Abstract
Micrometer-sized polycrystalline anatase particles are widely used in materials and life sciences, serving as essential components in photocatalytic materials. The ability to tailor their composition, shape, morphology, and functionality holds significant importance. In this study, we identified and examined the non-destructive route of Copper(II) implantation at the surface of polycrystalline TiO2. The [Cu(en)(Im)2]2+ complex ion demonstrated a remarkable affinity to concentrate and bind with the semiconductor’s surface, such as anatase, forming a surface-bound adduct: ≡TiO2 + [Cu(en)(Im)2]2+ → ≡TiO2//[Cu(en)(Im)2]2+. The misalignment of Fermi levels in TiO2//[Cu(en)(Im)2]2+ triggered electron transfer, leading to the reduction of the metal center, releasing Copper(I) in the process. Although less efficient, the released Copper(I) encountered a highly favorable environment, resulting in the formation of the surface complex TiO2:CuIIsc. The implanted Cu(I) was converted back into Cu(II) due to re-oxidation by dissolved oxygen. The penetration of the metal ion into the surface level of the polycrystalline TiO2 lattice was influenced by surface residual forces, making surface grafting of the Cu(II) ion inevitable due to surface chemistry. FTIR, UV–vis, Raman, XRD, EPR, and surface morphological (SEM, EDAX, and HRTEM) analyses identified the typical surface grafting of the Cu(II) cluster complex on the anatase surface matrix. Moreover, the XRD results also showed the formation of an impure phase. The TiO2 polycrystalline materials, modified by the incorporation of copper complexes, demonstrated an enhanced visible-light photocatalytic capability in the degradation of Rhodamine B dye in aqueous solutions. This modification significantly improved the efficiency of the photocatalytic process, expanding the applicability of TiO2 to visible light wavelengths. These studies open up the possibility of using copper complexes grafted on metal oxide surfaces for visible-light active photocatalytic applications. Moreover, this investigation not only showcases the improved visible-light photocatalytic behavior of copper-modified TiO2 polycrystalline materials, but also underscores the broader implications of this improvement in the advancement of sustainable and efficient water treatment technologies.
1. Introduction
In recent years, the quest for innovative materials has led to the discovery of a fascinating new class of surface-tunable microparticles [1,2,3]. These microparticles possess unique properties and hold immense promise for a wide range of applications [4]. Among this group of novel microparticles, semiconductor lattices have been developed, which serve as host structures for sub-surface transition metal ions [5]. This intriguing combination of semiconductor and transition metal elements opens up a world of possibilities for tailoring the properties and behavior of these microparticles [6,7].
The ability to tune the surface characteristics of these microparticles is of particular significance, as it allows researchers and engineers to precisely control their performance in various settings [8,9]. By manipulating the interaction between the surface transition metal ions and the semiconductor lattice, specific functionalities can be engineered, making these microparticles versatile building blocks for advanced technologies [10,11,12,13]. Moreover, the exploration of their unique features will unveil opportunities for transformative advances in diverse fields, ranging from electronics and energy to catalysis and biomedicine [14]. As we embark on this journey of discovery, these surface-tunable microparticles promise to revolutionize materials science and open up new horizons for cutting-edge technologies [15].
The pursuit of new functional materials stands as a critical imperative in contemporary science and technology. The search for novel mechanical, electrical, magnetic, chemical, biological, and optical devices has led to the discovery of interesting materials, fostering their manufacture [16,17,18,19]. A burgeoning field in surface science is transition metal oxide semiconductors, which has recently gained considerable attraction and interest [20]. Progress in surface science requires a transition towards more realistic composites and comprehensive model systems, given their significant contributions to various solid surface applications [21]. In particular, studies have been carried out on remote oxygen plasma treatment with a majority carrier concentration in polycrystalline TiO2, Lennard-Jones-type interaction potentials between ionic liquids and anatase [22], metal–ion implantation in oxides [23,24], and metal ion impregnation [25,26].
