Abstract
Herein, a green biocatalytic approach using lipase as a catalyst has been developed for the synthesis of 3-selanyl-isoflavones through the selenylation/cyclization of 2-hydroxyphenyl enaminones and diphenyl di-selenide under mild conditions. The environmentally friendly method reached high yields of 87–95% in a short time at 30 °C, with 17 examples of 3-selanyl-isoflavones successfully prepared. Furthermore, we have investigated the possible mechanisms underlying this reaction.
1. Introduction
Flavonoids, although sporadically found in nature, represent one of the most prevalent heterocycles in the pharmaceutical and agrochemical industries [1,2,3]. Flavonoid derivatives exhibit anti-inflammatory, anti-Alzheimer’s, anti-oxidant, and anti-tumor properties [4,5]. The incorporation of selenium moieties into the molecular structures of flavonoids holds immense potential in generating compounds with enhanced biological activity. Consequently, various strategies have been established for constructing organo-selenium compounds [6,7]. Owing to these unique properties, organic researchers have devoted their efforts to integrating selenium elements into flavonoid scaffolds, aiming to establish an efficient strategy for constructing selenium-containing flavonoid derivatives with improved pharmacological features.
As a highly promising compound across multiple fields, substantial research efforts have been devoted to developing various synthesis methods for 3-selanyl-isoflavones. In 2018, Zhu et al. introduced a synthesis method involving the interaction of flavone analogs and various aromatic iodoarene, utilizing KSeCN as a cheap selenium source to obtain ArSe-substituted flavones in good yields (Scheme 1a) [8]. In the same year, Ding’s group reported on the synthesis of 3-selanyl-isoflavones using NIS (N-iodosuccinimide) as a catalyst, along with flavone derivatives and diphenyl di-selenide as key reactants (Scheme 1b) [9]. In 2021, Liu et al. demonstrated a visible-light-promoted synthesis of 3-selanyl-4H-chromen-4-one compounds via the reaction between enaminones and di-selenides (Scheme 1c) [10]. Following that, Xu and colleagues devised a highly effective approach for synthesizing ArSe-substituted chromone derivatives in 2022. This method involved combining selenium powder and aromatic iodides in a one-pot reaction under simple conditions (Scheme 1d) [11]. Subsequently in 2023, Xia’s group also utilized enaminones and di-selenides to establish a synthesis of 3-selenylated chromones, employing select-fluor as a gentle oxidant (Scheme 1e) [12]. Furthermore, Doerner and his team successfully synthesized 3-selanyl-isoflavones in the same year, via a one-pot reaction using the eco-friendly reagent trichloro-iso-cyanuric acid and ethanol as a solvent under mild conditions, with 22 examples in high yields (Scheme 1f) [13]. Despite these advancements, the majority of these approaches rely on costly and environmentally harmful reagents, like metal catalysts, and are conducted under harsh conditions, which contradict the principles of green chemistry. Consequently, there is an urgent demand for the creation of new, mild reaction conditions and highly efficient methods for the synthesis of 3-selanyl-isoflavones [14].

Scheme 1.
Previous works on the synthesis of 3-selanyl-isoflavones. (a) Zhu’s work; (b) Ding’s work; (c) Liu’s work; (d) Xu’s work; (e) Xia’s work; (f) Doerner’s work.
Over the past decades, the biocatalytic oxidation method has emerged as a mature and practical strategy with wide applicability [15]. Compared to conventional methods, the biocatalytic system offers reduced energy consumption and can be conducted under mild reaction conditions. Under this background, urea hydrogen peroxide (UHP), as a biological green oxidant, has captured the interest of numerous researchers, due to its stability at room temperature, affordability, and user-friendly nature compared with traditional oxidants, such as H2O2 [16]. Research indicates that UHP can be utilized in various oxidation reactions in anhydrous organic solvents when combined with carboxylic anhydrides [17], which has also prompted some researchers to delve into its potential influence in related selenization reactions [18,19].
