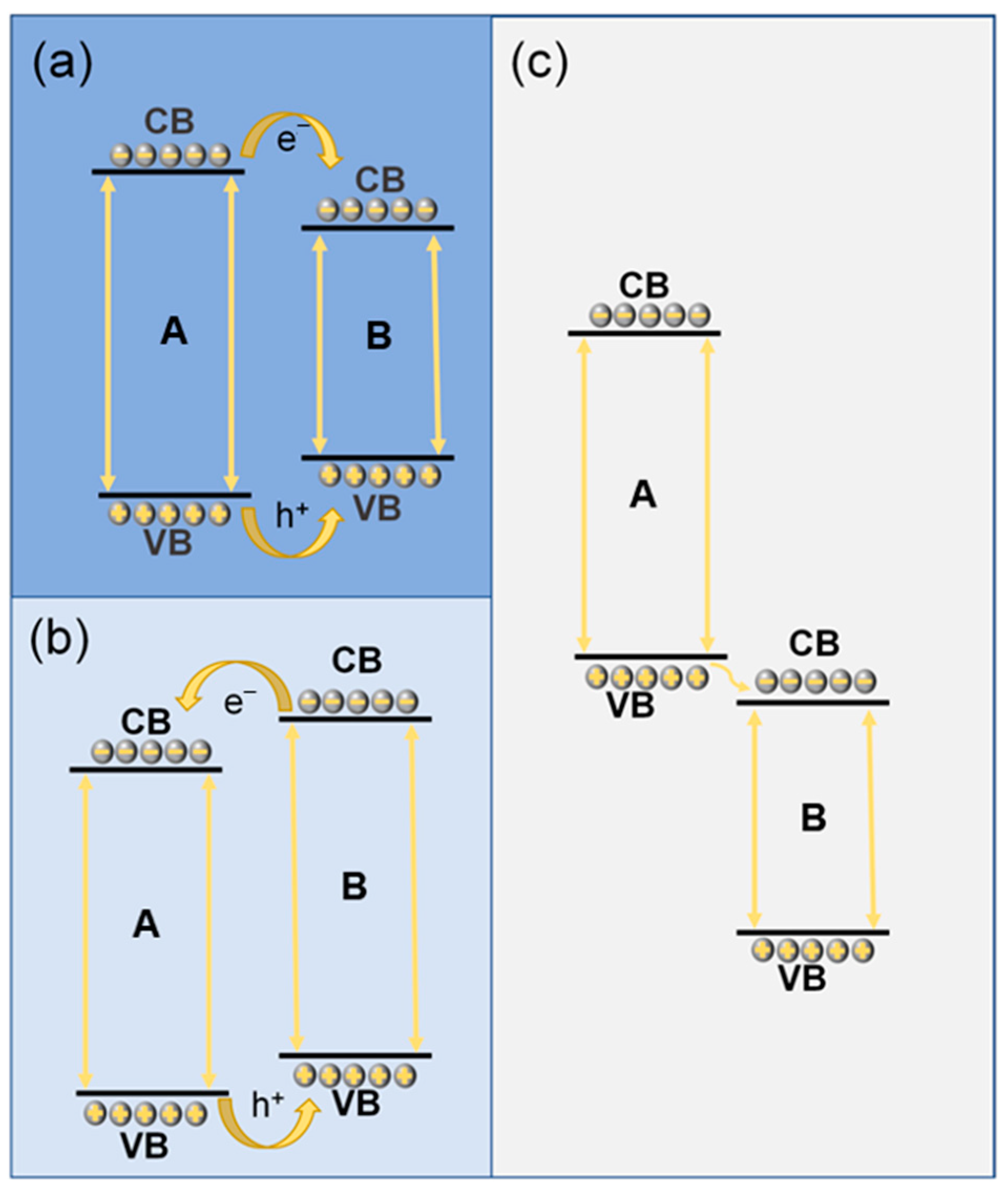
3.3. Common Heterojunction Design
3.3.1. Conventional Heterojunctions
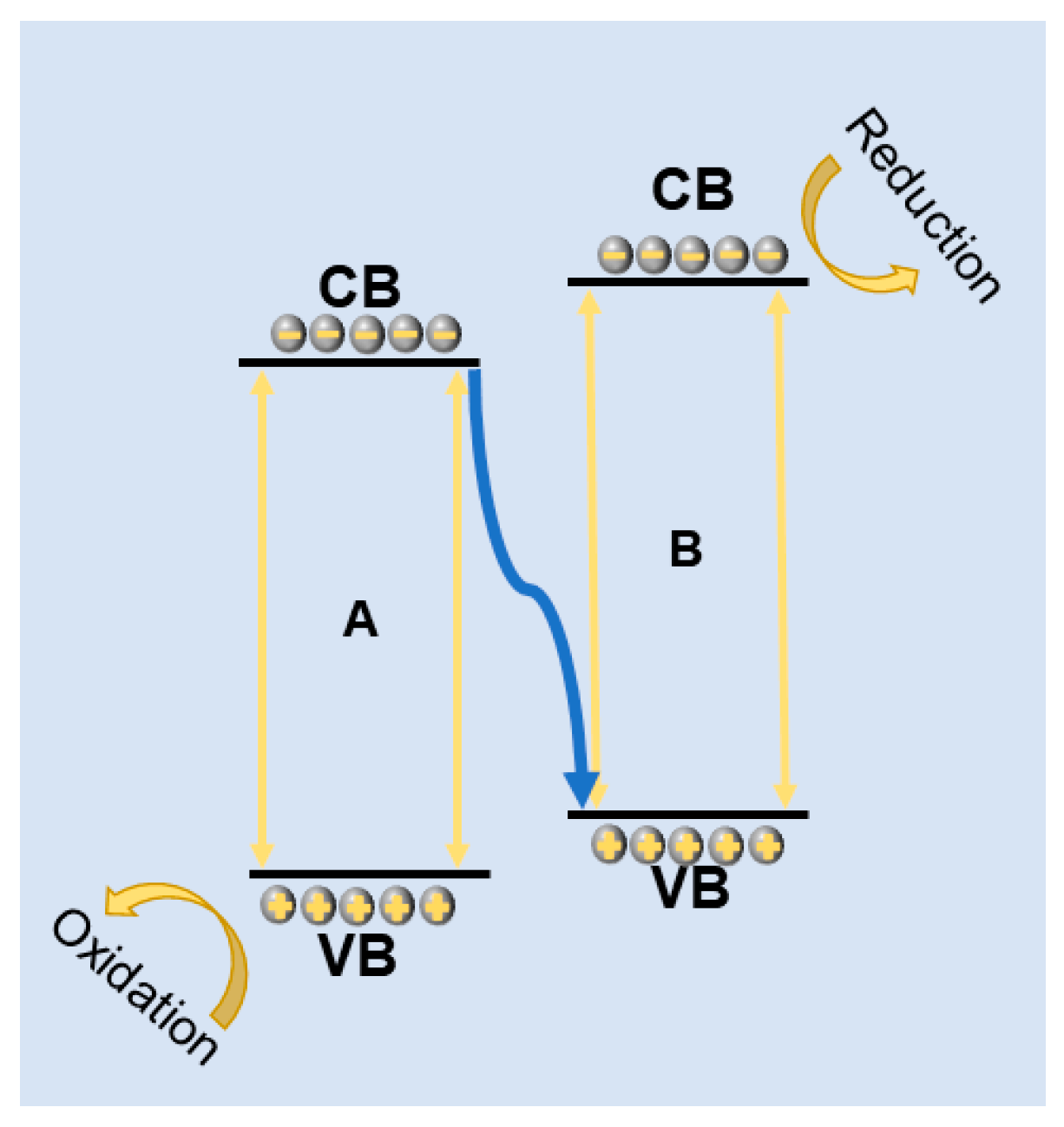
3.3.2. Type “Z” Heterojunction Design
4. Clay Mineral-Based Photocatalyst
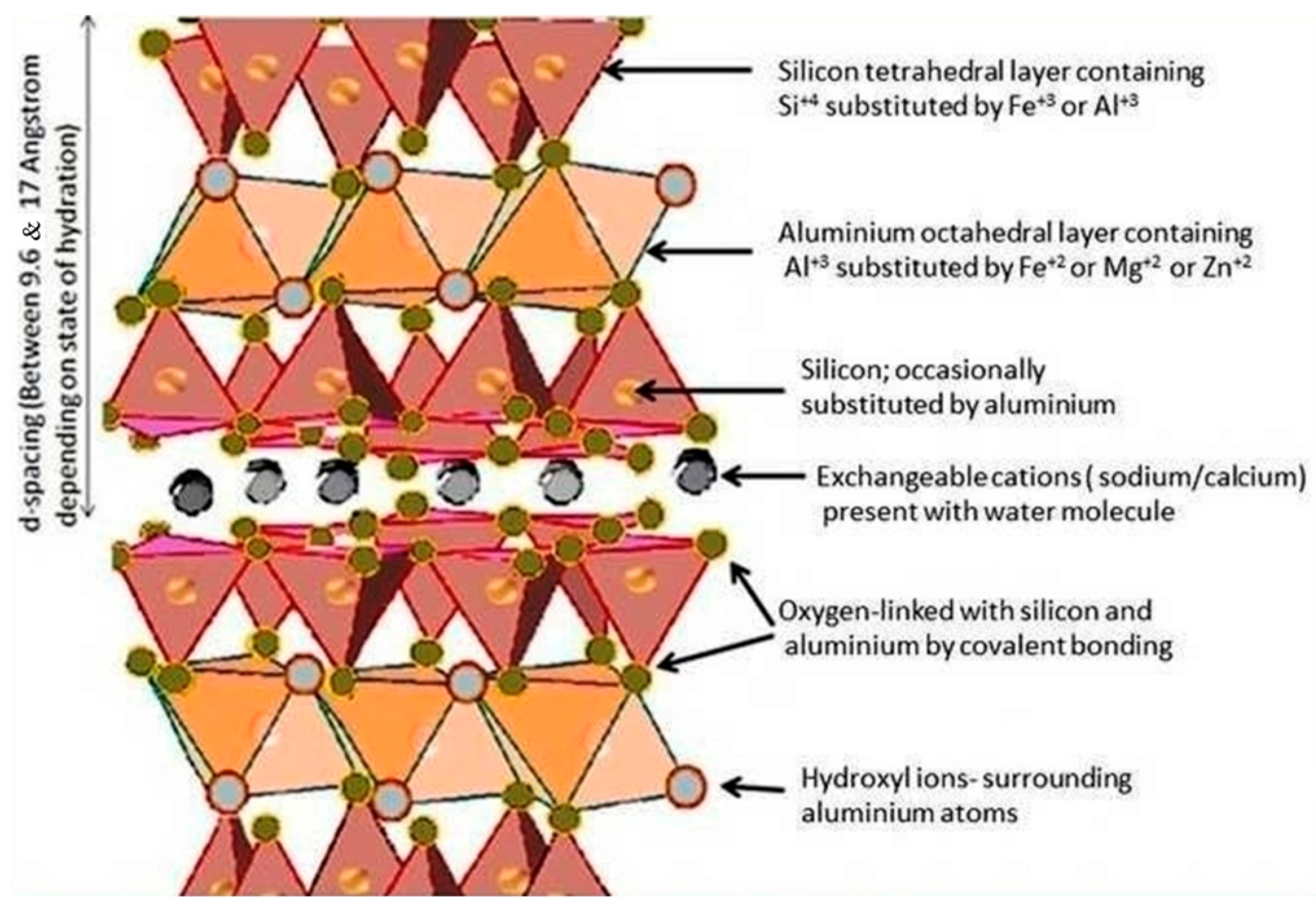
4.1. Layered-Structure Clay Mineral-Based Photocatalyst
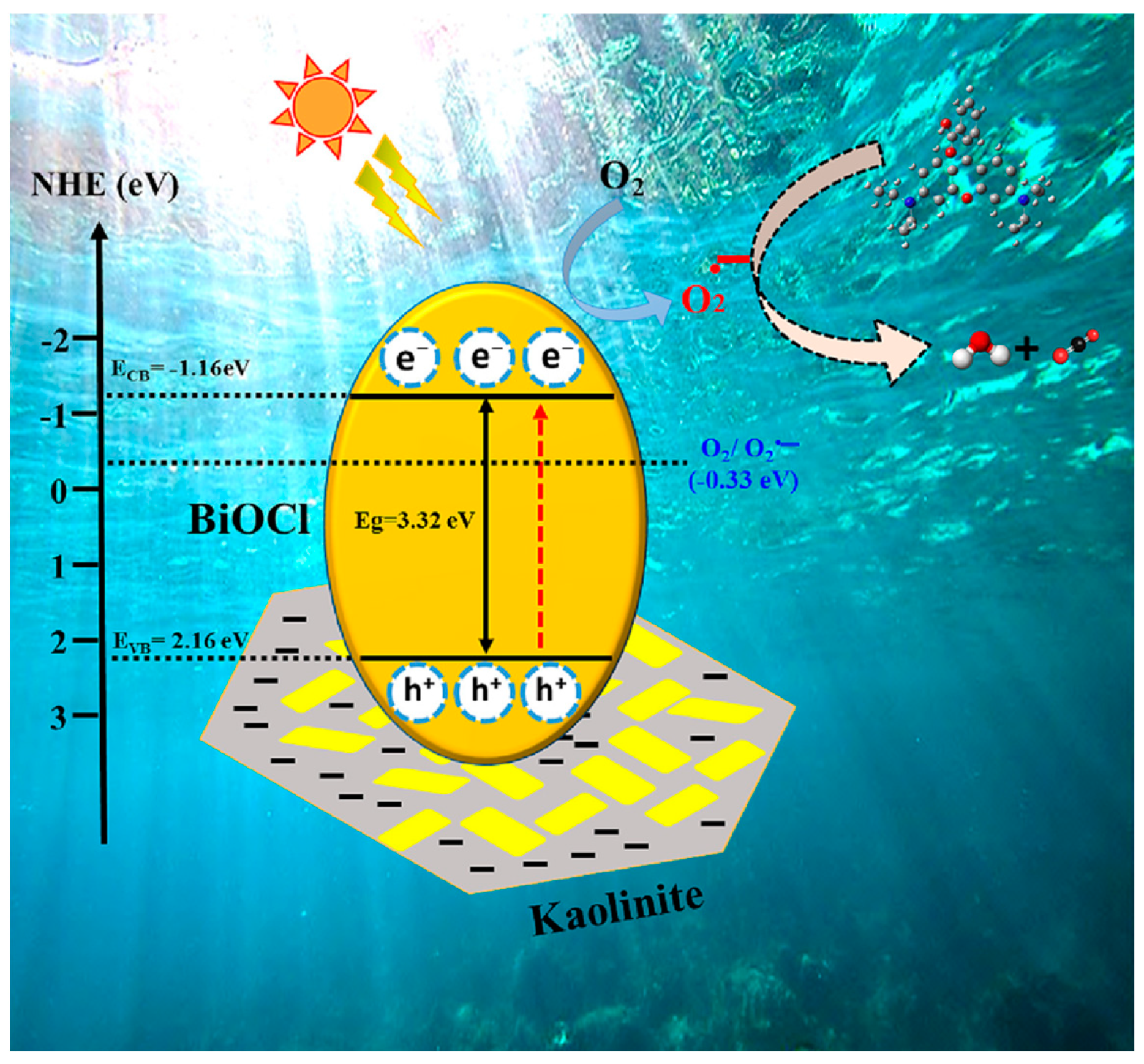
4.1.1. Kaolinite-Based Photocatalyst
4.1.2. Montmorillonite-Based Photocatalyst
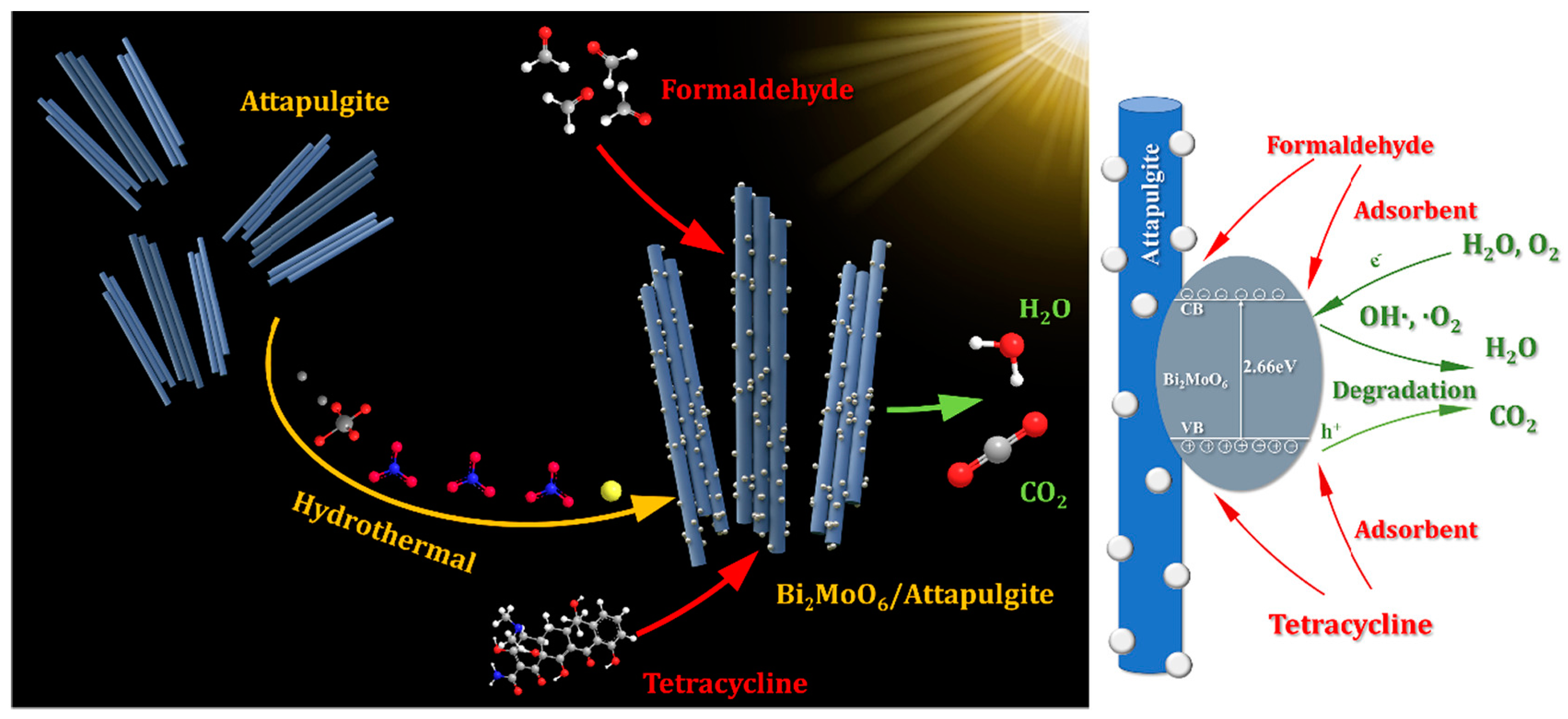
4.1.3. Attapulgite-Based Photocatalyst
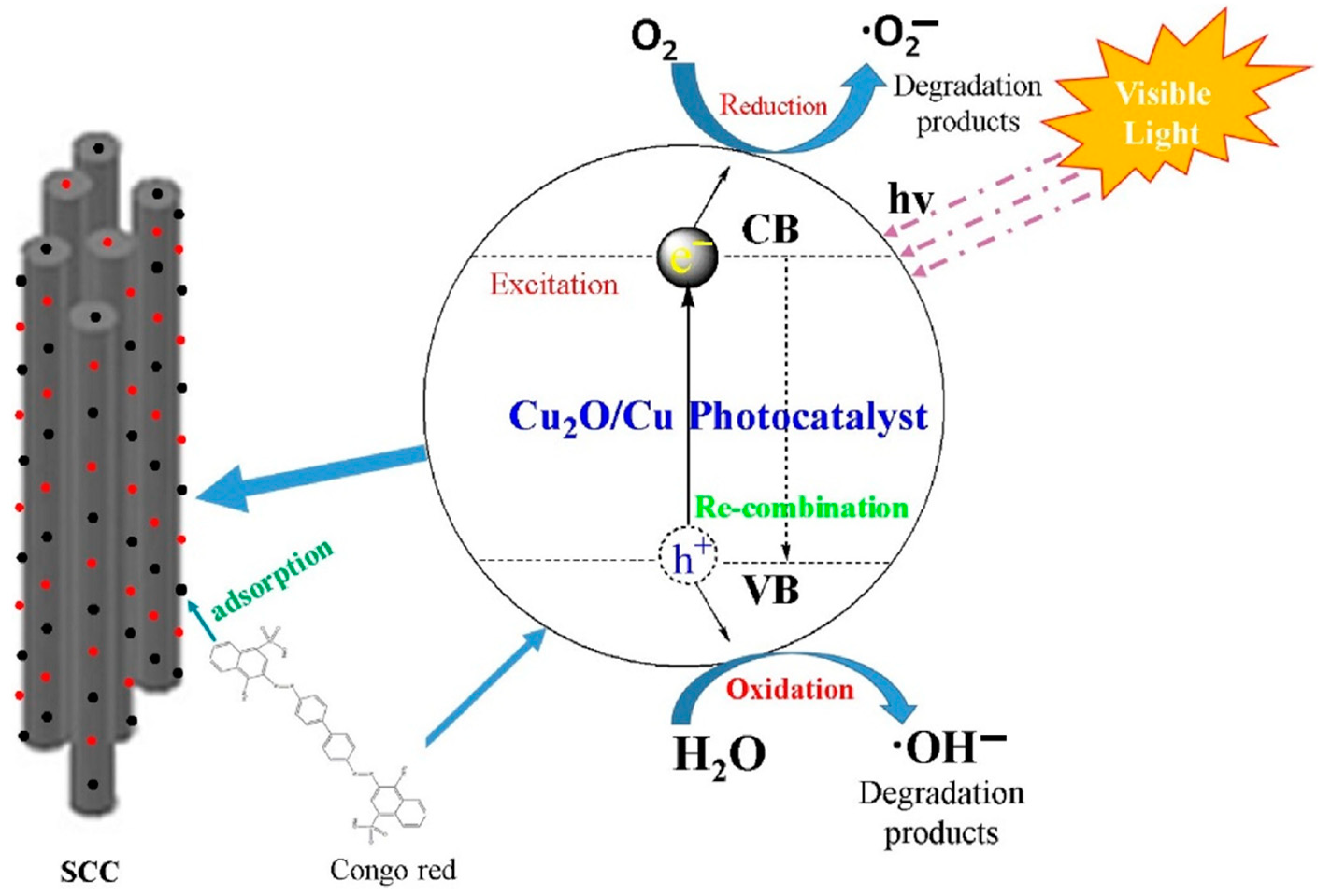
4.1.4. Sepiolite-Based Photocatalyst
4.2. Porous-Structure Clay Mineral-Based Photocatalyst
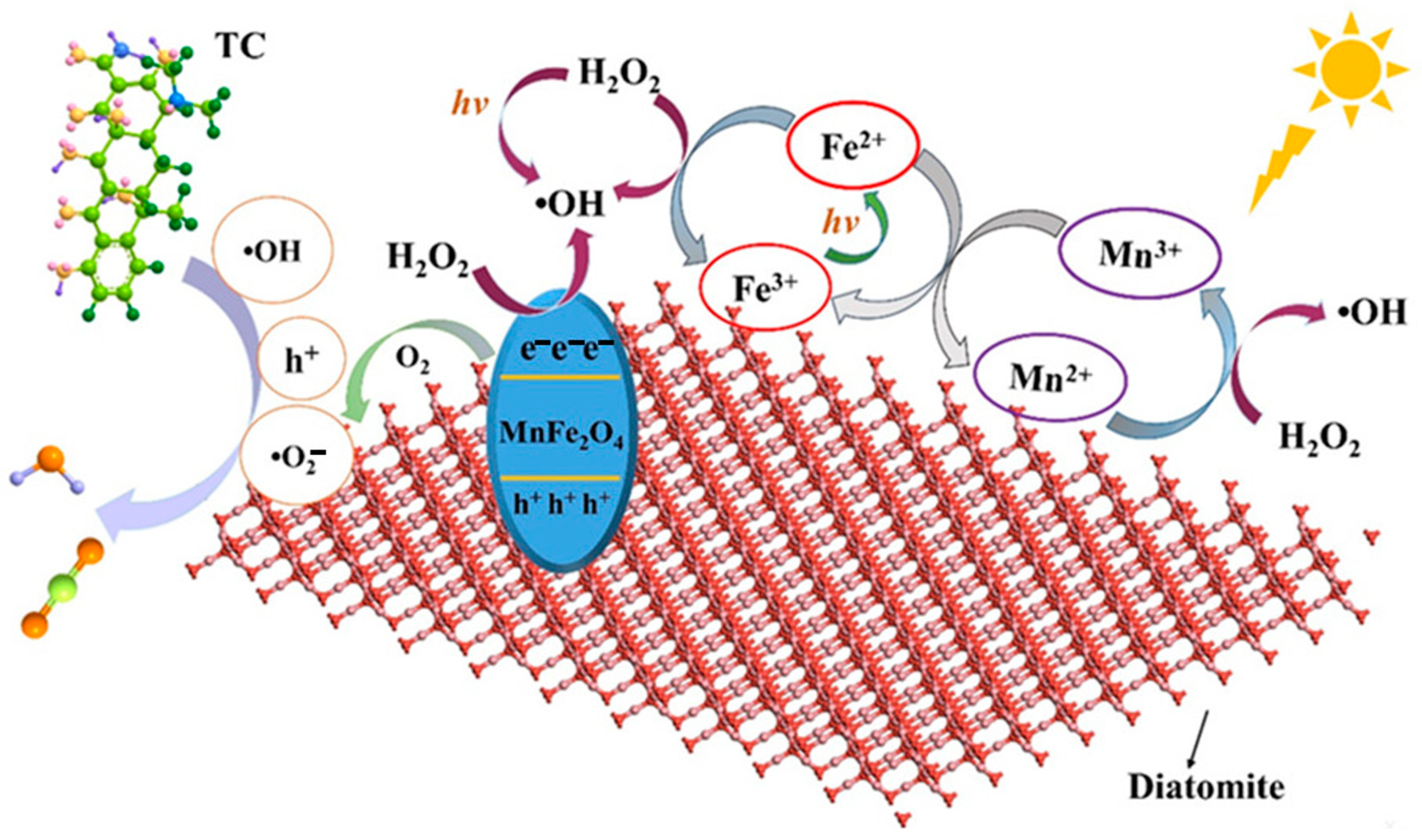
4.2.1. Diatomite-Based Photocatalyst
4.2.2. Zeolite-Based Photocatalyst
4.3. Differences among Clay-Based Materials
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, Y.; Zhang, N.; Chu, C.; Xiao, Y.; Niu, R.; Shao, C. Health impact assessment of the surface water pollution in China. Sci. Total Environ. 2024, 933, 173040. [Google Scholar] [CrossRef]
- Chen, P. Unlocking policy effects: Water resources management plans and urban water pollution. J. Environ. Manag. 2024, 365, 121642. [Google Scholar] [CrossRef]
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty years of China’s water pollution control: Experiences and challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef]
- Qi, Y.; Shen, Y.; Zhao, S.; Jiang, X.; Ma, R.; Cui, B.; Zhao, Q.; Wei, D. Degradation of multiple xanthates using highly efficient visible light-responsive BiOBr-TiO2 composite photocatalysts. J. Ind. Eng. Chem. 2024, 132, 461–473. [Google Scholar] [CrossRef]
- Zhao, S.; Xiao, H.; Chen, Y.; Qi, Y.; Yan, C.; Ma, R.; Zhao, Q.; Liu, W.; Shen, Y. Photocatalytic degradation of xanthates under visible light using heterogeneous CuO/TiO2/montmorillonite composites. Green Smart Min. Eng. 2024, 1, 67–75. [Google Scholar] [CrossRef]
- He, J.; Kumar, A.; Khan, M.; Lo, I.M.C. Critical review of photocatalytic disinfection of bacteria: From noble metals- and carbon nanomaterials-TiO2 composites to challenges of water characteristics and strategic solutions. Sci. Total Environ. 2021, 758, 143953. [Google Scholar] [CrossRef]
- Saddique, Z.; Imran, M.; Javaid, A.; Latif, S.; Hussain, N.; Kowal, P.; Boczkaj, G. Band engineering of BiOBr based materials for photocatalytic wastewater treatment via advanced oxidation processes (AOPs)—A review. Water Resour. Ind. 2023, 29, 100211. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Yang, S.; He, X.; Zheng, S.; Sun, Z.; Dionysiou, D.D. A review of clay based photocatalysts: Role of phyllosilicate mineral in interfacial assembly, microstructure control and performance regulation. Chemosphere 2021, 273, 129723. [Google Scholar] [CrossRef]
- Cerrato, E.; Gaggero, E.; Calza, P.; Paganini, M.C. The role of Cerium, Europium and Erbium doped TiO2 photocatalysts in water treatment: A mini-review. Chem. Eng. J. Adv. 2022, 10, 100268. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.U.H. Modification strategies of TiO2 based photocatalysts for enhanced visible light activity and energy storage ability: A review. J. Environ. Chem. Eng. 2023, 11, 111532. [Google Scholar] [CrossRef]
- Sugita, T.; Mori, M.; Shimoyama, I. Conversion of clay minerals to photocatalysts for CrVI reduction and salicylic acid decomposition. Appl. Clay Sci. 2023, 243, 107074. [Google Scholar] [CrossRef]
- Abeyta, K.P.; da Silva, M.L.A.; Silva, C.L.S.; Pontes, L.A.M.; Teixeira, L.S.G. Clay-based catalysts applied to glycerol valorization: A review. Sustain. Chem. Pharm. 2024, 40, 101641. [Google Scholar] [CrossRef]
- Marsh, A.T.M.; Krishnan, S.; Bernal, S.A. Structural features of thermally or mechano-chemically treated montmorillonite clays as precursors for alkali-activated cements production. Cem. Concr. Res. 2024, 181, 107546. [Google Scholar] [CrossRef]
- Mansuri, A.; Trivedi, C.; Parikh, A.; Kumar, A. Mitigating phthalate toxicity: The protective role of humic acid and clay in zebrafish larvae. Chemosphere 2024, 354, 141756. [Google Scholar] [CrossRef]
