Constructing Polyphosphazene Microsphere-Supported Pd Nanocatalysts for Efficient Hydrogenation of Quinolines under Mild Conditions
Abstract
1. Introduction
2. Results and Discussion
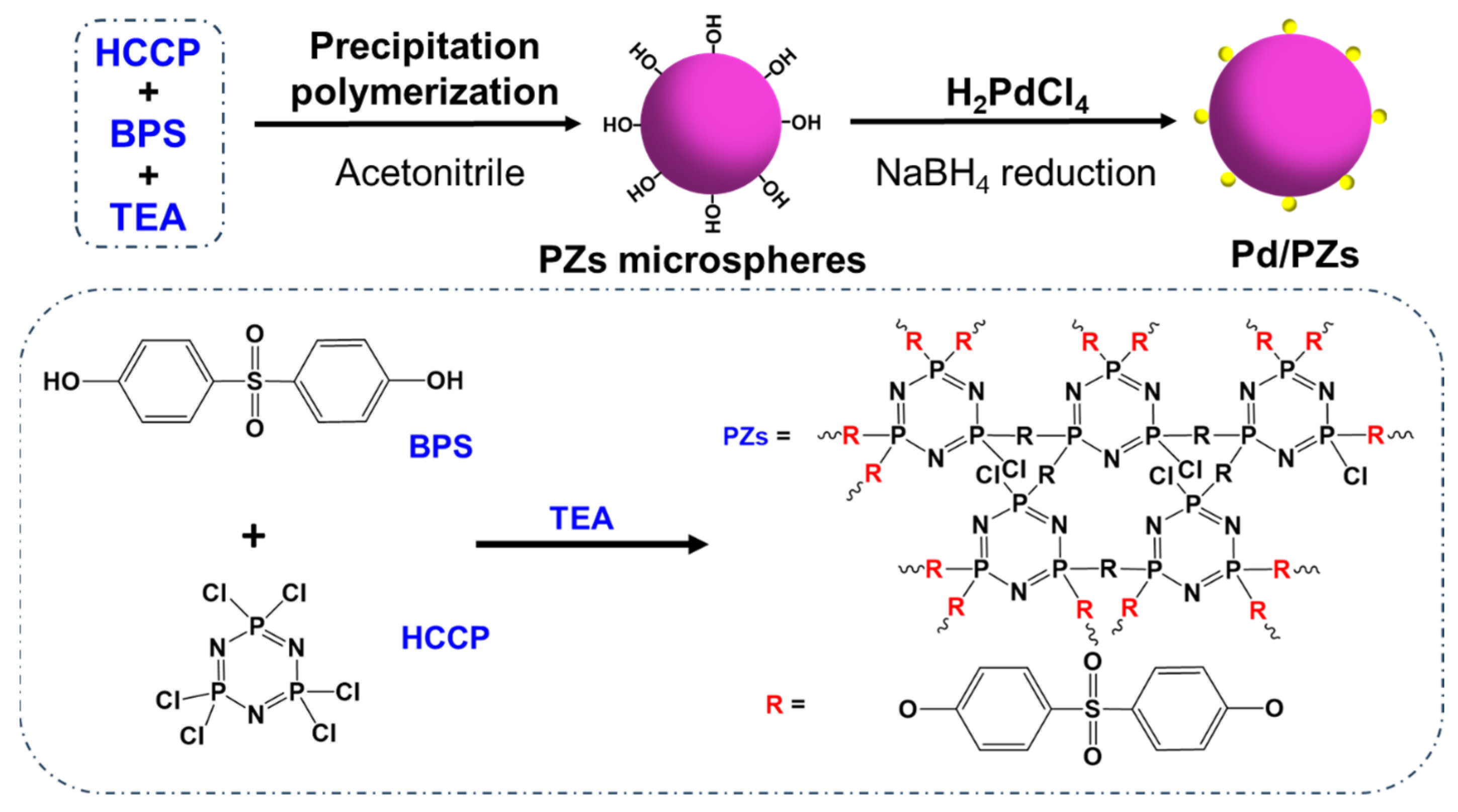
2.1. Fabrication Process of the Pd/PZs Catalyst
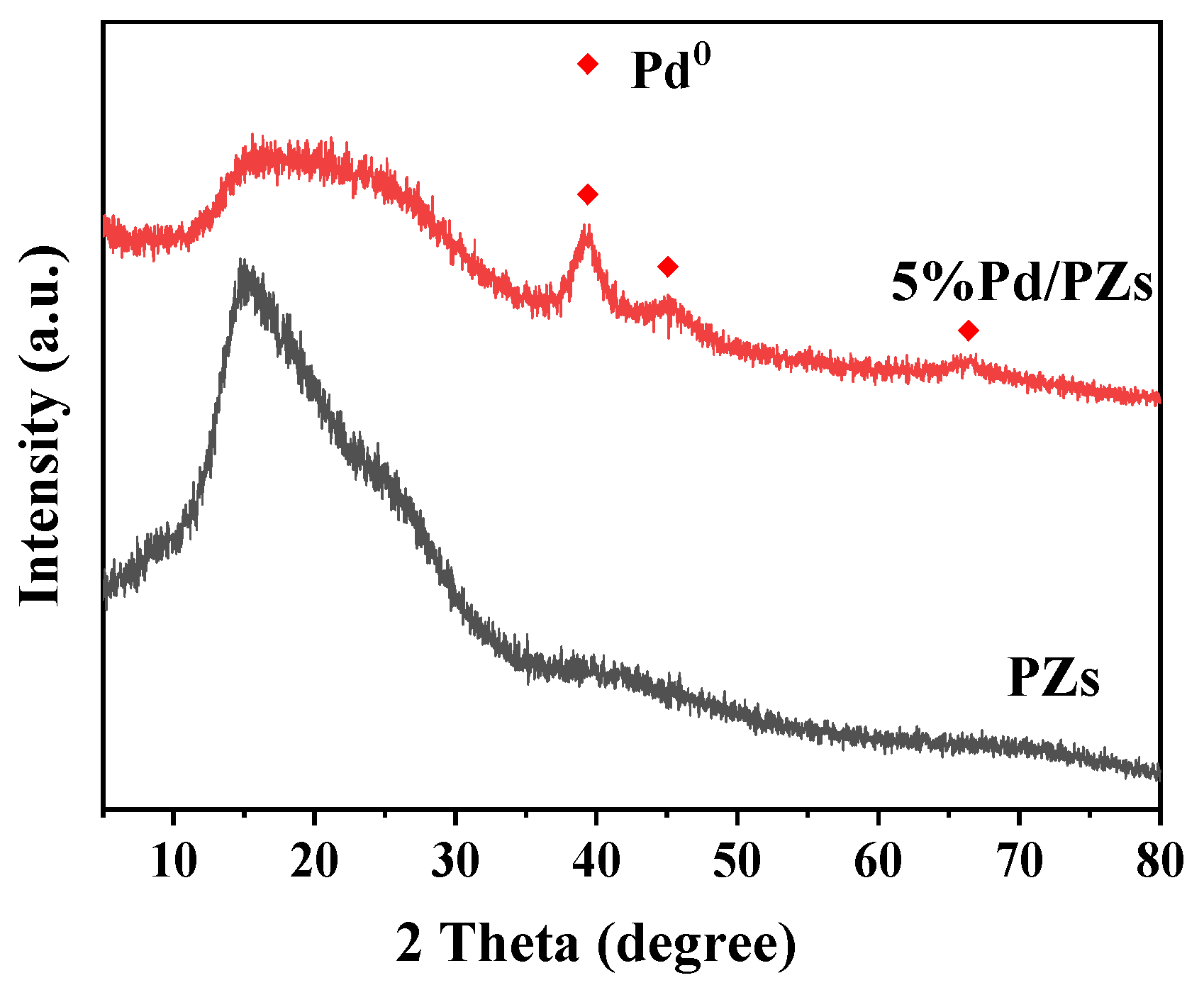
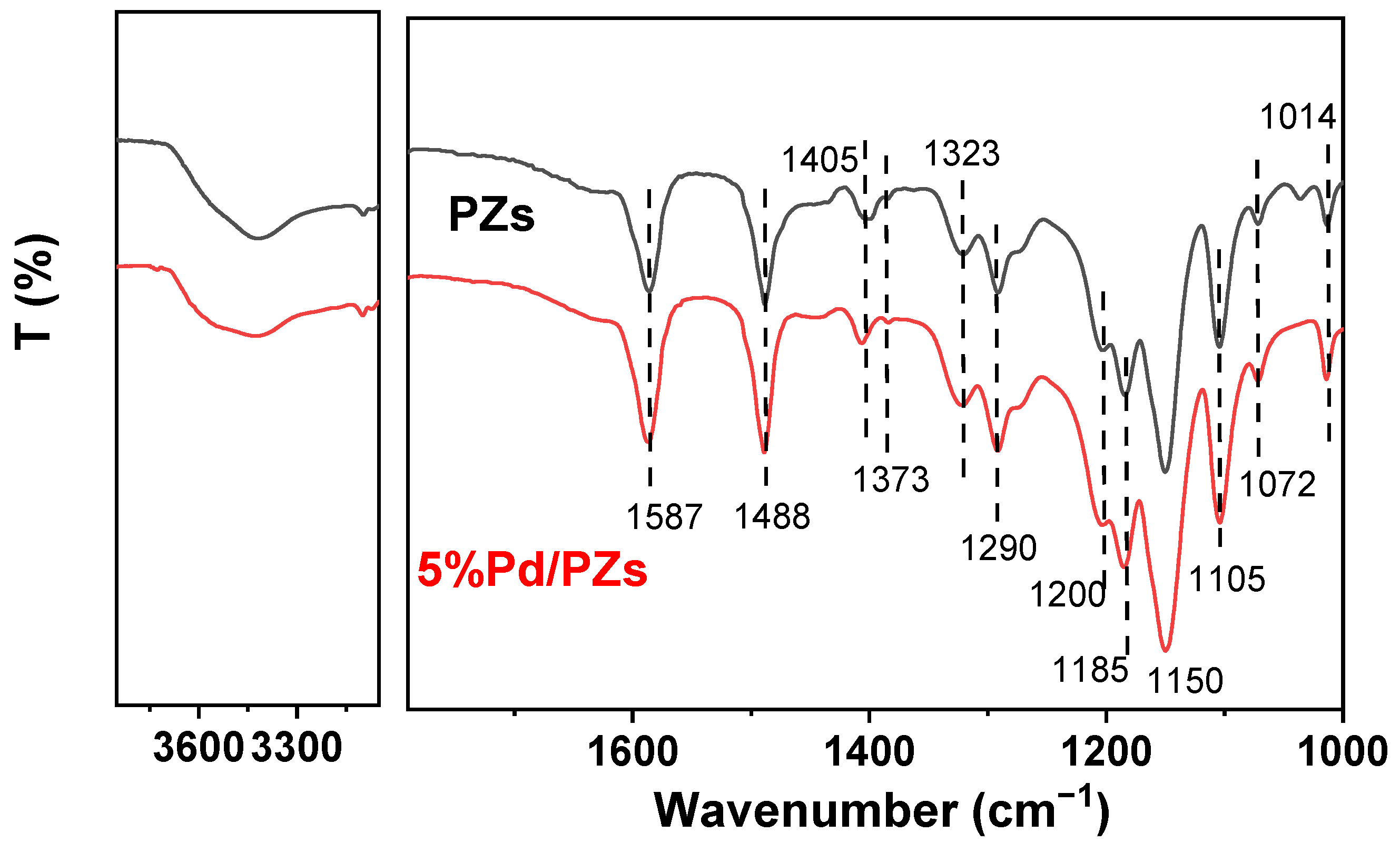
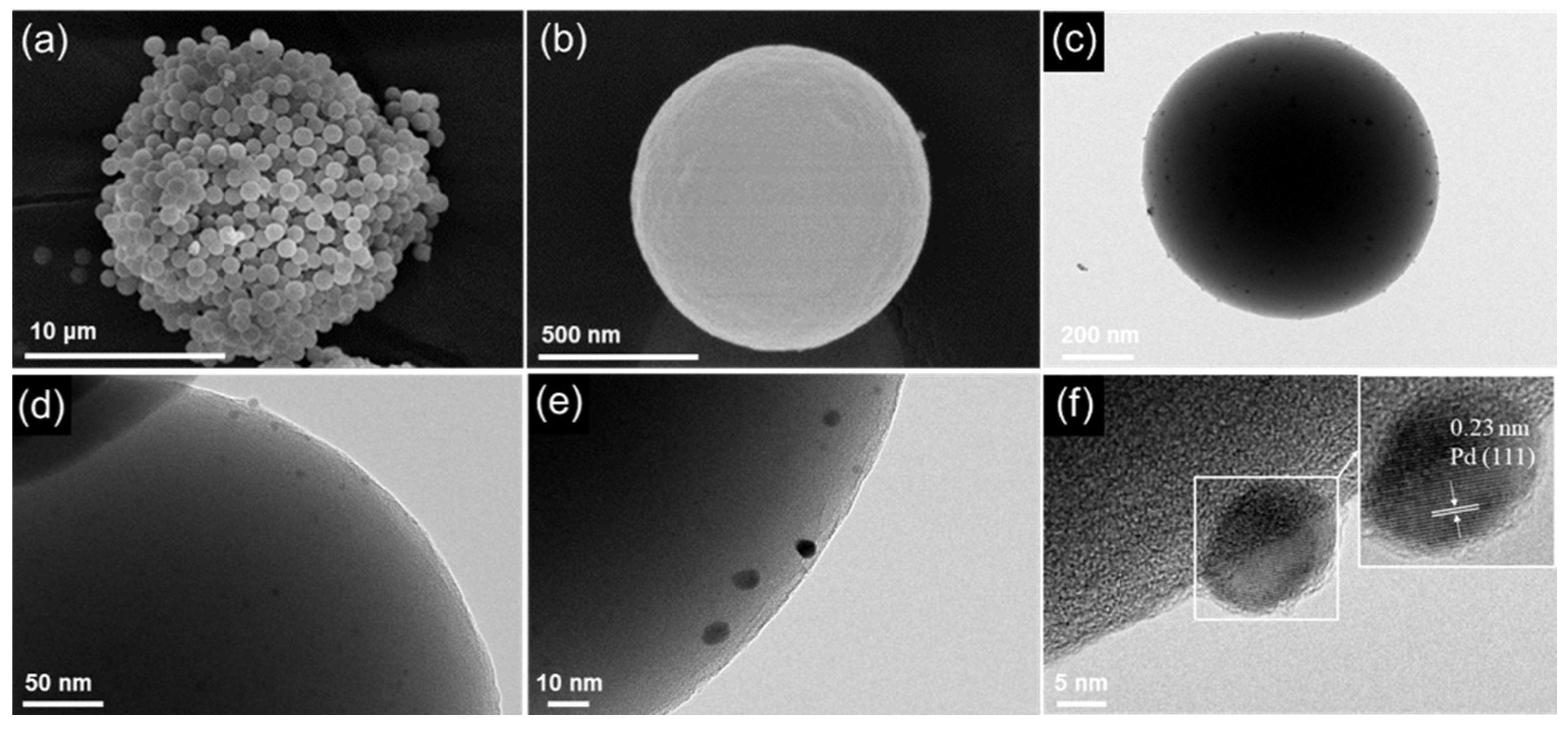
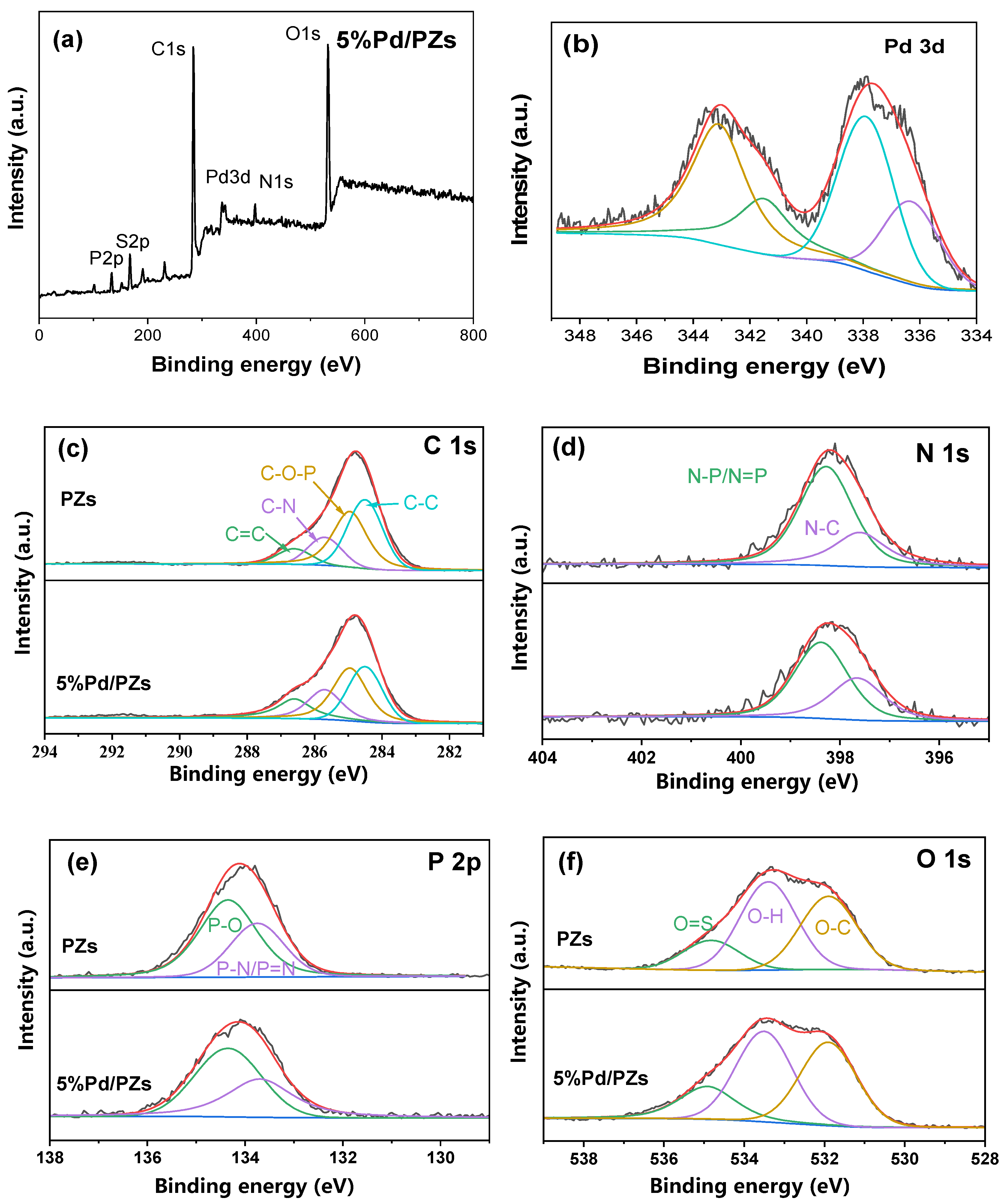
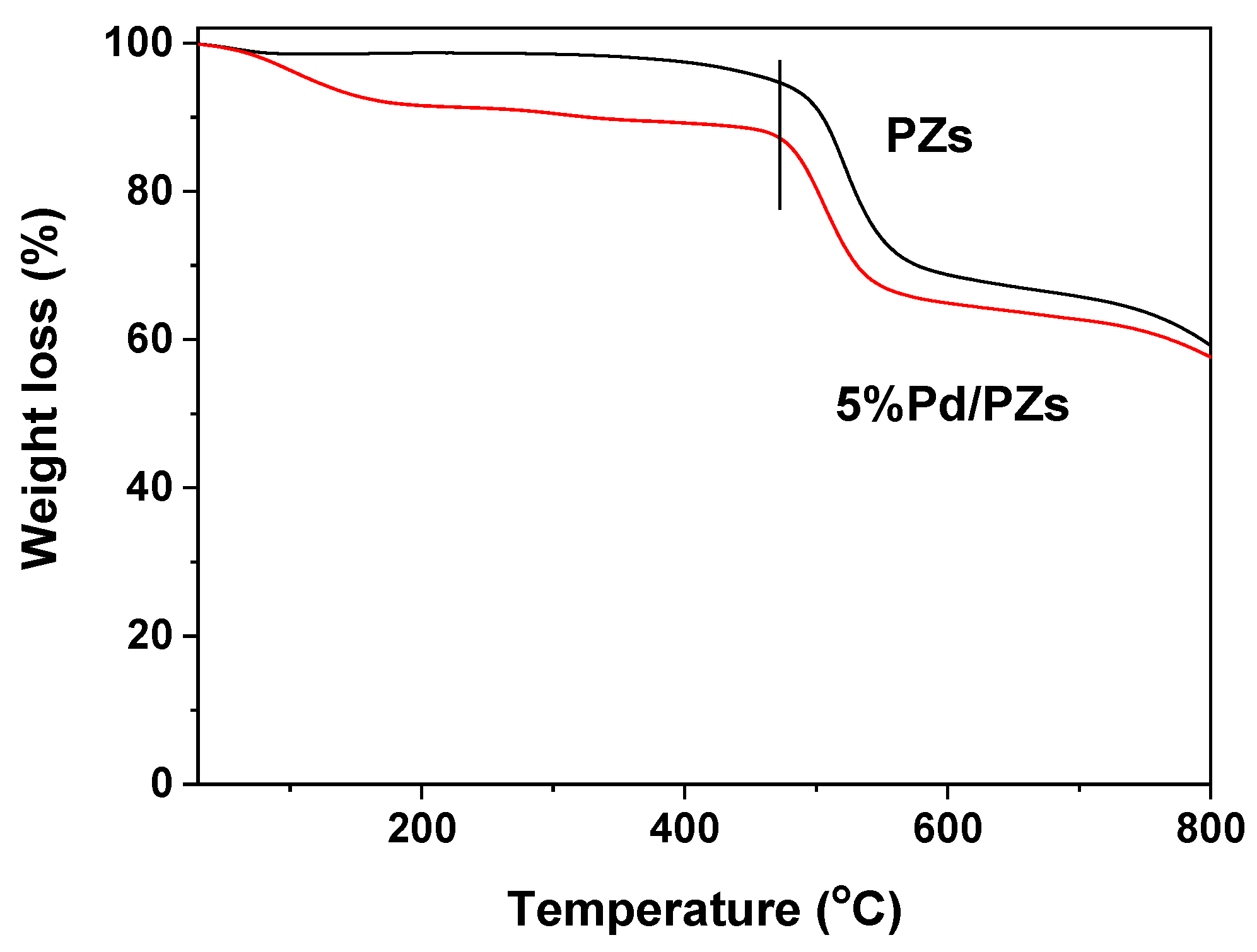
2.2. Composition and Structure
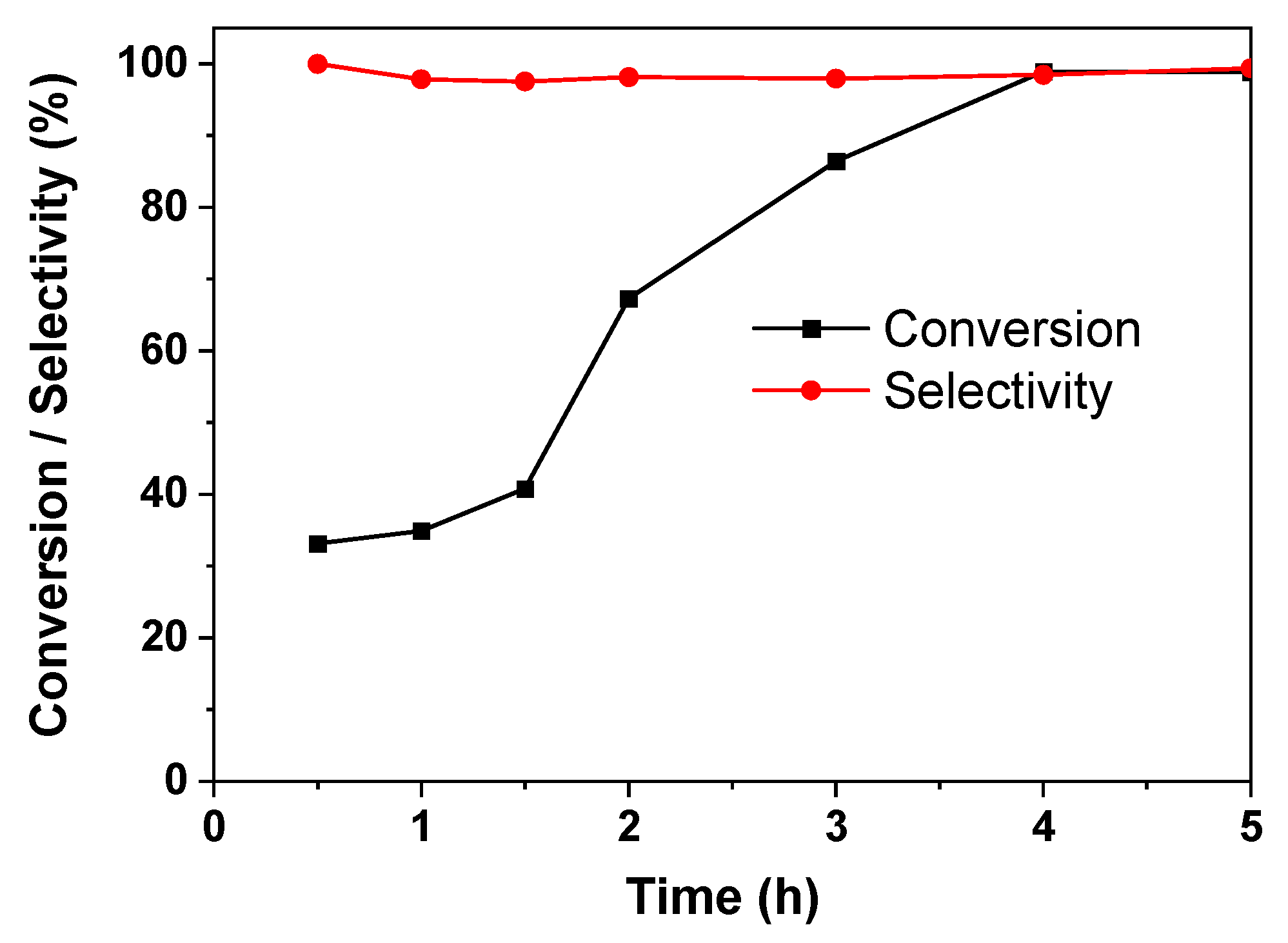
2.3. Catalytic Performance of the Pd/PZs Catalyst
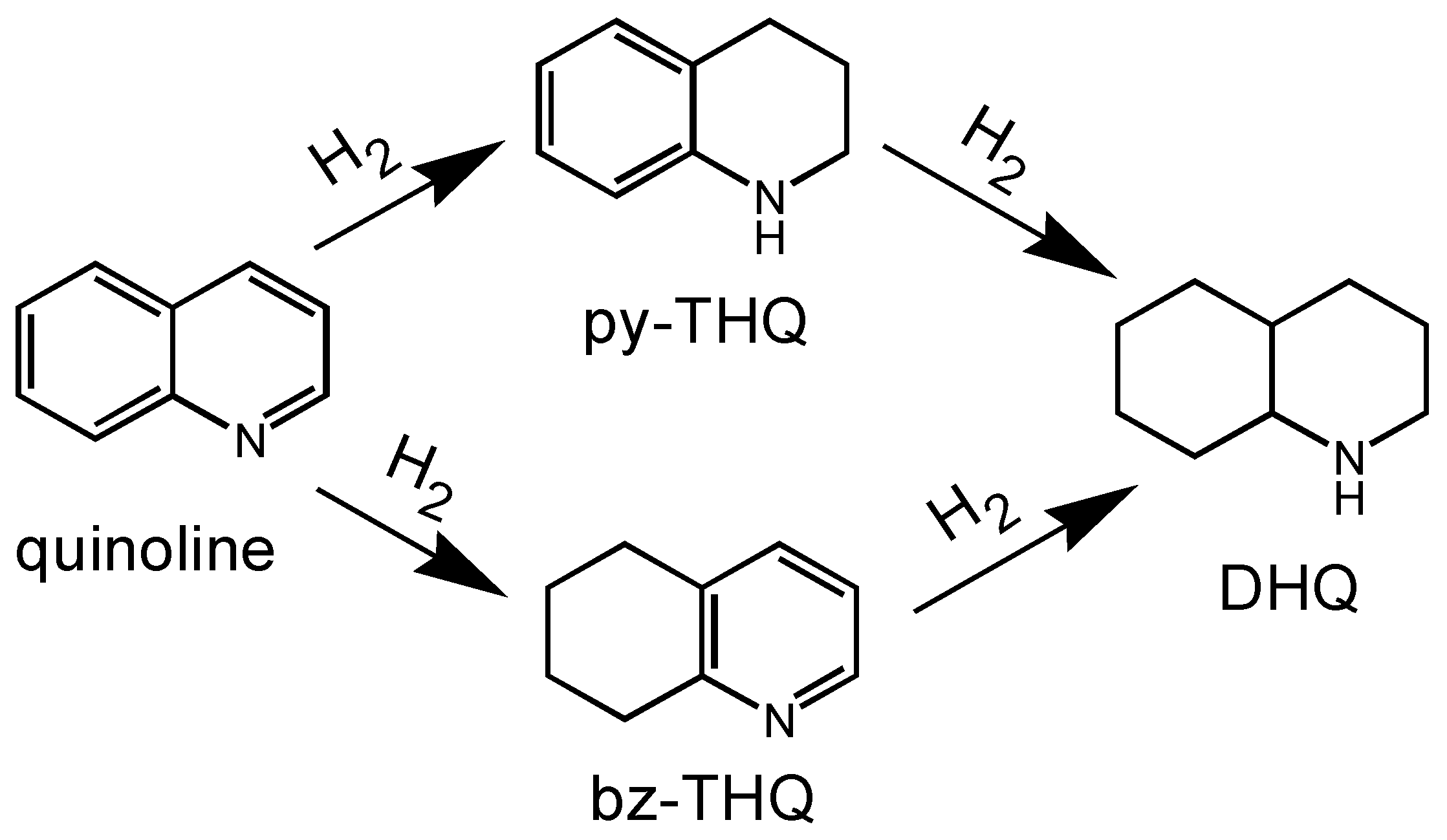
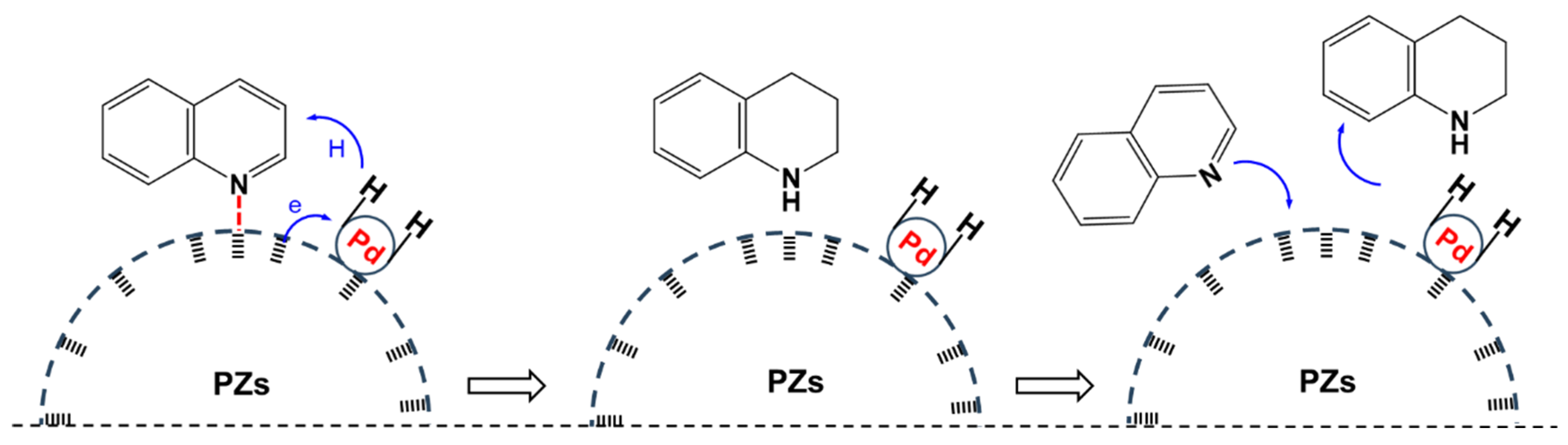
2.4. Possible Reaction Pathway
2.5. Hydrogenation of Other N/O/S-Heterocycles Compounds
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Poly-[Cyclotriphosphazene-co-(4,4′-Sulfonyldiphenol)] Microspheres
3.3. Preparation of the Pd/PZs Catalyst
3.4. Catalytic Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, S.; Brennessel, W.W.; Jones, W.D. A Molecular Iron Catalyst for the Acceptorless Dehydrogenation and Hydrogenation of N-heterocycles. J. Am. Chem. Soc. 2014, 136, 8564–8567. [Google Scholar] [CrossRef]
- Wang, D.S.; Chen, Q.A.; Lu, S.M.; Zhou, Y.G. Asymmetric Hydrogenation of Heteroarenes and Arenes. Chem. Rev. 2012, 112, 2557–2590. [Google Scholar] [CrossRef] [PubMed]
- Hegedűs, L.; Nguyen, T.T.T.; Lévay, K.; László, K.; Sáfrán, G.; Beck, A. Poisoning and Reuse of Supported Precious Metal Catalysts in the Hydrogenation of N-Heterocycles, Part II: Hydrogenation of 1-Methylpyrrole over Rhodium. Catalysts 2022, 12, 730. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Pang, S.F.; Wei, Z.H.; Jiao, H.J.; Dai, X.C.; Wang, H.L.; Shi, F. Synthesis of a Molecularly Defined Single-active Site Heterogeneous Catalyst for Selective Oxidation of N-heterocycles. Nat. Commun. 2018, 9, 1465. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Chen, Y.Q.; Wang, J.; Su, D.F.; Tang, M.H.; Mao, S.J.; Wang, Y. Cobalt Encapsulated in N-Doped Graphene Layers: An Efficient and Stable Catalyst for Hydrogenation of Quinoline Compounds. ACS Catal. 2016, 6, 5816–5822. [Google Scholar] [CrossRef]
- Zhu, Q.Z.; Yin, X.G.; Tan, Y.J.; Wei, D.D.; Li, Y.; Pei, X.L. Highly Dispersed Palladium Nanocatalyst Anchored on N-doped Nanoporous Carbon Microspheres Derived from Chitosan for Efficient and Stable Hydrogenation of Quinoline. Int. J. Biol. Macromol. 2024, 254, 127949. [Google Scholar] [CrossRef]
- Fish, R.H.; Thormodsen, A.D.; Cremer, G.A. Homogeneous Catalytic Hydrogenation. 1. Regiospecific Reductions of Polynuclear Aromatic and Polynuclear Heteroaromatic Nitrogen Compounds Catalyzed by Transition Metal Carbonyl Hydrides. J. Am. Chem. Soc. 1982, 104, 5234–5237. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, Z.M.; Ni, T.; Zhang, H.; Wu, C.; Qu, Y.Q. Tuning Chemical Compositions of Bimetallic AuPd Catalysts for Selective Catalytic Hydrogenation of Halogenated Quinolines. J. Mater. Chem. A 2017, 5, 3260–3266. [Google Scholar] [CrossRef]
- Lyons, T.W.; Leibler, I.N.; He, C.Q.; Gadamsetty, S.; Estrada, G.J.; Doyle, A.G. Broad Survey of Selectivity in the Heterogeneous Hydrogenation of Heterocycles. J. Org. Chem. 2024, 89, 1438–1445. [Google Scholar] [CrossRef]
- Chen, F.; Surkus, A.E.; He, L.; Pohl, M.M.; Radnik, J.; Topf, C.; Junge, K.; Beller, M. Selective Catalytic Hydrogenation of Heteroarenes with N-Graphene-Modified Cobalt Nanoparticles (Co3O4–Co/NGr@α-Al2O3). J. Am. Chem. Soc. 2015, 137, 11718–11724. [Google Scholar] [CrossRef]
