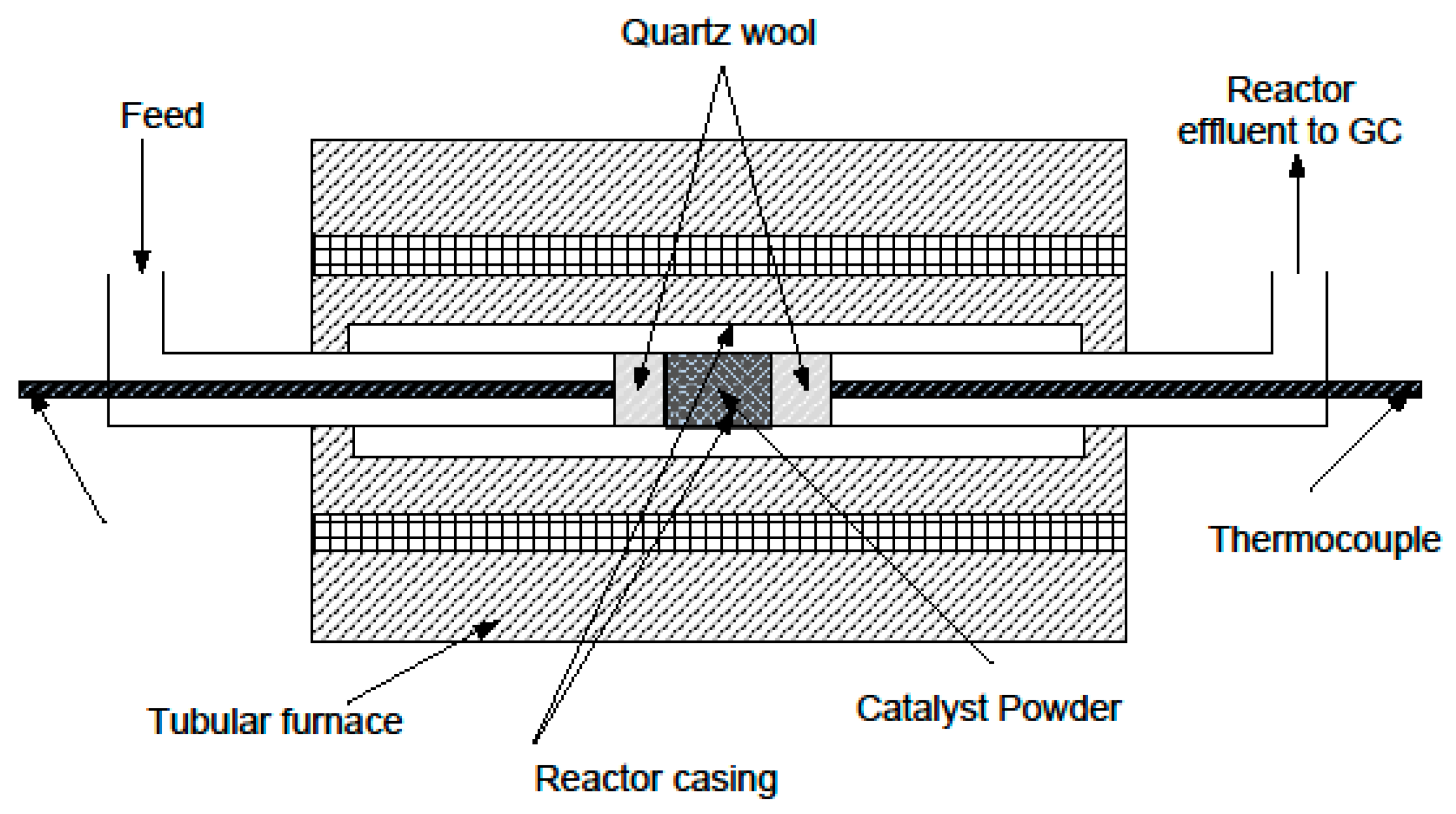
1. Introduction
Catalytic combustion is a flameless combustion that has received considerable attention over the last few decades, although it has been reported on for over one hundred years [
1,
2,
3]. The key advantages of catalytic combustion compared to conventional combustion are the elimination of the standard flammability limits and the ability to combust the reactants at a lower temperature. There are several factors that have been driving the interest in the catalytic combustion of methane in recent years, most of which are related to the solution of environmental problems. Methane is well known as a strong greenhouse gas, and there have been significant efforts made to reduce its emission, especially from such sources as the oil and gas industry, agriculture, and landfills. There is also an interest in the use of methane as an alternative fuel in internal combustion engines (ICEs) as a replacement for diesel or gasoline. Changing the fuel results in a large reduction in the emission of greenhouse gases, provided that the methane that is not burned in the engine is successfully destroyed in a catalytic converter. Even if a large part of the transportation energy is able to transition to electric vehicles, there will remain a need for ICEs in many applications. The aforementioned applications relate to what is referred to as secondary applications. There are also applications where the desire is to produce power from the combustion of natural gas, usually in a gas turbine, but to do so at a lower temperature than conventional homogeneous combustion [
4,
5,
6].