Simultaneously, numerous enzymes, such as hemoproteins [20,21,22,23,24], monooxygenases [25,26,27,28,29], laccases [30,31], and lipases [32,33], can catalyze oxidation reactions. Among these enzymes, lipases have been extensively studied due to their broad substrate compatibility, high stability under extreme conditions, excellent enantio-selectivity, and environmentally friendly characteristics. Our research group, along with other researchers, has reported on lipase-catalyzed oxidation reactions, including epoxidation [34], Dakin reaction [35], and oxidation of amines [36], using various solvents such as water, organic solvents, and ionic liquids [34,37,38]. These studies have clearly demonstrated the feasibility and promising potential of lipase-catalyzed oxidation reactions. The work presented here is part of our ongoing project on biocatalytic oxidation reactions and represents the first report on the lipase-mediated oxidative synthesis of 3-selanyl-isoflavones.
2. Results and Discussion
In this study, ethyl acetate served as the reaction medium and substrate for the in-situ generation of peroxyacetic acid, with urea-hydrogen peroxide (UHP) as the oxidant. Lipase-mediated oxidation utilized o-hydroxyphenyl enaminone 1a and diphenyl di-selenide 2 as the model substrates for synthesizing 3-hydroxy chromone 3a. As illustrated in Table 1, PPL, MML and CSL exhibited extremely poor catalytic activity with yields below 5% (entries 1–3), while Cal-B provided a moderate yield of 3a (67%) in this oxidation (entry 4). However, inactivated Cal-B, whether denatured by heating or PMSF, was unable to facilitate this oxidation (entries 5–6). On the other hand, a significantly high yield (90%) was achieved when Novozym 435, a commercial immobilized form of Cal-B, was utilized in this reaction (entry 7). It was interesting that there was a decline in yield when UHP was replaced with H2O2 (entry 8). At the same time, our control experiments on Novozym 435 demonstrated that the enzyme and UHP are essential components for the reaction, as the absence of either will prevent the reaction from proceeding (entries 9–11). Furthermore, experiments using mixed solvents were conducted, but the yield was significantly lower compared to that in pure ethyl acetate (entries 12–13). Thus, based on these results, Novozym 435 is determined to be the most effective biocatalyst when used in conjunction with UHP in ethyl acetate.

Table 1.
The effect of enzyme origin and oxidant on the synthesis of 3-selanyl-isoflavones.
Temperature plays a crucial role in determining the progress of lipase-catalyzed reactions. As shown in Figure 1, the reaction temperature varied from 20 °C to 80 °C to assess its impact. The results showed that enzyme activity continuously increased with the rise of temperature, reaching 90% yield at 30 °C. However, a minimal growth of yield could be observed, though the temperature had already grown up to 60 °C, followed by an obvious plunge over 60 °C. This can be attributed to the fact that higher temperatures enhance the thermal movement of substrate molecules, leading to more collisions and ultimately speeding up the enzyme-catalyzed reaction rate. Nonetheless, excessively high temperatures can also lead to enzyme inactivation, making the yield start to slump. Therefore, the optimal temperature was determined to be 30 °C, as the yield was almost no longer growing (only by about 1%) with increasing temperature.
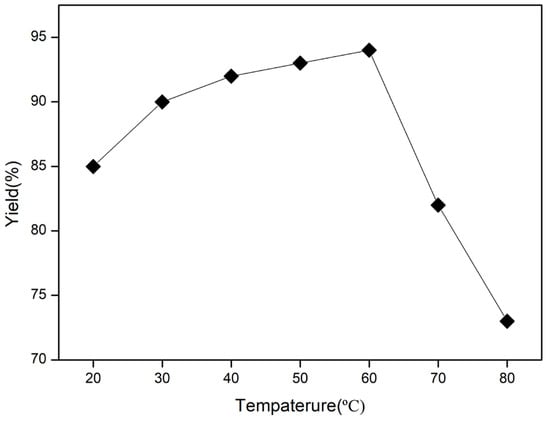
Figure 1.