Over the past years, multifaceted and innovative research on TiO2 has given rise to numerous reviews covering various aspects and topics [27,28,29,30,31,32]. Additionally, the investigation on the construction of dye-sensitized solar cells (DSSCs) has kindled a growing fascination with the adsorption properties of large molecules on oxide surfaces [33,34]. The anchoring of light-harvesting molecules on substrates plays a pivotal role in the efficiency of charge transfer to the electrode and, consequently, in the overall light-to-electron conversion efficiency of DSSCs. Of particular interest is the influence of the transition metal in the polycrystalline anatase titanium dioxide (TiO2) matrix on the modification of particle size and optical and electronic properties [35,36]. Such implantation could also generate oxygen vacancies or intermediate species on the TiO2 surface [37].
Photocatalysis, the process of using light to catalyze chemical reactions, has emerged as a promising and sustainable approach to address various environmental and energy challenges [38,39]. Among various photocatalytic materials, TiO2 stands out as an efficient and widely investigated semiconductor photocatalyst due to its excellent photoactivity and chemical stability [40,41]. However, to improve its photocatalytic performance and expand its applications, researchers have been exploring strategies to modify the surface of TiO2 [42,43]. In this context, the generation of novel surface complexes by introducing transition metal ions, such as Copper(II), into the TiO2 surface matrix has garnered considerable attention [44,45]. These modified surfaces, known as Cu-TiO2 surface complexes, exhibit unique properties that can significantly influence photocatalytic behavior. The controlled introduction of CuII ions into the TiO2 surface matrix can cause changes in the electronic structure, charge transfer dynamics, and surface properties, resulting in improved photocatalytic efficiency and selectivity.
This study focuses on a photocatalytic investigation of the Cu-TiO2 surface complex, with the aim of elucidating its role as an advanced photocatalyst. Through a series of experimental analyses and spectroscopic techniques, we aim to gain insights into the surface chemistry, electronic interactions, and photophysical processes of this surface complex. Additionally, this work explores the impact of different CuII surface grafting concentrations on photocatalytic performance, shedding light on the relationship between the surface complex and its photocatalytic activity. By comprehensively understanding the photocatalytic properties of the Cu-TiO2 surface complex, we can harness this knowledge to design innovative photocatalytic materials with improved efficiency and tailored functionalities.
2. Results and Discussion
2.1. Formation of Copper Complex Grafted TiO2
In this study, we employ a feasible ionic adsorption-based approach to develop a transition metal complex bound to a solid surface, namely, the host–dopant precursor: [Cu(en)(Im)2]2+, on a polycrystalline TiO2 semiconductor surface. To balance the reactivity of the host and grafting precursor, we carefully selected coordinating ligands for the metal cation based on their intrinsic Lewis acidity. The binding of the complex ion to the surface was investigated spectrally at various contact time intervals, and the results are shown in Figure 1.
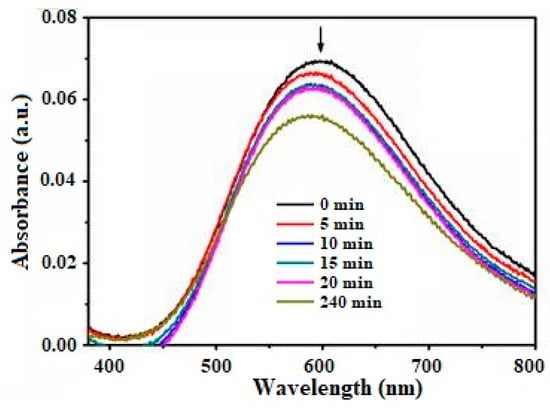
Figure 1.
Electronic absorption spectra measured for the complex:surface adduct, ≡TiO2//Cu(en)(Im)22+ in water at various contact times. Complex concentration = 1.65 × 10−3 M, ionic strength = 1M NaClO4, pH = 7.3 and at 298 K. Note: The arrow indicates a decrease in absorption resulting from an increase in the contact time interval.
Figure 1 presents repeatedly scanned spectra, illustrating the extent of the uptake of the copper(II) complex by the polycrystalline TiO2 in water at specific time intervals. A decrease in the absorbance at ~593 nm (λmax) was observed, indicating a reduction in the concentration of the complex in solution due to its adherence to the surface. For optimal performance, a strong binding energy between the elements constituting the complex ion and the surface of the crystals is essential.
This study facilitates an investigation of the interaction between the metal complex and polycrystalline TiO2 particles with different densities of deep trap states. The surface structure of TiO2 consists of rows with 2-fold coordinated top O atoms and Ti atoms, creating channels between the rows. The Ti atoms in the rows and channels are bonded to 2-fold coordinated bridging O atoms, resulting in a distinctive “zigzag” pattern [30]. This geometric arrangement allows the complex ion to explore (as illustrated in Scheme 1) the active sites near titanium atoms in an adjacent row. Thus, the integration of the complex ion at the interfacial layer with a specific coordination environment can lead to improvements in selectivity and adsorption (Equation (1)).

Scheme 1.
Schematic illustration of the grafting mechanism of metal complex ions onto hydroxyl (-OH) groups present on the surface of TiO2. A: adsorbed complex ion, Sta: stable structure, Unsta: unstable structure, and D: desorbed species.
Surface-bound adduct:
≡TiO2 + [Cu(en)(Im)2]2+ → ≡TiO2//[Cu(en)(Im)2]2+
Thermodynamic equilibrium takes place at the interface when semiconductor particles like TiO2 come into contact with an aqueous solution of the copper precursor complex. A mechanism involving the complex ion on the TiO2 surface results in (a) adsorbed stable structures, (b) adsorbed unstable structures, and (c) desorbed ions. In particular, bound ions with stable structures are selectively removed by desorption, while the bound ions with unstable structures undergo bond cleavage. This process provides the possibility of constructing a material using the represented structural unit as a host-grafting precursor, as is described in Equation (2).
Host-grafting precursor:
≡TiO2//[Cu(en)(Im)2]2+ (in water) → [Cu(en)(Im)2]2+:TiO2
In the host-grafting precursor, [Cu(en)(Im)2]2+:TiO2, the bond length, bond angle, and energy of the complex ion differ from those of the free complex ions in solution [46,47]. The presence of the added ion in the precursor creates solvated electrochemical species, where the Fermi energy difference drives electrons from the anatase valence band toward the electronic acceptor levels of the bound species. The environment of the bound complex ion facilitates the attraction of electrons to the acceptor levels at the interface, which subsequently leads to the accumulation of semiconductor holes and reduced species [48].
The strength of the bonds between the ligands and the metal center plays a pivotal role. If the binding forces are weaker, the precursor may undergo complex non-selective decomposition. This decomposition pathway significantly influences the composition and structure of the final material, and can even induce subtle changes in the lattice.
Interestingly, the coexistence of the complex ion and the anatase matrix in the same precursor has been the primary strategy to introduce grafting ions (such as CuI) into the surface matrix of anatase. However, this approach has been proven to have little success. Alternative methods and strategies are crucial to overcome these challenges and achieve the successful incorporation of grafting ions into the anatase surface matrix, thereby enhancing the potential for advanced materials and functional devices.
Core–dopant nucleation:
[CuII(en)(Im)2]2+:TiO2 (surface electron generated by surface tension) →
CuIsurf:TiO2
CuIsurf:TiO2 (presence of dissolved O2 in water) → TiO2:CuIIsc
The active species, CuI, generated as described in Equation (3), demonstrates a lower efficiency compared to CuII. However, its unique properties enable it to intelligently exploit highly favorable conditions, facilitating penetration into the surface level of the TiO2 lattice, driven by surface residual forces [49,50]. In this way, the incorporation of Cu(I) ions re-oxidized to Cu(II) and onto the surface of the TiO2 matrix contributes to the formation of the TiO2:CuIIsc surface complex, as is shown in Equation (4). Henceforth, our prepared samples, denoted as TiO2:CuIIsc, will be referred to as Cu-TiO2 (contact time), such as Cu-TiO2 (0, 5, 10, 15, 20, or 240 min). For example, the designation Cu-TiO2 (240 min) means the isolation of the copper complex grafted onto TiO2 with a contact time interval of 240 min during the adsorption method.
Understanding and harnessing the subtleties of surface chemistry and the interactions of active species in the complex bound-surface system are essential to optimize and tailor the properties of TiO2-based materials for various applications. The insights gained from this study will open up new avenues for the design and engineering of advanced materials with enhanced functionalities and performance characteristics, advancing in the field of photocatalysis and its related applications.
2.2. Characterization of Cu-TiO2 Material
The structures of the Cu-TiO2 compounds were confirmed by XRD, FTIR, Raman, and EPR spectral analyses. The composition was identified from EDAX and XRF analyses. The morphology was investigated by SEM, AFM, and HRTEM microscopes. The weak ferromagnetic behavior was identified by VSM analysis, the emission properties were investigated by photoluminescence analysis, and the results are shown in the following sections.
2.2.1. Phase Purity of the Product
Both the pure form of polycrystalline TiO2 and the sub-surface-implanted compound, Cu-TiO2, isolated at different time intervals, exhibited characteristic patterns (see Figure 2), corresponding to the anatase crystal phase (JCPDS file numbers 01-071-1166, 00-002-0387). The anatase structure of the polycrystalline TiO2 was confirmed by the presence of diffraction peaks corresponding to the (101), (004), (200), (105), and (211) planes. The XRD patterns of anatase showed the characteristic peak at 2θ = 25.2°, corresponding to the (101) plane (JCPDS 21-1272), confirming the phase purity of the crystalline solid. Notably, the XRD patterns of both the polycrystalline TiO2 and Cu-TiO2 samples primarily belonged to the anatase form and closely resembled each other.

Figure 2.
Powder X-ray diffraction patterns: (a) pristine TiO2, (b) Cu-TiO2 (0 min), (c) Cu-TiO2 (5 min), (d) Cu-TiO2 (10 min), (e) Cu-TiO2 (15 min), (f) Cu-TiO2 (20 min), and (g) Cu-TiO2 (240 min), respectively.
The qualitative phase analysis of the diffractograms indicated that all the polycrystalline TiO2 samples [51], including those of the Cu-TiO2 compound, belonged to the anatase phase, with no evidence of impurity phases like rutile. The crystal parameters a and c, presented in Table 1, further support the conclusion that both the polycrystalline TiO2 and Cu-TiO2 samples exhibited pure anatase phase characteristics.

Table 1.
Electronic absorption spectral data and lattice parameter from XRD data for pristine TiO2 and for the prepared Cu-TiO2 samples.
The XRD pattern obtained for Cu-TiO2 closely resembled the patterns observed for the pure polycrystalline TiO2 powders. Additionally, there were no significant differences in the values of the a and c parameters between the two solids. This observation confirms that the dopant metal did not penetrate the polycrystalline TiO2 crystal lattice, causing deformation, but rather, it was adsorbed on the particle surface and subsequently grafted at the surface level. Both the polycrystalline TiO2 and Cu-TiO2 crystalline solids belonged to the pure anatase phase, supporting the successful incorporation of Cu(II) ions on the surface and sub-surface of the TiO2 lattice without causing major structural alterations. The consistent phase purity and similar crystal parameters indicate the preservation of the anatase crystal structure, which is essential for maintaining the desired photocatalytic and electronic properties of the material.
2.2.2. Identification of Surface Complex
Upon exposure to the copper(II) complex ion, discernible chemical transformations occurred on the metal oxide surface, as corroborated by FTIR spectroscopy. The vibrational spectra (Figure S1a) unmistakably reveal the existence of the Cu-TiO2 surface complex. This complex is characterized by a broad IR band spanning 3434–3465 cm−1, narrow bands at 1638 and 1402 cm−1, and a faint absorption band at 442 cm−1. The broad band at 3434–3465 cm−1 corresponds to the stretching mode ν(O-H) of hydroxyl (OH) groups, while the bands at 1638 and 1432 cm−1 represent the bending mode δ(O-H) of OH groups [52,53]. The absorption at 442 cm−1 is attributed to the stretching mode ν(Ti-O) of the Ti-O bond, typically found in a higher-energy region around 534 cm−1 [54,55].
Significantly, the intensity of the band at 507 cm−1, representing the vibration of Ti-O bonds, demonstrates an enhancement with an increasing copper content on the solid. Notably, this band at 507 cm−1 undergoes a shift to a lower-energy region [56], providing compelling evidence for the successful incorporation of Cu(II) ions into the TiO2 matrix.
However, the concentration of Cu(II) in the matrix, as determined through EDAX and XRF elemental analyses, remained relatively modest, ranging approximately between 0.05–0.32 at.% and 0.48–0.73 at.%. Remarkably, no discernible organic molecules were observed in the FTIR spectra of the Cu-TiO2 compounds due to the low content of copper species detected in the EDAX and XRF measurements. Consequently, there was no ligand moiety present on the TiO2 surface for isolated samples. This absence suggests that copper was grafted in the form of an oxide onto the surface, covalently interacting with the –OH, H2O, and COOH groups present on the TiO2 surface.
The observed alterations in the FTIR spectra not only substantiate the formation of the Cu-TiO2 surface complex, but also confirm the effective grafting of Cu(II) ions into the TiO2 surface matrix. Despite the relatively low concentration of Cu(II), these surface modifications carry significant implications for the material’s photocatalytic performance and potential applications in catalysis, sensors, and other functional devices. The FTIR analysis provides invaluable insights on the structural changes induced by exposure to Cu(II) complex ions, setting the stage for informed advancements in the utilization of Cu-TiO2 for tailored applications.
2.2.3. Raman Spectral Analyses
The discerned Raman modes in the powder unmistakably aligned with the polycrystalline TiO2 anatase phase within the Cu-TiO2 matrix. Figure 3 provides strong support for the presence of Raman frequencies distinctive from the anatase phase while affirming the absence of the rutile phase [57]. The anticipated Raman active modes for the anatase phase, including Eg: 135 cm−1; Eg: 184 cm−1; B1g: 387 cm−1; A1g and B1g: 511 cm−1; and Eg: 632 cm−1, are all observed, with the B1g: 387 cm−1 peak serving as a reliable calibration marker for the anatase phase in both the pristine TiO2 and Cu-TiO2 polycrystals (Table S1).
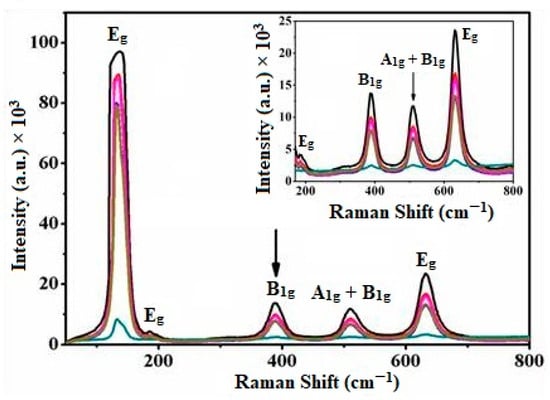
Figure 3.
Raman spectra of pure polycrystalline TiO2 and Cu-TiO2 (0, 5, 10, 20, and 240 min) reveal a decrease in peak intensity with increasing contact time. The arrows indicate this trend. The inset displays the Raman spectra in the range from 200 to 800 nm.
Notably, the absence of Raman vibrational modes indicative of secondary phases, such as metallic copper, copper oxides, or Cu-Ti oxide species, rules out the formation of segregated copper oxide on the polycrystalline TiO2. Nevertheless, within the experimental accuracy of a few wave numbers, discernible shifts and broadening are noted in the B1g: 387 cm−1 and Eg: 632 cm−1 peaks with higher grafting concentrations, indicating limited copper solubility and alterations in the surface structure. The substantial decrease in the signal intensity of the prominent peak at Eg: 135 cm−1 aligns with considerable shifts of less intense and broader peaks, suggesting the formation of the Cu-TiO2 matrix. These results underscore the significant impact of Cu(II) ions on the vibrations of the polycrystalline TiO2 crystal lattice, confirming the incorporation of grafting species.
Moreover, the observed shifts and broadening of the Raman peaks are attributed to the nonstoichiometric character of Cu-TiO2, potentially induced by the presence of oxygen vacancies. This comprehensive Raman analysis offers crucial insights into the structural modifications brought about by the integration of Cu(II) ions into the TiO2 surface matrix, further substantiating the successful formation of the Cu-TiO2 surface complex. These findings significantly contribute to our holistic understanding of the material’s structural changes, playing a pivotal role in optimizing its photocatalytic and electronic properties for a diverse range of practical applications. The Raman analysis serves as a valuable tool for characterizing the structural nuances induced by Cu(II) ion incorporation, affirming the formation of the Cu-TiO2 surface complex. These insights lay a solid foundation for advancing our understanding of the material’s properties, essential for tailoring their functionalities to meet the demands of varied practical applications.
2.2.4. Chemical Analyses of the Surface
Figure S2 presents the EDAX spectra of the Cu-TiO2 samples. Such findings reveal that the initial Cu content in the TiO2 was notably low (0.05 atom %) when the sample was isolated at 0 min, indicating a contact time of less than 30 s for the copper complex with TiO2 in water. However, it progressively increased during the adsorption period, which spanned from 5 to 240 min. Table 2 illustrates the relationship between the density of Cu in atomic percent (at.%) and the time of adsorption. It shows that the copper content in the anatase matrix increased from 0.08 at.% at 5 min to a maximum of 0.32 at.% at 240 min. Furthermore, the XRF spectra in Figure S3 exhibit two prominent peaks. The primary peak at 4.5 keV (kα) and the secondary peak at 4.9 keV (kβ) correspond to titanium, while the peaks at 8.05 keV (kα) and 8.92 keV (kβ) are attributed to copper. These findings provide valuable insights on the adsorption behavior of Cu(II) in the TiO2 matrix at different time intervals.

Table 2.
Chemical content of pristine TiO2 and Cu-TiO2 samples observed from Energy Dispersive X-ray and X-ray Fluorescence analysis.
2.2.5. Surface Topography
The SEM images of Cu-TiO2 (Figure S4) reveal a relatively rough particle surface, composed of tiny units. The particles exhibit large distributions of dimensions and shapes, with an average diameter of 160 nm. On the other hand, the images of Cu-TiO2 show a completely altered surface compared to that of the pure TiO2. Prior to adsorption, the particles were amorphous, irregularly and loosely arranged. However, the adsorption of the complex ion resulted in the formation of a packed form of polycrystalline TiO2, with occasional voids within the structure (Figure S4). The surface appearance is polygonal-like with voids, and it appears to be covered with spheres of various sizes. Generally, complex surface particles were highly agglomerated, and some exhibited non-spherical characteristics. These differences in morphology, particle shape, and size indicate the successful grafting of Cu on the TiO2 surface.
In Figure 4, HRTEM images of Cu(II):polycrystalline TiO2 isolated at 0 and 20 min adsorption time intervals are presented. The dominant dark images with black spherical appearances indicate the presence of grafting ions on the surface of the anatase. Therefore, it can be confirmed that polycrystalline TiO2 particles with Cu(II) grafting at the surface level of anatase were successfully produced. A clear boundary is observed between the grafted anatase particles, which have different shapes, sizes (ranging from 114 to 136 nm), and shades. These findings support the generation of copper(II)-grafted polycrystalline TiO2 particles. The HRTEM images exhibit a similar morphology, with particles showing a nearly spherical distribution, and the observed average particle size is consistent with the size calculated from the XRD peaks.

Figure 4.
HRTEM images of Cu-TiO2: (a) 0 min and (b) 20 min duration of uptake.
The AFM images of pure polycrystalline TiO2 and Cu-TiO2 (0, 10, 20, and 240 min) (Figures S5 and S6) demonstrate uniformly distributed grains with average heights ranging from ~0.19 to ~0.59 μm and distances from ~1.40 to ~4.51 nm. The features of the grains, measured at various angles, indicate near equidistance, relative smoothness, and alignment in a specific direction, showing some resemblance in properties [58]. The 3D images confirm that the particles form a packed surface rather than a sheet-packed surface, with compact islands. Furthermore, an enhancement in the Rms roughness is observed, which is a measure of the extension of microfacets and implies the presence of defects. The increase in the roughness of the samples is accompanied by an improvement in the grafting of copper(II) ions into the surface matrix. Consequently, from the evaluation of the roughness and grain size, it indicates an oblate spheroid shape of the grains. