Pushing the Operational Barriers for g-C3N4: A Comprehensive Review of Cutting-Edge Immobilization Strategies
Abstract
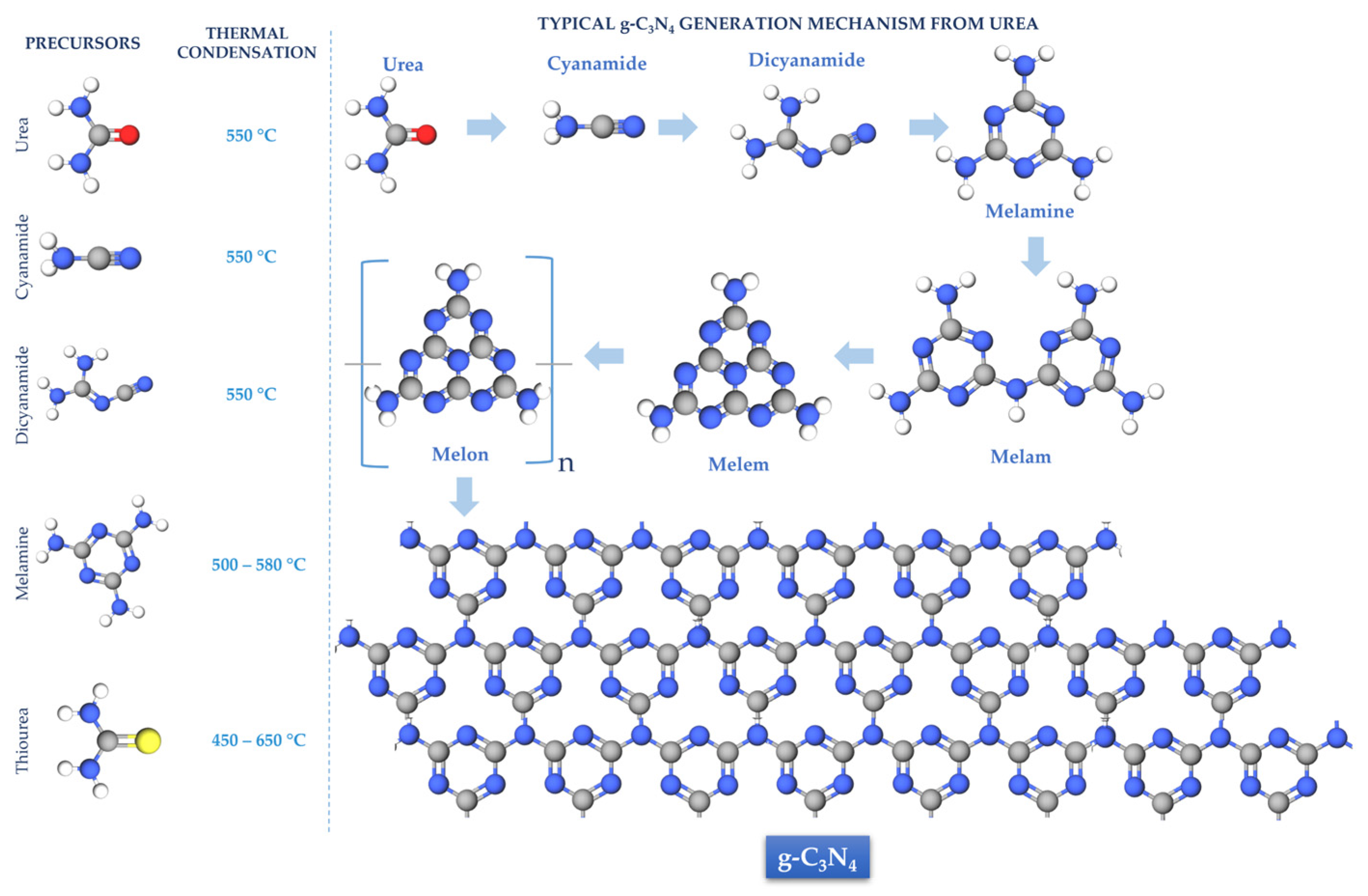
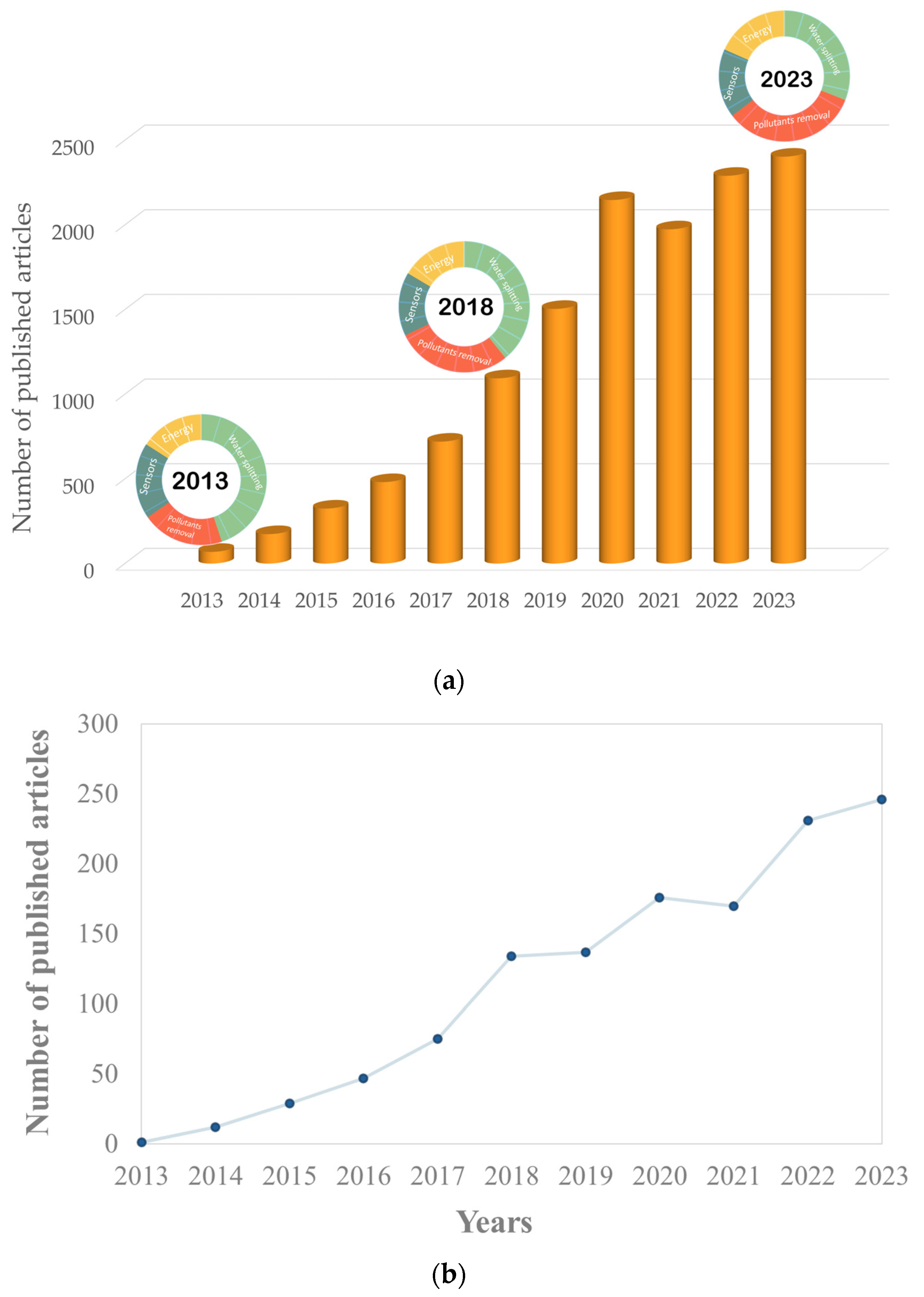
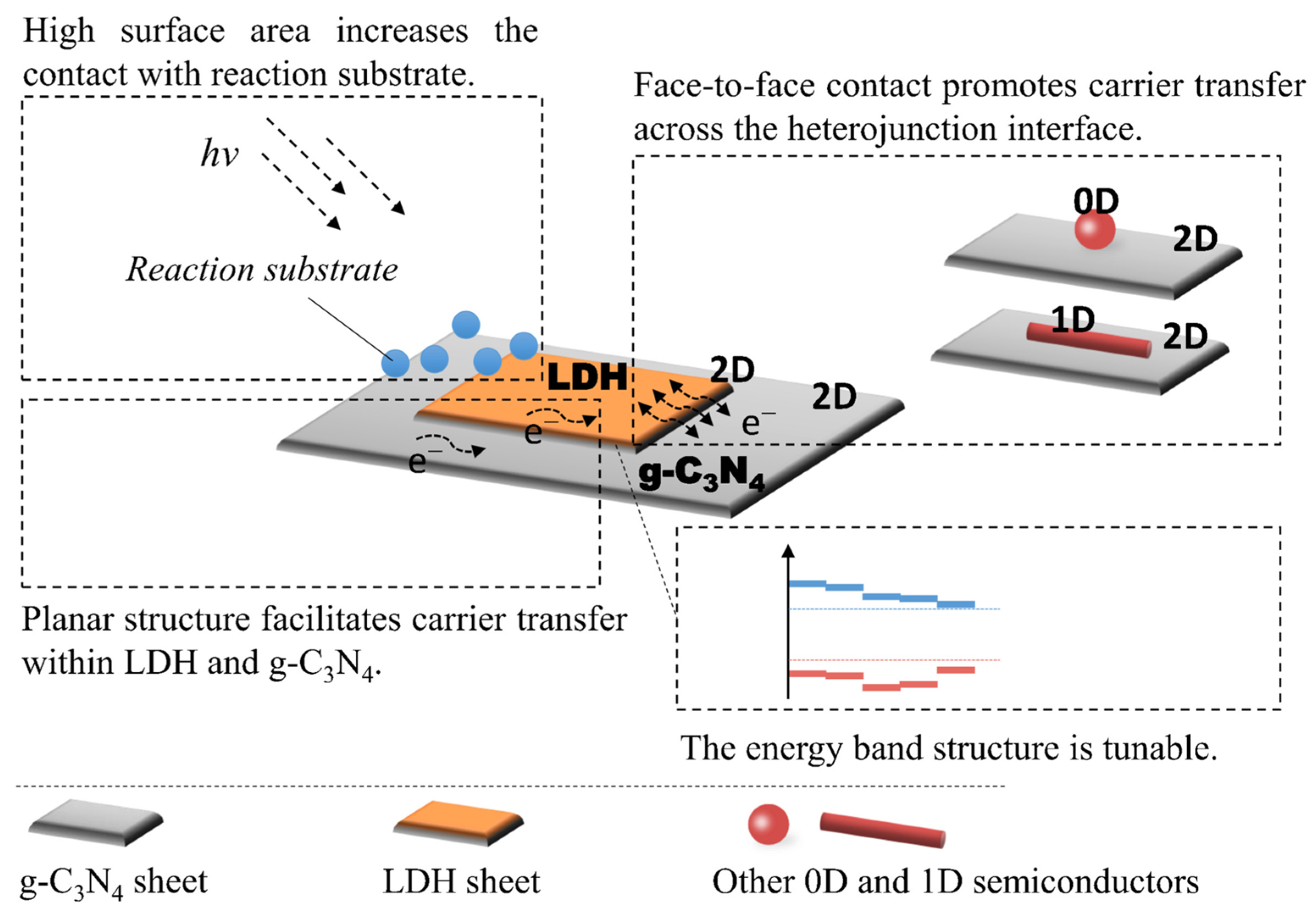
1. Introduction

2. Encapsulation
2.1. Alginate
| Material | Immobilization Technique | Target Pollutant | Removal (%) | Reuses | Ref. |
|---|---|---|---|---|---|
| Alginate | Encapsulation | Diclofenac Rhodamine B Isoproturon | 99 99 90 | 5 cycles, Rhodamine B 92% | [22] |
| Alginate | Encapsulation | Methylene Blue Rhodamine B | 80 50 | 5 cycles, Methylene Blue 70% | [34] |
| 3D kaolinite/alginate | Encapsulation | Brilliant Green | 97 | 10 cycles, 82% | [35] |
| Alginate | Encapsulation | Pb(II), Ni(II) Cu(II) | 383.4 mg g−1 306.3 mg g−1 168.2 mg g−1 | 5 cycles, capacity loss: 15.2%, 16.5%, 15.5% | [36] |
| Alginate | Encapsulation | Rhodamine B | 99 | 5 cycles, 99% | [37] |
| Alginate | Encapsulation-3D printing | Methylene Blue | 95 | 3 cycles, 95% | [38] |
| Chitosan | Encapsulation | Methylene Blue | 99 | 5 cycles, 97% | [39] |
| Cellulose/graphene oxide hybrid aerogels | Encapsulation | Methylene Blue | 99.9 | 5 cycles, 83% | [40] |
| Graphene aerogel | Encapsulation | Methylene Blue | 83 | 4 cycles, 78% | [41] |
| Cellulose | Encapsulation | Methylene Blue | 99.8 | 4 cycles, 95% | [42] |
| Hydroxyethyl cellulose | Cross-linking and gelation | Bisphenol A | 98 | - | [43] |
| Polyacrylonitrile (PAN)/polyaniline | Electrospinning | Methylene Blue Methyl Violet Ciprofloxacin Acetamiprid | 97.0 94.3 88.9 87.6 | - 5 cycles, 94% 5 cycles, 88% 5 cycles, 87% | [44] |
| PAN | Electrospinning | As(III) As(V) | 97 98 | 5 cycles, As(III) 80% 5 cycles, As(V) 85% | [45] |
| Polyvinyl alcohol (PVA)/poly(dopamine) | Electrospinning | Methylene Blue Escherichia coli | 98.7 93.1 | 5 cycles, 90% - | [46] |
| Silica nanofiber | Electrospinning | Tetracycline | 90.0 | 5 cycles, 83.7% | [47] |
| Polycaprolactone | Electrospinning | Aflatoxin B1 | 96.9 | 5 cycles, 96% | [48] |
| Cellulose nanofibers | Casting | Rhodamine B | 98 | - | [49] |
| Nb2O5 embedded polyethersulfone | Casting | Tetracycline | 88 | - | [50] |
| PAN nanofibers | Electrospinning and casting | Congo red Methyl blue | 92 87 | - | [51] |
| Polyurethane foam immobilized with Reduced Graphene Oxide (rGO)/TiO2/ultrathin-g-C3N4 (PRTCN) | Dip-coating and UV-light ageing process | Norfloxacin | 95.4 | 6 cycles, 95.4% | [52] |
| Aluminum-plastic supported | 3D printing and coating | Tetracycline hydrochloride | 93.6 | - | [53] |
| Exfoliated g-C3N4 | Electrophoretic deposition | Acid Orange 7 4-chlorophenol | 60 40 | - | [54] |
| Ni–Fe LDH Modified Sulphur Doping | Layer-by-layer assembly | 2,4-dinitrophenol | 98 | 5 cycles, 90% | [55] |
| Fe2O3/Ni-Fe LDH | Layer-by-layer assembly | Rhodamine B Methylene Blue | 88.5 91.2 | 5 cycles, <30% 5 cycles, <40% | [56] |
| Mg/Al-LDH | Layer-by-layer assembly | Pyrene | 79 | - | [57] |
| ZnCr LDH | Layer-by-layer assembly | Rhodamine B | 99.8 | 3 cycles, 84.5% | [58] |
2.2. Chitosan
| Material | Immobilization Technique | Observation and Application | Ref. |
|---|---|---|---|
| Chitosan and PVA | Encapsulation | Preparation of film for food packaging | [61] |
| Chitosan and PVA | Electrospinning | Nanofiber membranes with light catalytic antibacterial activity | [62] |
| Natural latex foam | Brush coating | PVA/MXene and protonated-g-C3N4. Solar steam generation. Good structural stability over 6 cycles | [65] |
| rGO/indium tin oxide (ITO) | Drop-casting | The MoS2–g-C3N4 immobilized on the surface of the rGO/ITO electrode enables dopamine detection with a linear response ranging from 0.005 to 1271.93 μM and a detection limit of 1.6 nM. | [66] |
| Glassy carbon | Coating | Nitrobenzene contaminant detection ranges from 10 μM to 1 mM, with a detection limit of 1.3 μM using g-C3N4 immobilized on glassy carbon | [67] |
| Amorphous Ni-imidazole framework | Ultrasonication | The amorphous Ni-imidazole framework in g-C3N4 enhances photocatalytic H2 production by around 2272.6 μmol/g/h | [68] |
| CdS quantum dots with Ni decorated | Ultrasonication and chemical deposition | A dual functionalization of g-C3N4 was carried out with Ni atoms and CdS quantum dots to enhance H2 production, achieving an evolution rate of 9.5 mmol/g/h | [69] |
| Boron and graphene quantum dots co-doping in ITO | Impregnation and coating | A highly sensitive photoelectrochemical sensor for dopamine detection, with a broad linear range (0.001–800 μM) and low detection limit (0.96 nM), was achieved by co-doping boron and graphene quantum dots into g-C3N4 in an ITO electrode | [70] |
| Screen-printed electrodes | Drop-casting | Incorporating 2D-carbonylated g-C3N4 into the screen-printed electrode improved glucose determination with a linear range of 0 to 5 mmol L−1 and a detection limit of 0.43 mmol L−1. | [71] |
