A First-Principles Study on the Reaction Mechanisms of Electrochemical CO2 Reduction to C1 and C2 Products on Cu(110)
Abstract
1. Introduction
2. Results and Discussion
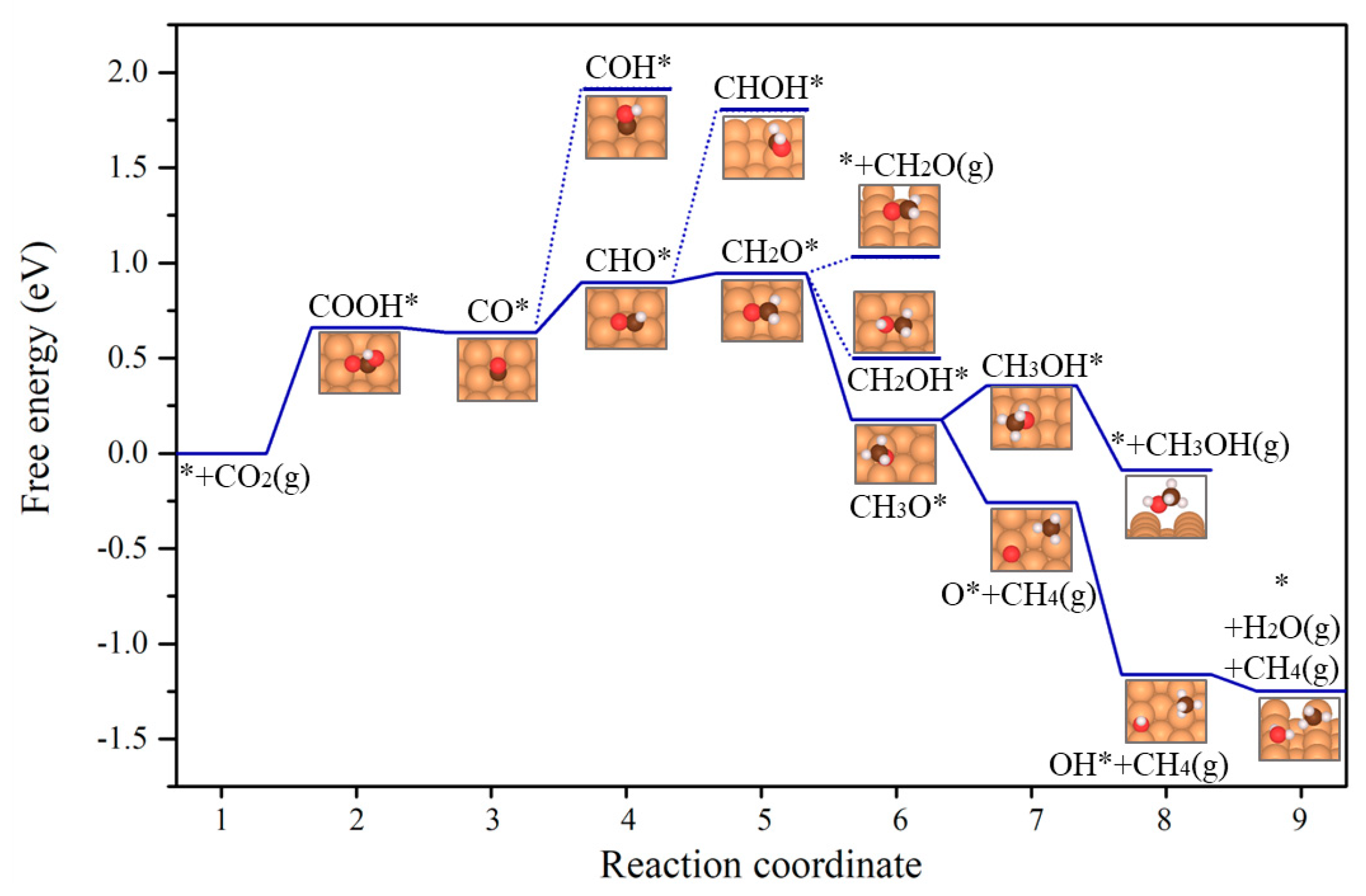
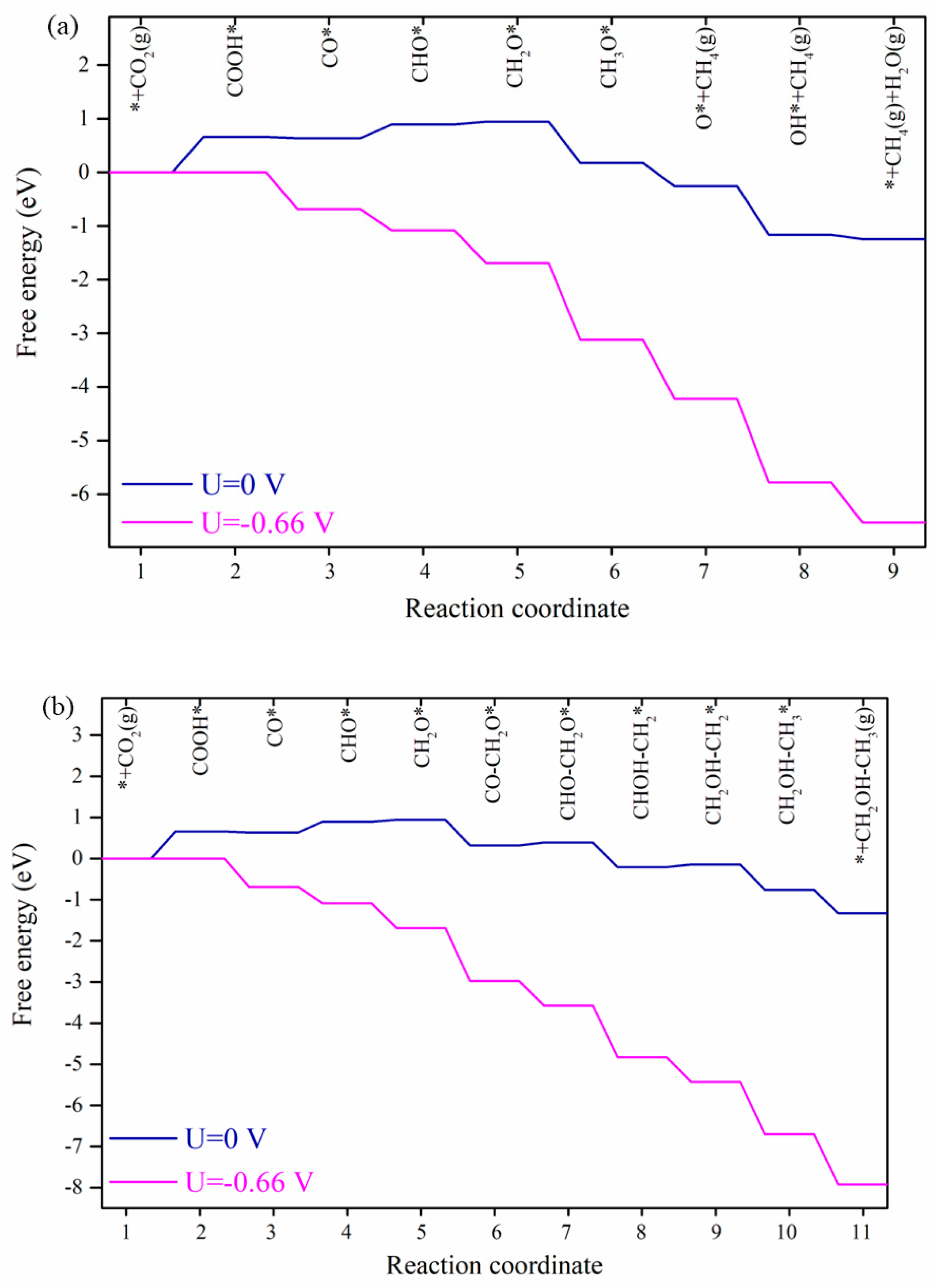
2.1. CO2 Reduction to CH4
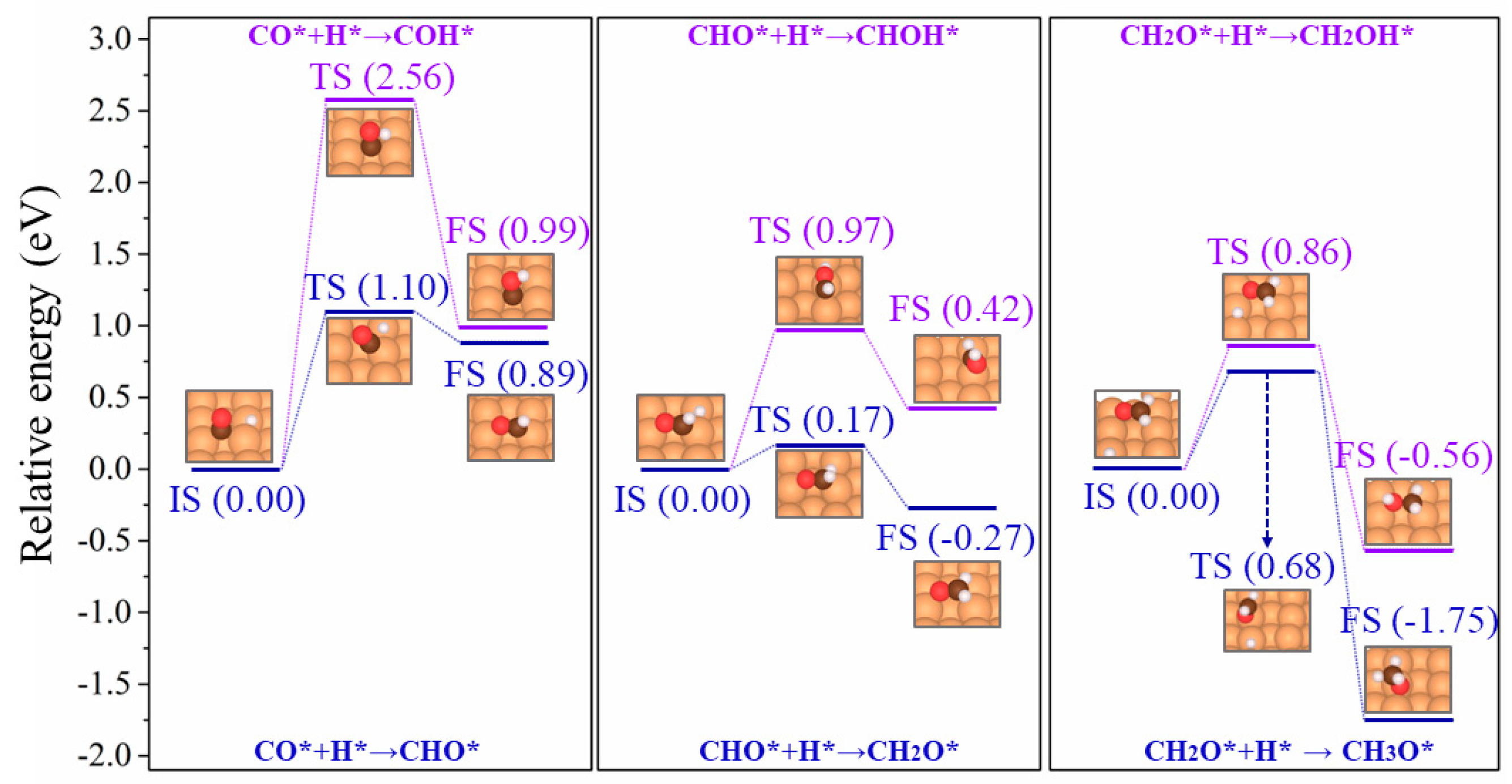
2.2. The Activation Energies from CO* Hydrogenation to CHxO*
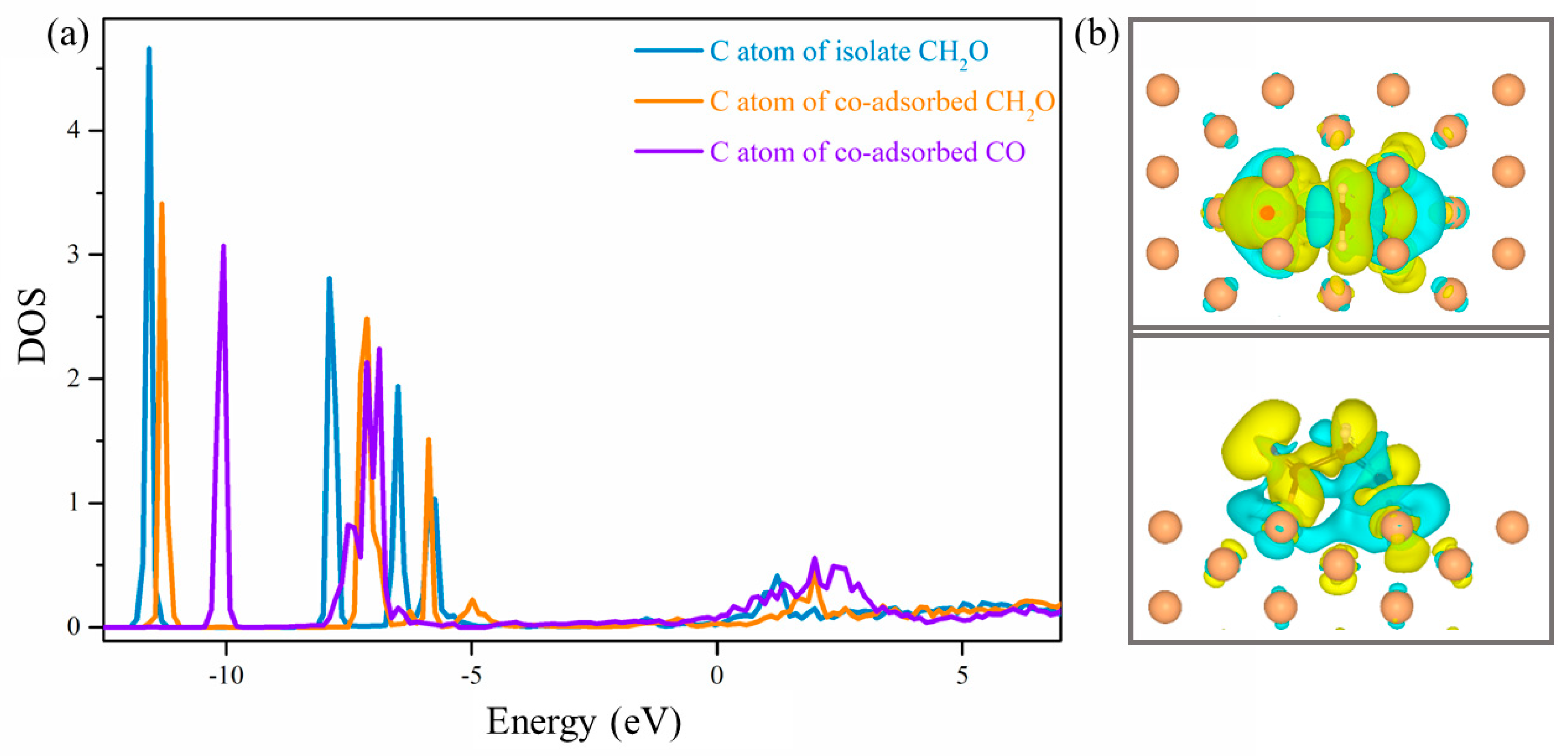
2.3. C-C Coupling Pathway
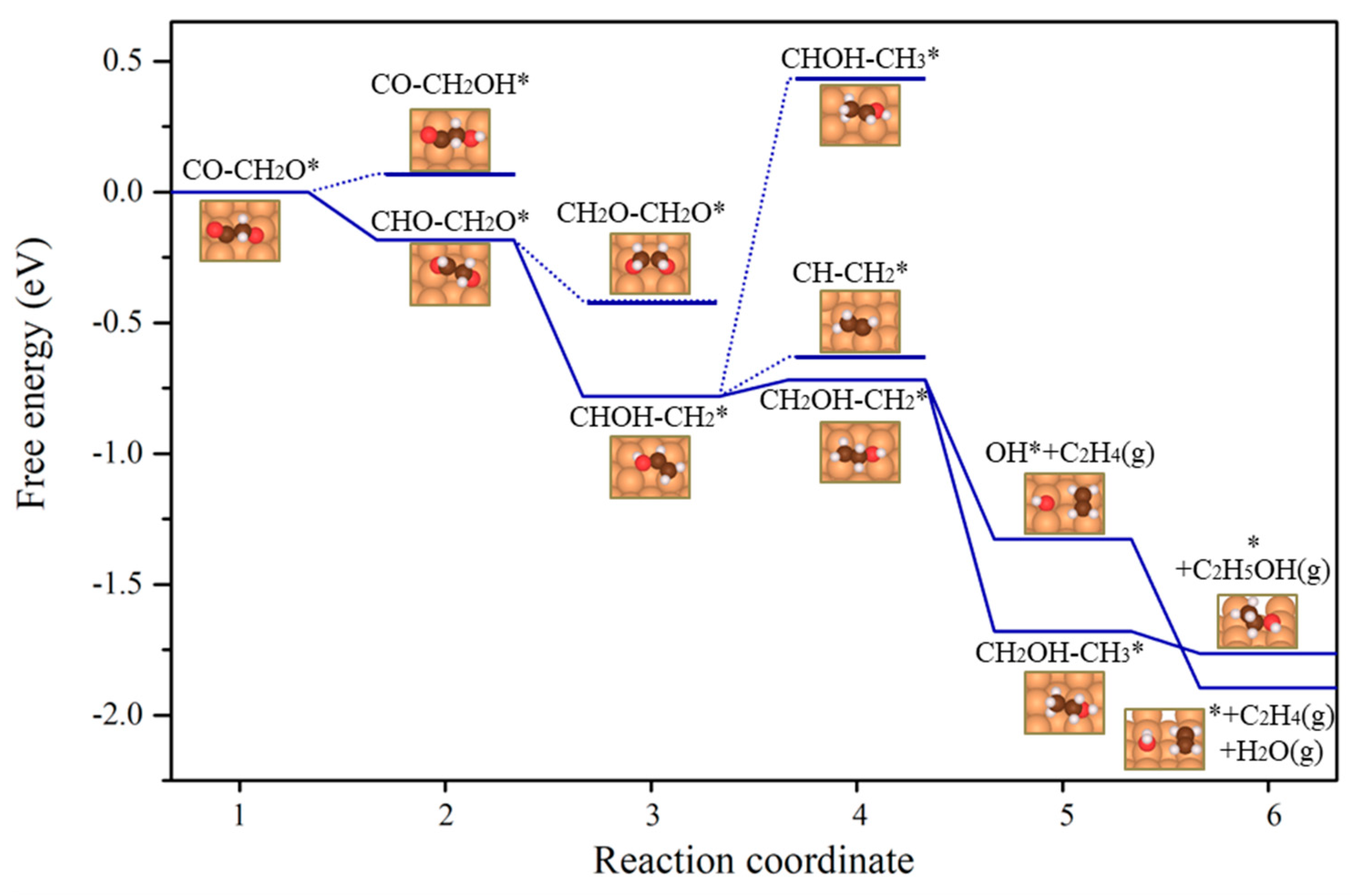
2.4. C2H5OH Production Pathway
2.5. The Analysis of Applied Potential
3. Computation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barberis, L.; Versteeg, C.I.; Meeldijk, J.D.; Stewart, J.A.; Vandegehuchte, B.D.; de Jongh, P.E. K and Na promotion enables high-pressure low-temperature reverse water gas shift over copper-based catalysts. ACS Catal. 2024, 14, 9188–9197. [Google Scholar] [CrossRef]
- Zhong, C.Y.; Yang, Y.F.; Chen, J.; Feng, B.M.; Wang, H.B.; Yao, Y.X. Nickel nanoparticles supported on lanthanum oxycarbonate with interfacial oxygen vacancies as catalysts for CO2 hydrogenation to methane. ACS Appl. Nano Mater. 2024, 7, 14057–14068. [Google Scholar] [CrossRef]
- Khalil, M.; Gunlazuardi, J.; Ivandini, T.A.; Umar, A. Photocatalytic conversion of CO2 using earth-abundant catalysts: A review on mechanism and catalytic performance. Renew. Sustain. Energy Rev. 2019, 113, 109246. [Google Scholar] [CrossRef]
- Wu, H.L.; Li, X.B.; Tung, C.H.; Wu, L.Z. Semiconductor quantum dots: An emerging candidate for CO2 photoreduction. Adv. Mater. 2019, 31, 1900709. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Y.; Wu, M.; Li, Y. The decomposition of total-factor CO2 emission efficiency of 97 contracting countries in Paris Agreement. Energy Econ. 2019, 78, 365–378. [Google Scholar] [CrossRef]
- Kar, S.; Goeppert, A.; Prakash, G.S. Combined CO2 capture and hydrogenation to methanol: Amine immobilization enables easy recycling of active elements. ChemSusChem 2019, 12, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yu, H.; He, T.; Zuo, S.; Liu, X.; Yang, H.; Ni, B.; Li, H.; Gu, L.; Wang, D.; et al. Visible-light-switched electron transfer over single porphyrin-metal atom center for highly selective electroreduction of carbon dioxide. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Jing, X.C.; Liu, R.H.; Guo, P.Q.; Yin, Z.Y. Plasmon-enhanced perovskite photocatalysts for CO2 reduction: A mini review. Energy Fuels 2024, 38, 4966–4979. [Google Scholar] [CrossRef]