- Dhar, M.; Bishnoi, S. Influence of calcination temperature on the physical and chemical characteristics of kaolinitic clays for use as supplementary cementitious materials. Cem. Concr. Res. 2024, 178, 107464. [Google Scholar] [CrossRef]
- Tole, I.; Delogu, F.; Qoku, E.; Habermehl-Cwirzen, K.; Cwirzen, A. Enhancement of the pozzolanic activity of natural clays by mechanochemical activation. Constr. Build. Mater. 2022, 352, 128739. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Recent progress of utilization of activated kaolinitic clay in cementitious construction materials. Compos. Part B Eng. 2021, 211, 108636. [Google Scholar] [CrossRef]
- Fatimah, I.; Ardianti, S.; Sahroni, I.; Purwiandono, G.; Sagadevan, S.; Doong, R.-A. Visible light sensitized porous clay heterostructure photocatalyst of zinc-silica modified montmorillonite by using tris(2,2′-bipyridyl) dichlororuthenium. Appl. Clay Sci. 2021, 204, 106023. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Jefri, O.A.; Alam, M.G.; Al-Faze, R.; Kooli, F. organo acid-activated clays for water treatment as removal agent of Eosin-Y: Properties, regeneration, and single batch design absorber. Heliyon 2024, 10, e30530. [Google Scholar] [CrossRef]
- Dodoo, D.; Appiah, G.; Acquaah, G.; Junior, T.D. Fixed-bed column study for the remediation of the bauxite-liquid residue using acid-activated clays and natural clays. Heliyon 2023, 9, e14310. [Google Scholar] [CrossRef]
- Trabelsi, W.; Tlili, A. Phosphoric acid purification through different raw and activated clay materials (Southern Tunisia). J. Afr. Earth Sci. 2017, 129, 647–658. [Google Scholar] [CrossRef]
- Fashtali, A.R.; Payan, M.; Ranjbar, P.Z.; Najafi, E.K.; Chenari, R.J. Attenuation of Zn(II) and Cu(II) by low-alkali activated clay-fly ash liners. Appl. Clay Sci. 2024, 250, 107298. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Provis, J.L.; Cizer, Ö.; Ye, G. Autogenous shrinkage of alkali-activated slag: A critical review. Cem. Concr. Res. 2023, 172, 107244. [Google Scholar] [CrossRef]
- Georgopoulos, G.; Aspiotis, K.; Badogiannis, E.; Tsivilis, S.; Perraki, M. Influence of mineralogy and calcination temperature on the behavior of palygorskite clay as a pozzolanic supplementary cementitious material. Appl. Clay Sci. 2023, 232, 106797. [Google Scholar] [CrossRef]
- Marsh, A.T.M.; Brown, A.P.; Freeman, H.M.; Walkley, B.; Pendlowski, H.; Bernal, S.A. Determining aluminium co-ordination of kaolinitic clays before and after calcination with electron energy loss spectroscopy. Appl. Clay Sci. 2024, 255, 107402. [Google Scholar] [CrossRef]
- Kashif, M.; Kang, C.; Siddhartha, T.R.; Che, C.A.; Su, Y.; Heynderickx, P.M. Investigating the effects of calcination temperature on porous clay heterostructure characteristics. Vacuum 2024, 227, 113415. [Google Scholar] [CrossRef]
- Mañosa, J.; Alvarez-Coscojuela, A.; Marco-Gibert, J.; Maldonado-Alameda, A.; Chimenos, J.M. Enhancing reactivity in muscovitic clays: Mechanical activation as a sustainable alternative to thermal activation for cement production. Appl. Clay Sci. 2024, 250, 107266. [Google Scholar] [CrossRef]
- Yin, Y.; Kang, X.; Han, B. Two-dimensional materials: Synthesis and applications in the electro-reduction of carbon dioxide. Chem. Synth. 2022, 2, 19. [Google Scholar] [CrossRef]
- D’Elia, A.; Pinto, D.; Eramo, G.; Giannossa, L.C.; Ventruti, G.; Laviano, R. Effects of processing on the mineralogy and solubility of carbonate-rich clays for alkaline activation purpose: Mechanical, thermal activation in red/ox atmosphere and their combination. Appl. Clay Sci. 2018, 152, 9–21. [Google Scholar] [CrossRef]
- Luzu, B.; Duc, M.; Djerbi, A.; Gautron, L. High performance illitic clay-based geopolymer: Influence of thermal/mechanical activation on strength development. Appl. Clay Sci. 2024, 258, 107445. [Google Scholar] [CrossRef]
- Huang, A.; He, J.; Feng, J.; Huang, C.; Yang, J.; Mo, W.; Su, X.; Zou, B.; Ma, S.; Lin, H.; et al. A high-efficiency clay mineral based organic photocatalyst towards photodegradation of butyl xanthate in mineral processing wastewater. Sep. Purif. Technol. 2024, 349, 127880. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Wijekoon, A.S.K.; Manoratne, C.H. TiO2-kaolinite composite photocatalyst for industrial organic waste decontamination. Next Mater. 2024, 3, 100065. [Google Scholar] [CrossRef]