Effect of different temperature on the synthesis of 3-selanyl-isoflavones. Reaction conditions: 1a (0.2 mmol), 2 (0.1 mmol), Novozym 435 (5 mg), UHP (0.5 mmol), ethyl acetate (1 mL), 2 h.
Novozym 435 is a commonly used immobilized lipase known for its excellent reusability in various practical applications. In this work, the reusability of Novozym 435 was assessed under optimized reaction conditions. After each batch, the filtered Novozym 435 was washed with ethyl acetate and reused in the same reaction. As shown in Figure 2, the yield of 3a remained relatively high (86%) after six cycles, but the yield witnessed a drop afterwards, possibly due to enzyme leakage from the carrier during washing, leading to enzyme loss in the subsequent cycle. Enzyme activity may also be decreased during the biocatalytic process, which is another reason for the decrease of yield.

Figure 2.
The reusability of Novozym 435 on the synthesis of 3-selanyl-isoflavones. Reaction conditions: 1a (0.2 mmol), 2 (0.1 mmol), Novozym 435 (5 mg), UHP (0.5 mmol), ethyl acetate (1 mL), 30 °C, 2 h.
Under the optimized conditions, the enzymatic method was systematically evaluated using 2-hydroxyphenyl enaminones (1) and diphenyl di-selenide (2), as outlined in Scheme 2. The reactions proceeded smoothly and were minimally affected by electronic or steric factors, resulting in the formation of products 3a–3q with yields ranging from 88% to 95%. The presence of either electron-withdrawing or electron-donating substituents on the phenyl group of 1 did not significantly impact the product yields. These results definitively demonstrate that compound 1, bearing diverse substituents at the 2-, 3-, 4-, and 5-positions of the aromatic rings, effectively participated in the reactions.
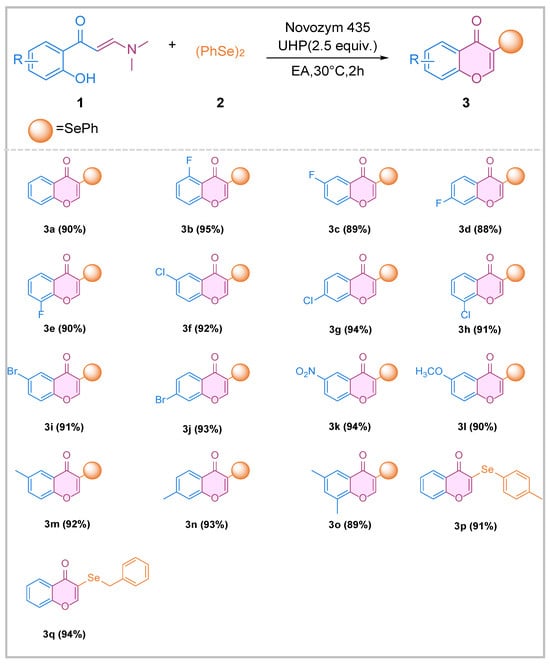
Scheme 2.
Scope of selenylation/cyclization reaction with enaminones and di-selenide via lipase. Reaction conditions: 1 (0.2 mmol), 2 (0.1 mmol), Novozym 435 (5 mg), UHP (0.5 mmol), ethyl acetate (1 mL), 30 °C, 2 h.
Based on the findings of these results and previous references [18,39], we proposed a potential mechanism for the lipase-catalyzed reaction. As shown in Scheme 3, ethyl acetate is transformed in situ into peracetic acid by UHP and lipase, serving as a strong oxidant to convert 2 into organo-seleninic acid I. Then, I can be correspondingly transformed into peroxy-seleninic acid II with further oxidation, which can generate organo-selenenic acid III subsequently. After that, selenic acid III is protonated and converted into highly reactive substance IV, which can promptly attack the C=C bond of 1a, producing selenium ion V. Following this, selenium ion V undergoes nucleophilic substitution and intramolecular cyclization to form VI, ultimately generating the target product 3a through the elimination of dimethylamine.