- Foster, B.M.; Paris, A.R.; Frick, J.J.; Blasini-Pérez, D.A.; Cava, R.J.; Bocarsly, A.B. Catalytic mismatching of CuInSe2 and Ni3Al demonstrates selective photoelectrochemical CO2 reduction to methanol. ACS Appl. Energy Mater. 2020, 3, 109–113. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Y.; Wang, Z.Y. Plasmonic-assisted electrocatalysis for CO2 reduction reaction. ChemElectroChem 2024, 11, e202300805. [Google Scholar] [CrossRef]
- Han, G.H.; Bang, J.; Park, G.; Choe, S.; Jang, Y.J.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Recent advances in electrochemical, photochemical, and photoelectrochemical reduction of CO2 to C2+ products. Small 2023, 19, 2205765. [Google Scholar] [CrossRef]
- Liu, B.; Wang, T.; Wang, S.J.; Zhang, G.; Zhong, D.Z.; Yuan, T.H.; Dong, H.; Wu, B.; Gong, J.L. Back-illuminated photoelectrochemical flow cell for efficient CO2 reduction. Nat. Commun. 2022, 13, 7111. [Google Scholar] [CrossRef]
- Zhang, W.J.; Jin, Z.; Chen, Z.P. Rational-designed principles for electrochemical and photoelectrochemical upgrading of CO2 to value-added chemicals. Adv. Sci. 2022, 9, 2105204. [Google Scholar] [CrossRef]
- Wang, P.L.; Wang, S.C.; Wang, H.Q.; Wu, Z.B.; Wang, L.Z. Recent progress on photo-electrocatalytic reduction of carbon dioxide. Part. Part. Syst. Charact. 2018, 35, 1700371. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical conversion of carbon dioxide (CO2) into fuels and value-added products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Gyawali, S.; Tirumala, R.T.A.; Loh, H.; Andiappan, M.; Bristow, A.D. Photocarrier recombination dynamics in highly scattering Cu2O nanocatalyst clusters. J. Phys. Chem. C 2024, 125, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, S.; Tirumala, R.T.A.; Andiappan, M.; Bristow, A.D. Size- and shape-dependent charge-carrier dynamics in sub-micron cuprous oxide nanoparticles. Front. Opt. 2022, JTu4A.86. [Google Scholar]
- Liu, G.H.; Wang, K.Y.; Hoivik, N.; Jakobsen, H. Progress on free-standing and flow-through TiO2 nanotube membranes. Sol. Sol. Energy Mater. Sol. Cells 2012, 98, 24–38. [Google Scholar] [CrossRef]
- Schreier, M.; Luo, J.S.; Gao, P.; Moehl, T.; Mayer, M.T.; Grätzel, M. Covalent immobilization of a molecular catalyst on Cu2O photocathodes for CO2 reduction. J. Am. Chem. Soc. 2016, 138, 1938–1946. [Google Scholar] [CrossRef]
- Tirumala, R.T.A.; Gyawali, S.; Wheeler, A.; Ramakrishnan, S.B.; Sooriyagoda, R.; Mohammadparast, F.; Khatri, N.; Tan, S.S.; Kalkan, A.K.; Bristow, A.D.; et al. Structure-property-performance relationships of cuprous oxide nanostructures for dielectric Mie resonance-enhanced photocatalysis. ACS Catal. 2022, 12, 7975–7985. [Google Scholar] [CrossRef]
- Liu, J.; Xia, C.F.; Zaman, S.; Su, Y.Q.; Tan, L.; Chen, S.H. Surface plasmon assisted photoelectrochemical carbon dioxide reduction: Progress and perspectives. J. Mater. Chem. A 2023, 11, 16918–16932. [Google Scholar] [CrossRef]
- Zhou, L.N.; Lou, M.H.; Bao, J.L.; Zhang, C.; Liu, J.G.; Martirez, J.M.P.; Tian, S.; Yuan, L.; Swearer, D.F.; Robatjazi, H.; et al. Hot carrier multiplication in plasmonic photocatalysis. PNAS 2021, 118, e2022109118. [Google Scholar] [CrossRef]
- Huang, L.; Zaman, S.; Tian, X.L.; Wang, Z.T.; Fang, W.S.; Xia, B.Y. Advanced platinum-based oxygen reduction electrocatalysts for fuel cells. Acc. Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Mohammadparast, F.; Tirumala, R.T.A.; Ramakrishnan, S.B.; Dadgar, A.P.; Andiappan, M. Operando UV-Vis spectroscopy as potential in-line PAT system for size determination of functioning metal nanocatalysts. Chem. Eng. Sci. 2020, 225, 115821. [Google Scholar] [CrossRef]
- Du, W.; Li, M.; Liu, Q.; Chen, R. Improving the electrocatalytic CO2 reduction performance of Bi catalysts for formic acid production via size control, morphology regulation and carbon complexation. New J. Chem. 2024, 48, 6000. [Google Scholar] [CrossRef]
- Cui, Y.J.; Cheng, Y.H.; Yang, C.L.; Su, Y.S.; Yao, D.F.; Liufu, B.P.; Li, J.L.; Fang, Y.W.; Liu, S.Y.; Zhong, Z.Y. High-performance electrocatalytic CO2 reduction for CO generation using hydrophobic porous carbon supported Au. ACS Sustain. Chem. Eng. 2023, 11, 11229–11238. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Li, Y.; Li, C. The origins of catalyst selectivity for the electrochemical conversion of carbon dioxide to methanol. Nano Res. 2024, 17, 5–17. [Google Scholar] [CrossRef]
- Lal, D.; Konnur, T.; Verma, A.M.; Shaneeth, M.; Rajan, A.G. Unraveling low overpotential pathways for electrochemical CO2 reduction to CH4 on pure and doped MoS2 edges. Ind. Eng. Chem. Res. 2023, 62, 21191–21207. [Google Scholar] [CrossRef]
