Expediting Corrosion Engineering for Sulfur-Doped, Self-Supporting Ni-Fe Layered Dihydroxide in Efficient Aqueous Oxygen Evolution
Abstract
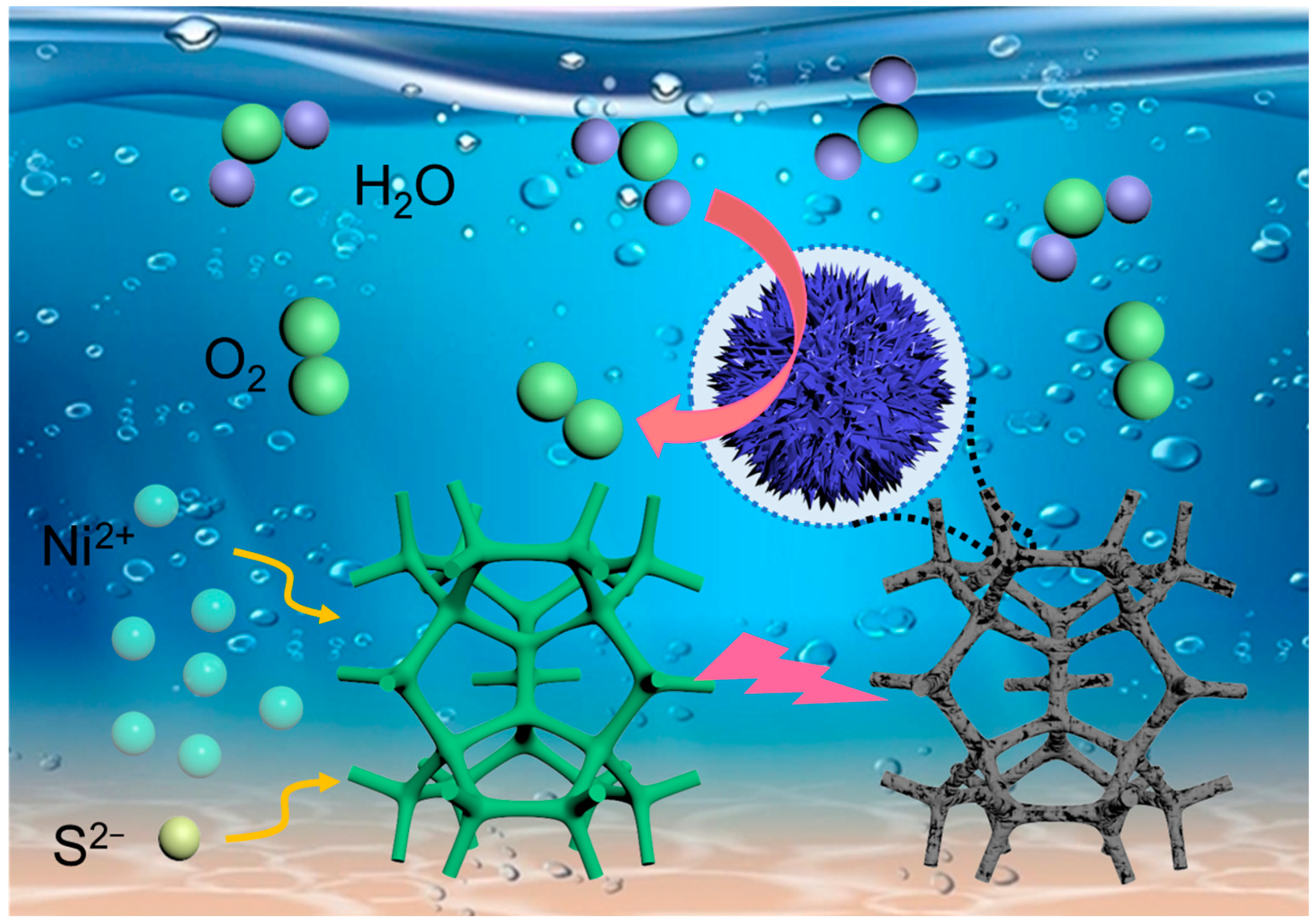
1. Introduction
2. Results and Discussion
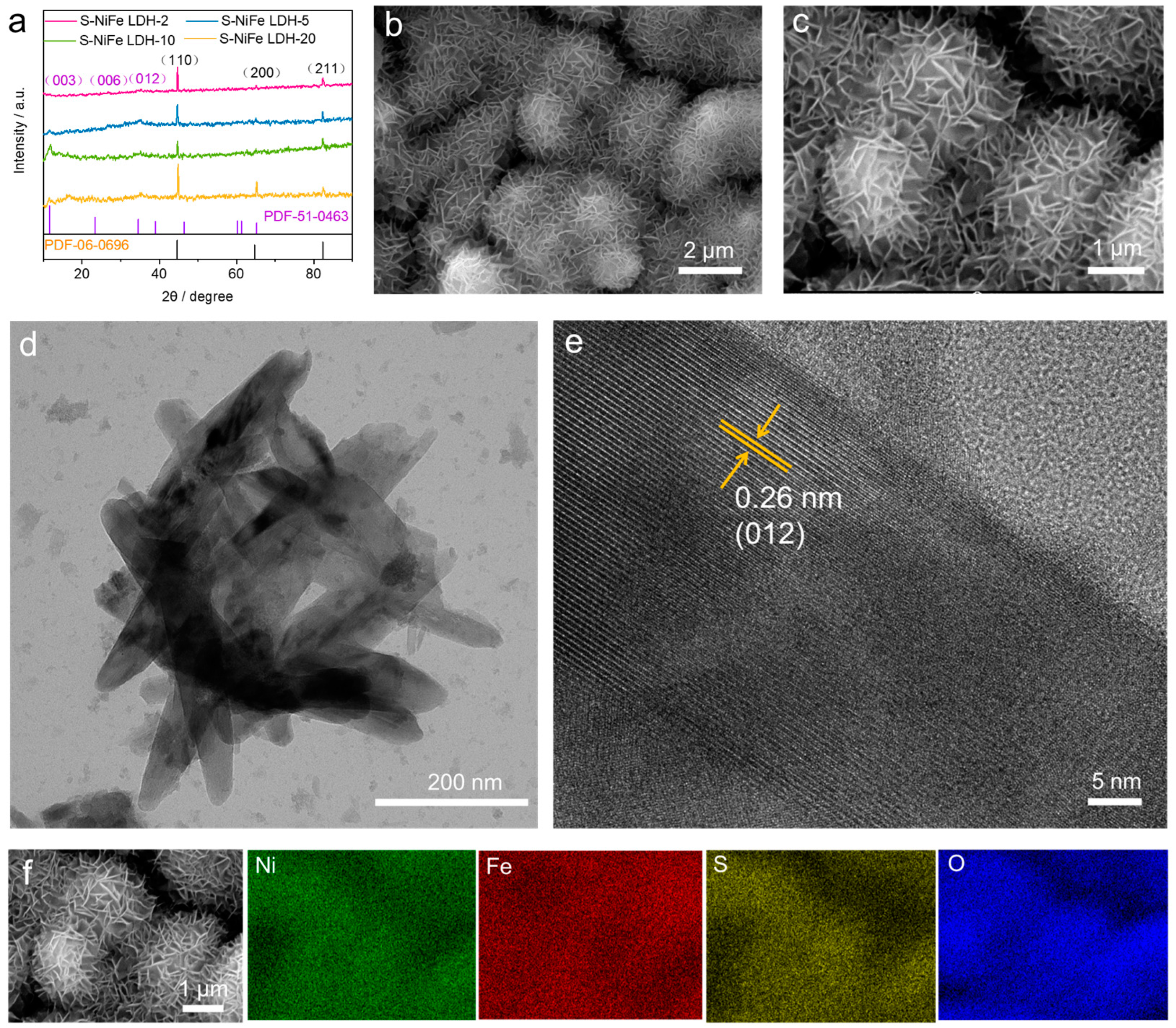
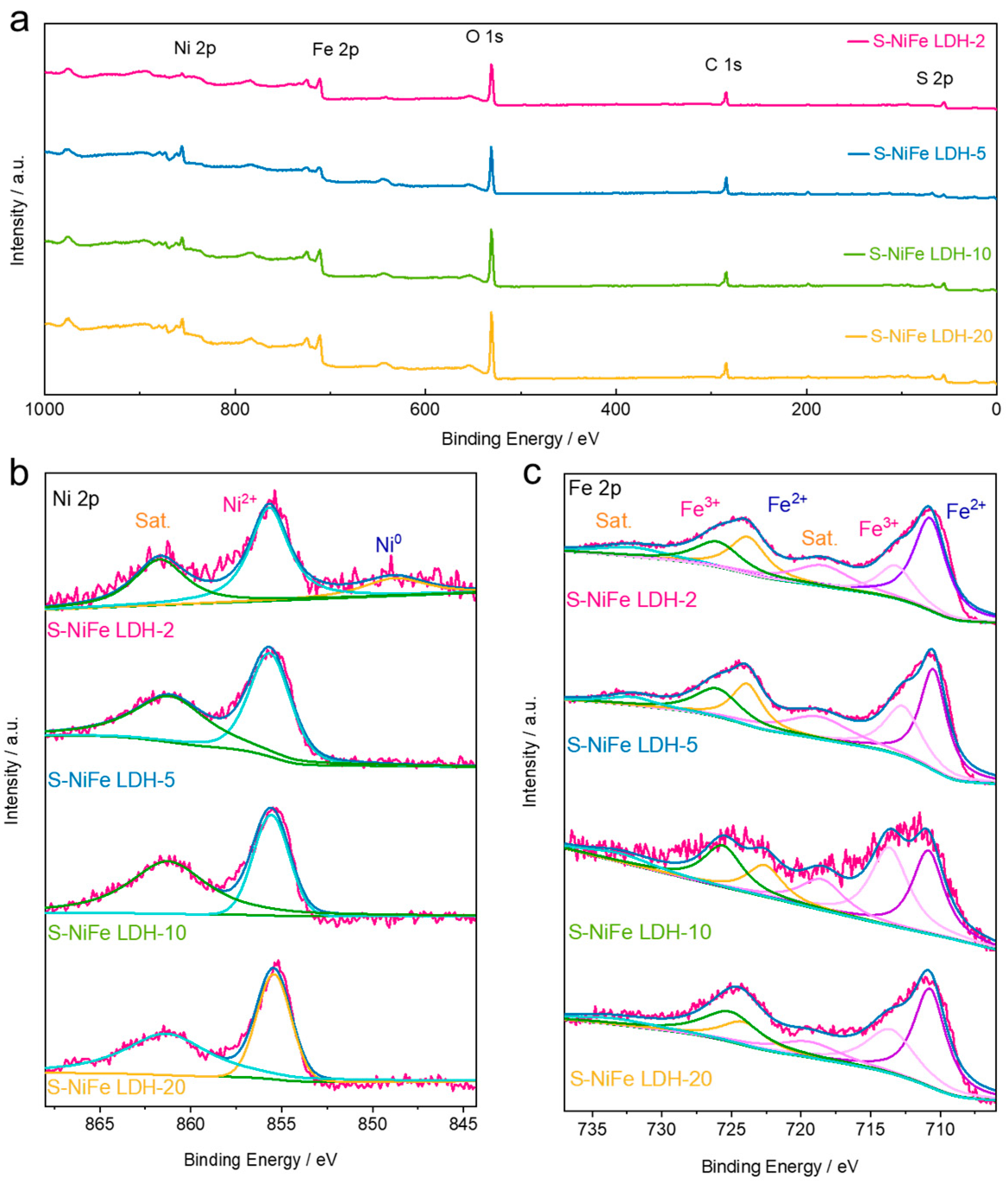
2.1. Structural Characterization
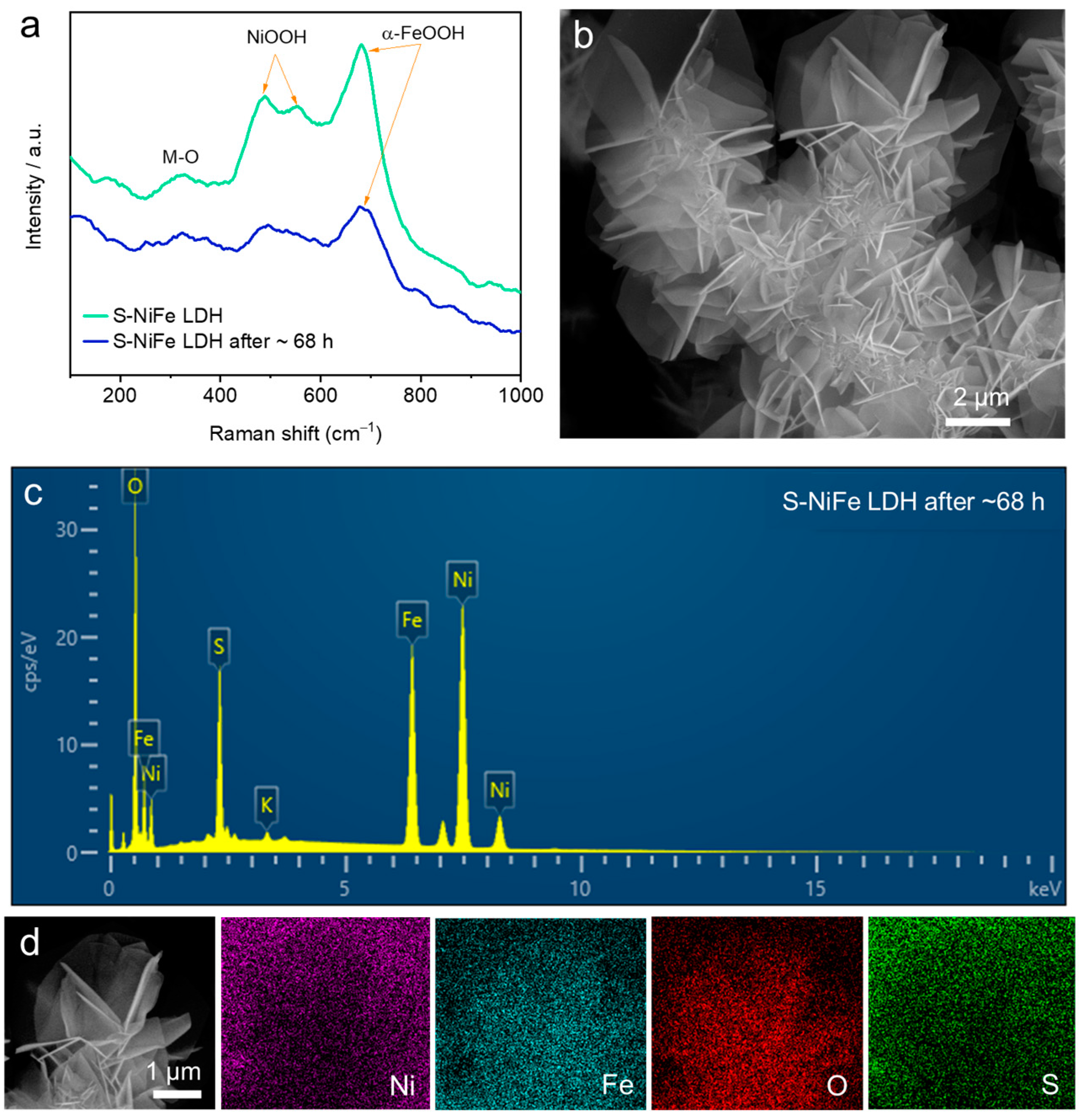
2.2. Electrochemical Performance
3. Materials and Methods
3.1. Materials and Agents
3.2. Preparation of Sulfur-Doped NiFe LDH Catalysts
3.3. Material Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. Advances in carbon capture. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar]
- Shahsavari, A.; Akbari, M. Potential of solar energy in developing countries for reducing energy-related emissions. Renew. Sustain. Energy Rev. 2018, 90, 275–291. [Google Scholar] [CrossRef]
- Zou, X.; Liu, Y.P.; Li, G.D.; Wu, Y.Y.; Liu, D.P.; Li, W.; Li, H.W.; Wang, D.J.; Zhang, Y.; Zou, X.X. Ultrafast Formation of Amorphous Bimetallic Hydroxide Films on 3D Conductive Sulfide Nanoarrays for Large-Current-Density Oxygen Evolution Electrocatalysis. Adv. Mater. 2017, 29, 1700404. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Xie, J.Y.; Wang, N.; Gao, W.K.; Ma, Y.; Chen, T.S.; Yan, X.T.; Li, Q.Z.; Zhou, Y.L.; Chai, Y.M. Zinc ion induced three-dimensional Co9S8 nano-neuron network for efficient hydrogen evolution. Renew. Energy 2020, 157, 415–423. [Google Scholar] [CrossRef]
- Ding, Y.; Miao, B.Q.; Li, S.N.; Jiang, Y.C.; Liu, Y.Y.; Yao, H.C.; Chen, Y. Benzylamine oxidation boosted electrochemical water-splitting: Hydrogen and benzonitrile co-production at ultra-thin Ni2P nanomeshes grown on nickel foam. Appl. Catal. B Environ. 2020, 268, 118393. [Google Scholar] [CrossRef]
- Zhang, R.R.; Wang, L.; Pan, L.; Chen, Z.C.; Jia, W.Y.; Zhang, X.W.; Zou, J.J. Solid-acid-mediated electronic structure regulation of electrocatalysts and scaling relation breaking of oxygen evolution reaction. Appl. Catal. B Environ. 2020, 277, 119237. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Yan, Y.; Mei, B.B.; Qi, R.J.; He, T.; Wang, Z.T.; Fang, W.S.; Zaman, S.D.; Su, Y.Q.; Ding, S.J.; et al. Local spin-state tuning of cobalt–iron selenide nanoframes for the boosted oxygen evolution. Energy Environ. Sci. 2021, 14, 365–373. [Google Scholar] [CrossRef]
- Ma, T.F.; Xu, W.W.; Li, B.R.; Chen, X.; Zhao, J.J.; Wan, S.S.; Jiang, K.; Zhang, S.X.; Wang, Z.F.; Tian, Z.Y.; et al. The Critical Role of Additive Sulfate for Stable Alkaline Seawater Oxidation on Nickel-Based Electrodes. Angew. Chem. Int. Ed. 2021, 60, 22740–22926. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.F.; Wu, Y.Y.; Chen, H.; Liu, Y.P.; Yang, L.; Ai, X.; Zou, X.X. High-performance oxygen evolution electrocatalysis by boronized metal sheets with self-functionalized surfaces. Energy Environ. Sci. 2019, 12, 684–692. [Google Scholar] [CrossRef]
- Wang, B.; Tang, C.; Wang, H.F.; Chen, X.; Cao, R.; Zhang, Q. A Nanosized CoNi Hydroxide@Hydroxysulfide Core–Shell Heterostructure for Enhanced Oxygen Evolution. Adv. Mater. 2018, 31, 1805658. [Google Scholar] [CrossRef]
- Yang, H.; Gong, L.Q.; Wang, H.M.; Dong, C.L.; Wang, J.L.; Qi, K.; Liu, H.F.; Guo, X.P.; Xia, B.Y. Preparation of nickel-iron hydroxides by microorganism corrosion for efficient oxygen evolution. Nat. Commun. 2020, 11, 5075. [Google Scholar] [CrossRef]
- Tang, Y.H.; Liu, Q.; Dong, L.; Wu, H.B.; Yu, X.Y. Activating the hydrogen evolution and overall water splitting performance of NiFe LDH by cation doping and plasma reduction. Appl. Catal. B Environ. 2020, 266, 118627. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, F.T.; Dong, Y.W.; Dong, B.; Dai, F.N.; Liu, C.G.; Chai, Y.M. Dynamic anion regulation to construct S-doped FeOOH realizing 1000 mAcm−2-level-current-density oxygen evolution over 1000 h. Appl. Catal. B Environ. 2022, 315, 121571. [Google Scholar] [CrossRef]
- Rao, R.R.; Stephens, I.E.L.; Durrant, J.R. Understanding What Controls the Rate of Electrochemical Oxygen Evolution. Joule 2021, 5, 16–18. [Google Scholar] [CrossRef]
- Yuan, W.Y.; Wang, S.Y.; Ma, Y.Y.; Qiu, Y.; An, Y.R.; Cheng, L.F. Interfacial Engineering of Cobalt Nitrides and Mesoporous Nitrogen-Doped Carbon: Toward Efficient Overall Water-Splitting Activity with Enhanced Charge-Transfer Efficiency. ACS Energy Lett. 2020, 5, 692–700. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.X.; Yu, S.J.; Wen, T.; Zhu, X.W.; Yang, F.X.; Sun, X.N.; Wang, X.K.; Hu, W.P. Ternary NiCo2PxNanowires as pH-Universal Electrocatalysts for Highly Efficient Hydrogen Evolution Reaction. Adv. Mater. 2016, 29, 1605502. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, R.J.; Gao, C.C.; Bian, L.Y.; Pu, Y.; Zhu, X.B.; Li, X.A.; Huang, W. Cation-Modulated HER and OER Activities of Hierarchical VOOH Hollow Architectures for High-Efficiency and Stable Overall Water Splitting. Small 2019, 15, 1904688. [Google Scholar] [CrossRef]
- Riyajuddin, S.; Tarik Aziz, S.K.; Kumar, S.; Nessim, G.D.; Ghosh, K. 3D-Graphene Decorated with g-C3N4/Cu3P Composite: A Noble Metal-free Bifunctional Electrocatalyst for Overall Water Splitting. ChemCatChem 2020, 12, 1394–1402. [Google Scholar] [CrossRef]
- Yue, S.; Wang, S.S.; Jiao, Q.Z.; Feng, X.T.; Zhan, K.; Dai, Y.Q.; Feng, C.H.; Li, H.S.; Feng, T.Y.; Zhao, Y. Preparation of Yolk–Shell-Structured CoxFe1−xP with Enhanced OER Performance. ChemSusChem 2019, 12, 4461–4470. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.X.; Gao, M.R.; Yu, S.H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Wang, H.M.; Chen, S. A Cluster-Type Self-Healing Catalyst for Stable Saline–Alkali Water Splitting. Catalysts 2024, 14, 81. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Dongfang, N.C.; Triana, C.A.; Huang, C.; Erni, R.; Wan, W.C.; Li, J.; Stoian, D.; Pan, L.; Zhang, P.; et al. Dynamics and control of active sites in hierarchically nanostructured cobalt phosphide/chalcogenide-based electrocatalysts for water splitting. Energy Environ. Sci. 2022, 15, 727–739. [Google Scholar] [CrossRef]
- Zhu, S.H.; Liu, T.T.; Yu, S.; Yang, H.J.; Sun, Q.M.; Zheng, J.Y. Constructing Stable MoOx-NiSx Film via Electrodeposition and Hydrothermal Method for Water Splitting. Catalysts 2023, 13, 1426. [Google Scholar] [CrossRef]
- Ma, Y.J.; Wang, L.; Wang, J.; Sun, C.H.; Ma, C.L. In Situ Construction of Cobalt-Doped High-Dispersive Heazlewoodite for Efficient Oxygen Evolution. Energy Fuels 2023, 37, 5441–5447. [Google Scholar] [CrossRef]
- Yan, B.; Krishnamurthy, D.; Hendon, C.H.; Deshpande, S.; Surendranath, Y.; Viswanathan, V. Surface Restructuring of Nickel Sulfide Generates Optimally Coordinated Active Sites for Oxygen Reduction Catalysis. Joule 2017, 1, 600–612. [Google Scholar] [CrossRef]
- Hung, T.F.; Yin, Z.W.; Betzler, S.B.; Zheng, W.J.; Yang, J.W.; Zheng, H.M. Nickel sulfide nanostructures prepared by laser irradiation for efficient electrocatalytic hydrogen evolution reaction and supercapacitors. Chem. Eng. J. 2019, 367, 115–122. [Google Scholar] [CrossRef]
- Wang, M.M.; Lin, M.T.; Li, J.T.; Huang, L.; Zhuang, Z.C.; Lin, C.; Zhou, L.; Mai, L.Q. Metal–organic framework derived carbon-confined Ni2P nanocrystals supported on graphene for an efficient oxygen evolution reaction. Chem. Commun. 2017, 53, 8372–8375. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, R.; Xiong, Y.; DiSalvo, F.J.; Abruña, H.D. Cobalt-Based Nitride-Core Oxide-Shell Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2019, 141, 19241–19245. [Google Scholar] [CrossRef]
- Fan, Y.C.; Ida, S.; Staykov, A.; Akbay, T.; Hagiwara, H.; Matsuda, J.; Kaneko, K.; Ishihara, T. Ni-Fe Nitride Nanoplates on Nitrogen-Doped Graphene as a Synergistic Catalyst for Reversible Oxygen Evolution Reaction and Rechargeable Zn-Air Battery. Small 2017, 13, 1700099. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.X.; Shi, P.H.; Fan, J.C.; Min, Y.L.; Xu, Q.J. Nickel Nitride Particles Supported on 2D Activated Graphene–Black Phosphorus Heterostructure: An Efficient Electrocatalyst for the Oxygen Evolution Reaction. Small 2019, 15, 1901530. [Google Scholar] [CrossRef]
- Ibraheem, S.; Chen, S.G.; Li, J.; Wang, Q.M.; Wei, Z.D. In situ growth of vertically aligned FeCoOOH-nanosheets/nanoflowers on Fe,N co-doped 3D-porous carbon as efficient bifunctional electrocatalysts for rechargeable zinc–O2 batteries. J. Mater. Chem. A 2019, 7, 9497–9502. [Google Scholar] [CrossRef]
- Zang, N.; Wu, Z.X.; Wang, J.; Jin, W. Rational design of Cu–Co thiospinel ternary sheet arrays for highly efficient electrocatalytic water splitting. J. Mater. Chem. A 2020, 8, 1799–1807. [Google Scholar] [CrossRef]
- Li, S.; Wan, J.X.; Liu, Z.Y.; Zhuang, M.D.; Ren, P.Y.; Shi, W.L.; Zeng, X.J.; Yang, J. Cyanogel-Based Preparation of Amorphous NiFe Nanoaggregates with Enhanced Activity and Stability for OER. Catalysts 2023, 13, 1261. [Google Scholar] [CrossRef]
- Lin, J.; Ding, Y.H.; Jin, H.L.; Zeng, T.B. Optimized Ni, Co, Mn Oxides Anchored on Graphite Plates for Highly Efficient Overall Water Splitting. Catalysts 2023, 13, 1031. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J. A review on NiFe-based electrocatalysts for efficient alkaline oxygen evolution reaction. J. Power Sources 2020, 448, 227375. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D.P. Recent Development of Ni/Fe-Based Micro/Nanostructures toward Photo/Electrochemical Water Oxidation. Adv. Energy Mater. 2019, 10, 1900954. [Google Scholar] [CrossRef]
- Kang, B.K.; Hussain, M.B.; Cheng, X.X.; Peng, C.; Wang, Z.Q. Green electrodeposition synthesis of NiFe-LDH/MoOx/BiVO4 for efficient photoelectrochemical water splitting. J. Colloid Interface Sci. 2022, 626, 146–155. [Google Scholar] [CrossRef]
- Ma, Y.M.; Wang, K.; Liu, D.U.; Yang, X.X.; Wu, H.; Xiao, C.H.; Ding, S.J. Surface dual-oxidation induced metallic copper doping into NiFe electrodes for electrocatalytic water oxidation. J. Mater. Chem. A 2019, 7, 22889. [Google Scholar] [CrossRef]
- Li, X.G.; Liu, C.; Fang, Z.T.; Xu, L.; Lu, C.L.; Hou, W.H. Ultrafast Room-Temperature Synthesis of Self-Supported NiFe-Layered Double Hydroxide as Large-Current–Density Oxygen Evolution Electrocatalyst. Small 2021, 18, 2104354. [Google Scholar] [CrossRef]
- Du, S.C.; Ren, Z.Y.; Wang, X.L.; Wu, J.; Meng, H.Y.; Fu, H.G. Controlled Atmosphere Corrosion Engineering toward Inhomogeneous NiFe-LDH for Energetic Oxygen Evolution. ACS Nano 2022, 16, 7794–7803. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.X.; Guo, X.Y.; Shen, T.; Xuan, C.J.; Tian, B.L.; Wen, Z.R.; Zhu, Y.; Wang, D.L. Synergistic regulation of nickel doping/hierarchical structure in cobalt sulfide for high performance zinc-air battery. Appl. Catal. B Environ. 2021, 298, 120539. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.Y.; Du, X.q.; Liang, J.n.; Wu, J.z.; Zhao, G.m.; Li, X.G.; Gui, S.W.; Zheng, F.Y.; Zhao, J.; et al. Revealing the complex lithiation pathways and kinetics of core-shell NiO@CuO electrode. Energy Storage Mater. 2022, 51, 11–18. [Google Scholar] [CrossRef]
- Ren, Z.X.; Li, X.H.; Peng, Y.J.; Wang, G.Z.; Yin, J.; Zhao, X.C.; Wang, W.; Wang, X.B. FeNiS2/reduced graphene oxide electrocatalysis with reconstruction to generate FeNi oxo/hydroxide as a highly-efficient water oxidation electrocatalyst. Rare Met. 2022, 41, 4127–4137. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, J.H.; Liu, T.T.; You, S.H.; Liu, P.; Li, F.J.; Gao, M.Q.; Chen, S.G.; Zhang, F.F. Constructing robust NiFe LDHs–NiFe alloy gradient hybrid bifunctional catalyst for overall water splitting: One-step electrodeposition and surface reconstruction. Rare Met. 2023, 42, 2272–2283. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Yu, W.L.; Cao, Y.N.; Zhao, J.; Dong, B.; Ma, Y.; Wang, F.L.; Fan, R.Y.; Zhou, Y.L.; Chai, Y.M. S-doped nickel-iron hydroxides synthesized by room-temperature electrochemical activation for efficient oxygen evolution. Appl. Catal. B-Environ 2021, 292, 120150. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.X.; Hu, Y.C.; Liu, Y.C.; Chen, S.L. A potential-driven switch of activity promotion mode for the oxygen evolution reaction at Co3O4/NiOxHy interface. eScience 2022, 2, 438–444. [Google Scholar] [CrossRef]
- Hao, M.; Wang, H.; Zhang, X.; Qu, Y.; Xuan, C.; Wu, Z.; Cui, M.; Wang, J. In situ construction of self-supporting Ni–Fe sulfide for high-efficiency oxygen evolution. New J. Chem. 2022, 46, 8250–8255. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, S.; Lin, H.; Han, S.; Zhong, W.; Xie, Y.; Hu, J.; Yang, S. NaBH4 induces a high ratio of Ni3+/Ni2+ boosting OER activity of the NiFe LDH electrocatalyst. RSC Adv. 2020, 10, 33475–33482. [Google Scholar] [CrossRef]
- Samal, A.; Kori, D.K.K.; Jain, A.; Das, A.K. A nickel-doped two-dimensional covalent organic polymer (2D-COP) for electrocatalytic hydrogen evolution reaction. ACS Appl. Energy Mater. 2024, 7, 2715–2725. [Google Scholar] [CrossRef]
- Zhou, C.H.; Chen, X.; Liu, S.; Han, Y.; Meng, H.B.; Jiang, Q.Y.; Zhao, S.M.; Wei, F.; Sun, J.; Tian, T.; et al. Superdurable Bifunctional Oxygen Electrocatalyst for HighPerformance Zinc–Air Batteries. J. Am. Chem. Soc. 2022, 144, 2694–2704. [Google Scholar] [CrossRef]
- Gao, X.Y.; Chen, J.; Sun, X.Z.; Wu, B.F.; Li, B.; Ning, Z.C.; Li, J.; Wang, N. Ru/RuO2 Nanoparticle Composites with N-Doped Reduced Graphene Oxide as Electrocatalysts for Hydrogen and Oxygen Evolution. ACS Appl. Nano Mater. 2020, 3, 12269–12277. [Google Scholar] [CrossRef]
- Li, J.W.; Xu, W.M.; Zhou, D.; Luo, J.X.; Zhang, D.W.; Xu, P.M.; Wei, L.C.; Yuan, D.S. Synthesis of 3D flower-like cobalt nickel phosphate grown on Ni foam as an excellent electrocatalyst for the oxygen evolution reaction. J. Mater. Sci. 2018, 53, 2077–2086. [Google Scholar] [CrossRef]
- Xu, J.C.; Li, Z.L.; Chen, D.; Yang, S.X.; Zheng, K.W.; Ruan, J.X.; Wu, Y.L.; Zhang, H.; Chen, J.; Xie, F.Y.; et al. Porous Indium Tin Oxide-Supported NiFe LDH as a Highly Active Electrocatalyst in the Oxygen Evolution Reaction and Flexible Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 48774–48783. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wang, H.; Peng, C.K.; Bu, L.M.; Chiang, C.L.; Tian, K.; Zhao, Y.; Zhao, J.Q.; Lin, Y.G.; Lee, J.M.; et al. Co-Induced Electronic Optimization of Hierarchical NiFe LDH for Oxygen Evolution. Small 2021, 16, 2002426. [Google Scholar] [CrossRef]
- Chen, J.S.; Ren, J.; Shalom, M.; Fellinger, T.; Antonietti, M. Stainless Steel Mesh-Supported NiS Nanosheet Array as Highly Efficient Catalyst for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 5509–5516. [Google Scholar] [CrossRef]
- Gao, L.; Guo, C.; Liu, X.; Ma, X.; Zhao, M.; Kuang, X.; Yang, H.; Zhu, X.; Sun, X.; Wei, Q. Co-Doped FeS2 with a porous structure for efficient electrocatalytic overall water splitting. New J. Chem. 2020, 44, 1711–1718. [Google Scholar] [CrossRef]
- Wu, S.W.; Liu, S.Q.; Tan, X.H.; Zhang, W.Y.; Cadien, K.; Li, Z. Ni3S2-embedded NiFe LDH porous nanosheets with abundant heterointerfaces for high-current water electrolysis. Chem. Eng. J. 2022, 442, 136105. [Google Scholar] [CrossRef]
- Zhao, M.H.; Wang, Y.N.; Mi, W.L.; Wu, J.T.; Zou, J.J.; Zhu, X.D.; Gao, J.; Zhang, Y.C. Surface-modified amorphous FeOOH on NiFe LDHs for high efficiency electrocatalytic oxygen evolution. Electrochim. Acta 2023, 458, 142513. [Google Scholar] [CrossRef]
- Hu, L.Y.; Zeng, X.; Wei, X.Q.; Wang, H.J.; Wu, Y.; Gu, W.L.; Shi, L.; Zhu, C.Z. Interface engineering for enhancing electrocatalytic oxygen evolution of NiFe LDH/NiTe heterostructures. Appl. Catal. B-Environ. 2020, 273, 119014. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, J.; Liu, H.; Wang, L.; Sun, C.; Gong, L.; Zhang, X.; Zhu, J. Expediting Corrosion Engineering for Sulfur-Doped, Self-Supporting Ni-Fe Layered Dihydroxide in Efficient Aqueous Oxygen Evolution. Catalysts 2024, 14, 394. https://doi.org/10.3390/catal14070394
Ma Y, Wang J, Liu H, Wang L, Sun C, Gong L, Zhang X, Zhu J. Expediting Corrosion Engineering for Sulfur-Doped, Self-Supporting Ni-Fe Layered Dihydroxide in Efficient Aqueous Oxygen Evolution. Catalysts. 2024; 14(7):394. https://doi.org/10.3390/catal14070394
Chicago/Turabian StyleMa, Yingjun, Jie Wang, Hangning Liu, Lin Wang, Changhui Sun, Liangyu Gong, Xiaogang Zhang, and Jiefang Zhu. 2024. "Expediting Corrosion Engineering for Sulfur-Doped, Self-Supporting Ni-Fe Layered Dihydroxide in Efficient Aqueous Oxygen Evolution" Catalysts 14, no. 7: 394. https://doi.org/10.3390/catal14070394
APA StyleMa, Y., Wang, J., Liu, H., Wang, L., Sun, C., Gong, L., Zhang, X., & Zhu, J. (2024). Expediting Corrosion Engineering for Sulfur-Doped, Self-Supporting Ni-Fe Layered Dihydroxide in Efficient Aqueous Oxygen Evolution. Catalysts, 14(7), 394. https://doi.org/10.3390/catal14070394