The broad range of applications alluded to in the foregoing implies a plethora of possible reactor feed conditions. Generally speaking, the feed type can be divided into those containing significant quantities of water (wet feed) and those that do not (dry feed). It is often the case that the methane concentration is low, and there is an excess of oxygen (lean feed). Many fugitive streams and those from compression ignition (CI) engines fall into this category. On the other hand, the feed may be stoichiometric, as for spark ignition (SI) engines, or even methane-rich, as for some fugitive emission streams. Furthermore, the feed temperature can be at ambient or elevated temperature. In addition to the nature of the feed, another key factor is the allowable or possible operating temperature of the catalyst section in the reactor. In some cases, the internal recuperation of energy may be used to maintain a high reactor temperature even with a low feed temperature [
7,
8,
9,
10,
11].
Many catalysts have been used for hydrocarbon combustion in general and for methane in particular [
12]. Several metals have been reported, both non-noble (or earth common) as well as metals from the platinum group metals (PGMs), especially platinum and palladium. With cost being a major factor in catalyst selection, there is obviously a large interest in the more earth-abundant and lower-cost metals [
13,
14,
15,
16,
17,
18,
19,
20,
21]. A common disadvantage of earth common metals is that they have a lower activity, which can then result in the reactor having a large physical footprint. Furthermore, a lower activity implies a higher reactor operating temperature, which is not always desirable. Because of their higher activity, PGMs are more commonly employed in applications where either or both a small reactor and low ignition temperature are desired [
22,
23,
24,
25,
26].
Platinum is one of the major PGM components of the standard automotive catalytic converters and is often considered to be the most effective choice for the combustion of non-methane hydrocarbons. It has, however, also been reported in methane combustion applications, with the catalyst taking various forms [
27,
28,
29,
30,
31,
32,
33,
34,
35,
36]. Although platinum does play a role, the most widely used PGM catalyst for methane combustion is palladium, and research on this metal likely accounts for the majority of papers extant on this subject.
As with most catalytic systems, the role of the support is also important, and its selection can affect both the catalytic mechanism and activity and the resulting rate equation, as reported in, for example, references [
37,
38,
39,
40,
41]. Reported supports include, for example, alumina [
42,
43,
44,
45,
46,
47,
48,
49], zirconia [
50,
51,
52,
53,
54], silica [
55,
56,
57], aluminosilicates [
58], cobalt oxide [
59,
60], ceria [
61,
62], nickel compounds [
63], and tin [
64,
65]. In addition to the choice of support, the preparation method [
66,
67,
68] and the use of promoters [
69,
70,
71] can also have a significant impact on the catalytic activity.
Using combinations of PGMs for methane combustion catalysts is common, and such additions include rhodium [
72,
73,
74,
75,
76] and gold [
77,
78,
79]. By far, the most common combination is palladium and platinum, with example references being [
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91,
92,
93].
It is clear that with this large variety of possible catalysts, even restricting ourselves to those based on PGMs, there exists the possibility for different rate expressions and reaction mechanisms. In this paper, we provide a review of kinetic modelling studies that have been reported in the literature with a view to summarizing the current state of knowledge. The focus is on the type of catalysts typically used for emission control applications, rather than those for power applications, which are usually carried out at elevated temperatures. We then present the results of a detailed investigation into kinetic studies for some methane oxidation catalysts with PGMs as the primary active ingredient. We use a leading commercial catalyst as a benchmark and then show results from five PGM catalysts that were prepared in our own laboratories. This paper should make a valuable contribution to those contemplating kinetic studies for these types of catalysts.
2. Review of Reaction Mechanism and Kinetic Models
As noted in the Introduction, the primary active ingredient in catalysts used to promote the oxidation of methane is often palladium. The active form of the metal is palladium oxide (PdO), and it has been shown [
94,
95] that PdO is readily formed when supported Pd is heated in an oxygen environment above temperatures of 523 to 573 K. Although PdO is more active in the oxidation of methane than Pd, PdO begins to decompose to form Pd at higher temperatures [
96]. The transition temperature depends on the oxygen partial pressure above the catalyst, and at 20% oxygen, the transition temperature is about 1023 K [
3] and decreases as the oxygen partial pressure is reduced. This transition temperature can become very important when methane is combusted under stoichiometric conditions, such as what often occurs in a spark ignition natural gas engine.