Scheme 3.
Proposed mechanism for lipase synthesis of 3-selanyl-isoflavones.
3. Materials and Methods
3.1. Materials
Cal-B (C. antarctica lipase B), MML (Lipase from Aspergillus niger), CSL (Lipase from Candida sp. 99–125), PPL (Porcine pancreatic lipase). Novozym 435 (protein content: 10%) was purchased from Novo Nordisk Co., Ltd. (Beijing, China). The quantity (per gram of carrier) of protein bound to the carrier in the commercial immobilized lipases were 100 mg for Novozym 435 when determined using a conventional Kjeldahl method. In addition, 2-Hydroxyphenyl enaminones were prepared in the laboratory. Diphenyl di-selenide, urea hydrogen peroxide (UHP) and all other chemical reagents were bought from Bide Pharmatech. Ltd. and Energy-Chemical Ltd. (Shanghai, China). All commercially available reagents and solvents were used as received without additional purification.
Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a 400 MHz spectrometer. Chemical shifts for protons are reported in parts per million downfield from tetra-methyl-silane (TMS) and are referenced to the residual protium in the NMR solvent (CHCl3 = δ 7.26 ppm). 1H NMR data are presented as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad), coupling constant in Hertz (Hz), integration. Mass spectra were recorded on the Bruker MicrOTOF Q II and an Orbitrap FusionTM TribridTM mass spectrometer (Thermo Scientific, San Jose, CA, USA) coupled with HESI ion source. A 100 MHz spectrometer was used to report 13C NMR spectra, while a 76 MHz spectrometer and 376 MHz spectrometer was used to record 77Se NMR and 19F NMR spectra data separately. Data for 13C NMR, 77Se NMR and 19F NMR were reported in terms of chemical shifts (δ ppm). High resolution mass spectra (HRMS) were obtained by use of an Orbitrap Fusion Tribrid mass spectrometer (Thermo Scientific, San Jose, CA, USA) coupled with HESI ion source.
3.2. General Procedure for Lipase-Catalyzed Synthesis of 3-Selanyl-Isoflavones (3)
Novozym 435 (5 mg) was added into a mixture of 2-hydroxyphenyl enaminones (1, 0.2 mmol), diphenyl di-selenide (2, 0.1 mmol) and UHP (0.5 mmol) in ethyl acetate (1 mL) at 30 °C for 2 h.
Following the completion of the reaction, which was monitored by TLC, the mixture was filtered and the residue was washed with ethyl acetate. Subsequently, the mixture underwent separation by TLC with ethyl acetate/petroleum ether as the mobile phase and was dried in a vacuum to obtain the pure solid product (3). All isolated products were well characterized through 1H NMR spectroscopy, and the results were found to be essentially in agreement with previously published literature [9,12,13,40], where the 13C NMR data also could be found. As for the compounds that were not reported before, we further performed analyses on these compounds utilizing 13C NMR, 77Se NMR, and 19F NMR spectroscopy techniques. Moreover, we tested the data for HRMS of all the compounds. The details can be found in our Supplementary Materials (SM).
4. Conclusions
In summary, we have developed a highly efficient synthesis of 3-selanyl-isoflavones through lipase-mediated selenylation/cyclization of 2-hydroxyphenyl enaminones and diphenyl di-selenide in high yields (87–95%). Compared with chemical catalysis, our method is more environmentally friendly, using lipase as a green catalyst, and the reaction can occur under mild conditions (ethyl acetate as a solvent, lipase as a green catalyst, 2 h at 30 °C). Overall, the research conducted on the development of 3-selanyl-isoflavones could lead to the exploration of novel possibilities in enzymatic organic synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14070413/s1, Figure S1: Experimental Section; Figure S2: Data of Products; Figure S3: 1H NMR, 13C NMR, 19F NMR and 77Se NMR Spectra of Products.
Author Contributions
Investigation, methodology, visualization, writing—original draft, and formal analysis, W.K.; methodology, Y.P.; visualization, W.S.; formal analysis, W.K., C.W., Y.P., W.S. and X.C.; supervision, conceptualization, funding acquisition, and writing review and editing, Z.W. and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Development Program of Jilin Province (No. 20230101135JC).
Data Availability Statement
Data presented in this study are available in the Supplementary Materials. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, Y.; Cheng, F.-B.; Wu, X.-R.; Zhu, W.; Liao, J.-W.; Jiang, Y.; Zhang, C.; Niu, W.-Y.; Yu, Y.; Duan, H.-Q.; et al. Flavonoid derivatives synthesis and anti-diabetic activities. Bioorg. Chem. 2020, 95, 103501. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, B.; Chen, H.; Huang, H.; Hu, Y. Promiscuous enzyme-catalyzed cascade reaction: Synthesis of xanthone derivatives. Bioorg. Chem. 2018, 80, 555–559. [Google Scholar] [CrossRef]
- Li, H.; Lyv, Y.; Zhou, S.; Yu, S.; Zhou, J. Microbial cell factories for the production of flavonoids–barriers and opportunities. Bioresour. Technol. 2022, 360, 127538. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.R.; Sobarzo-Sánchez, E.; Ashraf, G.M.; et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Altunay, N.; Tuzen, M. A simple and green ultrasound liquid–liquid microextraction method based on low viscous hydrophobic deep eutectic solvent for the preconcentration and separation of selenium in water and food samples prior to HG-AAS detection. Food Chem. 2021, 364, 130371. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; He, Z.; Zhang, J.; Cao, Y.; Mao, D.; Feng, C.; Tian, B.; Chen, G. Molecular docking-assisted design and synthesis of an anti-tumor quercetin–Se(iv) complex. New J. Chem. 2020, 44, 8434–8441. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, B.; Yu, J.; Ren, Y.; Wang, J.; Xie, P.; Pittman, C.U.; Zhou, A. Copper-catalyzed generation of flavone selenide and thioether derivatives using KSeCN and KSCN via C–H functionalization. Org. Biomol. Chem. 2018, 16, 5999–6005. [Google Scholar] [CrossRef]
- Ding, C.; Yu, Y.; Yu, Q.; Xie, Z.; Zhou, Y.; Zhou, J.; Liang, G.; Song, Z. NIS/TBHP Induced Regioselective Selenation of (Hetero)Arenes via Direct C−H Functionalization. ChemCatChem 2018, 10, 5397–5401. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Zhang, J.-R.; Huang, G.-B.; Zhou, Y.-H.; Chen, Y.-Y.; Xu, Y.-L. Visible Light-Promoted Selenylation/Cyclization of Enaminones toward the Formation of 3-Selanyl-4H-Chromen-4-Ones. Adv. Synth. Catal. 2021, 363, 1656–1661. [Google Scholar] [CrossRef]
- Xu, P.; Zhong, Z.; Huang, H.; Zhou, A. Selenation of 2-Hydroxyphenyl Enaminones with Se Powder to Generate ArSe-subsituted Chromone Derivatives. Chem. Select 2022, 7, e202202854. [Google Scholar] [CrossRef]
- Xia, J.-H.; Chen, Q.; Yuan, J.-W.; Shi, W.-S.; Yang, L.-R.; Xiao, Y.-M. Selectfluor-mediated tandem cyclization of enaminones with diselenides toward the synthesis of 3-selenylated chromones. RSC Adv. 2023, 13, 26948–26959. [Google Scholar] [CrossRef]
- Doerner, C.V.; Neto, J.S.S.; Cabreira, C.R.; Saba, S.; Sandjo, L.P.; Rafique, J.; Braga, A.L.; de Assis, F.F. Synthesis of 3-selanyl-isoflavones from 2-hydroxyphenyl enaminones using trichloroisocyanuric acid (TCCA): A sustainable approach. New J. Chem. 2023, 47, 5598–5602. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 46. [Google Scholar] [CrossRef]
- Varma, R.S.; Naicker, K.P. The Urea−Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles. Org. Lett. 1999, 1, 189–192. [Google Scholar] [CrossRef]
- Verma, P.; Chauhan, S.; Singh, V.; Singh, S.; Srivastava, V. Urea hydrogen peroxide-initiated synthesis of pyranopyrazoles through oxidative coupling under base- and metal-free conditions by physical grinding method. Mol. Divers. 2022, 26, 1769–1777. [Google Scholar] [CrossRef]
- Moraes, C.A.O.; Santos, R.B.C.; Cavalcante, M.F.O.; Guilhermi, J.S.; Ali, M.A.; Botteselle, G.V.; Frizon, T.E.A.; Shah, M.I.A.; Lião, L.M.; Beatriz, A.; et al. Urea Hydrogen Peroxide and Ethyl Lactate, an Eco-Friendly Combo System in the Direct C(sp2)–H Bond Selenylation of Imidazo [2,1-b]thiazole and Related Structures. ACS Omega 2023, 8, 39535–39545. [Google Scholar] [CrossRef]
- Page, P.C.B.; Buckley, B.R.; Elliott, C.; Chan, Y.; Dreyfus, N.; Marken, F. Chemoselective Oxidation of Sulfides to Sulfoxides with Urea-Hydrogen Peroxide Complex Catalysed by Diselenide. Synlett 2016, 27, 80–82. [Google Scholar] [CrossRef]
- Bordeaux, M.; Tyagi, V.; Fasan, R. Highly Diastereoselective and Enantioselective Olefin Cyclopropanation Using Engineered Myoglobin-Based Catalysts. Angew. Chem. Int. Ed. 2015, 54, 1744–1748. [Google Scholar] [CrossRef]
- Xie, H.; Li, F.; Xu, Y.; Wang, C.; Xu, Y.; Wu, J.; Li, Z.; Wang, Z.; Wang, L. Vitreoscilla hemoglobin: A natural carbene transfer catalyst for diastereo- and enantioselective synthesis of nitrile-substituted cyclopropanes. Green Chem. 2023, 25, 6853–6858. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Xu, Y.; Xie, H.; Wu, J.; Wang, C.; Li, Z.; Wang, Z.; Wang, L. Engineering of Dual-Function Vitreoscilla Hemoglobin: A One-Pot Strategy for the Synthesis of Unnatural α-Amino Acids. Org. Lett. 2023, 25, 7115–7119. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Xu, Y.; Ma, J.; Xie, H.; Yang, H.; Han, W.; Wang, C.; Li, Z.; Wang, L. Novel dual-enzyme system for synthesis of 2-alkyl and 2-arylbenzoxazoles via aerobic oxidation. Org. Chem. Front 2023, 10, 3509–3514. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, N.; Li, F.; Wang, C.; Xie, H.; Wu, J.; Cheng, L.; Wang, L.; Wang, Z. Application of Vitreoscilla Hemoglobin as a Green and Efficient Biocatalyst for the Synthesis of Benzoxazoles in Water. ChemBioChem 2024, 25, e202300609. [Google Scholar] [CrossRef]
- Xu, X.; Hilberath, T.; Hollmann, F. Selective oxyfunctionalisation reactions catalysed by P450 monooxygenases and peroxygenases—A bright future for sustainable chemical synthesis. Curr. Opin. Green Sustainable Chem. 2023, 39, 100745. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Jiang, Y.; Qin, X.; Ma, N.; Yao, F.; Dong, S.; Liu, C.; Feng, Y.; Jin, L.; et al. Engineering Cytochrome P450BM3 Enzymes for Direct Nitration of Unsaturated Hydrocarbons. Angew. Chem. Int. Ed. 2023, 62, e202217678. [Google Scholar] [CrossRef]
- Fan, S.; Cong, Z. Emerging Strategies for Modifying Cytochrome P450 Monooxygenases into Peroxizymes. Acc. Chem. Res. 2024, 57, 613–624. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, B.; Wang, F.; Yu, X.; Ma, L.; Li, A.; Reetz, M.T. Chemo- and Regioselective Dihydroxylation of Benzene to Hydroquinone Enabled by Engineered Cytochrome P450 Monooxygenase. Angew. Chem. Int. Ed. 2019, 58, 764–768. [Google Scholar] [CrossRef]
- Bornadel, A.; Hatti-Kaul, R.; Hollmann, F.; Kara, S. A Bi-enzymatic Convergent Cascade for ε-Caprolactone Synthesis Employing 1,6-Hexanediol as a ‘Double-Smart Cosubstrate’. ChemCatChem 2015, 7, 2442–2445. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, S.; Miao, S.; Suo, H.; Xu, H.; Hu, Y. Co-immobilization of laccase and ABTS onto amino-functionalized ionic liquid-modified magnetic chitosan nanoparticles for pollutants removal. J. Hazard. Mater. 2021, 401, 123353. [Google Scholar] [CrossRef]
- Könst, P.; Kara, S.; Kochius, S.; Holtmann, D.; Arends, I.W.C.E.; Ludwig, R.; Hollmann, F. Expanding the Scope of Laccase-Mediator Systems. ChemCatChem 2013, 5, 3027–3032. [Google Scholar] [CrossRef]
- Ding, X.; Dong, C.-L.; Guan, Z.; He, Y.-H. Concurrent Asymmetric Reactions Combining Photocatalysis and Enzyme Catalysis: Direct Enantioselective Synthesis of 2,2-Disubstituted Indol-3-ones from 2-Arylindoles. Angew. Chem. Int. Ed. 2019, 58, 118–124. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Zhu, H.; Shen, Y.; Le, Z.; Xie, Z. Combining enzyme and photoredox catalysis for the synthesis of quinazolines. Mol. Catal. 2023, 550, 113549. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Tang, Y.; Wu, J.; Wang, C.; Du, C.; Wang, Z.; Wang, L. Synthesis of functionalized 1,4-dihydropyridines containing benzosultams catalyzed by lipase. New J. Chem. 2023, 47, 9708–9713. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Wang, C.; Zhang, L.; Li, F.; Zhang, W.; Chen, P.; Wang, L. A mild and efficient Dakin reaction mediated by lipase. Green Chem. Lett. Rev. 2017, 10, 269–273. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Wang, C.; Zheng, L.; Wang, Z.; Zhao, R.; Wang, L. Lipase-Mediated Amidation of Anilines with 1,3-Diketones via C–C Bond Cleavage. Catalysts 2017, 7, 115. [Google Scholar] [CrossRef]
- Suo, H.; Geng, H.; Zhang, L.; Liu, G.; Yan, H.; Cao, R.; Zhu, J.; Hu, Y.; Xu, L. Covalent immobilization of lipase on an ionic liquid-functionalized magnetic Cu-based metal–organic framework with boosted catalytic performance in flavor ester synthesis. J. Mater. Chem. B 2023, 11, 1302–1311. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, Z.; Fang, K.; He, X.; Xu, H.; Hu, Y. Enzymatic approach to cascade synthesis of bis(indolyl)methanes in pure water. RSC Adv. 2020, 10, 10848–10853. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Xu, Y.; Li, F.; Liu, Y.; Sun, Y.; Wang, C.; Chen, P.; Wang, L. Efficient synthesis of 3-hydroxy chromones via oxidative cyclization mediated by lipase. Green Chem. Lett. Rev. 2022, 15, 689–694. [Google Scholar] [CrossRef]
- Guo, T. Ammonium iodide-mediated regioselective chalcogenation of chromones with diaryl disulfides and diselenides. Synth. Commun. 2017, 47, 2053–2061. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