- Liu, Y.M.; Chen, S.; Quan, X.; Yu, H.T. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Luo, G.; Zhang, J.B.; Chen, M.H.; Wang, Z.Q.; Sham, T.K.; Zhang, L.J.; Li, Y.F.; Zheng, G.F. Double sulfur vacancies by lithium tuning enhance CO2 electroreduction to n-propanol. Nat. Commun. 2021, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.S.; Liang, X.Y.; Zhang, Q.; Ren, X.F.; Gao, L.G.; Ma, T.L.; Liu, A.M.; Pasti, I.A. Density functional theory study of CuAg bimetal electrocatalyst for CO2RR to produce CH3OH. Catalysts 2024, 14, 7. [Google Scholar] [CrossRef]
- Zhang, Z.; Bian, L.; Tian, H.; Liu, Y.; Bando, Y.; Yamauchi, Y.; Wang, Z.-L. Tailoring the surface and interface structures of copper-based catalysts for electrochemical reduction of CO2 to ethylene and ethanol. Small 2022, 18, 2107450. [Google Scholar] [CrossRef]
- Borovinskaya, E.S.; Trebbin, S.; Alscher, F.; Breitkopf, C. Synthesis, modification, and characterization of CuO/ZnO/ZrO2 mixed metal oxide catalysts for CO2/H2 conversion. Catalysts 2019, 9, 1037. [Google Scholar] [CrossRef]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Cuenya, B.R. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Song, Z.; Wang, X.; Ren, Z.; Fu, H. Relationships between structural design and synthesis engineering of Cu-based catalysts for CO2 to C2 electroreduction. Chem. Eng. J. 2024, 479, 147606. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Zhang, X.-L.; Niu, Z.-Z.; Gao, F.-Y.; Yang, P.-P.; Chi, L.-P.; Shi, L.; Wei, W.-S.; Liu, R.; Chen, Z.; et al. Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction. J. Am. Chem. Soc. 2022, 144, 259–269. [Google Scholar] [CrossRef]
- Jiang, K.; Sandberg, R.B.; Akey, A.J.; Liu, X.; Bell, D.C.; Nørskov, J.K.; Chan, K.; Wang, H. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 2018, 1, 111–119. [Google Scholar] [CrossRef]
- Guo, S.Y.; Liu, Y.C.; Murphy, E.; Ly, A.; Xu, M.J.; Matanovic, I.; Pan, X.Q.; Atanassov, P. Robust palladium hydride catalyst for electrocatalytic formate formation with high CO tolerance. Appl. Catal. B 2022, 16, 121659. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, B.S.; Zhang, L.H.; Sun, J. Rational design strategies of Cu-based electrocatalysts for CO2 electroreduction to C2 products. J. Energy Chem. 2022, 71, 63–82. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Gallent, E.P.; Koper, M.T.M. Structure sensitivity of the electrochemical reduction of carbon monoxide on copper single crystals. ACS Catal. 2013, 3, 1292–1295. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Kwon, Y.; van der Ham, C.J.M.; Qin, Z.; Koper, M.T.M. A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2011, 2, 1902. [Google Scholar] [CrossRef]
- Karapinar, D.; Creissen, C.E.; de la Cruz, J.G.R.; Schreiber, M.W.; Fontecave, M. Electrochemical CO2 reduction to ethanol with copper-based catalysts. ACS Energy Lett. 2021, 6, 694–706. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Adsorption of CO accompanied with simultaneous charge transfer on copper single crystal electrodes related with electrochemical reduction of CO2 to hydrocarbons. Surf. Sci. 1995, 335, 258–263. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes. J. Mol. Catal. A Chem. 2003, 199, 39–47. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Perez-Gallent, E.; Figueiredo, M.C.; Calle-Vallejo, F.; Koper, M.T.M. Spectroscopic observation of a hydrogenated CO dimer intermediate during CO reduction on Cu(100) electrodes. Angew. Chem. Int. Ed. 2017, 56, 3621–3624. [Google Scholar] [CrossRef]
- Ou, L.H.; He, Z.X.; Yang, H.; Chen, Y.D. Theoretical insights into potential-dependent C-C bond formation mechanisms during CO2 electroreduction into C2 products on Cu(100) at simulated electrochemical interfaces. ACS Omega 2021, 6, 17839–17847. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, X.; Wang, B. Insight into the preference mechanism of CHx(x = 1–3) and C-C chain formation involved in C2 oxygenate formation from syngas on the Cu(110) surface. J. Phys. Chem. C 2013, 117, 6594–6606. [Google Scholar] [CrossRef]
- Kuo, T.-C.; Chou, J.-W.; Shen, M.-H.; Hong, Z.-S.; Chao, T.-H.; Lu, Q.; Cheng, M.-J. First-principles study of C-C coupling pathways for CO2 electrochemical reduction catalyzed by Cu(110). J. Phys. Chem. C 2021, 125, 2464–2476. [Google Scholar] [CrossRef]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 reduction: Classifying Cu facets. ACS Catal. 2019, 9, 7894–7899. [Google Scholar] [CrossRef]
- Takahashi, I.; Koga, O.; Hoshi, N.; Hori, Y. Electrochemical reduction of CO2 at copper single crystal Cu(S)-[n(111)×(111)] and Cu(S)-[n(110)×(100)] electrodes. J. Electroanal. Chem. 2002, 533, 135–143. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef]
- Luo, W.J.; Nie, X.W.; Janik, M.J.; Asthagiri, A. Facet dependence of CO2 reduction paths on Cu electrodes. ACS Catal. 2016, 6, 219–229. [Google Scholar] [CrossRef]
- Jo, D.Y.; Ham, H.C.; Lee, K.Y. Facet-dependent electrocatalysis in the HCOOH synthesis from CO2 reduction on Cu catalyst: A density functional theory study. Appl. Surf. Sci. 2020, 527, 146857. [Google Scholar] [CrossRef]
- van Rensburg, W.J.; Petersen, M.A.; Datt, M.S.; van den Berg, J.A.; van Helden, P. On the kinetic interpretation of DFT-derived energy profiles: Cu-catalyzed methanol synthesis. Catal. Lett. 2015, 145, 559–568. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, P.B.; Li, A.W.; Wei, B.; Si, K.P.; Wei, Y.; Wang, X.G.; Zhu, G.D.; Chen, Q.; Gu, X.K.; et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 2022, 13, 1877. [Google Scholar] [CrossRef]
- Xue, Q.; Qi, X.; Li, K.; Zeng, Y.; Xu, F.; Zhang, K.; Yang, T.; Qi, X.; Jiang, J. DFT study of CO2 reduction reaction to CH3OH on low-index Cu surfaces. Catalysts 2023, 13, 722. [Google Scholar] [CrossRef]
- Shin, D.Y.; Jo, J.H.; Lee, J.Y.; Lim, D.H. Understanding mechanisms of carbon dioxide conversion into methane for designing enhanced catalysts from first-principles. Comput. Theor. Chem. 2016, 1083, 31–37. [Google Scholar] [CrossRef]
- Montoya, J.H.; Shi, C.; Chan, K.; Norskov, J.K. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 2015, 6, 2032–2037. [Google Scholar] [CrossRef]
- Guo, S.Y.; Liu, Y.C.; Huang, Y.; Wang, H.S.; Murphy, E.; Delafontaine, L.; Chen, J.L.; Zenyuk, I.V.; Atanassov, P. Promoting electrolysis of carbon monoxide toward acetate and 1-propanol in flow electrolyzer. ACS Energy Lett. 2023, 8, 935–942. [Google Scholar] [CrossRef]
- Jouny, M.; Hutchings, G.S.; Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2019, 2, 1062–1070. [Google Scholar] [CrossRef]
- Karamad, M.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Mechanistic pathway in the electrochemical reduction of CO2 on RuO2. ACS Catal. 2015, 5, 4075–4081. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, L.; Zhang, Z.; Cui, P. Prediction of a kinetic pathway for fabricating the narrowest zigzag graphene nanoribbons on Cu(111). J. Phys. Chem. C 2021, 125, 21933–21942. [Google Scholar] [CrossRef]
- Mo, Y.; Zhu, W.; Kaxiras, E.; Zhang, Z. Electronic nature of step-edge barriers against adatom descent on transition-metal surfaces. Phys. Rev. Lett. 2018, 101, 216101. [Google Scholar] [CrossRef] [PubMed]
- Phatak, A.A.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Density functional theory comparison of water dissociation steps on Cu, Au, Ni, Pd, and Pt. J. Phys. Chem. C 2009, 113, 7269–7276. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, N.; Wang, N.; Zhou, B.; Yin, P.; Song, B.Y.; Yu, J.; Yang, Y.S. Mechanism investigations on water gas shift reaction over Cu(111), Cu(100), and Cu(211) surfaces. ACS Omega 2022, 7, 3514–3521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.C.; Chen, J.C.; Zhao, M.D.; Yu, Q.; Wang, Y.G.; Li, J. Rational design of copper-based single-atom alloy catalysts for electrochemical CO2 reduction. Nano Res. 2022, 15, 7116–7123. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9901. [Google Scholar] [CrossRef]
- Nie, X.W.; Jiang, X.; Wang, H.Z.; Luo, W.J.; Janik, M.J.; Chen, Y.G.; Guo, X.W.; Song, C.S. Mechanistic understanding of alloy effect and water promotion for Pd-Cu bimetallic catalysts in CO2 hydrogenation to methanol. ACS Catal. 2018, 8, 4873–4892. [Google Scholar] [CrossRef]
- Bai, H.; Ma, M.M.; Bai, B.; Zuo, J.P.; Cao, H.J.; Zhang, L.; Zhang, Q.F.; Vinokurov, V.A.; Huang, W. The active site of syngas conversion into ethanol over Cu/ZnO/Al2O3 ternary catalysts in slurry bed. J. Catal. 2019, 380, 68–82. [Google Scholar] [CrossRef]
- Durand, W.J.; Peterson, A.A.; Studt, F.; Abild-Pedersen, F.; Nørskov, J.K. Structure effects on the energetics of the electrochemical reduction of CO2 by copper surfaces. Surf. Sci. 2011, 605, 1354–1359. [Google Scholar] [CrossRef]
- Vegge, T.; Rasmussen, T.; Leffers, T.; Pedersen, O.B.; Jacobsen, K.W. Atomistic simulations of cross-slip of jogged screw dislocations in copper. Philos. Mag. Lett. 2001, 81, 137–144. [Google Scholar] [CrossRef]
- Dong, H.L.; Li, Y.Y.; Jiang, D.E. First-principles insight into electrocatalytic reduction of CO2 to CH4 on a copper nanoparticle. J. Phys. Chem. C 2018, 122, 11392–11398. [Google Scholar] [CrossRef]
- Maulana, A.L.; Putra, R.I.D.; Saputro, A.G.; Agusta, M.K.; Nugrahaab; Dipojono, H.K. DFT and microkinetic investigation of methanol synthesis via CO2 hydrogenation on Ni(111)-based surfaces. Phys. Chem. Chem. Phys. 2019, 21, 20276. [Google Scholar] [CrossRef]
- Reichenbach, T.; Mondal, K.; Jäger, M.; Vent-Schmidt, T.; Himmel, D.; Dybbert, V.; Bruix, A.; Krossing, I.; Walter, M.; Moseler, M. Ab initio study of CO2 hydrogenation mechanisms on inverse ZnO/Cu catalysts. J. Catal. 2018, 360, 168–174. [Google Scholar] [CrossRef]
- Chang, C.C.; Ku, M.S. Role of high-index facet Cu(711) surface in controlling the C2 selectivity for CO2 reduction reaction—A DFT study. J. Phys. Chem. C 2021, 125, 10919–10925. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.G.; Bodappa, N.; Yang, W.M.; Liang, Q.; Radjenovica, P.M.; Wang, Y.H.; Zhang, Y.J.; Dong, J.C.; Tian, Z.Q.; et al. Elucidating electrochemical CO2 reduction reaction processes on Cu(hkl) single-crystal surfaces by in situ Raman spectroscopy. Energy Environ. Sci. 2022, 15, 3968–3977. [Google Scholar] [CrossRef]
- Gao, S.T.; Xiang, S.Q.; Shi, J.L.; Zhang, W.; Zhao, L.B. Theoretical understanding of the electrochemical reaction barrier: A kinetic study of CO2 reduction reaction on copper electrodes. Phys. Chem. Chem. Phys. 2020, 22, 9607–9615. [Google Scholar] [CrossRef] [PubMed]
- Grabow, L.C.; Mavrikakis, M. Mechanism of Methanol Synthesis on Cu through CO2 and CO Hydrogenation. ACS Catal. 2011, 1, 365–384. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, G.C. A Systematic Theoretical study of water gas shift reaction on Cu(111) and Cu(110): Potassium effect. ACS Catal. 2019, 9, 2261–2274. [Google Scholar] [CrossRef]
- Mandal, S.C.; Rawat, K.S.; Garg, P.; Pathak, B. Hexagonal Cu(111) Monolayers for selective CO2 hydrogenation to CH3OH: Insights from density functional theory. ACS Appl. Nano Mater. 2020, 2, 7686–7695. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Z.; Fan, G.; Li, F. Critical role of Cu nanoparticle-loaded Cu(100) surface structures on structured copper-based catalysts in boosting ethanol generation in CO2 electroreduction. ACS Appl. Mater. Interfaces 2024, 16, 35143–35154. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhang, L. A First-Principles Study on the Reaction Mechanisms of Electrochemical CO2 Reduction to C1 and C2 Products on Cu(110). Catalysts 2024, 14, 468. https://doi.org/10.3390/catal14070468
Xu Y, Zhang L. A First-Principles Study on the Reaction Mechanisms of Electrochemical CO2 Reduction to C1 and C2 Products on Cu(110). Catalysts. 2024; 14(7):468. https://doi.org/10.3390/catal14070468
Chicago/Turabian StyleXu, Yangyang, and Lixin Zhang. 2024. "A First-Principles Study on the Reaction Mechanisms of Electrochemical CO2 Reduction to C1 and C2 Products on Cu(110)" Catalysts 14, no. 7: 468. https://doi.org/10.3390/catal14070468
APA StyleXu, Y., & Zhang, L. (2024). A First-Principles Study on the Reaction Mechanisms of Electrochemical CO2 Reduction to C1 and C2 Products on Cu(110). Catalysts, 14(7), 468. https://doi.org/10.3390/catal14070468