Many studies have been carried out to elucidate the reaction mechanism and thus propose rate models for PGM-based methane combustion in the presence and absence of water. Some early work is reviewed in [
22]. The reaction is not straightforward to study, and the catalyst activity can be a strong function of the support type, preparation method, and operating history [
97,
98].
First-principle investigations [
99] reported that dry methane combustion on the PdO(101) facet occurred via two routes depending on the temperature; in the 500–630 K range, C-H bond cleavage occurred via reaction with the OH groups, while in the 900–1000 K temperature range, C-H bond cleavage occurred through reaction with the lattice oxygen atoms. At the higher temperature ranges, the rate-determining step (RDS) was proposed to be the dissociative adsorption of methane on the PdO surface, while in the lower temperature ranges, water adsorption on the undercoordinated Pd sites was the RDS [
97]. Utilizing microcalorimetry studies, Xin et al. [
100] proposed similar conclusions, with methane combustion following a pseudo-first-order reaction in terms of methane kinetics for dry methane combustion on PdO. Contrary to these findings, Specchia et al. [
101] proposed that dry methane combustion on PdO/Ce
xZr
1−xO
2 occurred via a Mars–van Krevelen mechanism. In a study by Müller et al. [
102], dry methane combustion over
18O-labelled ZrO
2-supported PdO was proposed to occur partially via a redox Mars–van Krevelen mechanism. However, the overall conversion was found to be influenced by the coaction between the surface reaction of the adsorbed reactants and the redox mechanism [
102]. Following this hypothesis, a study by Fujimoto et al. [
52] on the kinetics of dry methane combustion at low temperatures on the Pd/ZrO
2 catalyst characterized by the Mars–van Krevelen mechanism detected the involvement of methane activation in site pairs comprising Pd, which functions as an oxygen vacancy and PdO
x that serves as a source for the oxygen atoms. Methane was found to be dissociatively adsorbed on metallic Pd, thereby producing H and CH
x species, while oxidation occurred on the adjacent PdO [
53,
103].
Wet methane combustion on Pd-based catalysts has been reported to be independent of O
2 and CO
2 concentrations and was determined to have a reaction order of 1 and −1 for methane and water, respectively [
52,
104]. The RDS for wet methane combustion on monometallic Pd at lower temperatures (below 723 K) was determined to be water desorption from the catalyst surface [
105], while at higher temperatures, the RDS was determined to be methane activation on the Pd sites [
52]. Regarding the mechanism for methane combustion, using a PdO
x/ZrO
2 catalyst, Fujimoto et al. [
52] reported that the reaction occurred in three steps. Initially, methane molecules were physically adsorbed onto vacant metallic Pd sites, followed by sequential hydrogen-atom abstraction from the adsorbed methane backbone, which resulted in the formation of surface hydroxyl. The proposed rate-determining step for this reaction was determined to be the H-atom abstraction from the methane molecule when the OH* species were the most abundant surface intermediate species [
52]. Later, it was proposed by Ribeiro et al. [
106] and Burch et al. [
107] that the decomposition of the surface hydroxyl (Pd-OH) was the determining factor for methane activation.
Based on the above mechanistic observations, the rate expression for the catalytic combustion of methane for the dry case is often reported in the form of a power-law-type equation as follows:
As noted above, water has a strong effect on the reaction rate. The inhibition effect of water may be caused by the transition of palladium oxide to palladium hydroxide [
106,
108,
109] according to the following reaction:
Although Pd(OH)
2 has been reported to decompose to PdO at 520 K [
110], it has been suggested that the ability of the support to retain water can stabilize Pd(OH)
2 at higher temperatures [
37,
109].
The classical power law model incorporating the strong inhibition effect of water for Pd-based catalysts was proposed as follows [
106,
111,
112]:
The order of the reaction with respect to methane is often reported to be one or slightly less, whilst the order of reaction with respect to oxygen is zero or close to zero [
111,
112], although other orders have been reported [
113,
114]. Variation in the values of
n and
m for such a power law model can be explained using the theory of kinetics over non-uniform surfaces, where
. DFT studies by Qi et al. [
115] revealed that for dry methane combustion on a Pd catalyst, the RDS was the dissociation of methane on matched pair sites. The authors proposed a rate equation in the form of Equation (1), with
m = 1,
n = 0 and
l = −1.
There is a lack of agreement on the effect of carbon dioxide on methane oxidation over palladium. Some investigators [
37,
108,
109] reported negligible inhibition, whilst others [
106] reported significant inhibition with about 0.5 volume % carbon dioxide.
Utilizing the Mars–van Krevelen mechanism, Golodets et al. [
116] proposed a rate equation for methane oxidation incorporating the water inhibition term as follows:
Mezaki and Watson [
117] proposed a simplified rate equation for the scenario if adsorbed water was the most abundant surface intermediate, which is as follows:
Modelling studies by Hayes et al. [
104] for methane combustion over Pd catalysts in the presence and absence of water revealed that the water inhibition term had to be included in the rate equation, as illustrated in Equation (5). In another study by Kikuchi et al. [
118], the same rate expression as in Equation (5) was utilized to model wet methane combustion on Pd/Al
2O
3 and Pd/SnO
2 catalysts and found a good agreement between the modelling and experimental results.
Several catalytic methane combustion studies on Pd/Al
2O
3 catalysts revealed a first order to CH
4 and a negative first order to H
2O [
106,
119,
120]. Utilizing a steady-state mole balance equation, depicted in Equation (6), Alyani et al. [
120] revealed that the activation energy associated with splitting the first C-H bond for Pd/Al
2O
3 was in the range of 60 to 72 kJ/mol, while the heat of water adsorption was determined to be around −81 kJ/mol.
Kinetic studies for lean-burn wet methane combustion on SnO
2-supported Pd-based catalysts by Kikuchi et al. [
118] revealed that the catalyst followed a rate equation similar to that illustrated in Equation (5). In this case, the activation energy was determined to be around 111 kJ/mol, and the heat of water adsorption value was −31 kJ/mol [
117]. Contrary to this, the wet methane combustion studies by Kumar et al. [
121] on a Pd/SnO
2 catalyst revealed a partial order of −0.11 to water. Considering the partial order to water, the authors proposed an additive reaction rate equation, as depicted below [
121].
The first site was considered to be unaffected by water, while the second site was considered to be affected by water with a reaction order of −1 to it. Keeping the observed activation energy on the site affected by water similar to that observed in the case of the conventional Pd/Al
2O
3 catalyst, the activation energy on the site unaffected by water was determined to be 108 kJ/mol [
120]. Similar studies by Keller et al. [
122] revealed an activation energy (E
a0) of 144 kJ/mol and 117 kJ/mol for the Pd/Al
2O
3 catalyst and Pd/SnO
2 catalyst, respectively. However, the authors utilized a rate equation that incorporated an inhibition factor, as depicted below.
While Equation (8) is similar to Equation (5), Keller et al. determined that the order to water, that is, the value of
for the Pd/Al
2O
3 catalyst and Pd/SnO
2 catalyst, was 1.99 and 0.87, respectively [
122]. Studies by Nasr et al. [
123] for lean wet methane combustion on a Pd/Co
3O
4 catalyst also revealed a partial order of −0.37 to water. The authors proposed a rate model with additive contributions from the Pd and Co counterparts as follows:
Their kinetic study revealed that the higher water tolerance on the Co
3O
4-supported catalyst was due to synergism resulting from strong metal–support interactions between Pd and Co [
123].
Several studies have been conducted to define rate equations for methane combustion on Pt-only catalysts as well [
124,
125,
126]. While Pt is known to be less active than Pd/PdO for methane combustion under dry feed conditions, Pt is found to be active to catalyze methane combustion under wet feed conditions in its metallic state [
23,
127]. Considering the first H-atom abstraction from the C-H backbone by chemisorbed oxygen on Pt as the rate-determining step [
115], the generally accepted rate equation for methane combustion on Pt-based catalysts is illustrated as follows [
126,
128]:
Utilizing a rate equation like Equation (3), DFT studies by Qi et al. [
115] for methane combustion on Pd-Pt catalysts with varying Pd:Pt ratios revealed reaction orders of −1.05, −0.98, and −0.79 to water for Pd
0.75Pt
0.25, Pd
0.5Pt
0.5, and Pd
0.25Pt
0.75 catalysts, respectively. Similar studies by Abbasi et al. [
124] for bimetallic Pd:Pt catalysts in the presence and absence of water revealed a rate equation as follows:
K2 essentially had a value of zero, and the value of
n was determined to be 1 [
124]. In another study by Habibi et al. [
129] for SiO
2-encapsulated Pd-Pt catalysts, the authors revealed that the catalyst followed different rate equations in the presence and absence of water. While under dry feed conditions, the catalyst followed a rate equation as that of Equation (5), under wet feed conditions, the catalyst followed a rate model given by [
29].
Equation (12) was proposed by accounting for the water-inhibiting oxygen exchange effect on the support surface [
129]. Lean-burn wet methane combustion kinetic studies on Al
2O
3 and SnO
2-supported Pd:Pt catalysts by Kumar et al. [
121] revealed that both catalysts exhibited a −1 order to water. While the Pd/SnO
2 catalyst exhibited a partial order to water, different mechanisms of Pt and SnO
2 actions resulted in bimetallic catalysts exhibiting a −1 order to water. Interestingly, while the Pd-Pt/Al
2O
3 catalyst and Pd-Pt/SnO
2 catalyst revealed similar activation energies in the range of 134 kJ/mol, the presence of different active sites on either catalyst resulted in the Pd-Pt/SnO
2 catalyst exhibiting a higher turnover frequency, almost two-fold in magnitude as that observed on the Pd:Pt/Al
2O
3 catalyst [
121]. Utilizing a rate equation like that of Equation (3), Yang et al. [
130] revealed that while the Pd/Al
2O
3 catalyst exhibited a −1 order to water, the Al
2O
3-supported Pd-Pt catalyst exhibited an order of −0.23 to water. The authors also revealed that the addition of Pt resulted in a reduction in the activation energy from 127 kJ/mol in the Pd catalyst to 58 kJ/mol in the Pd-Pt catalyst in the presence of water [
130].
It has also been observed that there can be a significant change in the activation energy of the reaction at higher temperatures. An early work [
22] on methane oxidation over Pd catalysts in the temperature range of 500 to 800 K reported a sharp change in the activation energy of the reaction. The temperature at which the change occurred varied from 654 to 720 K and was a function of support material and reactant concentration. The low-temperature range activation energy varied from 75 to 95 kJ/mol and decreased to a value between 23 and 45 kJ/mol at high temperatures. In another work [
131], an activation energy of 131 kJ/mol was reported below 770 K and 19 kJ/mol at higher temperatures. The authors attributed the decline to heat and mass transfer effects, although these effects are likely to be the major cause [
132].
Sakai et al. [
133] studied a Pd catalyst for methane combustion and observed a transition at about 690 K, with the activation energy dropping from 76.5 kJ/mol to 61 kJ/mol. Liu et al. [
134] also observed a change in apparent activation energy. In their model, all heat and mass transfer steps were accounted for explicitly. These observations for the transition of methane catalytic oxidation activity are summarized in
Table 1. Finally, in [
10], the transition from 104 kJ/mol to 47.5 kJ/mol was observed to happen at about 811 K. In this work, all heat and mass transfer effects were explicitly accounted for. Clearly, there is some complexity and variation with this reaction.
5. Kinetic Modelling
Kinetic modelling was performed on all of the reported catalysts. The modelling assumed an isothermal reactor, plug flow, and the absence of heat and mass transfer limitations. The maximum temperature rise across the catalyst bed was five degrees. The calculations validating the plug flow and heat and mass transfer assumptions are shown in detail in the
Supplementary Information.
The main conclusion from the ignition curves is that the reaction is not overall first-order in the absence of added water for most of the catalysts. For Catalyst 1, with the addition of two percent by volume water, the reaction is apparently first-order overall. Clearly, the rate equation must involve a water inhibition term but one that dominates the denominator at high water concentrations. For the other catalysts, we have previously reported their dependence on water [
121,
136]. In this section, we first present a detailed modelling study for Catalyst 1 with pseudo-first-order kinetics followed by a model that explicitly includes water inhibition. We then show the results of the same analysis for Catalysts 2 to 6.
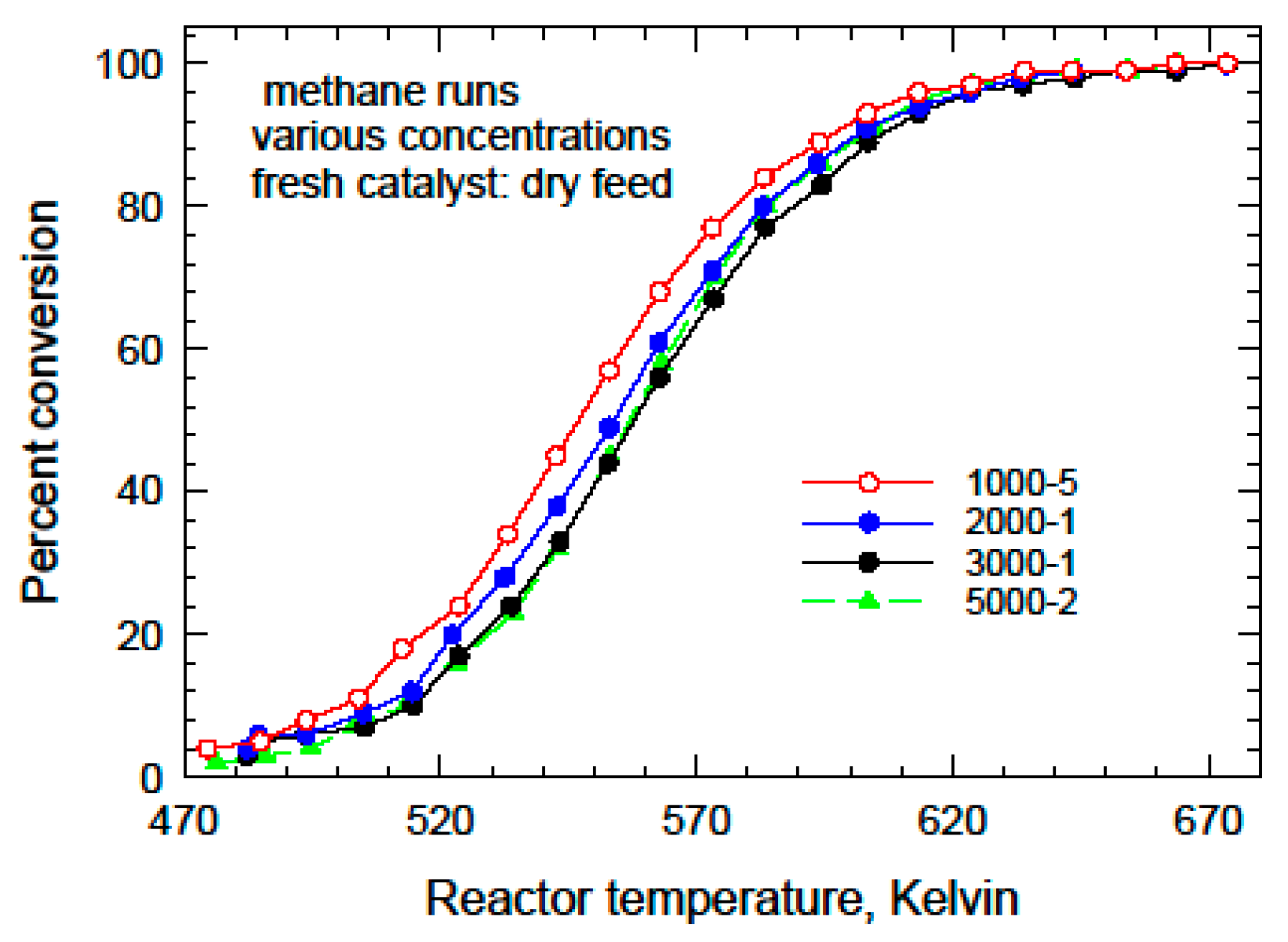
As a preliminary step, the dry results were analyzed using a pseudo-first-order reaction, with the data at each feed concentration level being modelled separately. We assume that the reactor operated under isothermal plug flow conditions, and the rate constant is governed by an Arrhenius-type expression as follows:
where
A is a constant, and
E is the activation energy in kJ/mol. We include the constant
A inside the exponential to improve the performance of the optimizer, which is a standard modelling trick. The Arrhenius plots for the dry runs for each methane concentration are given in
Figure 6. There is an increasing trend in the apparent activation energy, which is indicative of the potential influence of water. The parameter values are given in
Table 4.
Now consider the effect of water explicitly, using an equation in the form of Equation (5).
For dry feed, the water concentration is directly related to the methane concentration and the fraction conversion, giving a rate model of the following form:
where
F denotes the molar flow rate, and
Q indicates the volumetric flow rate. If we assume plug flow and isothermal operation, then we can generate an analytical solution for fraction conversion. In terms of catalyst mass, the PFR mole balance is as follows:
The integration gives the following expression for the fraction conversion:
An optimizer was built using MATLAB R2023b to determine the parameter values. Two optimization strategies were used, a genetic algorithm and one based on the gradient method. Both tools essentially gave the same results. Only the conversion data between 15% and 85% were used in the optimization. There were seven experiments performed under dry conditions on the fresh catalyst, and all seven were used to determine the optimal rate parameters. These parameters were found to be as follows:
A comparison of the experimental ignition curves and the model predictions for each inlet concentration of methane is shown in
Figure 7. The agreement is satisfactory, especially in the conversion region above 20%.
The same optimization procedure was also performed for the dry runs obtained after HTA-1 (before the six-week break), HTA-2, and HTA-3. As before, only the data between 15% and 85% conversion were used. Five optimizations were performed in each case. In the first one, all four parameters in the kinetic model were allowed to vary. Then, the parameters were fixed successively to those obtained in the case of the fresh catalyst; that is, first
H was fixed; then
H and
K0; then
H,
K0, and
E; and finally
H and
E. The parameters are summarized in
Table 5. The table gives the values when all parameters were free, as well as the values obtained when the values of
E and
H were fixed to be equal to the same value computed for the fresh catalyst, whilst the complete set is given in the
Supplementary Information.
Graphs of the agreements are presented in the Supplementary Information. The agreement between model and prediction is good in all cases. Although there are small differences obtained with each of the parameter sets, these differences are within the experimental uncertainty.
We can draw some overall conclusions regarding these dry runs. The first is that there is a range of parameter values that will give an acceptable fit, so the values shown should not necessarily be considered absolute. In particular, the model is fairly insensitive to the value of
H, provided that the other parameters are allowed to adjust. Finally, it is interesting to observe that there is a set of common values for
E,
K0, and
H that gives an acceptable fit for the four levels of catalyst activity, with only the value of
A being adjusted to reflect the deactivation. The comparisons are shown in
Figures S22–S24.
We now consider the results when water was added to the feed. As shown earlier, the ignition curves obtained with 2% added water were essentially independent of the inlet methane concentration. Therefore, a first-order rate model is a close approximation for the result, because the water inhibition term in the denominator dominates the result. The first set of results to which a kinetic analysis was performed included the wet runs obtained after HTA-2. In the first instance, the kinetic parameters obtained using the Arrhenius analysis shown earlier were used to generate the ignition curves. The result is shown in
Figure 8, denoted Model (a). As a second step, the first-order rate parameters were optimized using all of the data, including those at low and high concentrations. However, note that for this optimization, absolute errors were used to reduce the influence of the low and high conversion data. The resulting parameters were very similar, and the predicted ignition curve is also shown in
Figure 8 as Model (b). The parameters for the two models are given in
Table 6. As seen from both the graphs and the table of data, the results are very close in both cases.
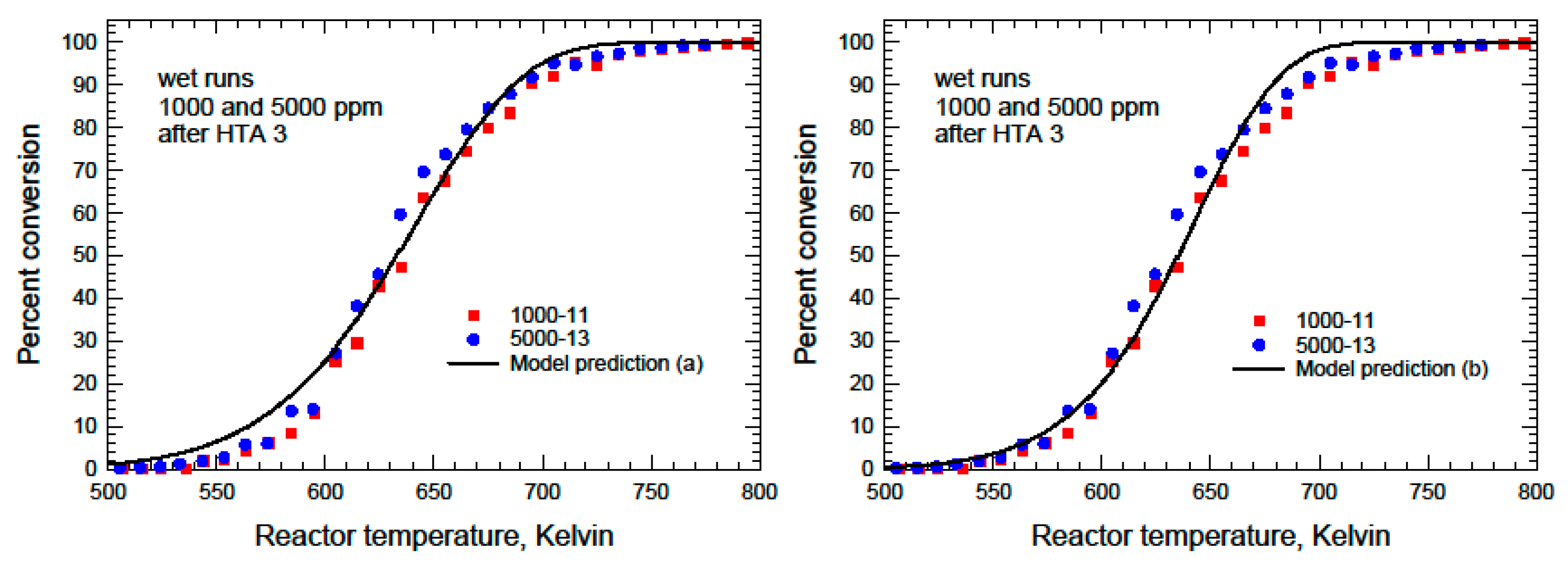
A similar analysis was performed on the wet ignition curves obtained after HTA-3, and the results are shown in
Figure 9. In the first instance, the values obtained from the Arrhenius analysis performed earlier were used, denoted Model (a). Because the apparent activation energy obtained from the Arrhenius plot appeared to be much lower than expected, the data were optimized again by fixing the apparent activation energy to 104.59 kJ/mol, the value obtained for the wet runs after HTA-2. The optimization was carried out using the absolute error to calculate the objective function and both the data between 15% and 85% conversion and the entire dataset. The result was the same in both cases. This result is also shown in
Figure 8 as Model (b). The parameter values are given in
Table 7.
We then repeated the analysis for Catalysts 2 to 6. The ignition data shown in
Figure 5 were first analyzed in the same manner as for Catalyst 1, using in the first instance the pseudo-first-order approximation. The resulting Arrhenius style plots are shown in
Figure 10; see also
Figures S27–S31. The first-order rate parameters were determined by regression analysis for each methane concentration separately and for all of the data at all concentrations. The parameters thus obtained are shown in
Table 8. The complex model with water inhibition, represented by Equation (14), was then optimized using the MATLAB program. The resulting parameters are given in
Table 9.
Note that these parameters should not necessarily be considered unique. As seen for Catalyst 1 with the same complex rate model for the dry runs at different levels of catalyst activity, there are multiple sets of parameters that can give ignition curves that are virtually indistinguishable by the naked eye and have very small differences in the values of the objective function. For Catalysts 2 to 6, we let the MATLAB optimizer find the best values of the objective function and did not perform any sensitivity analysis on the parameter values. We are simply trying to show the differences between the fits that are possible with this complex model compared to the pseudo-first-order model.
Figure 11 shows plots of the agreement of the model with the experimental results. For each catalyst,
Figure 10 shows the agreement at a single concentration for both the first-order model and the complex model. In addition, the ignition curves at all of the concentration values were plotted, with either the first-order model (Catalysts 2, 4, and 5) or the complex model (Catalysts 3 and 6) fit. All of the curves are shown in the
Supplementary Information, Figures S31–S35.
As mentioned earlier, detailed kinetic analysis for Catalysts 2 to 6 under high water feed conditions was reported in references [
121,
135]. We must emphasize that under high water conditions, all of the catalysts tested here showed strong water inhibition effects. To assist the discussion, we present a brief summary of the results below. For all of these catalysts, under conditions of either 5 or 10% water, there was negligible dependence on the methane concentration. This observation indicates that with large amounts of water in the feed, the relatively small amount of water produced by methane combustion does not exert a noticeable effect within the limits of the experimental error. In other words, at each water concentration level, the reaction appears to be first-order in methane. The apparent reaction order with respect to water can be determined by the analysis of the results at different water concentrations. These results are given in
Table 10, where Model C1 refers to one with the form of Equation (5), while Model C2 has the form of Equation (9). We also show one result at 5 and 10% water for Catalyst 4, which shows apparent first-order behaviour at methane concentrations up to and including 5000 ppm by volume.
Figure 12 shows the ignition curves.
6. Conclusions and Recommendations
It is evident from the literature presented here that there have been a wide variety of kinetic models presented for the catalytic combustion of methane over PGMs. Obviously, this literature covers a wide variety of catalyst formulations, including support types, promoters, and preparation techniques. The issue is complicated by the fact that complete catalyst details are sometimes lacking, and characterization data may not be available. Nevertheless, some observations can be made.
Water has an inhibiting effect on the catalyst activity; however, for some catalysts under dry feed conditions with low methane concentrations, a first-order model is sufficient. For the catalysts that we synthesized, the common denominator for the lack of water dependence at low methane concentration was the presence of cobalt in the support, that is, Catalysts 2, 4, and 5. That observation is consistent with the results presented in
Table 10, where it is seen that the experiments conducted at high water concentrations showed a relatively low dependence on water, compared to Catalysts 3 and 6. At high water concentrations, a first-order model is often sufficient, although only at a specified feed water concentration. In other cases, even at low methane concentrations, the water effect is easily observable. What is certainly evident is that it is necessary to perform detailed kinetic measurements on any given catalyst to be certain that the result is valid, especially if one wishes to design a reactor based on the results.
One surprising result from the ageing studies on Catalyst 1 was the essentially complete recovery of the dry feed activity after the first hydrothermal ageing experiment when the catalyst was left for six weeks at ambient conditions. The recovery of activity after exposure to wet feed has been observed previously but never complete recovery. From a reactor operational standpoint, this observation may not be so significant, because operation would likely be either dry or wet only. However, it does emphasize the importance of the time scale when performing a mixture of wet and dry experiments to determine kinetics under deactivating conditions.
Considering Catalyst 1, we observed that for the dry and wet feed conditions, there was a common set of activation energies that gave an acceptable fit to the results at each deactivation level. However, better fits could be obtained when all parameters were allowed to vary, which is to be expected. One should always treat the actual values of parameters obtained in this manner with some level of caution and not state that one parameter set is necessarily better, simply because it gave a smaller value of the objective function.
As a final note, we emphasize the importance of computationally and experimentally determining the absence of mass and heat transfer limitation effects. While this advice is clearly well known, it can be difficult to achieve with rapid, highly exothermic combustion reactions, so special care should be taken.