| NiFe LDH */ sulphur-doping | Layer-by-layer assembly | Designed a Ni-Fe LDH using a deep eutectic solvent-based fabrication method for the detection of dimetridazole, an antiprotozoal drug. The assay exhibits a linear range from 0.0008 to 110.77 μM with a detection limit of 1.6 nM | [72] |
| CoAl-LDH * | Layer-by-layer assembly | This material is added to the cement to generate a photocatalytic cement mortar for NOx degradation, achieving an efficiency of around 42% | [73] |
| Carbon nanotubes and lignin | 3D-printing solid support | Vertical 3D printing creates an eco-friendly electrode with a H2 yield of up to 4.36 µmol/cm2 h, surpassing g-C3N4 films 41.6 times. | [74] |
| Nafion | Drop-casting | This electrocatalysts for water splitting show impressive O2 evolution reaction performance with a low overpotential of 355 mV and a Tafel slope of 46.8 mV dec−1. | [75] |
2.3. Cellulose-Based Materials
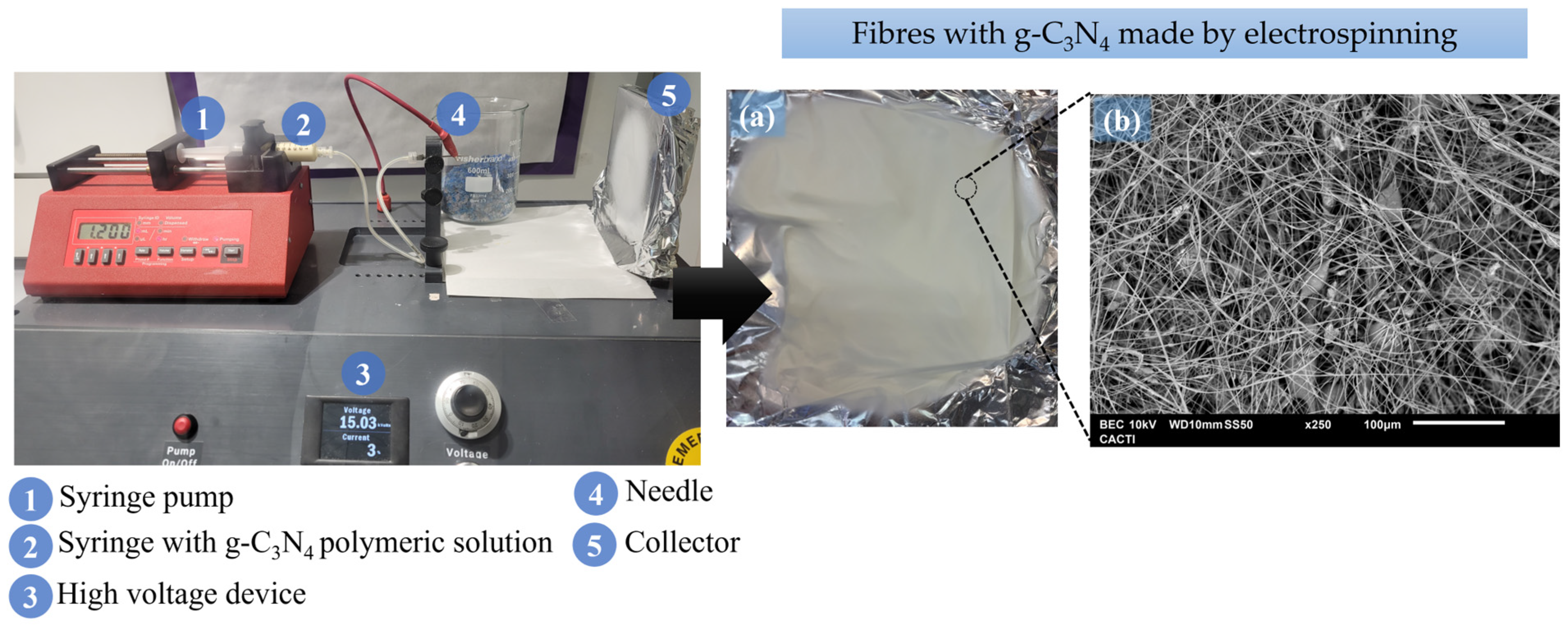
3. Electrospinning
3.1. Membrane Based on PAN Fibers
3.2. Enhanced Applications and Properties of PVA Membranes
3.3. Membrane with Incorporation of Carbon Quantum Dots
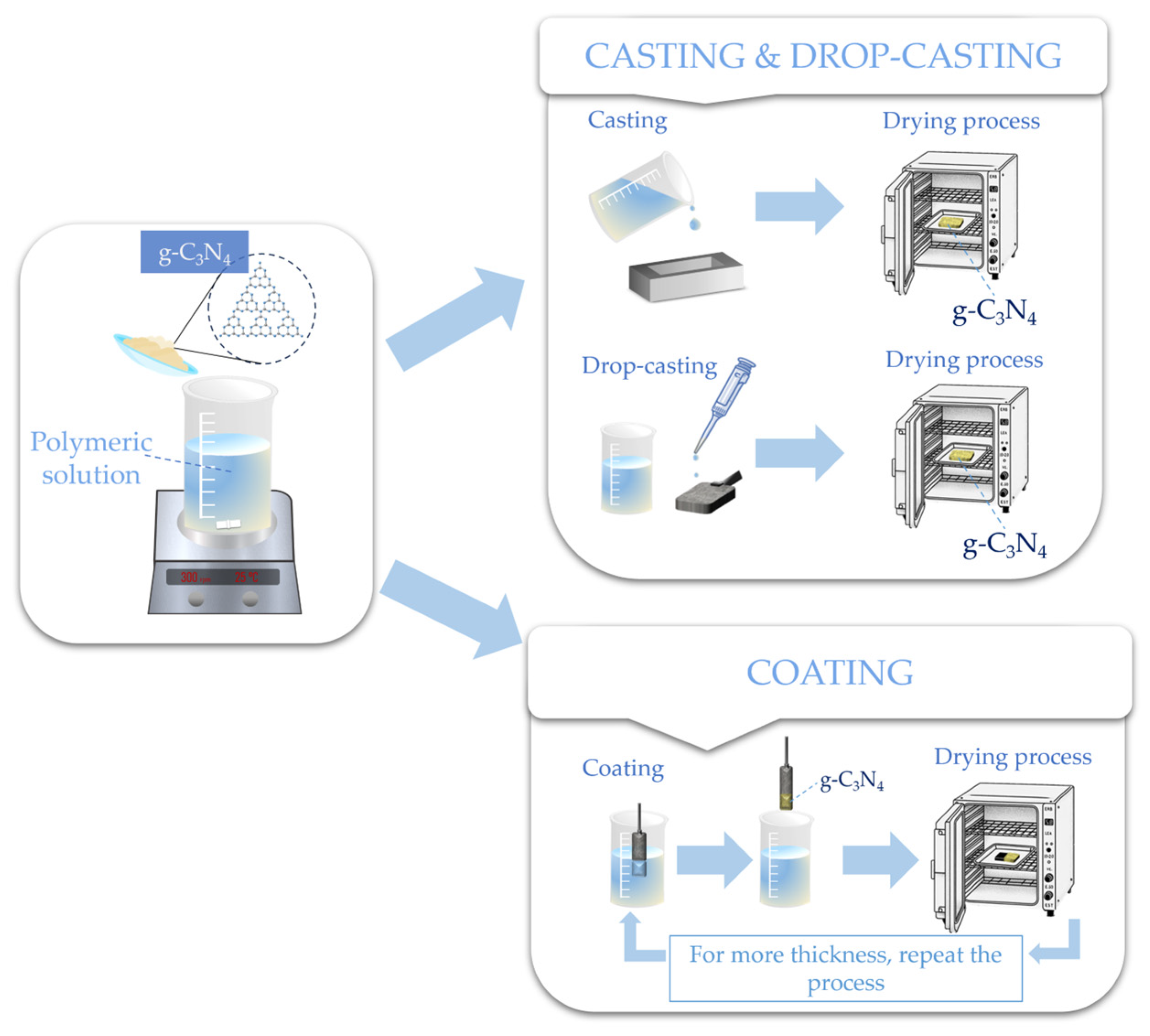
4. Casting
5. Coating
6. Layer-by-Layer Assembly
7. Future Horizons in the Immobilization of g-C3N4
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Cates, E.L. Photocatalytic Water Treatment: So Where Are We Going with This? Environ. Sci. Technol. 2017, 51, 757–758. [Google Scholar] [CrossRef]
- Li, X.; Huang, G.; Chen, X.; Huang, J.; Li, M.; Yin, J.; Liang, Y.; Yao, Y.; Li, Y. A Review on Graphitic Carbon Nitride (g-C3N4) Based Hybrid Membranes for Water and Wastewater Treatment. Sci. Total Environ. 2021, 792, 148462. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wu, Z.P.; Huang, K.W.; Ye, J.; Zhang, H. Surface Modification of 2D Photocatalysts for Solar Energy Conversion. Adv. Mater. 2022, 34, e2200180. [Google Scholar] [CrossRef] [PubMed]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahsheh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of Graphitic Carbon Nitride (g-C3N4) for Photocatalysis: A Critical Review. Arab. J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Hasija, V.; Patial, S.; Singh, P.; Nguyen, V.H.; Van Le, Q.; Thakur, V.K.; Hussain, C.M.; Selvasembian, R.; Huang, C.W.; Thakur, S.; et al. Photocatalytic Inactivation of Viruses Using Graphitic Carbon Nitride-Based Photocatalysts: Virucidal Performance and Mechanism. Catalysts 2021, 11, 1448. [Google Scholar] [CrossRef]
- Borges, R.A.; Pedrosa, M.F.; Manrique, Y.A.; Silva, C.G.; Silva, A.M.T.; Faria, J.L.; Sampaio, M.J. 3D Structured Photocatalysts for Sustainable H2O2 Generation from Saccharides Derivatives. Chem. Eng. J. 2023, 470, 144066. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M. Comprehensive Review on Advanced Reusability of G-C3N4 Based Photocatalysts for the Removal of Organic Pollutants. Chemosphere 2022, 297, 134190. [Google Scholar] [CrossRef]
- Sun, Y.; Kumar, V.; Kim, K.-H. The Assessment of Graphitic Carbon Nitride (g-C3N4) Materials for Hydrogen Evolution Reaction: Effect of Metallic and Non-Metallic Modifications. Sep. Purif. Technol. 2023, 305, 122413. [Google Scholar] [CrossRef]
- Velo-Gala, I.; Torres-Pinto, A.; Silva, C.G.; Ohtani, B.; Silva, A.M.T.; Faria, J.L. Graphitic Carbon Nitride Photocatalysis: The Hydroperoxyl Radical Role Revealed by Kinetic Modelling. Catal. Sci. Technol. 2021, 11, 7712–7726. [Google Scholar] [CrossRef]
- Wang, L.; Tong, Y.; Feng, J.; Hou, J.; Li, J.; Hou, X.; Liang, J. G-C3N4-Based Films: A Rising Star for Photoelectrochemical Water Splitting. Sustain. Mater. Technol. 2019, 19, e00089. [Google Scholar] [CrossRef]
- Mao, X.; Guo, R.; Chen, Q.; Zhu, H.; Li, H.; Yan, Z.; Guo, Z.; Wu, T. Recent Advances in Graphitic Carbon Nitride Based Electro-Catalysts for CO2 Reduction Reactions. Molecules 2023, 28, 3292. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)-Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Chang, X.; Fan, H.; Lei, L.; Wu, X.; Wang, W.; Ma, L. Generation Mechanism of the Defects in G-C3N4 Synthesized in N2 Atmosphere and the Method for Improving Photocatalysis Activity. Catalysts 2023, 13, 269. [Google Scholar] [CrossRef]
- Iqbal, O.; Ali, H.; Li, N.; Al-Sulami, A.I.; F Alshammari, K.; Abd-Rabboh, H.S.M.; Al-Hadeethi, Y.; Din, I.U.; Alharthi, A.I.; Altamimi, R.; et al. A Review on the Synthesis, Properties, and Characterizations of Graphitic Carbon Nitride (g-C3N4) for Energy Conversion and Storage Applications. Mater. Today Phys. 2023, 34, 101080. [Google Scholar] [CrossRef]
- Du, C.; Xu, J.; Ding, G.; He, D.; Zhang, H.; Qiu, W.; Li, C.; Liao, G. Recent Advances in LDH/g-C3N4 Heterojunction Photocatalysts for Organic Pollutant Removal. Nanomaterials 2023, 13, 3066. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, B.; An, X.; Li, Z.; Zhu, H.; Mao, B.; Calatayud, D.G.; James, T.D. A Practical Graphitic Carbon Nitride (g-C3N4) Based Fluorescence Sensor for the Competitive Detection of Trithiocyanuric Acid and Mercury Ions. Dye. Pigment. 2019, 170, 107476. [Google Scholar] [CrossRef]
- Wu, C.; Xue, S.; Qin, Z.; Nazari, M.; Yang, G.; Yue, S.; Tong, T.; Ghasemi, H.; Hernandez, F.C.R.; Xue, S.; et al. Making G-C3N4 Ultra-Thin Nanosheets Active for Photocatalytic Overall Water Splitting. Appl. Catal. B Environ. 2021, 282, 119557. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Díez, A.M.; Silva, C.G.; Faria, J.L.; Sanromán, M.Á.; Silva, A.M.T.; Pazos, M. Photoelectrocatalytic Degradation of Pharmaceuticals Promoted by a Metal-Free G-C3N4 Catalyst. Chem. Eng. J. 2023, 476, 146761. [Google Scholar] [CrossRef]
- Thomas, S.A.; Pallavolu, M.R.; Khan, M.E.; Cherusseri, J. Graphitic Carbon Nitride (g-C3N4): Futuristic Material for Rechargeable Batteries. J. Energy Storage 2023, 68, 107673. [Google Scholar] [CrossRef]
- Joseph, A.; Vijayanandan, A. Review on Support Materials Used for Immobilization of Nano-Photocatalysts for Water Treatment Applications. Inorganica Chim. Acta 2023, 545, 121284. [Google Scholar] [CrossRef]
- Falletta, E.; Longhi, M.; Di, A.; Boffito, D.C.; Bianchi, C.L. Floatable Graphitic Carbon Nitride/Alginate Beads for the Photodegradation of Organic Pollutants under Solar Light Irradiation. J. Clean. Prod. 2022, 371, 133641. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Gopalram, K. Immobilized TiO2/Chitosan Beads for Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid. Int. J. Biol. Macromol. 2020, 161, 282–291. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Giannakoudakis, D.A.; Prekodravac, J.R.; Nair, V.; Colmenares, J.C. Role of Catalyst Supports in Biocatalysis. J. Chem. Technol. Biotechnol. 2023, 98, 7–21. [Google Scholar] [CrossRef]
- Meng, F.; Tian, Z.; Tian, W.; Zhang, H. Advances in Carbon Nitride Supported Single-Atom Photocatalysts for Hydrogen Evolution. Curr. Opin. Chem. Eng. 2023, 41, 100941. [Google Scholar] [CrossRef]
- Das, S.; Mahalingam, H. Dye Degradation Studies Using Immobilized Pristine and Waste Polystyrene-TiO2/RGO/g-C3N4 Nanocomposite Photocatalytic Film in a Novel Airlift Reactor under Solar Light. J. Environ. Chem. Eng. 2019, 7, 103289. [Google Scholar] [CrossRef]
- Hu, C.; Lin, Y.R.; Yang, H.C. Recent Developments in Graphitic Carbon Nitride Based Hydrogels as Photocatalysts. ChemSusChem 2019, 12, 1794–1806. [Google Scholar] [CrossRef]
- Fang, X.; Feng, C.; Li, T.; Wang, Y.; Zhu, S.; Ren, H.; Huang, H. G-C3N4/Polyvinyl Alcohol-Sodium Alginate Aerogel for Removal of Typical Heterocyclic Drugs from Water. Environ. Pollut. 2023, 319, 121057. [Google Scholar] [CrossRef]
- Nasir, A.M.; Jaafar, J.; Aziz, F.; Yusof, N.; Norhayati, W.; Salleh, W.; Ismail, A.F.; Aziz, M. A Review on Floating Nanocomposite Photocatalyst: Fabrication and Applications for Wastewater Treatment. J. Water Process Eng. 2020, 36, 101300. [Google Scholar] [CrossRef]
- Rana, A.; Sudhaik, A.; Raizada, P.; Nguyen, V.-H.; Xia, C.; Parwaz Khan, A.A.; Thakur, S.; Nguyen-Tri, P.; Nguyen, C.C.; Kim, S.Y.; et al. Graphitic Carbon Nitride Based Immobilized and Non-Immobilized Floating Photocatalysts for Environmental Remediation. Chemosphere 2022, 297, 134229. [Google Scholar] [CrossRef]
- Guo, H.; Qin, Q.; Chang, J.; Lee, D. Modified Alginate Materials for Wastewater Treatment: Application Prospects. Bioresour. Technol. 2023, 387, 129639. [Google Scholar] [CrossRef]
- Rodríguez Couto, S.; Domínguez, A.; Sanromán, A. Photocatalytic Degradation of Dyes in Aqueous Solution Operating in a Fluidised Bed Reactor. Chemosphere 2002, 46, 83–86. [Google Scholar] [CrossRef]
- Dalponte, I.; Mathias, A.L.; Jorge, R.M.M.; Weinschutz, R. Degradação Fotocatalítica de Tartrazina Com TiO2 Imobilizado Em Esferas de Alginato. Quim. Nova 2016, 39, 1165–1169. [Google Scholar] [CrossRef]
- Hao, D.; Huang, Q.; Wei, W.; Bai, X.; Ni, B.J. A Reusable, Separation-Free and Biodegradable Calcium Alginate/g-C3N4 Microsphere for Sustainable Photocatalytic Wastewater Treatment. J. Clean. Prod. 2021, 314, 128033. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M.; Kumar, R. 3D Kaolinite/g-C3N4 -Alginate Beads as an Affordable and Sustainable Photocatalyst for Wastewater Remediation. Carbohydr. Polym. 2024, 323, 121420. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; An, Q.; Xiao, Z.; Zhai, S.; Hao, J.; Tong, Y. Alginate Modified Graphitic Carbon Nitride Composite Hydrogels for Efficient Removal of Pb (II), Ni (II) and Cu (II) from Water. Int. J. Biol. Macromol. 2020, 148, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
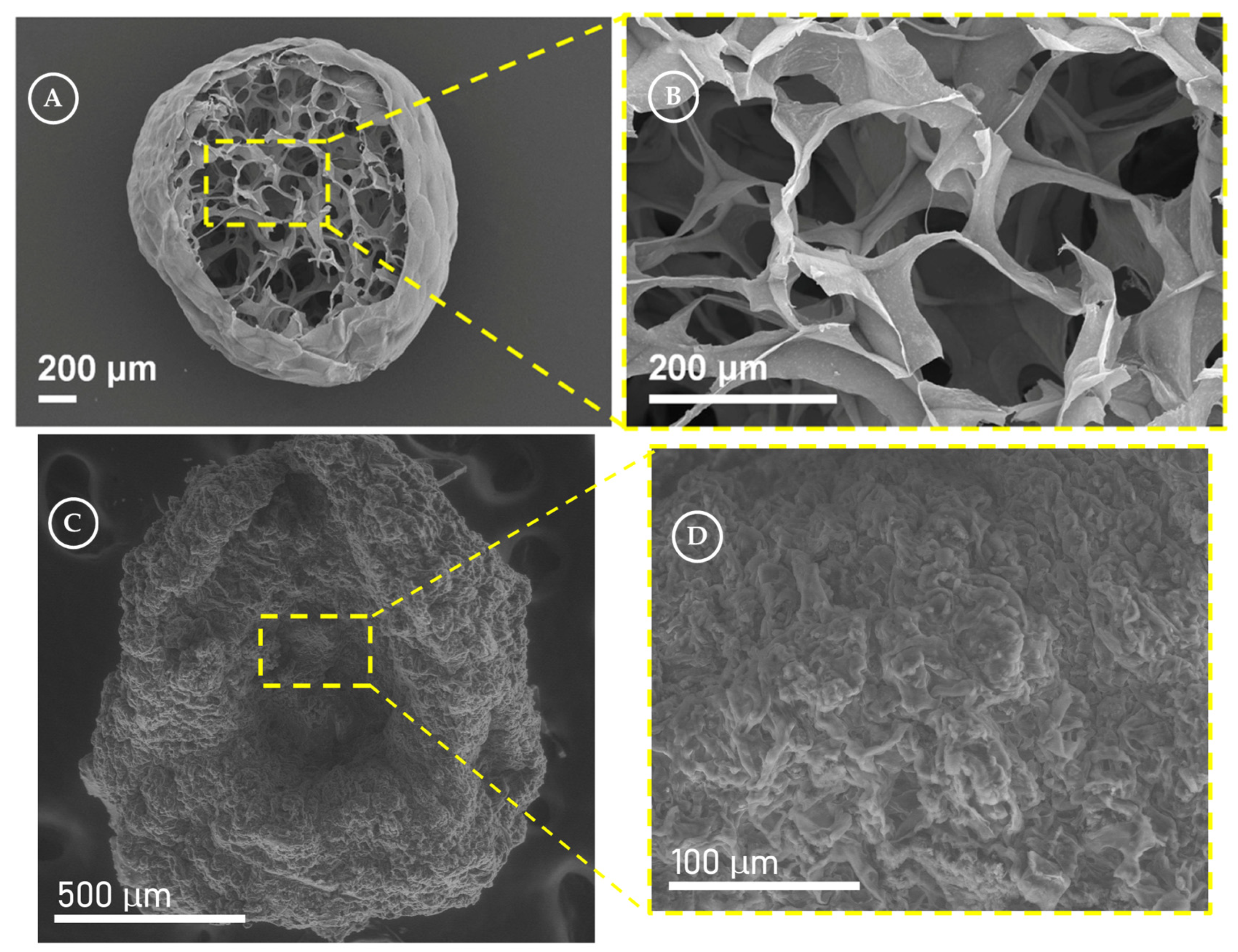
- Fu, G.B.; Xie, R.; Qin, J.W.; Deng, X.B.; Ju, X.J.; Wang, W.; Liu, Z.; Chu, L.Y. Facile Fabrication of Photocatalyst-Immobilized Gel Beads with Interconnected Macropores for the Efficient Removal of Pollutants in Water. Ind. Eng. Chem. Res. 2021, 60, 8762–8775. [Google Scholar] [CrossRef]
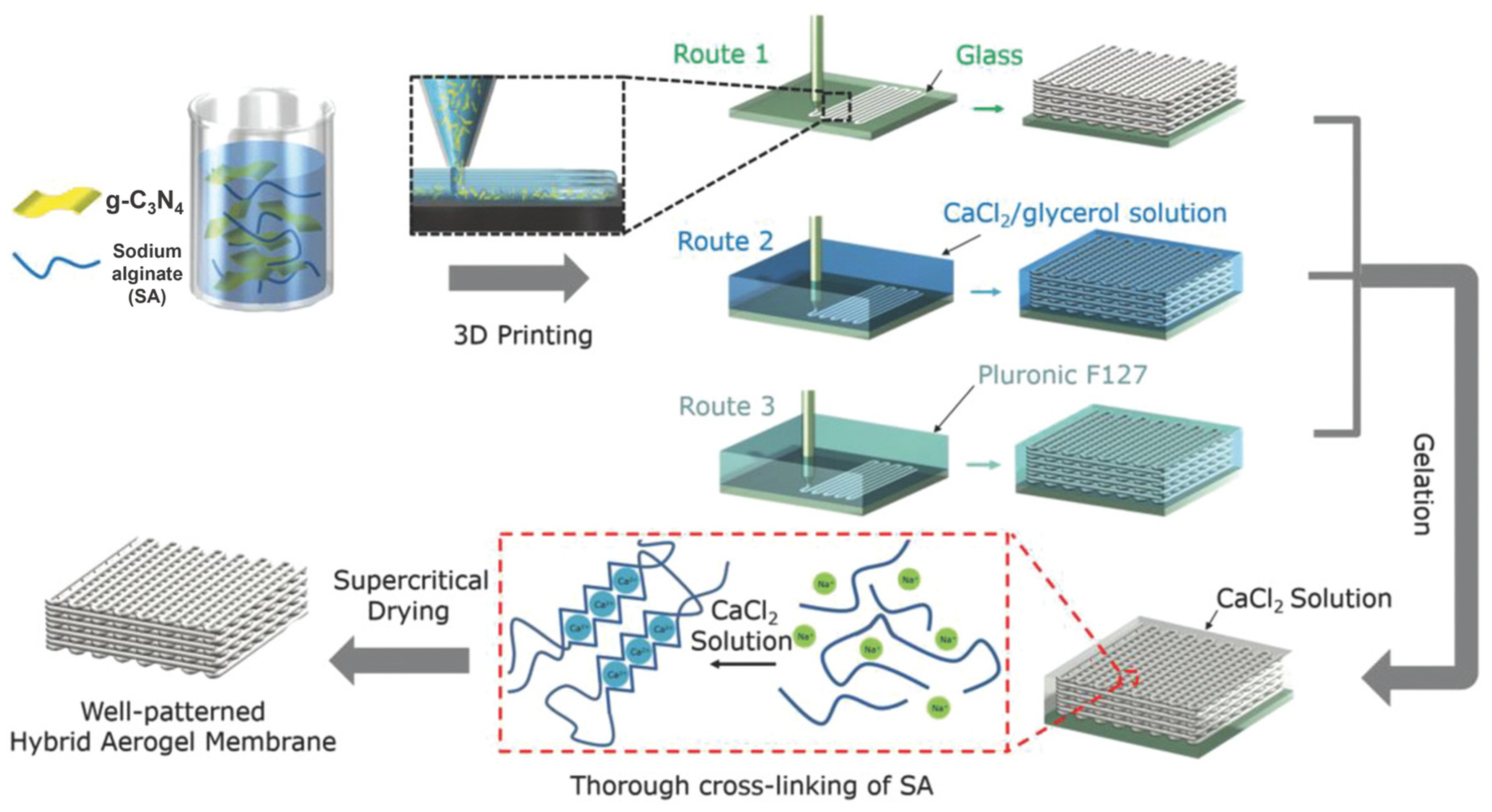
- He, P.; Tang, X.; Chen, L.; Xie, P.; He, L.; Zhou, H.; Zhang, D.; Fan, T. Patterned Carbon Nitride—Based Hybrid Aerogel Membranes via 3D Printing for Broadband Solar Wastewater Remediation. Adv. Funct. Mater. 2018, 28, 1801121. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, Q.; Wang, S.; Dong, P.; Zhang, L. Regenerable G-C3N4-Chitosan Beads with Enhanced Photocatalytic Activity and Stability. RSC Adv. 2018, 8, 27516–27524. [Google Scholar] [CrossRef]
- Cai, X.; Tan, X.; Jiao, D.; Li, H.; Zhang, D.; Wang, Q. Rational Construction of 3D Recyclable G-C3N4 When Encapsulated by Cellulose/Graphene Oxide Hybrid Aerogels for Efficient Contaminant Removal. Ind. Crops Prod. 2023, 202, 117096. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhang, S.H.; Li, J.; Zheng, X.C.; Guan, X.X. Constructing of 3D Graphene Aerogel-g-C3N4 Metal-Free Heterojunctions with Superior Purification Efficiency for Organic Dyes. J. Mol. Liq. 2020, 310, 113242. [Google Scholar] [CrossRef]
- Bai, W.; Yang, X.; Du, X.; Qian, Z.; Zhang, Y.; Liu, L.; Yao, J. Robust and Recyclable Macroscopic G-C3N4/Cellulose Hybrid Photocatalysts with Enhanced Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2020, 504, 144179. [Google Scholar] [CrossRef]
- Ruan, X.; Wang, L.; Liang, D.; Shi, Y. Environmental Applications of 3D G-C3N4-Based Hydrogel with Synergistic Effect of Adsorption and Photodegradation. Langmuir 2023, 39, 3371–3379. [Google Scholar] [CrossRef]
- Mao, Y.; Lin, L.; Chen, Y.; Yang, M.; Zhang, L.; Dai, X.; He, Q.; Jiang, Y.; Chen, H.; Liao, J.; et al. Preparation of Site-Specific Z-Scheme g-C3N4/PAN/PANI@LaFeO3 Cable Nanofiber Membranes by Coaxial Electrospinning: Enhancing Filtration and Photocatalysis Performance. Chemosphere 2023, 328, 138553. [Google Scholar] [CrossRef]
- Tripathy, M.; Padhiari, S.; Ghosh, A.K.; Hota, G. Polyacrylonitrile Support Impregnated with Amine-Functionalized Graphitic Carbon Nitride/Magnetite Composite Nanofibers towards Enhanced Arsenic Remediation: A Mechanistic Approach. J. Colloid Interface Sci. 2023, 640, 890–907. [Google Scholar] [CrossRef]
- Luan, J.; Bai, X.; Liu, Y.; Song, T.; Sun, H.; Dai, Y.; Yu, J. PVA/PDA@g-C3N4 Composite Nanofiber Membranes for Enhanced Photocatalytic Bacteriostasis and Degradation. J. Polym. Environ. 2023, 4. [Google Scholar] [CrossRef]
- Huang, F.; Guo, S.; Yan, Y.; Zhang, W.; Cao, J.; Li, G.; Ji, Y. Integrated Flexible Photocatalytic Composite Nanofiber Membranes Combined In-Situ Grown CQDs/g-C3N4 with Thermally Etched Porous Silica. Sep. Purif. Technol. 2023, 325, 124672. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Enhancement of AFB1 Removal Efficiency via Adsorption/Photocatalysis Synergy Using Surface-Modified Electrospun PCL-g-C3N4/CQDs Membranes. Biomolecules 2023, 13, 550. [Google Scholar] [CrossRef]
- Anusuyadevi, P.R.; Riazanova, A.V.; Hedenqvist, M.S.; Svagan, A.J. Floating Photocatalysts for Effluent Refinement Based on Stable Pickering Cellulose Foams and Graphitic Carbon Nitride (g-C3N4). ACS Omega 2020, 5, 22411–22419. [Google Scholar] [CrossRef]
- Letswalo, V.P.; Dlamini, L.N.; Malinga, S.P. Efficient Degradation of Tetracycline Using a Nanostructured G-C3N4/Nb2O5/HPEI/PES Photocatalytic Membrane. Environ. Adv. 2022, 10, 100322. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, M.; Yang, H.; Xu, Z. Novel Ultrafiltration Membrane Fabricated Using Nanofiber Layer as Integral Filler: Taking PAN and C3N4 as Example. J. Memb. Sci. 2023, 684, 121872. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Huang, Z.; Xu, Y.; Zhao, Q.; Wang, H.; Shi, M.; Li, X.; Jiang, K.; Wu, D. “Floating Catalytic Foam” with Prominent Heat-Induced Convection for the Effective Photocatalytic Removal of Antibiotics. J. Hazard. Mater. 2024, 463, 132879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.A.; Mao, J.; Lu, C.; Yang, S.; Qian, Q.; Chen, Q.; Xue, H.; Sun, X.; Yang, M.Q. Design and Fabrication of Self-Suspending Aluminum-Plastic/Semiconductor Photocatalyst Devices for Solar Energy Conversion. J. Environ. Sci. 2024, 136, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Rusek, J.; Paušová, Š.; Praus, P.; Krýsa, J. Immobilization of Exfoliated G-C3N4 for Photocatalytical Removal of Organic Pollutants from Water. Catalysts 2021, 11, 203. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P.; Nguyen, V.-H. Synthesis and Photocatalytic Activity of Ni–Fe Layered Double Hydroxide Modified Sulphur Doped Graphitic Carbon Nitride (SGCN/Ni–Fe LDH) Photocatalyst for 2,4-Dinitrophenol Degradation. Top. Catal. 2020, 63, 1030–1045. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Abbasi-Asl, H.; Sabzehmeidani, M.M.; Ghaedi, M.; Moradi, Z. Interface Engineering of a Magnetic 2D-C3N4/Fe2O3/NiFe-LDH Heterostructure for Efficient Photocatalytic Degradation of Methylene Blue and Rhodamine B Dyes under Visible Light. Appl. Clay Sci. 2023, 246, 107182. [Google Scholar] [CrossRef]
- Cong, X.; Shang, Y.; Zhao, L.; Jiang, H.; Tian, W.; Wu, J.; Zhang, S.; Tian, D.; Lu, S.; Tan, Y. Binary Heterojunctions of Mg/Al-LDH and Carbon-Based Matrixes Derived from Self-Assembly Synthesis for Inhibition of Pyrene Photopolymerizing: Elucidation of LDHs Protecting Bitumen against UV Aging. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 677, 132313. [Google Scholar] [CrossRef]
- Khamesan, A.; Esfahani, M.M.; Ghasemi, J.B.; Farzin, F.; Parsaei-Khomami, A.; Mousavi, M. Graphitic-C3N4/ZnCr-Layered Double Hydroxide 2D/2D Nanosheet Heterojunction: Mesoporous Photocatalyst for Advanced Oxidation of Azo Dyes with in Situ Produced H2O2. Adv. Powder Technol. 2022, 33, 103777. [Google Scholar] [CrossRef]
- Zheng, Q.; Aiello, A.; Choi, Y.S.; Tarr, K.; Shen, H.; Durkin, D.P.; Shuai, D. 3D Printed Photoreactor with Immobilized Graphitic Carbon Nitride: A Sustainable Platform for Solar Water Purification. J. Hazard. Mater. 2020, 399, 123097. [Google Scholar] [CrossRef]
- Anush, S.M.; Raju, S.N.; Gayathri, B.H.; Ajeya, K.P.; Girish, Y.R.; Darshan, S.; Naveen, Y.P.; Prashantha, K.; Narendra, B.K.; Jayaram, A. Graphitic C3N4 Incorporated Chitosan-Poly (Vinyl Alcohol) Blend Nanocomposites for the Removal of Cu (II) and Cr (VI) Ions from Aqueous Solutions. Express Polym. Lett. 2024, 18, 102–115. [Google Scholar] [CrossRef]
- Liu, S.; Gao, X.; Fan, H.; Zhang, M.; Waterhouse, G.I.N.; Zhu, S. Green and Recyclable Graphitic Carbon Nitride/Chitosan/Polyvinyl Alcohol Photocatalytic Films with Efficient Antibacterial Activity for Fruit Packaging. Int. J. Biol. Macromol. 2023, 236, 123974. [Google Scholar] [CrossRef]
- Bai, X.; Song, T.; Luan, J.; Chen, M.; Yu, J.; Tian, H. Polyvinyl Alcohol/Chitosan Nanofiber Membranes Loaded with Oxygenated Graphitic Carbon Nitride Nanosheets for Enhanced Photocatalytic Bacteriostasis. J. Appl. Polym. Sci. 2023, 140, e54081. [Google Scholar] [CrossRef]
- Fan, G.; Du, B.; Zhou, J.; Yan, Z.; You, Y.; Luo, J. Porous Self-Floating 3D Ag2O/g-C3N4 Hydrogel and Photocatalytic Inactivation of Microcystis Aeruginosa under Visible Light. Chem. Eng. J. 2021, 404, 126509. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Ginting, R.T.; Abdullah, H.; Fauzia, V. Facile Preparation of MXene and Protonated-g-C3N4 on Natural Latex Foam for Highly Efficient Solar Steam Generation. Mater. Lett. 2022, 313, 131779. [Google Scholar] [CrossRef]
- Velmurugan, S.; Yang, T.C.K. Fabrication of High-Performance Molybdenum Disulfide-Graphitic Carbon Nitride p-n Heterojunction Stabilized RGO/ITO Photoelectrode for Photoelectrochemical Determination of Dopamine. ACS Appl. Electron. Mater. 2020, 2, 2845–2856. [Google Scholar] [CrossRef]
- Gowri, V.M.; John, S.A. Fabrication of Electrically Conducting Graphitic Carbon Nitride Film on Glassy Carbon Electrode with the Aid of Amine Groups for the Determination of an Organic Pollutant. J. Electroanal. Chem. 2020, 879, 114787. [Google Scholar] [CrossRef]
- Rajan, A.; Neppolian, B. Coordinative Integration of Amorphous Nickel-Imidazole Framework with Graphitic Carbon Nitride for Enhanced Photocatalytic Hydrogen Production. Appl. Mater. Today 2022, 28, 101524. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, X.; Yang, C.; Feng, X.; Zhang, Z.F.; Song, K.; Wu, S.; Li, L.; Jiang, T.; Shi, J.W. Insight into the Role of Ni Atoms at the Interface of G-C3N4/CdS in Photocatalytic H2 Evolution. Sep. Purif. Technol. 2023, 327, 124996. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, H.; Won, M.; Kim, E.; Li, M.; Kim, J.S. Codoping G-C3N4 with Boron and Graphene Quantum Dots: Enhancement of Charge Transfer for Ultrasensitive and Selective Photoelectrochemical Detection of Dopamine. Biosens. Bioelectron. 2023, 224, 115050. [Google Scholar] [CrossRef]
- Martimiano do Prado, T.; Catunda, L.G.d.S.; Calegaro, M.L.; Correa, D.S.; Machado, S.A.S. Synthesis and Characterization of 2D-Carbonylated Graphitic Carbon Nitride: A Promising Organic Semiconductor for Miniaturized Sensing Devices. Electrochim. Acta 2022, 431, 2–10. [Google Scholar] [CrossRef]
- Sriram, B.; Baby, J.N.; Wang, S.F.; Ranjitha, M.R.; Govindasamy, M.; George, M. Eutectic Solvent-Mediated Synthesis of NiFe-LDH/Sulfur-Doped Carbon Nitride Arrays: Investigation of Electrocatalytic Activity for the Dimetridazole Sensor in Human Sustenance. ACS Sustain. Chem. Eng. 2020, 8, 17772–17782. [Google Scholar] [CrossRef]
- Lu, L.; Yang, Z.; Huang, M.; Xu, J.; Zhou, J.; Briseghella, B.; Marano, G.C. Microstructural and Mechanical Properties of Photocatalytic Cement Mortar with G-C3N4/CoAl-LDH Nanoflowers. J. Build. Eng. 2023, 74, 106900. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, H.; Gong, W.; Gu, X.; Liu, T.; Zhang, J.; Qin, W.; Chen, H.; Jin, Y.; Liang, Z.; et al. Wood-Inspired Binder Enabled Vertical 3D Printing of g-C3N4/CNT Arrays for Highly Efficient Photoelectrochemical Hydrogen Evolution. Adv. Funct. Mater. 2021, 31, 2105045. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Díez, A.M.; Silva, C.G.; Faria, J.L.; Sanromán, M.Á.; Silva, A.M.T.; Pazos, M. Tuning Graphitic Carbon Nitride (g-C3N4) Electrocatalysts for Efficient Oxygen Evolution Reaction (OER). Fuel 2024, 360, 130575. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, A.; Zhang, Y.; Tian, Z.; Cheng, X.; Gao, Y.; Zhou, X.; Wu, X.; Chen, K.; Ning, X. A Photoactive Self-Healing Carboxymethyl Chitosan-Based Hydrogel for Accelerated Infected Wound Healing through Simultaneously Modulating Multiple Critical Tissue Repair Factors. Int. J. Biol. Macromol. 2023, 242, 124631. [Google Scholar] [CrossRef]
- Shan, T.; Li, J.; Wu, S.; Wu, H.; Zhang, F. Boosting H2O2 Production over Carboxymethyl Cellulose Modified G-C3N4 via Hydrogen-Bonding-Assisted Charge Transfer. Chem. Eng. J. 2023, 478, 147509. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; Kang, Y.; Li, Q.; Xia, Y.; Zhu, Y.; Hou, H.; Uddin, H.; Gengenbach, T.R.; Xia, D.; et al. Simultaneously Tuning Charge Separation and Oxygen Reduction Pathway on Graphitic Carbon Nitride by Polyethylenimine for Boosted Photocatalytic Hydrogen Peroxide Production. ACS Catal. 2020, 10, 3697–3706. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Wang, D.; Zhang, F.; Zhao, Y.; Yan, M.; Zheng, C.; Wang, Q.; Long, M.; Chen, C. Modification of G-C3N4 with Hydroxyethyl Cellulose as Solid Proton Donor via Hydrogen Bond to Enhance H2O2 Production. Appl. Catal. B Environ. 2022, 318, 121749. [Google Scholar] [CrossRef]
- Smýkalov, A.; Kinnertov, E. Metal-Free Hybrid Nanocomposites of Graphitic Carbon Nitride and Char: Synthesis, Characterisation and Photocatalysis under Visible Irradiation. J. Taiwan Inst. Chem. Eng. 2023, 2013, 104864. [Google Scholar] [CrossRef]
- Sultan, M.; Mansor, E.S.; Adeeb, Z.; Elsayed, H. Fabrication of Highly Efficient Nano-Composite Films Based on ZnO-g-C3N4 @ PAA-g-(HEC/PVA)-Fe3+ for Removal of Methylene Blue Dye from Water. J. Water Process Eng. 2021, 42, 102184. [Google Scholar] [CrossRef]
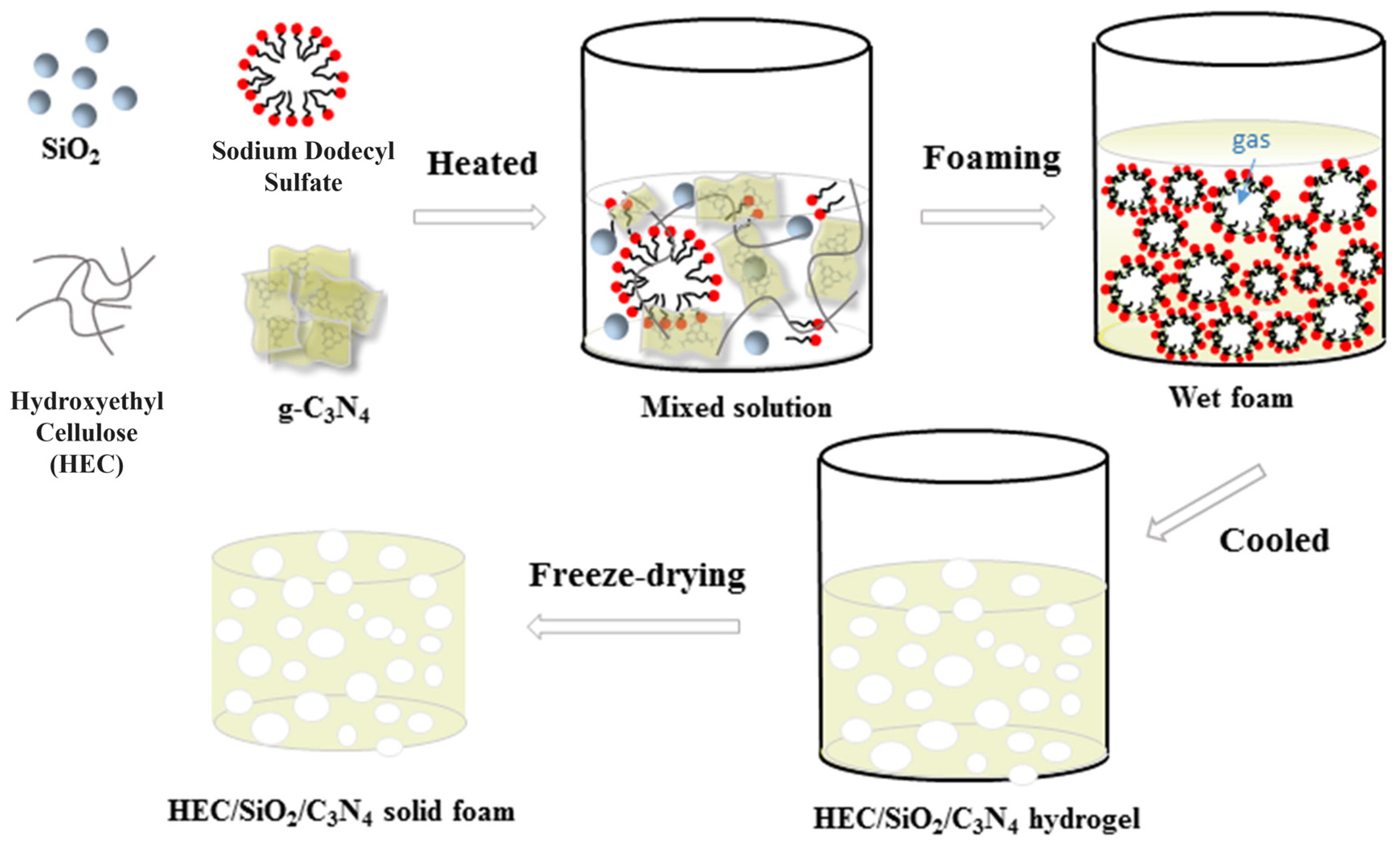
- Gao, Z.; Qi, N.; Chen, W.; Zhao, H. Construction of Hydroxyethyl Cellulose/Silica/Graphitic Carbon Nitride Solid Foam for Adsorption and Photocatalytic Degradation of Dyes. Arab. J. Chem. 2022, 15, 104105. [Google Scholar] [CrossRef]
- Syed, M.H.; Khan, M.M.R.; Zahari, M.A.K.M.; Beg, M.D.H.; Abdullah, N. A Review on Current Trends and Future Prospectives of Electrospun Biopolymeric Nanofibers for Biomedical Applications. Eur. Polym. J. 2023, 197, 112352. [Google Scholar] [CrossRef]
- Mazur, F.; Pham, A.-H.; Chandrawati, R. Polymer Materials as Catalysts for Medical, Environmental, and Energy Applications. Appl. Mater. Today 2023, 35, 101937. [Google Scholar] [CrossRef]
- Si, Y.; Shi, S.; Hu, J. Applications of Electrospinning in Human Health: From Detection, Protection, Regulation to Reconstruction. Nano Today 2023, 48, 101723. [Google Scholar] [CrossRef]
- Švára, D.; Filipová, B.; Jelínek, P.; Mikeš, P.; Kluk, A.; Šoóš, M. The Impact of Polymer Mixture Composition on the Properties of Electrospun Membranes for Drug Delivery Applications. Int. J. Pharm. 2023, 647, 123548. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, D.-G.; Li, X.; Williams, G.R. The Development and Bio-Applications of Multifluid Electrospinning. Mater. Highlights 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Huang, C.-C. Electrospinning PVA Solution-Rheology and Morphology Analyses. Fibers Polym. 2012, 13, 44–50. [Google Scholar] [CrossRef]
- Liu, S.; Liang, P.; Liu, J.; Xin, J.; Li, X.; Shao, C.; Li, X.; Liu, Y. Anchoring Bismuth Oxybromo-Iodide Solid Solutions on Flexible Electrospun Polyacrylonitrile Nanofiber Mats for Floating Photocatalysis. J. Colloid Interface Sci. 2022, 608, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shao, C.; Yang, S.; Li, X.; Guo, X.; Wang, X.; Li, X.; Liu, Y. Heterojunction of G-C3N4/BiOI Immobilized on Flexible Electrospun Polyacrylonitrile Nanofibers: Facile Preparation and Enhanced Visible Photocatalytic Activity for Floating Photocatalysis. ACS Sustain. Chem. Eng. 2018, 6, 2316–2323. [Google Scholar] [CrossRef]
- Zhang, F.; Xin, J.; Wu, X.; Liu, J.; Niu, L.; Wang, D.; Li, X.; Shao, C.; Li, X.; Liu, Y. Floating Metal Phthalocyanine@polyacrylonitrile Nanofibers for Peroxymonosulfate Activation: Synergistic Photothermal Effects and Highly Efficient Flowing Wastewater Treatment. J. Hazard. Mater. 2023, 459, 132228. [Google Scholar] [CrossRef]
- Chai, P.; Luo, C.; Gu, J.; Yu, F.; Chao, M.; Chen, X.; Yan, L. Euplectella Aspergillum Inspired PPAN@g-C3N4 Fibrous Membrane with Abundant Micro/Nano Pores for High-Throughput Wastewater Purification. J. Environ. Chem. Eng. 2023, 11, 110347. [Google Scholar] [CrossRef]
- Ziyadi, H.; Baghali, M.; Bagherianfar, M.; Mehrali, F.; Faridi-Majidi, R. An Investigation of Factors Affecting the Electrospinning of Poly (Vinyl Alcohol)/Kefiran Composite Nanofibers. Adv. Compos. Hybrid Mater. 2021, 4, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Ayodeji, O.J.; Khyum, M.M.O.; Afolabi, R.T.; Smith, E.; Kendall, R.; Ramkumar, S. Preparation of Surface-Functionalized Electrospun PVA Nanowebs for Potential Remedy for SARS-CoV-2. J. Hazard. Mater. Adv. 2022, 7, 100128. [Google Scholar] [CrossRef] [PubMed]
- Yavuzturk Gul, B.; Orhun Teber, O.; Tuncay, G.; Pekgenc, E.; Arabi, N.; Hemmati-Eslamlu, P.; Habibi-Yangjeh, A.; Vatanpour, V.; Koyuncu, I. Modification of PAN Electrospun Nanofiber Membranes with G-C3N4 Nanotubes/Carbon Dots to Enhance MBR Performance. Chemosphere 2024, 349, 140866. [Google Scholar] [CrossRef] [PubMed]
- Abdinejad, M.; Tang, K.; Dao, C.; Saedy, S.; Burdyny, T. Immobilization Strategies for Porphyrin-Based Molecular Catalysts for the Electroreduction of CO2. J. Mater. Chem. A 2022, 10, 7626–7636. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.; Chittor Neelakantan, S. Immobilization of Molecular Complexes on Graphitized Carbon Cloth as Stable and Hybrid Electrocatalysts for Enhanced Hydrogen Evolution Reaction and Oxygen Evolution Reaction. Mater. Adv. 2022, 3, 8201–8210. [Google Scholar] [CrossRef]
- Salgaonkar, K.N.; Kale, S.R.; Nalajala, N.; Mansuri, S.; Gopinath, C.S. Selective and Generic Photocatalytic Oxidation of Alcohol with Pd−TiO2 Thin Films: Butanols to Butanal/Butanone with Different Morphologies of Pd and 0.5θPt-Pd Counterparts. Chem. Asian J. 2023, 18, e202201239. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Wang, X.; Hu, J.; Zhang, J.; Zheng, L.; Mao, L.; Zheng, H.; Zhu, M. Protruding Pt Single-Sites on Hexagonal ZnIn2S4 to Accelerate Photocatalytic Hydrogen Evolution. Nat. Commun. 2022, 13, 1287. [Google Scholar] [CrossRef]
- Hegedűs, P.; Szabó-Bárdos, E.; Horváth, O.; Szabó, P.; Horváth, K. Investigation of a TiO2 Photocatalyst Immobilized with Poly(Vinyl Alcohol). Catal. Today 2017, 284, 179–186. [Google Scholar] [CrossRef]
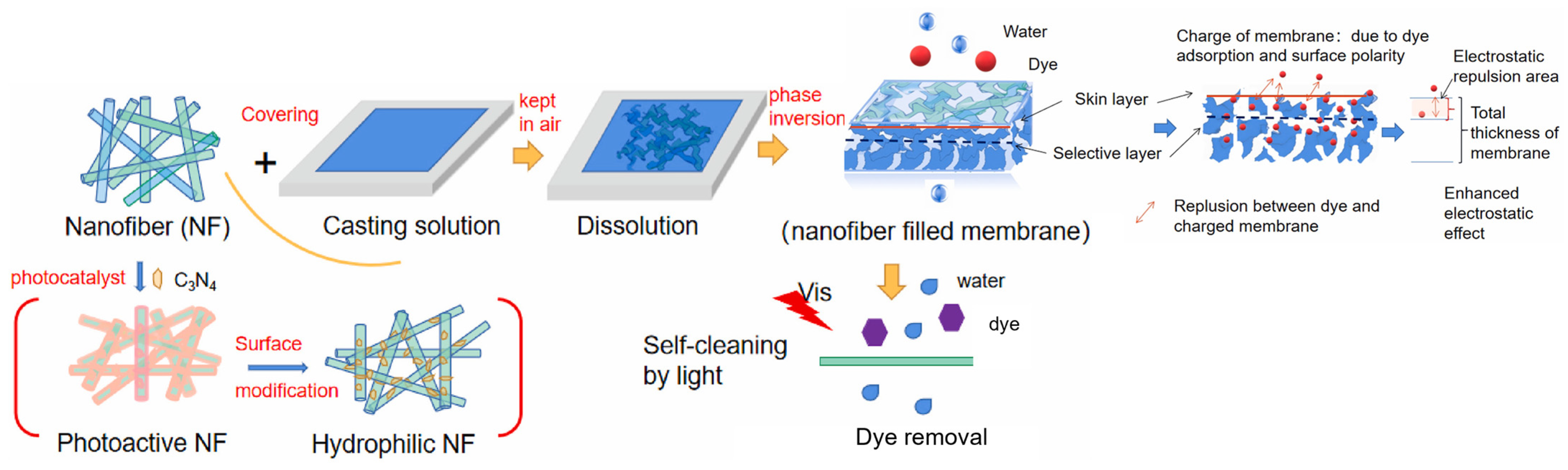
- Zhou, C.; Wang, J.; Zhang, Q.; Dou, M.; Huo, K.; Han, C.; Gao, B. Explication of Polydopamine Modified G-C3N4 as Effective Additive in PVDF Nanocomposite Membrane Fabrication for Enhanced Ultrafiltration and Self-Cleaning Performance. J. Environ. Chem. Eng. 2023, 11, 110577. [Google Scholar] [CrossRef]
- Gowri, V.M.; Ajith, A.; John, S.A.; Chang, W.S.; Gowthaman, N.S.K. Different Modes of Attachment of Graphitic Carbon Nitrides on Glassy Carbon Electrode and Their Electrocatalytic Activity. Microchem. J. 2023, 191, 108818. [Google Scholar] [CrossRef]
- Marhoon, I.I.; Atiyah, I.A.; Rasheed, A.K. 3D G-C3N4 Porous Nanoribbons Pillared-MXene/PVA Nanocomposite: An Architecture with High Dielectric, Breakdown Strength, Thermal Conductivity, and Mechanical Strength Characteristics. J. Alloys Compd. 2023, 969, 172229. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Letswalo, V.P.; Dlamini, L.N.; Malinga, S.P. A 2D/1D g-C3N4/Nb2O5 Heterostructure in HPEI Template with the Potential for Water Treatment. Mater. Lett. X 2021, 12, 100100. [Google Scholar] [CrossRef]
- Bouarioua, A.; Zerdaoui, M. Photocatalytic Activities of TiO2 Layers Immobilized on Glass Substrates by Dip-Coating Technique toward the Decolorization of Methyl Orange as a Model Organic Pollutant. J. Environ. Chem. Eng. 2017, 5, 1565–1574. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.K.; Lee, S.C. Immobilization of Polymeric G-C3N4 on Structured Ceramic Foam for Efficient Visible Light Photocatalytic Air Purification with Real Indoor Illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef]
- Li, H.; Zang, L.; Shen, F.; Wang, L.; Sun, L.; Yuan, F. Tubular G-C3N4/Carbon Framework for High-Efficiency Photocatalytic Degradation of Methylene Blue. RSC Adv. 2021, 11, 18519–18524. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zeng, Z.; Zeng, G.; Gong, J.; Xiao, R.; Ye, S.; Chen, M. Powerful Combination of G-C3N4 and LDHs for Enhanced Photocatalytic Performance: A Review of Strategy, Synthesis, and Applications. Adv. Colloid Interface Sci. 2019, 272, 101999. [Google Scholar] [CrossRef] [PubMed]
- Arrabito, G.; Bonasera, A.; Prestopino, G.; Orsini, A.; Mattoccia, A.; Martinelli, E.; Pignataro, B.; Medaglia, P.G. Layered Double Hydroxides: A Toolbox for Chemistry and Biology. Crystals 2019, 9, 361. [Google Scholar] [CrossRef]
- Momin, Z.H.; Lingamdinne, L.P.; Kulkarni, R.; Pal, C.A.K.; Choi, Y.L.; Koduru, J.R.; Chang, Y.Y. Improving U(VI) Retention Efficiency and Cycling Stability of GCN-Supported Calcined-LDH Composite: Mechanism Insight and Real Water System Applications. Chemosphere 2024, 346, 140551. [Google Scholar] [CrossRef]
- Boumeriame, H.; Cherevan, A.; Eder, D.; Apaydin, D.H.; Chafik, T.; Da Silva, E.S.; Faria, J.L. Engineering G-C3N4 with CuAl-Layered Double Hydroxide in 2D/2D Heterostructures for Visible-Light Water Splitting. J. Colloid Interface Sci. 2023, 652, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Wang, S.F. Electrochemical Determination of Vanillin Using 2D/2D Heterostructure Based on ZnCr-Layered Double Hydroxide and g-CN. Microchim. Acta 2023, 190, 423. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Chandra, P.; Khan, M.E.; Choi, C.-H.; Yoon, T. Sulfur-Doped Graphitic Carbon Nitride: Tailored Nanostructures for Photocatalytic, Sensing, and Energy Storage Applications. Adv. Colloid Interface Sci. 2023, 322, 103048. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Fan, C.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Pan, S.; Zhang, B.; Ding, J.; Rong, S.; et al. Effective Mineralization and Detoxification of Tetracycline Hydrochloride Enabled by Oxygen Vacancies in G-C3N4/LDH Composites. Sep. Purif. Technol. 2023, 305, 122554. [Google Scholar] [CrossRef]
- Zou, X.; Sun, Z.; Hu, Y.H. G-C3N4-Based Photoelectrodes for Photoelectrochemical Water Splitting: A Review. J. Mater. Chem. A 2020, 8, 21474–21502. [Google Scholar] [CrossRef]
- Benedoue, S.; Benedet, M.; Gasparotto, A.; Gauquelin, N.; Orekhov, A.; Verbeeck, J.; Seraglia, R.; Pagot, G.; Rizzi, G.A.; Balzano, V.; et al. Insights into the Photoelectrocatalytic Behavior of GCN-Based Anode Materials Supported on Ni Foams. Nanomaterials 2023, 13, 1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Y.; Xu, D.; Chen, L.; Qiu, X.; Zhu, Y. Graphitic Carbon Nitride for Photoelectrochemical Detection of Environmental Pollutants. ACS ES T Eng. 2022, 2, 140–157. [Google Scholar] [CrossRef]
- Yuan, C.; He, Z.; Chen, Q.; Wang, X.; Zhai, C.; Zhu, M. Selective and Efficacious Photoelectrochemical Detection of Ciprofloxacin Based on the Self-Assembly of 2D/2D g-C3N4/Ti3C2 Composites. Appl. Surf. Sci. 2021, 539, 148241. [Google Scholar] [CrossRef]
- Jang, J.; Kang, S.; Pawar, R.C.; Lee, C.S. Electrospun One-Dimensional Graphitic Carbon Nitride-Coated Carbon Hybrid Nanofibers (GCN/CNFs) for Photoelectrochemical Applications. Curr. Appl. Phys. 2018, 18, 1006–1012. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Torres-Pinto, A.; Rosales, E.; Silva, C.G.; Faria, J.L.; Pazos, M.; Silva, A.M.T. Optimisation of a Photoelectrochemical System for the Removal of Pharmaceuticals in Water Using Graphitic Carbon Nitride. Catal. Today 2024, 432, 114578. [Google Scholar] [CrossRef]
- Azhdeh, A.; Mashhadizadeh, M.H.; Moazami, H.R. Developing a Photo-Electric-Field Wireless Electrochemical System for Highly Efficient Removal of Diazinon as an Organic Model Pollutant as a next-Generation Electrochemical Advanced Oxidation Process. J. Appl. Electrochem. 2023, 53, 1219–1243. [Google Scholar] [CrossRef]
- Maślana, K.; Kędzierski, T.; Żywicka, A.; Zielińska, B.; Mijowska, E. Design of Self-Cleaning and Self-Disinfecting Paper-Shaped Photocatalysts Based on Wood and Eucalyptus Derived Cellulose Fibers Modified with GCN/Ag Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100656. [Google Scholar] [CrossRef]
- Khezri, B.; Villa, K.; Novotný, F.; Sofer, Z.; Pumera, M. Smartdust 3D-Printed Graphene-Based Al/Ga Robots for Photocatalytic Degradation of Explosives. Small 2020, 16, e2002111. [Google Scholar] [CrossRef] [PubMed]
- Phang, S.J.; Wong, V.-L.; Cheah, K.H.; Tan, L.-L. Robust Graphitic Carbon Nitrite-Based Thermoset Coating for Synergistic Adsorption–Photocatalysis Process in 3D-Printed Photoreactors. Energy Rep. 2023, 9, 30–37. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, A. Pushing the Operational Barriers for g-C3N4: A Comprehensive Review of Cutting-Edge Immobilization Strategies. Catalysts 2024, 14, 175. https://doi.org/10.3390/catal14030175
Fdez-Sanromán A, Pazos M, Rosales E, Sanromán A. Pushing the Operational Barriers for g-C3N4: A Comprehensive Review of Cutting-Edge Immobilization Strategies. Catalysts. 2024; 14(3):175. https://doi.org/10.3390/catal14030175
Chicago/Turabian StyleFdez-Sanromán, Antia, Marta Pazos, Emilio Rosales, and Angeles Sanromán. 2024. "Pushing the Operational Barriers for g-C3N4: A Comprehensive Review of Cutting-Edge Immobilization Strategies" Catalysts 14, no. 3: 175. https://doi.org/10.3390/catal14030175
APA StyleFdez-Sanromán, A., Pazos, M., Rosales, E., & Sanromán, A. (2024). Pushing the Operational Barriers for g-C3N4: A Comprehensive Review of Cutting-Edge Immobilization Strategies. Catalysts, 14(3), 175. https://doi.org/10.3390/catal14030175









