Metal–Organic Framework Fe-BTC as Heterogeneous Catalyst for Electro-Fenton Treatment of Tetracycline
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Characterization
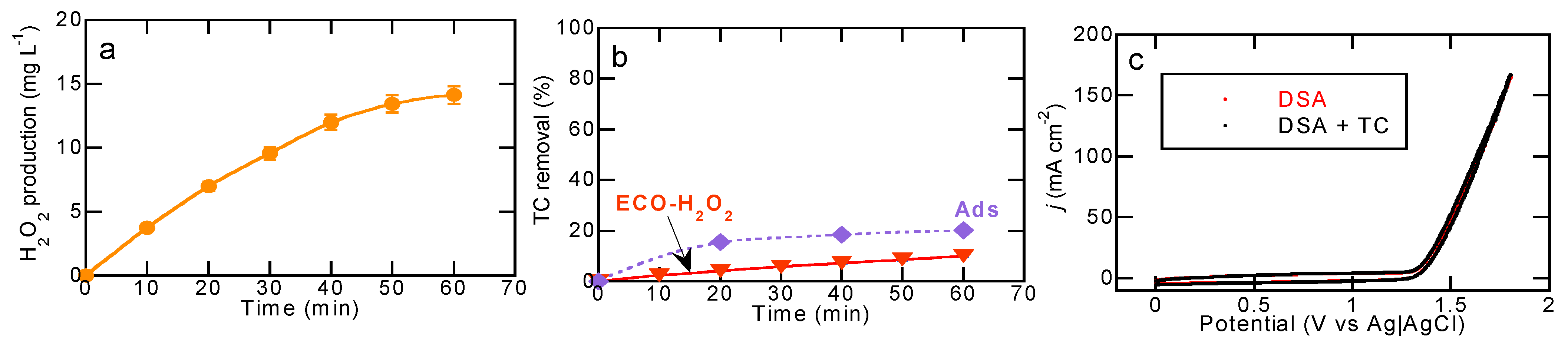
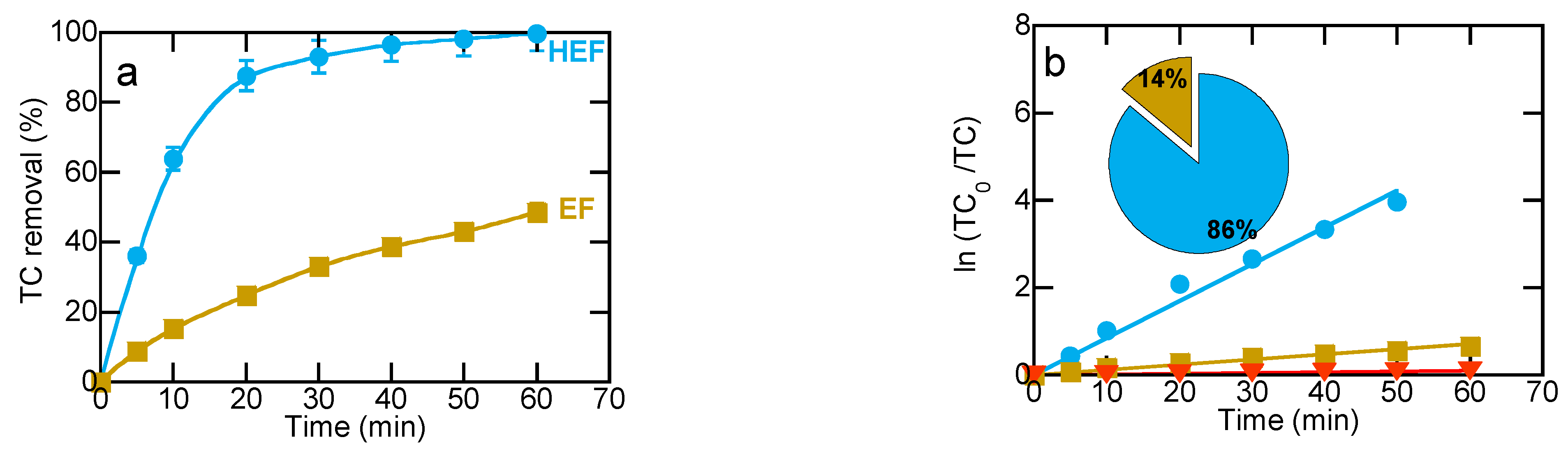
2.2. Degradation of TC by Electrochemical Methods
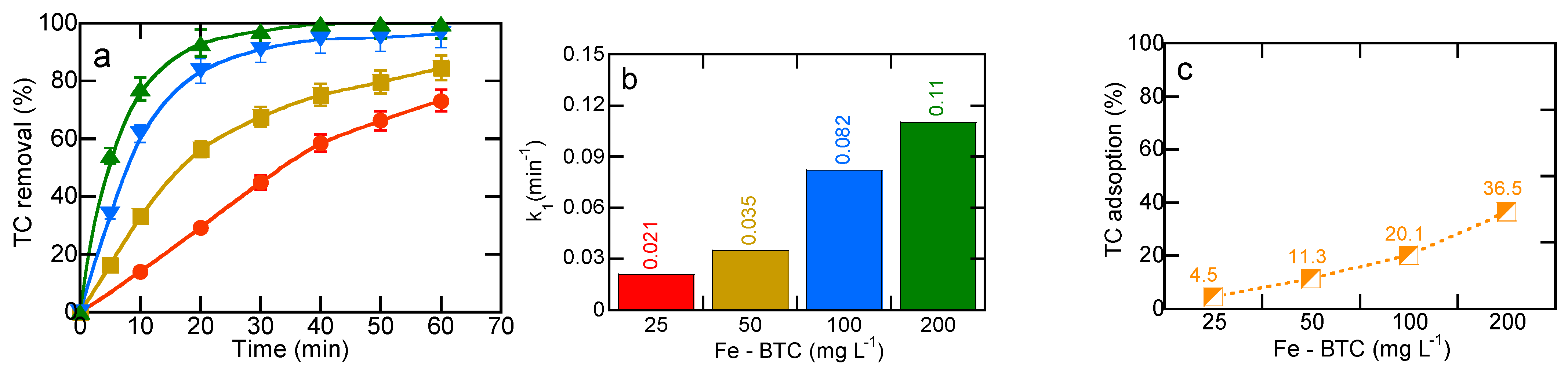
2.3. Effect of MOF Dose on Heterogeneous Electro-Fenton Performance
2.4. Effect of Applied Current as Key Parameter of Electrochemically-Driven Technologies
3. Experimental Section
3.1. Chemicals
3.2. Characterization Techniques
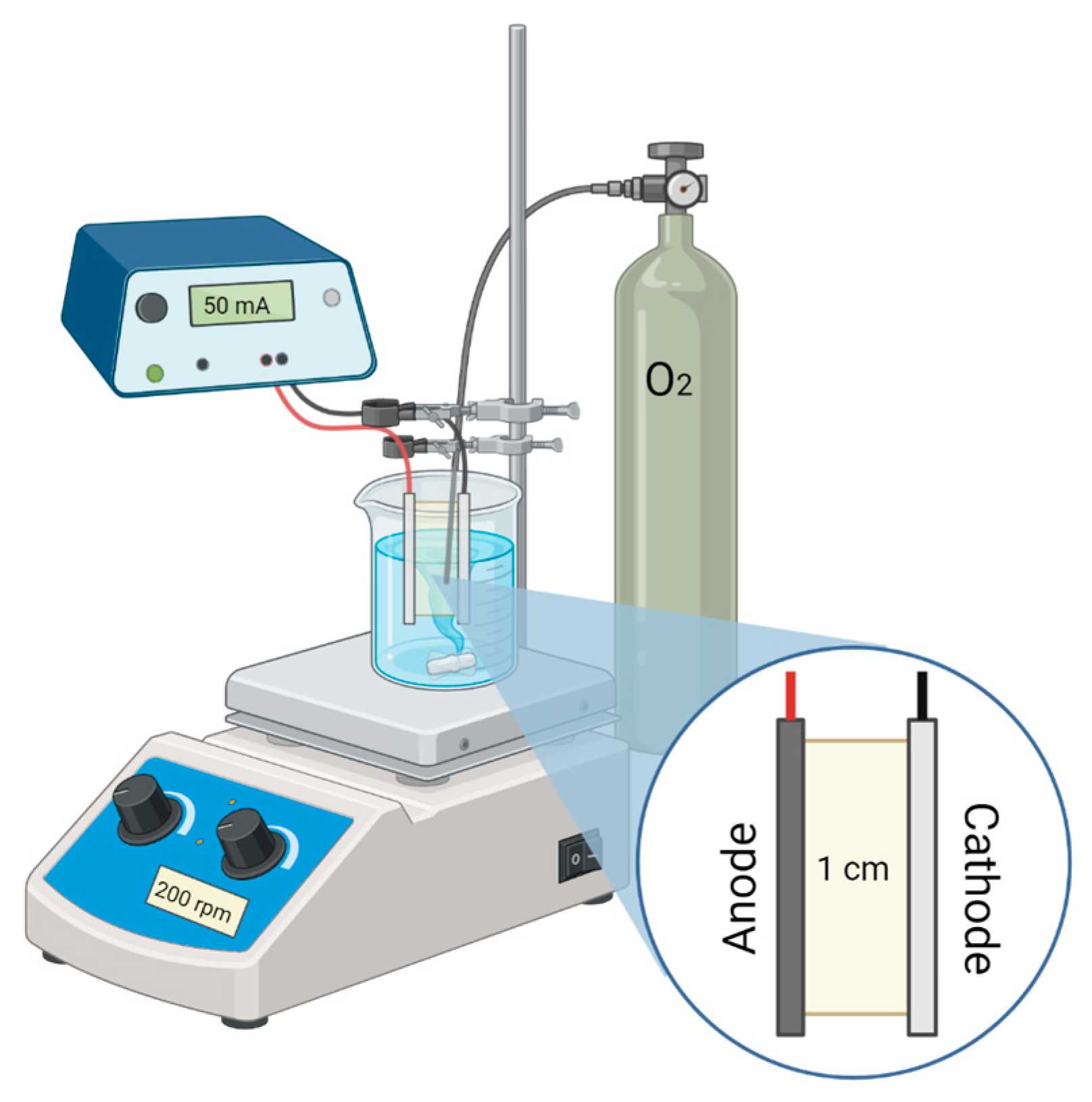
3.3. Electrochemical Setup
3.4. Analytical Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, H.; Hong, J.; Zhao, L. MOF-derived Fe2O3@C-coupled Bi2MoO6 heterojunctions for highly efficient Photo-Fenton degradation of tetracycline. J. Mol. Liq. 2023, 383, 122157. [Google Scholar] [CrossRef]
- Fiaz, A.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Tian, Y.; Liu, C.; Zhang, S.; Cao, L.; Zhou, Y.; Zhang, S. Effective removal of tetracycline antibiotics from water by magnetic functionalized biochar derived from rice waste. Environ. Pollut. 2023, 330, 121681. [Google Scholar] [CrossRef]
- Amor, C.; Marchão, L.; Lucas, M.S.; Peres, J.A. Application of Advanced Oxidation Processes for the Treatment of Recalcitrant Agro-Industrial Wastewater: A Review. Water 2019, 11, 205. [Google Scholar] [CrossRef]
- dos Santos, A.J.; Fortunato, G.V.; Kronka, M.S.; Vernasqui, L.G.; Ferreira, N.G.; Lanza MR, V. Electrochemical oxidation of ciprofloxacin in different aqueous matrices using synthesized boron-doped micro and nano-diamond anodes. Environ. Res. 2022, 204, 112027. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process Saf. Environ. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Lu, S.; Liu, L.; Demissie, H.; An, G.; Wang, D. Design and application of metal-organic frameworks and derivatives as heterogeneous Fenton-like catalysts for organic wastewater treatment: A review. Environ. Int. 2021, 146, 106273. [Google Scholar] [CrossRef]
- dos Santos, A.J.; Kronka, M.S.; Fortunato, G.V.; Lanza MR, V. Recent advances in electrochemical water technologies for the treatment of antibiotics: A short review. Curr. Opin. Electrochem. 2021, 26, 100674. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Olvera-Vargas, H.; Oturan, N.; Oturan, M.A. Heterogeneous electro-Fenton process: Principles and applications. Handb. Environ. Chem. 2018, 61, 85–110. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Tan, Y.; Xu, B.; Cai, J.; Zhang, Y.; Wang, Q.; Wu, Q.; Yang, B.; Huang, J. Recent Advances in Metal-Organic Framework (MOF)-Based Photocatalysts: Design Strategies and Applications in Heavy Metal Control. Molecules 2023, 28, 6681. [Google Scholar] [CrossRef]
- Mumtaz, N.; Javaid, A.; Imran, M.; Latif, S.; Hussain, N.; Nawaz, S.; Bilal, M. Nanoengineered metal-organic framework for adsorptive and photocatalytic mitigation of pharmaceuticals and pesticide from wastewater. Environ. Pollut. 2022, 308, 119690. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Hou, C.; Chen, W.; Li, S.; Ren, R.K.; Li, Z. Efficient degradation of perfluorooctanoic acid by solar photo-electro-Fenton like system fabricated by MOFs/carbon nanofibers composite membrane. Chem. Eng. J. 2021, 414, 128940. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Qiu, S.; Wan, J.; Ma, Y.; Yan, Z.; Xie, Q. In-situ fabrication from MOFs derived MnxCo3-x@C modified graphite felt cathode for efficient electro-Fenton degradation of ciprofloxacin. Appl. Surf. Sci. 2022, 586, 152804. [Google Scholar] [CrossRef]
- Ye, Z.; Padilla, J.A.; Xuriguera, E.; Brillas, E.; Sirés, I. Magnetic MIL(Fe)-type MOF-derived N-doped nano-ZVI@C rods as heterogeneous catalyst for the electro-Fenton degradation of gemfibrozil in a complex aqueous matrix. Appl. Catal. B Environ. 2020, 266, 118604. [Google Scholar] [CrossRef]
- Zheng, Y.; Du, X.; Song, G.; Gu, J.; Guo, J.; Zhou, M. Degradation of carbamazepine over MOFs derived FeMn@C bimetallic heterogeneous electro-Fenton catalyst. Chemosphere 2023, 312, 137353. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J. Degradation of Cephalosporin C using MOF-derived Fe-Co bimetal in carbon cages as electro-Fenton catalyst at natural pH. Sep. Purif. Technol. 2023, 323, 124388. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Zhang, Q.; Zhou, M. S-doped MIL-53 as efficient heterogeneous electro-Fenton catalyst for degradation of sulfamethazine at circumneutral pH. J. Hazard. Mater. 2022, 424, 127674. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.; Lopez-Ruiz, J.A.; Barpaga, D.; Garcia-Segura, S. The Surge of Metal–Organic-Framework (MOFs)-Based Electrodes as Key Elements in Electrochemically Driven Processes for the Environment. Molecules 2021, 26, 5713. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.L.; Zhou, W.; Qian, C.; Zhao, Y. Industrializing metal–organic frameworks: Scalable synthetic means and their transformation into functional materials. Mater. Today 2021, 47, 170–186. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, A.A.; Rojas-García, E.; López-Medina, R.; García-Martínez, D.C.; Nicolás- Antúnez, J.; Maubert-Franco, A.M. Magnetite nanoparticles into Fe-BTC MOF as adsorbent material for the remediation of metal (Cu(II), Pb(II, As(III) and Hg(II)) ions-contaminated water. Catal. Today 2022, 394–396, 94–102. [Google Scholar] [CrossRef]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Maubert Franco, A.M. Adsorption of azo-dye Orange II from aqueous solutions using a metal-organic framework material: Iron- benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing KS, W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Dalakoti, S.; Singh, N.; Wamba, H.N.; Kaishyop, J.; Divekar, S.; Arya, A.; Dasgupta, S. Rapid Aqueous Medium Organization of Trimesate Metal–Organic Frameworks of Cu, Fe: Exploring Suitability in Gas Separation. ACS Appl. Energy Mater. 2023, 1, 3309–3322. [Google Scholar] [CrossRef]
- Hu, X.; Lou, X.; Li, C.; Ning, Y.; Liao, Y.; Chen, Q.; Mananga, E.S.; Shen, M.; Hu, B. Facile synthesis of the Basolite F300-like nanoscale Fe-BTC framework and its lithium storage properties. RSC Adv. 2016, 6, 114483–114490. [Google Scholar] [CrossRef]
- Ploegmakers, J.; Japip, S.; Nijmeijer, K. Mixed matrix membranes containing MOFs for ethylene/ethane separation Part A: Membrane preparation and characterization. J. Membr. Sci. 2013, 428, 445–453. [Google Scholar] [CrossRef]
- Sapnik, A.F.; Bechis, I.; Collins, S.M.; Johnstone, D.N.; Divitini, G.; Smith, A.J.; Chater, P.A.; Addicoat, M.A.; Johnson, T.; Keen, D.A.; et al. Mixed hierarchical local structure in a disordered metal–organic framework. Nat. Commun. 2021, 12, 2062. [Google Scholar] [CrossRef]
- Byeon, A.; Yun, W.C.; Kim, J.M.; Lee, J.W. Recent progress in heteroatom-doped carbon electrocatalysts for the two-electron oxygen reduction reaction. Chem. Eng. J. 2023, 456, 141042. [Google Scholar] [CrossRef]
- Fortunato, G.V.; Bezerra, L.S.; Cardoso ES, F.; Kronka, M.S.; Santos, A.J.; Greco, A.S.; Júnior JL, R.; Lanza MR, V.; Maia, G. Using Palladium and Gold Palladium Nanoparticles Decorated with Molybdenum Oxide for Versatile Hydrogen Peroxide Electroproduction on Graphene Nanoribbons. ACS Appl. Mater. Interfaces. 2022, 14, 6777–6793. [Google Scholar] [CrossRef] [PubMed]
- Kronka, M.S.; Fortunato, G.V.; Mira, L.; dos Santos, A.J.; Lanza, M.R.V. Using Au NPs anchored on ZrO2/carbon black toward more efficient H2O2 electrogeneration in flow-by reactor for carbaryl removal in real wastewater. Chem. Eng. J. 2023, 452, 139598. [Google Scholar] [CrossRef]
- Peng, W.; Liu, J.; Liu, X.; Wang, L.; Yin, L.; Tan, H.; Hou, F.; Liang, J. Facilitating two-electron oxygen reduction with pyrrolic nitrogen sites for electrochemical hydrogen peroxide production. Nat. Commun. 2023, 14, 4430. [Google Scholar] [CrossRef] [PubMed]
- Fóti, G. Oxidation of Organics by Intermediates of Water Discharge on IrO2 and Synthetic Diamond Anodes. Electrochem. Solid-State Lett. 1999, 2, 228. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Oturan, N.; Bo, J.; Trellu, C.; Oturan, M.A. Comparative Performance of Ten Electrodes in Electro-Fenton Process for Removal of Organic Pollutants from Water. ChemElectroChem 2021, 8, 3294–3303. [Google Scholar] [CrossRef]
- Vernasqui, L.G.; dos Santos, A.J.; Fortunato, G.V.; Kronka, M.S.; Barazorda-Ccahuana, H.L.; Fajardo, A.S.; Ferreira, N.G.; Lanza MR, V. Highly porous seeding-free boron-doped ultrananocrystalline diamond used as high-performance anode for electrochemical removal of carbaryl from water. Chemosphere 2022, 305, 135497. [Google Scholar] [CrossRef]
- Barazorda-Ccahuana, H.L.; Fajardo, A.S.; dos Santos, A.J.; Lanza MR, V. Decentralized approach toward organic pollutants removal using UV radiation in combination with H2O2-based electrochemical water technologies. Chemosphere 2023, 342, 140079. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Sanromán, M.A.; Rosales, E. Heterogeneous electro-Fenton system using Fe-MOF as catalyst and electrocatalyst for degradation of pharmaceuticals. Chemosphere 2023, 340, 139942. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- dos Santos, A.J.; Sirés, I.; Alves AP, M.; Martínez-Huitle, C.A.; Brillas, E. Vermiculite as heterogeneous catalyst in electrochemical Fenton-based processes: Application to the oxidation of Ponceau SS dye. Chemosphere 2020, 240, 124838. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.; Garcia-Segura, S.; Cerrón-Calle, G.A.; Chen, F.-Y.; Tian, X.; Wang, X.; Huang, X.; Wang, H.; Alvarez, P.J.J.; Lou, J.; et al. Electrified water treatment: Fundamentals and roles of electrode materials. Nat. Rev. Mater. 2023, 8, 472–490. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, M.; Yuan, D.; Li, X.; Wang, Z.; Zhang, X.; Jiao, T.; Ke, J. Fe3O4 nanoparticles three-dimensional electro-peroxydisulfate for improving tetracycline degradation. Chemosphere 2021, 268, 129315. [Google Scholar] [CrossRef] [PubMed]
- Muzenda, C.; Nkwachukwu, O.V.; Arotiba, O.A. Synthetic Ilmenite (FeTiO3) Nanoparticles as a Heterogeneous Electro-Fenton Catalyst for the Degradation of Tetracycline in Wastewater. Ind Eng Chem Res 2022, 61, 11417–11428. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, S.; Feng, H.; Hu, T.; Wu, Y.; Luo, T.; Tang, W.; Wang, D. Efficient degradation of tetracycline using core–shell Fe@Fe2O3-CeO2 composite as novel heterogeneous electro-Fenton catalyst. Chem. Eng. J. 2022, 428, 131403. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Jonoush, Z.A.; Rezaee, A. Three-dimensional electro-Fenton system supplied with a nanocomposite of microbial cellulose/Fe3O4 for effective degradation of tetracycline. Chemosphere 2023, 317, 137890. [Google Scholar] [CrossRef]
- Barhoumi, N.; Olvera-Vargas, H.; Oturan, N.; Huguenot, D.; Gadri, A.; Ammar, S.; Brillas, E.; Oturan, M.A. Kinetics of oxidative degradation/mineralization pathways of the antibiotic tetracycline by the novel heterogeneous electro-Fenton process with solid catalyst chalcopyrite. Appl. Catal. B Environ. 2017, 209, 637–647. [Google Scholar] [CrossRef]
- Badagoppam Haroon, K.H.; Bhunia, S.K. Fenton-like Catalysts Based on Graphitic Carbon Nitride Nanosheets Decorated with Fe3O4 Nanoparticles for Removal of Colorless Tetracycline. ACS Appl. Nano Mater. 2024. [Google Scholar] [CrossRef]
- Magdaleno, A.L.; Brillas, E.; Garcia-Segura, S.; dos Santos, A.J. Comparison of electrochemical advanced oxidation processes for the treatment of complex synthetic dye mixtures. Sep. Purif. Technol. 2024, 345, 127295. [Google Scholar] [CrossRef]
- Atrashkevich, A.; Fajardo, A.S.; Westerhoff, P.; Walker, W.S.; Sánchez-Sánchez, C.A.; Garcia-Segura, S. Overcoming barriers for nitrate electrochemical reduction: By-passing water hardness. Water Res. 2022, 225, 119118. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.C.; Boaventura RA, R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Gopal, G.; Alex, S.A.; Chandrasekaran, N.; Mukherjee, A. A review on tetracycline removal from aqueous systems by advanced treatment techniques. RSC Adv. 2020, 10, 27081–27095. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Interaction between tetracycline and microorganisms during wastewater treatment: A review. Sci. Total Environ. 2021, 757, 143981. [Google Scholar] [CrossRef]


| Catalyst Material | Catalyst Conc. | TC Conc. | Percent Degradation | Time to Achieve Removal | Reference |
|---|---|---|---|---|---|
| Fe-BTC Basolite®F300 | 100 mg L−1 | 10 mg L−1 | 100% | 40 min | This publication |
| Fe3O4 nanoparticles | 200 mg L−1 | 25 mg L−1 | 86.53% | 60 min | [44] |
| Synthetic ilmenite (FeTiO3) nanoparticles | 15 mg L−1 | 10 mg L−1 | 61.4% | 120 min | [45] |
| Core–shell Fe@Fe2O3-CeO2 | 80 mg L−1 | 50 mg L−1 | 90.7% | 60 min | [46] |
|
Microbial cellulose/Fe3O4 nanocomposite | 500 mg L−1 | 50 mg L−1 | 100% | 20 min | [47] |
| Chalcopyrite | 1000 mg L−1 | 89 mg L−1 | 86% | 120 min | [48] |
| Graphitic carbon nitride nanosheets decorated with Fe3O4 | 20 mg L−1 | 10 mg L−1 | 90% | 60 min | [49] |
| Compound | Molecular Weight (g/mol) | Molecular Formula | Solubility (mol/L) | pkow | pka1 | pka2 |
|---|---|---|---|---|---|---|
| Tetracycline | 444.44 | C22H24N2O8 | 0.041 | −1.25 | 3.2 | 7.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, T.M.; dos Santos, A.J.; Garcia-Segura, S. Metal–Organic Framework Fe-BTC as Heterogeneous Catalyst for Electro-Fenton Treatment of Tetracycline. Catalysts 2024, 14, 314. https://doi.org/10.3390/catal14050314
Fisher TM, dos Santos AJ, Garcia-Segura S. Metal–Organic Framework Fe-BTC as Heterogeneous Catalyst for Electro-Fenton Treatment of Tetracycline. Catalysts. 2024; 14(5):314. https://doi.org/10.3390/catal14050314
Chicago/Turabian StyleFisher, Taylor Mackenzie, Alexsandro J. dos Santos, and Sergi Garcia-Segura. 2024. "Metal–Organic Framework Fe-BTC as Heterogeneous Catalyst for Electro-Fenton Treatment of Tetracycline" Catalysts 14, no. 5: 314. https://doi.org/10.3390/catal14050314
APA StyleFisher, T. M., dos Santos, A. J., & Garcia-Segura, S. (2024). Metal–Organic Framework Fe-BTC as Heterogeneous Catalyst for Electro-Fenton Treatment of Tetracycline. Catalysts, 14(5), 314. https://doi.org/10.3390/catal14050314