- Li, X.; Simon, U.; Bekheet, M.F.; Gurlo, A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies 2022, 15, 5607. [Google Scholar] [CrossRef]
- Ruzimuradov, O.; Musaev, K.; Mamatkulov, S.; Butanov, K.; Gonzalo-Juan, I.; Khoroshko, L.; Turapov, N.; Nurmanov, S.; Razzokov, J.; Borisenko, V.; et al. Structural and optical properties of sol-gel synthesized TiO2 nanocrystals: Effect of Ni and Cr (co)doping. Opt. Mater. 2023, 143, 114203. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, R.; Hou, T.; Zhang, S.; Wan, X.; Li, Y.; Liu, S.; Liu, J.; Zhang, J. Doping transition metal in PdSeO3 atomic layers by aqueous cation exchange: A new doping protocol for a new 2D photocatalyst. Chin. Chem. Lett. 2022, 33, 3739–3744. [Google Scholar] [CrossRef]
- Bashir, N.; Sawaira, T.; Jamil, A.; Awais, M.; Habib, A.; Afzal, A. Challenges and prospects of main-group metal-doped TiO2 photocatalysts for sustainable water remediation. Mater. Today Sustain. 2024, 27, 100869. [Google Scholar] [CrossRef]
- Sahoo, S.; Mahamallik, P.; Das, R.; Panigrahi, S. A critical review on non-metal doped g-C3N4 based photocatalyst for organic pollutant remediation with sustainability assessment by life cycle analysis. Environ. Res. 2024, 258, 119390. [Google Scholar] [CrossRef]
- Sultana, M.; Mondal, A.; Islam, S.; Khatun, M.A.; Rahaman, M.H.; Chakraborty, A.K.; Rahman, M.S.; Rahman, M.M.; Nur, A.S.M. Strategic development of metal doped TiO2 photocatalysts for enhanced dye degradation activity under UV–Vis irradiation: A review. Curr. Res. Green Sustain. Chem. 2023, 7, 100383. [Google Scholar] [CrossRef]
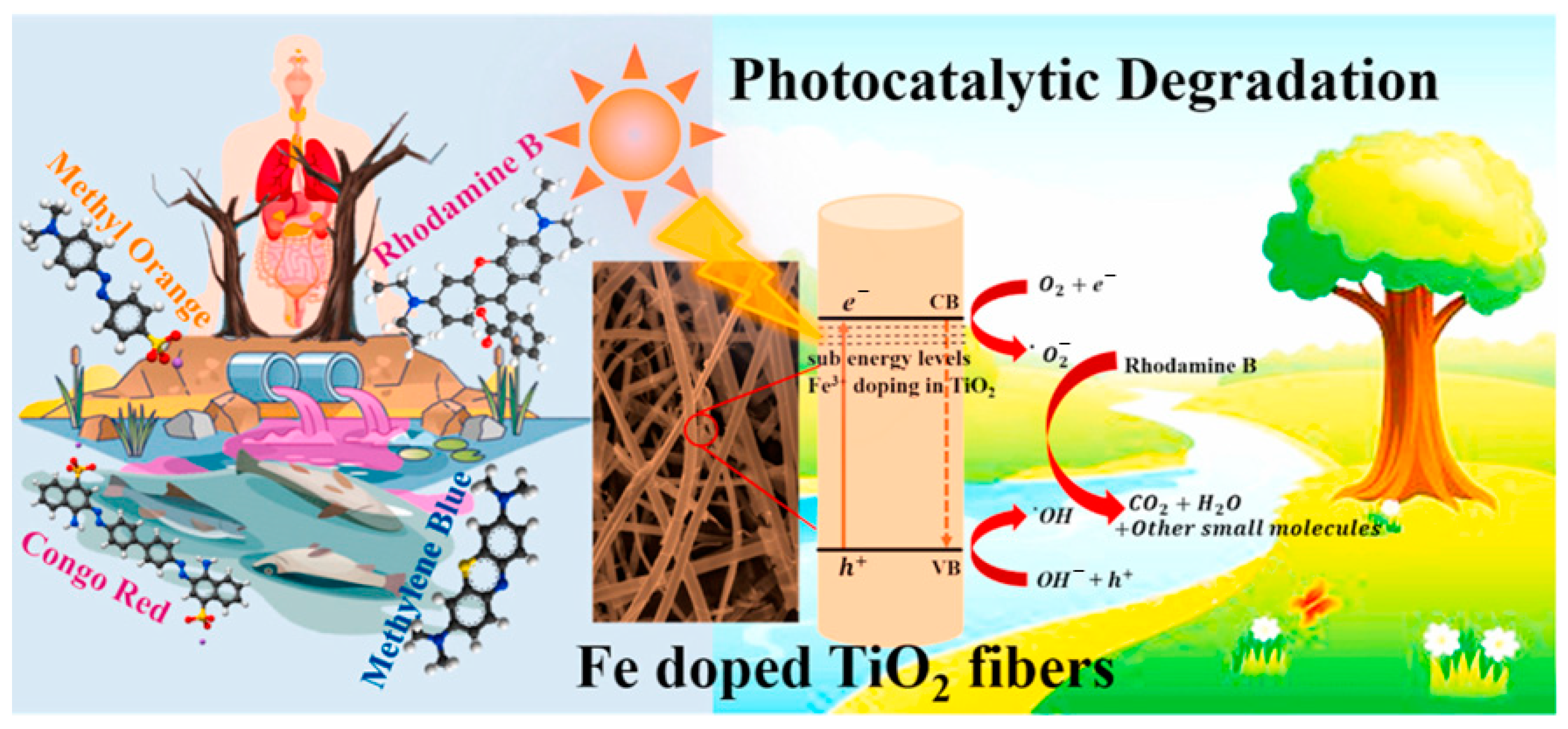
- Mahadadalkar, M.A.; Park, N.; Yusuf, M.; Nagappan, S.; Nallal, M.; Park, K.H. Electrospun Fe doped TiO2 fiber photocatalyst for efficient wastewater treatment. Chemosphere 2023, 330, 138599. [Google Scholar] [CrossRef]
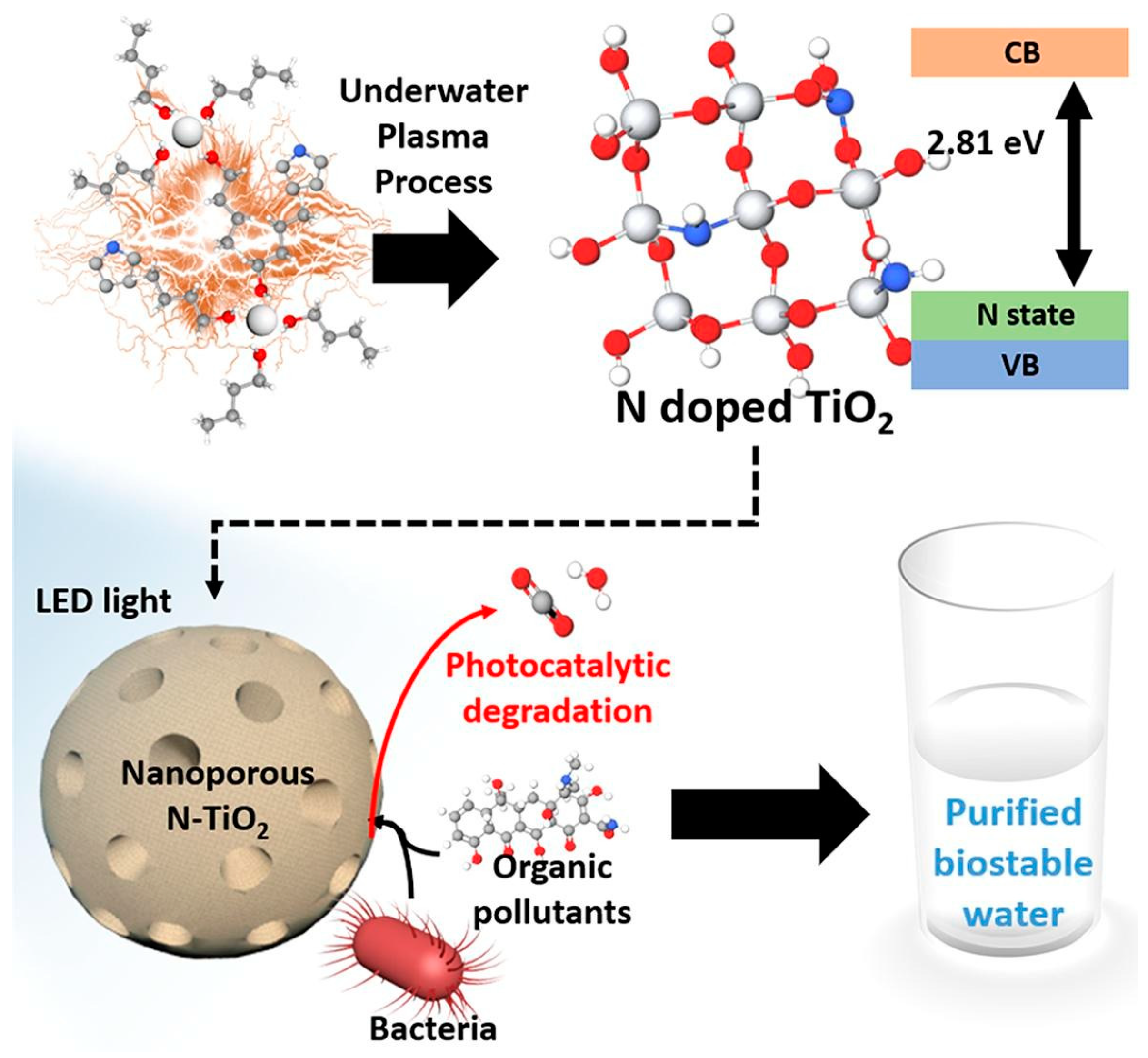
- Lim, C.; An, H.-R.; Ha, S.; Myeong, S.; Min, C.G.; Chung, H.-J.; Son, B.; Kim, C.-Y.; Park, J.-I.; Kim, H.; et al. Highly visible-light-responsive nanoporous nitrogen-doped TiO2 (N-TiO2) photocatalysts produced by underwater plasma technology for environmental and biomedical applications. Appl. Surf. Sci. 2023, 638, 158123. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, S.; Wang, B.; Yang, R.; Wang, R.; Zhu, X.; Song, Y.; Yuan, J.; Xu, H.; Li, H. A candy-like photocatalyst by wrapping Co, N-co-doped hollow carbon sphere with ultrathin mesoporous carbon nitride for boosted photocatalytic hydrogen evolution. Chin. Chem. Lett. 2023, 34, 107749. [Google Scholar] [CrossRef]
- Demarema, S.; Nasr, M.; Ookawara, S.; Abdelhaleem, A. New insights into green synthesis of metal oxide based photocatalysts for photodegradation of organic pollutants: A bibliometric analysis and techno-economic evaluation. J. Clean. Prod. 2024, 463, 142679. [Google Scholar] [CrossRef]
- Ahmad, I.; Li, G.; Al-Qattan, A.; Obaidullah, A.J.; Mahal, A.; Duan, M.; Ali, K.; Ghadi, Y.Y.; Ali, I. A review on versatile metal vanadates photocatalysts for energy conversion and environmental remediation. Mater. Today Sustain. 2024, 25, 100666. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Du, M.; Liu, W.; Liu, N.; Ling, Y.; Kang, S. Mechanisms of noble metal-enhanced ferroelectric spontaneous polarized photocatalysis. Nano Energy 2024, 124, 109495. [Google Scholar] [CrossRef]
- Li, F.; Zhu, G.; Jiang, J.; Yang, L.; Deng, F.; Li, X. A review of updated S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2024, 177, 142–180. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhang, J.; Dai, K. Metal-sulfide-based heterojunction photocatalysts: Principles, impact, applications, and in-situ characterization. Chin. J. Catal. 2023, 49, 42–67. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Chen, Y.; Gao, S.; Liu, W.; Wei, D. Hydrothermal synthesis of novel ternary hierarchical MoS2/TiO2/clinoptilolite nanocomposites with remarkably enhanced visible light response towards xanthates. Appl. Surf. Sci. 2021, 542, 148578. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Lei, T.; Yang, Z.; Yan, J.; Jiang, D. Preparation of ternary BiVO4/g-C3N4/diatomite composites for enhanced photodegradation of rhodamine B and formaldehyde. J. Photochem. Photobiol. A Chem. 2024, 457, 115906. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Wang, S.; Duan, R.; Wang, S.; Li, S.; Lan, B.; Gao, L. Synthesis of a double type-II CdS/Zn2In2S5/g-C3N4 heterojunction photocatalyst for the photodegradation of tetracycline under visible light. J. Environ. Chem. Eng. 2023, 11, 111315. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, H.; Alnuqaydan, A.M.; Siddeeg, S.M.; Abdullaeva, B.S.; Shawabkeh, A. Optimization of tetracycline antibiotic photocatalytic degradation from wastewater using a novel type-II α-Fe2O3-CeO2-SiO2 heterojunction photocatalyst: Performance, pathways, mechanism insights, reaction kinetics and toxicity assessment. J. Water Process Eng. 2024, 57, 104703. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, T.; Yang, T.; Pu, K.; Shi, J.; Zhou, A.; Li, H.; Wang, S.; Xue, J. Z-scheme heterojunction photocatalyst formed by MOF-derived C-TiO2 and Bi2WO6 for enhancing degradation of oxytetracycline: Mechanistic insights and toxicity evaluation in the presence of a single active species. J. Colloid Interface Sci. 2024, 665, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Y.; Duan, R.; Li, S.; Lan, B.; Ma, Z. Synthesis of Ag2ZnGeO4/g-C3N4 binary heterostructure: A Z-scheme heterojunction photocatalyst for enhanced tetracycline degradation under visible light. Chem. Eng. Sci. 2024, 296, 120253. [Google Scholar] [CrossRef]
- Hu, F.; Li, J.; Peng, X.; Xu, J.; Xu, J.; Zeng, Y.; Chen, H.; Hong, B.; Wang, X. Ag/α-Fe2O3/g-C3N4 nanocomposites as Z-scheme heterojunction photocatalysts for degradation of RhB. J. Alloys Compd. 2024, 983, 173893. [Google Scholar] [CrossRef]
- Shuaibu, A.S.; Hafeez, H.Y.; Mohammed, J.; Dankawu, U.M.; Ndikilar, C.E.; Suleiman, A.B. Progress on g-C3N4 based heterojunction photocatalyst for H2 production via Photocatalytic water splitting. J. Alloys Compd. 2024, 1002, 175062. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H.; Issa, M.A.; Ammar, S.H.; Ebrahim, S.E.; Khadim, H.J.; Okab, A.A. Photocatalytic degradation of Congo red dye using magnetic silica-coated Ag2WO4/Ag2S as Type I heterojunction photocatalyst: Stability and mechanisms studies. Mater. Sci. Semicond. Process 2023, 153, 107151. [Google Scholar] [CrossRef]
- Qi, K.; Imparato, C.; Almjasheva, O.; Khataee, A.; Zheng, W. TiO2-based photocatalysts from type-II to S-scheme heterojunction and their applications. J. Colloid Interface Sci. 2024, 675, 150–191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and catalytic mechanisms of heterojunction photocatalysts and the application of titanium dioxide (TiO2)-based heterojunctions in environmental remediation. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Hussain, M.K.; Khalid, N.R.; Tanveer, M.; Abbas, A.; Ali, F.; Hassan, W.; Rianna, M.; Rahman, S.; Hamza, M.; Aslam, M. Facile fabrication of Z-scheme ZnO/MoO3 heterojunction as an excellent visible-light responsive photocatalyst for the degradation of rhodamine B and alizarin yellow dyes. Opt. Mater. 2024, 148, 114794. [Google Scholar] [CrossRef]
- Muhammad, W.; Ali, W.; Khan, M.A.; Ali, F.; Zada, A.; Ansar, M.Z.; Yap, P.-S. Construction of visible-light-driven 2D/2D NiFe2O4/g-C3N4 Z-scheme heterojunction photocatalyst for effective degradation of organic pollutants and CO2 reduction. J. Environ. Chem. Eng. 2024, 12, 113409. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M.; Polagani, R.K. 3D kaolinite/g-C3N4-alginate beads as an affordable and sustainable photocatalyst for wastewater remediation. Carbohydr. Polym. 2024, 323, 121420. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Clay based nanocomposites for removal of heavy metals from water: A review. J. Environ. Manag. 2019, 232, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, T.; Han, J.; Zhang, Y.; Zhao, L.; Zhao, J.; Li, R.; Huang, Y.; Gu, Z.; Wu, J. The Application of Mineral Kaolinite for Environment Decontamination: A Review. Catalysts 2023, 13, 123. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Yang, J.; Ma, S.; Frost, R.L. The thermal behavior of kaolinite intercalation complexes—A review. Thermochim. Acta 2012, 545, 1–13. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, L.; Cheng, H. Rational design of kaolinite-based photocatalytic materials for environment decontamination. Appl. Clay Sci. 2021, 208, 106098. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Q.; Cheng, H. Recent advances in kaolinite-based material for photocatalysts. Chin. Chem. Lett. 2021, 32, 2617–2628. [Google Scholar] [CrossRef]
- Awad, M.E.; Farrag, A.M.; El-Bindary, A.A.; El-Bindary, M.A.; Kiwaan, H.A. Photocatalytic degradation of Rhodamine B dye using low-cost pyrofabricated titanium dioxide quantum dots-kaolinite nanocomposite. Appl. Organomet. Chem. 2023, 37, e7113. [Google Scholar] [CrossRef]
- Ighnih, H.; Haounati, R.; Malekshah, R.E.; Ouachtak, H.; Jada, A.; Addi, A.A. Photocatalytic degradation of RhB dye using hybrid nanocomposite BiOCl@Kaol under sunlight irradiation. J. Water Process Eng. 2023, 54, 103925. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Li, Y.; Yu, B.; Hu, Z.; Wang, H. Construction of direct Z-scheme SnS2@ZnIn2S4@kaolinite heterostructure photocatalyst for efficient photocatalytic degradation of tetracycline hydrochloride. Chem. Eng. J. 2022, 429, 132105. [Google Scholar] [CrossRef]
- Jayrajsinh, S.; Shankar, G.; Agrawal, Y.K.; Bakre, L. Montmorillonite nanoclay as a multifaceted drug-delivery carrier: A review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Bee, S.-L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Sarkar, A.; Mushahary, N.; Basumatary, F.; Das, B.; Basumatary, S.F.; Venkatesan, K.; Selvaraj, M.; Rokhum, S.L.; Basumatary, S. Efficiency of montmorillonite-based materials as adsorbents in dye removal for wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 112519. [Google Scholar] [CrossRef]
- Li, Y.; Tian, G.; Dong, G.; Bai, S.; Han, X.; Liang, J.; Meng, J.; Zhang, H. Research progress on the raw and modified montmorillonites as adsorbents for mycotoxins: A review. Appl. Clay Sci. 2018, 163, 299–311. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Y.; Wei, H.; Li, K. In situ growth of cube-like AgCl on montmorillonite as an efficient photocatalyst for dye (Acid Red 18) degradation. Appl. Surf. Sci. 2018, 456, 577–585. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, P.; Zhang, Y.; Sun, K.; Shi, X.; Li, L. The preparation of conjugated microporous polymer composite materials with montmorillonite template and its improvement in photocatalytic degradation for multiple antibiotics. Appl. Clay Sci. 2023, 231, 106752. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Sathishkumar, K.; Mohebi, S.; Balarak, D. Preparation of CuFe2O4/montmorillonite nanocomposite and explaining its performance in the sonophotocatalytic degradation process for ciprofloxacin. Colloid Interface Sci. Commun. 2021, 45, 100532. [Google Scholar] [CrossRef]
- Chen, H.; Jia, Y.; Li, J.; Ai, Y.; Zhang, W.; Han, L.; Chen, M. Enhanced efficiencies on purifying acid mine drainage in constructed wetlands based on synergistic adsorption of attapulgite-soda residue composites and microbial sulfate reduction. J. Hazard. Mater. 2024, 470, 134221. [Google Scholar] [CrossRef]
- Kong, M.; Ouyang, X.; Han, T.; Wang, W.; Yin, H.; Wang, Y. Combination of lanthanum-modified attapulgite and Vallisneria natans for immobilization of phosphorus in various types of sediments. Chem. Eng. J. 2024, 492, 152264. [Google Scholar] [CrossRef]
- Wu, T.; Tang, X.; Lin, Y.; Wang, Y.; Ma, S.; Xue, Y.; Ren, H.; Xu, K. Heterogeneous catalytic ozonation of atrazine with oxygen vacancy-rich cu-loaded and ce-loaded attapulgite: Efficiency, mechanism and environmental application. Chem. Eng. J. 2024, 486, 150079. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Jiang, J.; Yao, J. Designing of Recyclable Attapulgite for Wastewater Treatments: A Review. ACS Sustain. Chem. Eng. 2018, 7, 1855–1869. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, X.; Qiao, J.; Liu, Y.; Zhang, J.; Wang, Y. Recent developments in synthesis of attapulgite composite materials for refractory organic wastewater treatment: A review. RSC Adv. 2024, 14, 16300–16317. [Google Scholar] [CrossRef]
- Zhao, S.; Qi, Y.; Lv, H.; Jiang, X.; Wang, W.; Cui, B.; Liu, W.; Shen, Y. Effect of clay mineral support on photocatalytic performance of BiOBr-TiO2 for efficient photodegradation of xanthate. Adv. Powder Technol. 2024, 35, 104431. [Google Scholar] [CrossRef]
- Hakki, H.K.; Sillanpää, M. Comprehensive analysis of photocatalytic and photoreactor challenges in photocatalytic wastewater treatment: A case study with ZnO photocatalyst. Mater. Sci. Semicond. Process 2024, 181, 108592. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, Y.; Ma, Z.; Hu, Y.; Guan, H.; Zhang, W.; Jia, S. A readily synthesized ZnIn2S4/attapulgite as a high-performance photocatalyst for Cr(VI) reduction. Mater. Sci. Semicond. Process 2024, 178, 108446. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Li, S.; Xuan, K.; Guo, Y.; Li, J.; Wang, X.; Li, X.; Zhou, Z. Mesoporous sulfur-doped g-C3N4@attapulgite composite as an advanced photocatalyst for efficiently uranium(VI) recovery from aqueous solutions. J. Environ. Chem. Eng. 2024, 12, 112886. [Google Scholar] [CrossRef]
- Ma, J.; Zou, J.; Li, L.; Yao, C.; Kong, Y.; Cui, B.; Zhu, R.; Li, D. Nanocomposite of attapulgite–Ag3PO4 for Orange II photodegradation. Appl. Catal. B Environ. 2014, 144, 36–40. [Google Scholar] [CrossRef]
- Shang, J.; Deng, C.; Xue, Z.; Dong, Z.; Fu, L.; Fan, H. Kinetics of catalytic ozonation of methylene blue in wastewater with Fe/Ce co-doped in attapulgite. Desalination Water Treat. 2024, 317, 100023. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, C.; Zheng, S.; Di, Y.; Sun, Z.; Li, C. Design and controllable preparation of Bi2MoO6/attapulgite photocatalyst for the removal of tetracycline and formaldehyde. Appl. Clay Sci. 2021, 215, 106319. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, L.; Hursthouse, A.; Song, N.; Ren, B. Sepiolite-Based Adsorbents for the Removal of Potentially Toxic Elements from Water: A Strategic Review for the Case of Environmental Contamination in Hunan, China. Int. J. Environ. Res. Public Health 2018, 15, 1653. [Google Scholar] [CrossRef] [PubMed]
- Eren, E.; Cubuk, O.; Ciftci, H.; Eren, B.; Caglar, B. Adsorption of basic dye from aqueous solutions by modified sepiolite: Equilibrium, kinetics and thermodynamics study. Desalination 2010, 252, 88–96. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Mayoral, A.; Garcia-Hernandez, M.; Ben, H.A.A.; Ruiz-Hitzky, E. Sepiolite nanoplatform for the simultaneous assembly of magnetite and zinc oxide nanoparticles as photocatalyst for improving removal of organic pollutants. J. Hazard. Mater. 2017, 340, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Ren, X.; Meng, D.; Gao, D.; Guo, Q.; Hu, X.; Wang, L.; Song, J. Facile fabrication of S-scheme Bi2MoO6/g-C3N4/sepiolite ternary photocatalyst for efficient tetracycline degradation under visible light. Mater. Sci. Semicond. Process 2023, 166, 107712. [Google Scholar] [CrossRef]
- Wang, P.; Qi, C.; Hao, L.; Wen, P.; Xu, X. Sepiolite/Cu2O/Cu photocatalyst: Preparation and high performance for degradation of organic dye. J. Mater. Sci. Technol. 2019, 35, 285–291. [Google Scholar] [CrossRef]
- Ren, X.; Hu, G.; Guo, Q.; Gao, D.; Wang, L.; Hu, X. Ag/Ag3PO4 nanoparticles assembled on sepiolite nanofibers: Enhanced visible-light-driven photocatalysis and the important role of Ag decoration. Mater. Sci. Semicond. Process 2023, 156, 107272. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, X.; Li, T.; Gao, S.; Gao, D.; Guo, Q.; Wang, L.; Hu, X. Facile synthesis of nano CeO2/sepiolite composite as visible-light-driven photocatalyst for rapid tetracycline removal. J. Environ. Chem. Eng. 2024, 12, 112829. [Google Scholar] [CrossRef]
- Yi, B.; Zeng, J.; Zhang, W.; Cui, H.; Liu, H.; Au, C.-T.; Wan, Q.; Yang, H. Enhanced hydrophilicity and promoted charge transfer in covalent triazine frameworks/sepiolite complexed via hydrogen bonding for visible-light driven degradation of antibiotics. Appl. Clay Sci. 2023, 238, 106921. [Google Scholar]
- Lopes, R.P.; da Souza, M.S.; Pereira, J.C.; Raupp, S.V.; Tatumi, S.H.; Yee, M.; Dillenburg, S.R. Late Pleistocene-Holocene diatomites from the coastal plain of southern Brazil: Paleoenvironmental implications. Quat. Int. 2021, 598, 38–55. [Google Scholar] [CrossRef]
- Borrelli, M.; Perri, E.; Avagliano, D.; Coraggio, F.; Critelli, S. Paleogeographic and sedimentary evolution of North Calabrian basins during the Messinian Salinity Crisis (South Italy). Mar. Pet. Geol. 2022, 141, 105726. [Google Scholar] [CrossRef]
- Zahajská, P.; Opfergelt, S.; Fritz, S.C.; Stadmark, J.; Conley, D.J. What is diatomite? Quat. Res. 2020, 96, 48–52. [Google Scholar] [CrossRef]
- Sannikova, N.; Shulepova, O.; Bocharova, A.; Kostomakhin, N.; Ilyasov, O.; Kovaleva, O. Natural reserves of diatomite are as a component of organomineral fertilizers based on chicken manure. IOP Conf. Ser. Earth Environ. Sci. 2021, 937, 032093. [Google Scholar] [CrossRef]
- Li, B.; Huang, H.; Guo, Y.; Zhang, Y. Diatomite-immobilized BiOI hybrid photocatalyst: Facile deposition synthesis and enhanced photocatalytic activity. Appl. Surf. Sci. 2015, 353, 1179–1185. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zheng, L.; Lin, G.L.; Ni, L.F.; Song, X.C. Synthesis and photocatalytic activity of BiOCl/diatomite composite photocatalysts: Natural porous diatomite as photocatalyst support and dominant facets regulator. Adv. Powder Technol. 2020, 31, 339–350. [Google Scholar] [CrossRef]
- Tanniratt, P.; Wasanapiarnpong, T.; Mongkolkachit, C.; Sujaridworakun, P. Utilization of industrial wastes for preparation of high performance ZnO/diatomite hybrid photocatalyst. Ceram. Int. 2016, 42, 17605–17609. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Liu, Z.; Zhao, L.; Xiao, S.; Bi, F.; Wang, H.; Li, Y. One-pot synthesis of g-C3N4/diatomite composite with nitrogen vacancies for enhanced photocatalytic activity. Mater. Sci. Semicond. Process 2024, 177, 108341. [Google Scholar] [CrossRef]
- Zhu, H.; Ren, Q.; Ding, Y.; Zhu, C.; Zong, Y.; Hu, X.; Jin, Z. One-step synthesis of Ag3VO4/diatomite composite material for efficient degradation of organic dyes under visible light. Inorg. Chem. Commun. 2021, 131, 108759. [Google Scholar] [CrossRef]
- Van Viet, P.; Van Chuyen, D.; Hien, N.Q.; Duy, N.N.; Thi, C.M. Visible-light-induced photo-Fenton degradation of rhodamine B over Fe2O3-diatomite materials. J. Sci. Adv. Mater. Devices 2020, 5, 308–315. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Liu, D.; Liu, X.; Li, X.; Leng, C. Highly efficient removal of tetracycline hydrochloride under neutral conditions by visible photo-Fenton process using novel MnFe2O4/diatomite composite. J. Water Process Eng. 2021, 43, 102307. [Google Scholar] [CrossRef]
- SMITH, J.V. Topochemistry of zeolites and related materials. 1. Topology and geometry. Chem. Rev. 1988, 88, 149–182. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Li, G.; Cui, B.; Wei, D.; Shen, Y. Synthesis of clinoptilolite-supported BiOCl/TiO2 heterojunction nanocomposites with highly-enhanced photocatalytic activity for the complete degradation of xanthates under visible light. Chem. Eng. J. 2021, 407, 126697. [Google Scholar] [CrossRef]
- Rafiani, A.; Aulia, D.; Kadja, G.T.M. Zeolite-encapsulated catalyst for the biomass conversion: Recent and upcoming advancements. Case Stud. Chem. Environ. Eng. 2024, 9, 100717. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M. Synthesis of hierarchically porous ZSM-5 zeolite by self-assembly induced by aging in the absence of seeding-assistance. Microporous Mesoporous Mater. 2020, 303, 110296. [Google Scholar] [CrossRef]
- Ravi, M.; Sushkevich, V.L.; van Bokhoven, J.A. Towards a better understanding of Lewis acidic aluminium in zeolites. Nat. Mater. 2020, 19, 1047–1056. [Google Scholar] [CrossRef]
- Pfriem, N.; Hintermeier, P.H.; Eckstein, S.; Kim, S.; Liu, Q.; Shi, H.; Milakovic, L.; Liu, Y.; Halle, G.L.; Baráth, E.; et al. Role of the ionic environment in enhancing the activity of reacting molecules in zeolite pores. Science 2021, 372, 952–957. [Google Scholar] [CrossRef]
- Znad, H.; Abbas, K.; Hena, S.; Awual, M.R. Synthesis a novel multilamellar mesoporous TiO2/ZSM-5 for photo-catalytic degradation of methyl orange dye in aqueous media. J. Environ. Chem. Eng. 2018, 6, 218–227. [Google Scholar] [CrossRef]
- Badvi, K.; Javanbakht, V. Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean. Prod. 2021, 280, 124518. [Google Scholar] [CrossRef]
- Latha, P.; Karuthapandian, S. Novel, Facile and Swift Technique for Synthesis of CeO2 Nanocubes Immobilized on Zeolite for Removal of CR and MO Dye. J. Clust. Sci. 2017, 28, 3265–3280. [Google Scholar] [CrossRef]
- Liu, J.; Lin, H.; Dong, Y.; He, Y.; Liu, C. MoS2 nanosheets loaded on collapsed structure zeolite as a hydrophilic and efficient photocatalyst for tetracycline degradation and synergistic mechanism. Chemosphere 2022, 287 Pt 2, 132211. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, N.; Tabatabaie, T.; Ramavandi, B.; Amiri, F. Ibuprofen elimination from water and wastewater using sonication/ultraviolet/hydrogen peroxide/zeolite-titanate photocatalyst system. Environ. Res. 2021, 198, 111260. [Google Scholar] [CrossRef] [PubMed]
| Photocatalyst | Heterojunction Design | Pollutants | Pollutant Dosage (mg/L) | Degradation Rate | Irradiation Time (min) | Ref. |
|---|---|---|---|---|---|---|
| MoS2/TiO2/clinoptilolite | Type I | Xanthate | 20 | over 90% | 180 | [49] |
| BiVO4/g-C3N4/Diatomite | Type II | Rhodamine B | 20 | 99% | 60 | [50] |
| CdS/Zn2In2S5/g-C3N4 | Type II | Tetracycline | 20 | 94.7% | 60 | [51] |
| α-Fe2O3-CeO2-SiO2 | Type II | Tetracycline | 25.1 | 95.9% | 94.2 | [52] |
| Bi2WO6/C-TiO2 | Z-scheme | Oxytetracycline | 15 | 93.6% | 100 | [53] |
| Ag2ZnGeO4/g-C3N4 | Z-scheme | Tetracycline | 10 | 94.3% | 140 | [54] |
| Ag/α-Fe2O3/g-C3N4 | Z-scheme | Rhodamine B | 20 | 97.6% | 55 | [55] |
| Photocatalyst | Heterojunction Design | Radiative Type | Pollutants | Pollutant Dosage (mg/L) | Degradation Rate | Irradiation Time (min) | Ref. |
|---|---|---|---|---|---|---|---|
| TiO2/kaolinite | / | solar light | Rhodamine B | 5 | 91% | 120 | [70] |
| SnS2/ZnIn2S4/kaolinite | Z-scheme | solar light | Tetracycline | 40 | 97.8% | 60 | [73] |
| AgCl/montmorillonite | / | UV light | Acid red 18 | 50 | 90% | 4.5 | [78] |
| CuFe2O4/montmorillonite | / | sonophotocatalytic | Ciprofloxacin | 50 | Nearly 100% | 60 | [80] |
| Ag3PO4/attapulgite | / | visible light | Orange II | 70 | 99% | 90 | [90] |
| Bi2MoO₆/attapulgite | / | visible light | Tetracycline | 30 | 90% | 120 | [92] |
| Sepiolite/Cu2O/Cu | / | visible light | Congo red | 10 | 95.1% | 50 | [97] |
| CeO2/sepiolite | / | visible light | Tetracycline | 40 | 92.7% | 120 | [99] |
| Ag3VO4/diatomite | / | visible light | Rhodamine B | 10 | 96% | 40 | [109] |
| MnFe2O4/diatomite | / | photo-Fenton process | Tetracycline Hydrochloride | 80 | 91.8% | 60 | [111] |
| Ni/TiO2/zeolite | / | UV light and H2O2 | Methylene blue | 10 | 99% | 120 | [119] |
| MoS2/zeolites | / | visible light | Tetracycline | 200 | 87.2% | 180 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Zhao, S.; Shen, Y.; Jiang, X.; Lv, H.; Han, C.; Liu, W.; Zhao, Q. A Critical Review of Clay Mineral-Based Photocatalysts for Wastewater Treatment. Catalysts 2024, 14, 575. https://doi.org/10.3390/catal14090575
Qi Y, Zhao S, Shen Y, Jiang X, Lv H, Han C, Liu W, Zhao Q. A Critical Review of Clay Mineral-Based Photocatalysts for Wastewater Treatment. Catalysts. 2024; 14(9):575. https://doi.org/10.3390/catal14090575
Chicago/Turabian StyleQi, Yaozhong, Sikai Zhao, Yanbai Shen, Xiaoyu Jiang, Haiyi Lv, Cong Han, Wenbao Liu, and Qiang Zhao. 2024. "A Critical Review of Clay Mineral-Based Photocatalysts for Wastewater Treatment" Catalysts 14, no. 9: 575. https://doi.org/10.3390/catal14090575
APA StyleQi, Y., Zhao, S., Shen, Y., Jiang, X., Lv, H., Han, C., Liu, W., & Zhao, Q. (2024). A Critical Review of Clay Mineral-Based Photocatalysts for Wastewater Treatment. Catalysts, 14(9), 575. https://doi.org/10.3390/catal14090575