- Colliere, V.; Verelst, M.; Lecante, P.; Axet, M.R. Colloidal Ruthenium Catalysts for Selective Quinaldine Hydrogenation: Ligand and Solvent Effects. Chem.-Eur. J. 2024, 30, e202302131. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Che, Y.X.; Xia, J.; Zheng, L.R.; Lv, H.F.; Zhang, J.; Liang, H.W.; Meng, X.M.; Ma, D.; Song, W.G.; et al. Metal–Sulfur Interfaces as the Primary Active Sites for Catalytic Hydrogenations. J. Am. Chem. Soc. 2024, 146, 11542–11552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, H. Enantioselective Metal-free Hydrogenations of Disubstituted Quinolines. Org. Lett. 2015, 17, 6266–6269. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Ikeda, C.; Takahashi, Y.; Fujita, K. Homogeneous Catalytic System for Reversible Dehydrogenation—Hydrogenation Reactions of Nitrogen Heterocycles with Reversible Interconversion of Catalytic Species. J. Am. Chem. Soc. 2009, 131, 8410–8412. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.; Bastidas, L.J.; Gonzalez, B.; Vallejo, R.; Baricelli, P.J. Kinetics and Mechanisms of Homogeneous Catalytic Reactions. Part 11. Regioselective Hydrogenation of Quinoline Catalyzed by Rhodium Systems Containing 1,2-Bis(diphenylphosphino)ethane. Catal. Lett. 2011, 141, 1305–1310. [Google Scholar] [CrossRef]
- Facchetti, G.; Christodoulou, M.S.; Binda, E.; Fusè, M.; Rimoldi, I. Asymmetric Hydrogenation of 1-aryl substituted-3,4-Dihydroisoquinolines with Iridium Catalysts Bearing Different Phosphorus-Based Ligands. Catalysts 2020, 10, 914. [Google Scholar] [CrossRef]
- Wu, J.G.; Li, X.; Fu, K.; Cao, D.; Cheng, D.J. Constructing Fully Exposed Pt Atomically Dispersed Catalysts for Enhanced Multifunctional Selective Hydrogenation Reactions. Chem. Eng. J. 2024, 481, 148706. [Google Scholar] [CrossRef]
- Campanati, M.; Vaccari, A.; Piccolo, O. Mild Hydrogenation of Quinoline: 1. Role of Reaction Parameters. J. Mol. Catal. A Chem. 2002, 179, 287–292. [Google Scholar] [CrossRef]
- Hashimoto, N.; Takahashi, Y.; Hara, T.; Shimazu, S.; Mitsudome, T.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Fine Tuning of Pd0 Nanoparticle Formation on Hydroxyapatite and Its Application for Regioselective Quinoline Hydrogenation. Chem. Lett. 2010, 39, 832–834. [Google Scholar] [CrossRef]
- Campanati, M.; Casagrande, M.; Fagiolino, I.; Lenarda, M.; Storaro, L.; Battagliarin, M.; Vaccari, A. Mild Hydrogenation of Quinoline: 2. A Novel Rh-containing Pillared Layered Clay Catalyst. J. Mol. Catal. A Chem. 2002, 184, 267–272. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Capodiferro, V.F.; Mali, M.; Manno, D.; Cotugno, P.; Monopoli, A.; Mastrorilli, P. Highly Selective Hydrogenation of Quinolines Promoted by Recyclable Polymer Supported Palladium Nanoparticles under Mild Conditions in Aqueous Medium. Appl. Catal. A Gen. 2014, 481, 89–95. [Google Scholar] [CrossRef]
- He, Z.H.; Li, N.; Wang, K.; Wang, W.T.; Liu, Z.T. Selective Hydrogenation of Quinolines over a CoCu Bimetallic Catalyst at Low Temperature. Mol. Catal. 2019, 470, 120–126. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Fei, B.; Chen, X.; Zhang, J.; Mu, X. Tunable and Selective Hydrogenation of Furfural to Furfuryl alcohol and Cyclopentanone over Pt Supported on Biomass-derived Porous Heteroatom Doped Carbon. Faraday Discuss. 2017, 202, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.J.; Duan, Y.N.; Zhang, S.C.; Fei, B.H.; Chen, X.F.; Yang, Y. Selective Semihydrogenation of Alkynes Catalyzed by Pd Nanoparticles Immobilized on Heteroatom-doped Hierarchical Porous Carbon Derived from Bamboo Shoots. ChemSusChem 2017, 10, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cheng, L.L.; Yang, Y.G.; Chen, X.F.; Chen, F.T.; Lu, W.Y. Construction of High-Density Fe Clusters Embedded in a Porous Carbon Nitride Catalyst with Effectively Selective Transformation of Benzene. ACS Sus. Chem. Eng. 2023, 11, 1518–1526. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, S.; Chen, X.; Chen, F.; Chen, X.; Lu, W. Convenient Fabrication of Ultrafine VOx Decorated on Porous g-C3N4 for Boosting Photocatalytic Degradation of Pharmaceuticals with Peroxymonosulfate. Surf. Interfaces 2023, 42, 103300. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, L.; Hu, Y.; Xiao, Q.; Chen, X.; Lu, W. Robust Co3O4 Nanocatalysts Supported on Biomass-derived Porous N-doped Carbon Toward Low-Pressure Hydrogenation of Furfural. Front. Mater. Sci. 2023, 17, 230645. [Google Scholar] [CrossRef]
- Gong, Y.T.; Zhang, P.F.; Xu, X.; Li, Y.; Li, H.R.; Wang, Y. A Novel Catalyst Pd@ompg-C3N4 for Highly Chemoselective Hydrogenation of Quinoline under Mild Conditions. J. Catal. 2013, 297, 272–280. [Google Scholar] [CrossRef]
- Spitaleri, A.; Pertici, P.; Scalera, N.; Vitulli, G.; Hoang, M.; Turney, T.W.; Gleria, M. Supported Ruthenium Nanoparticles on Polyorganophosphazenes: Preparation, Structural and Catalytic Studies. Inorg. Chim. Acta 2003, 352, 61–71. [Google Scholar] [CrossRef]
- Sun, S.S.; Peng, X.Y.; Guo, X.C.; Chen, X.F.; Liu, D. Boosting Solvent-Free Aerobic Oxidation of Benzylic Compounds into Ketones over Au-Pd Nanoparticles Supported by Porous Carbon. Catalysts 2024, 14, 158. [Google Scholar] [CrossRef]
- Ge, N.Q.; Hu, X.M.; Pan, Z.J.; Cai, S.J.; Fu, F.Y.; Wang, Z.Q.; Yao, J.M.; Liu, X.D. Sustainable Fabrication of Cellulose Aerogel Embedded with ZnO@noble Metal (Ag, Au, Ag-Au) NPs for Sensitive and Reusable SERS Application. Colloid. Surface. A 2023, 671, 131650. [Google Scholar] [CrossRef]
- Shi, M.; Hu, N.N.; Liu, H.M.; Qian, C.; Lv, C.; Wang, S. Controlled Synthesis of Pt-loaded Yolk-shell TiO2@SiO2 Nanoreactors as Effective Photocatalysts for Hydrogen Generation. Front. Mater. Sci. 2022, 16, 220591. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Xu, Z.; Chen, J.Y.; Zhong, M.Q.; Wang, J.H.; Chew, J.W. A Review on Functional Applications of Polyphosphazenes as Multipurpose Material for Lithium-ion Batteries. J. Energy Storage 2024, 85, 111049. [Google Scholar] [CrossRef]
- Wang, M.H.; Fu, J.W.; Huang, D.D.; Zhang, C.; Xu, Q. Silver Nanoparticles-decorated Polyphosphazene Nanotubes: Synthesis and Applications. Nanoscale 2013, 5, 7913–7919. [Google Scholar] [CrossRef]
- Wang, M.H.; Fu, J.W.; Chen, Z.H.; Wang, X.Z.; Xu, Q. In Situ Growth of Gold Nanoparticles onto Polyphosphazene Microspheres with Amino-groups for Alcohol Oxidation in Aqueous Solutions. Mater. Lett. 2015, 143, 201–204. [Google Scholar] [CrossRef]
- Ahmad, M.; Nawaz, T.; Assiri, M.A.; Hussain, R.; Hussain, I.; Imran, M.; Ali, S.; Wu, Z.P. Fabrication of Bimetallic Cu-Ag Nanoparticle-Decorated Poly(cyclotriphosphazene-co-4,4’-sulfonyldiphenol) and Its Enhanced Catalytic Activity for the Reduction of 4-Nitrophenol. ACS Omega 2022, 7, 7096–7102. [Google Scholar] [CrossRef]
- Huang, M.H.; Li, Z.; Du, L.L.; Jin, Z.K.; Li, R.H. CuPd/MgO for Efficient Catalytic Hydrogen Production from Formaldehyde Solution at Room Temperature. Chinese J. Inorg. Chem. 2022, 38, 2452–2458. [Google Scholar]
- Chen, S.S.; Li, Z.W.; Yuan, W.B.; Duan, W.S.; Qiao, C.D.; Yao, J.S.; Zhang, C.B.; Zhao, H.; Li, M.; Yang, G.H. Polyphosphazene-Functionalized Microspheres as Efficient Catalysts for the Knoevenagel Reaction under Mild Conditions. ChemPlusChem 2022, 87, e202200249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Lin, X.Y.; Huang, X.Y.; Chen, Y.Q.; Lin, S.; Huang, X.; Xie, Z.L. The Role Identification of Nitrogen Dopant in Nanocarboncatalysis. Carbon Future 2024, 1, 9200008. [Google Scholar]
- Mukherjee, S.; Vannice, M.A. Solvent Effects in Liquid-phase Reactions: I. Activity and Selectivity during Citral Hydrogenation on Pt/SiO2 and Evaluation of Mass Transfer Effects. J. Catal. 2006, 243, 108–130. [Google Scholar] [CrossRef]
- Ren, Y.S.; Wang, Y.X.; Li, X.; Zhang, Z.H.; Chi, Q. Selective Hydrogenation of Quinolines into 1,2,3,4-Tetrahydroquinolines over a Nitrogen-doped Carbon-supported Pd catalyst. New. J. Chem. 2018, 42, 16694–16702. [Google Scholar] [CrossRef]
- Ren, D.; He, L.; Yu, L.; Ding, R.S.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. An Unusual Chemoselective Hydrogenation of Quinoline Compounds Using Supported Gold Catalysts. J. Am. Chem. Soc. 2012, 134, 17592–17598. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.M.; Wang, Y.H.; Chen, P.; Du, D.J.; Jiang, J.Y.; Jin, Z.L. Highly Efficient and Recyclable Rhodium Nanoparticle Catalysts for Hydrogenation of Quinoline and Its Derivatives. Catal. Sci. Technol. 2015, 5, 4746–4749. [Google Scholar] [CrossRef]
- Chen, Y.G.; Yu, Z.J.; Chen, Z.A.; Shen, R.; Wang, Y.; Cao, X.; Peng, Q.; Li, Y.D. Controlled One-pot Synthesis of RuCu Nanocages and Cu@Ru Nanocrystals for the Regioselective Hydrogenation of Quinoline. Nano. Res. 2016, 9, 2632–2640. [Google Scholar] [CrossRef]
- Sahoo, B.; Kreyenschulte, C.; Agostini, G.; Lund, H.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. A Robust Iron Catalyst for the Selective Hydrogenation of Substituted (iso) Quinolones. Chem. Sci. 2018, 9, 8134–8141. [Google Scholar] [CrossRef]
- Shaikh, M.N.; Abdelnaby, M.M.; Hakeem, A.S.; Nasser, G.A.; Yamani, Z.H. Co3O4/Nitrogen-doped Graphitic Carbon/Fe3O4 Nanocomposites as Reusable Catalysts for Hydrogenation of Quinoline, Cinnamaldehyde, and Nitroarenes. ACS Appl. Nano Mater. 2021, 4, 3508–3518. [Google Scholar] [CrossRef]
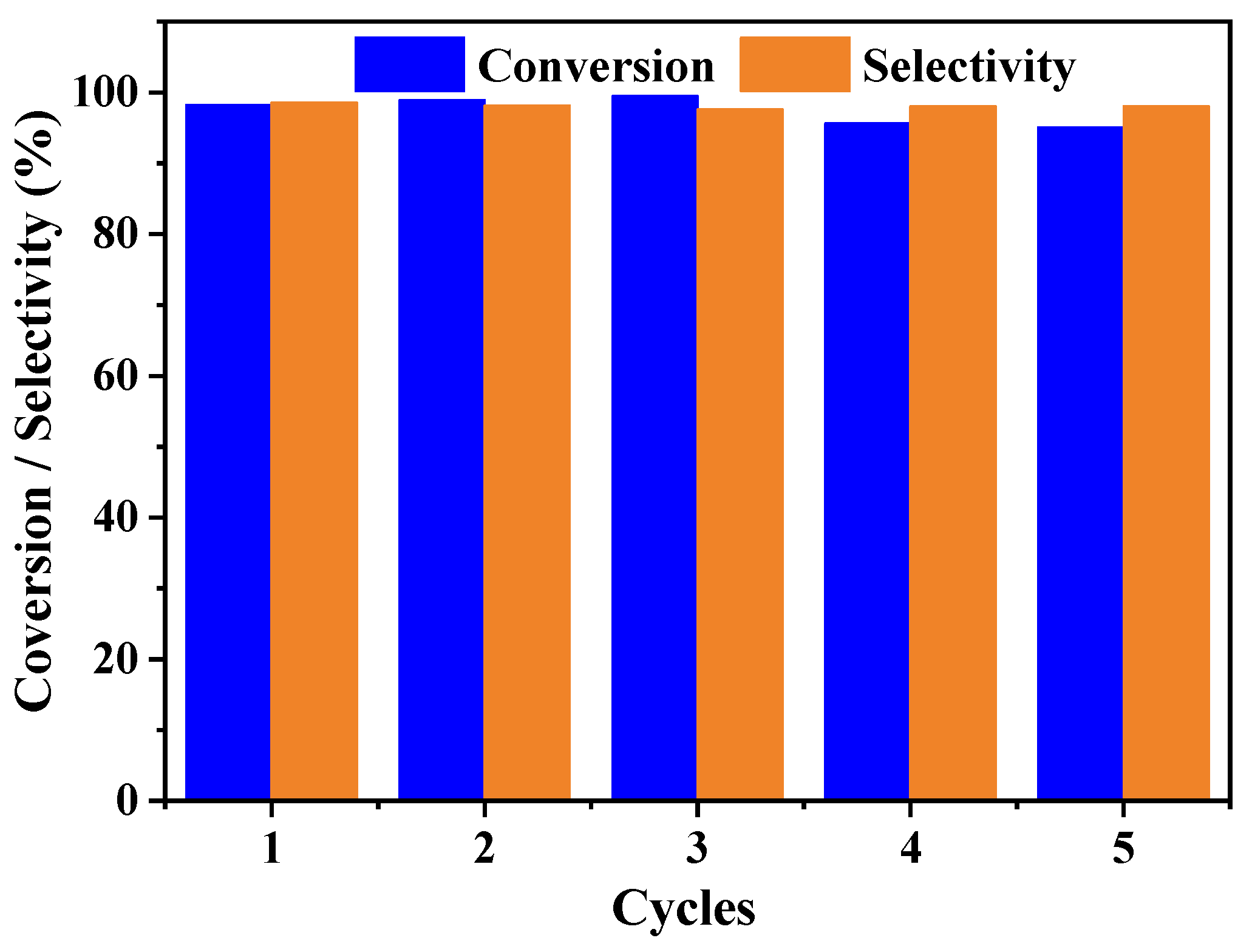
| Entry | Samples | T (°C) | Time (h) | Con. (%) | Sel. (%) |
|---|---|---|---|---|---|
| 1 | PZs | 40 | 2 | <1 | <1 |
| 2 | 1%Pd/PZs | 40 | 2 | 6.4 | 97.2 |
| 3 | 5%Pd/PZs | 40 | 2 | 67.3 | 98.2 |
| 4 | 5%Pd/PZs | 40 | 4 | 98.9 | 98.5 |
| 5 | 5%Pd/C | 40 | 2 | 99.0 | 80.2 |
| 6 | 5%Pd/PZs | 30 | 2 | 41.8 | 98.5 |
| 7 | 5%Pd/PZs | 40 | 2 | 67.3 | 98.2 |
| 8 | 5%Pd/PZs | 50 | 2 | 92.7 | 96.9 |
| Entry | Solvent | Con. (%) | Sel. (%) | Solvent Polarity |
|---|---|---|---|---|
| 1 | ethanol | 67.3 | 98.2 | 4.3 |
| 2 | water | 46.2 | 99.2 | 10.2 |
| 3 | THF | 40.3 | 98.1 | 4.2 |
| 4 | toluene | 90.7 | 97.9 | 2.4 |
| 5 | hexane | 51.6 | 98.5 | 0.06 |
| Entry | Substrates | Products | T (°C) | t (h) | Con. (%) | Sel. (%) |
|---|---|---|---|---|---|---|
| 1 | 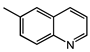 |  | 40 | 6 | 98.1 | >99 |
| 2 |  | 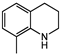 | 40 | 6 | 99.3 | >99 |
| 3 |  |  | 60 | 4 | 58.3 | >99 |
| 4 | 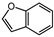 | 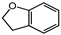 | 40 | 6 | 100 | >99 |
| 5 |  |  | 40 | 6 | 2.6 | >99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Xiao, Q.; Yang, Y.; Dong, B.; Zhao, Z. Constructing Polyphosphazene Microsphere-Supported Pd Nanocatalysts for Efficient Hydrogenation of Quinolines under Mild Conditions. Catalysts 2024, 14, 345. https://doi.org/10.3390/catal14060345
Chen X, Xiao Q, Yang Y, Dong B, Zhao Z. Constructing Polyphosphazene Microsphere-Supported Pd Nanocatalysts for Efficient Hydrogenation of Quinolines under Mild Conditions. Catalysts. 2024; 14(6):345. https://doi.org/10.3390/catal14060345
Chicago/Turabian StyleChen, Xiufang, Qingguang Xiao, Yiguo Yang, Bo Dong, and Zhengping Zhao. 2024. "Constructing Polyphosphazene Microsphere-Supported Pd Nanocatalysts for Efficient Hydrogenation of Quinolines under Mild Conditions" Catalysts 14, no. 6: 345. https://doi.org/10.3390/catal14060345
APA StyleChen, X., Xiao, Q., Yang, Y., Dong, B., & Zhao, Z. (2024). Constructing Polyphosphazene Microsphere-Supported Pd Nanocatalysts for Efficient Hydrogenation of Quinolines under Mild Conditions. Catalysts, 14(6), 345. https://doi.org/10.3390/catal14060345











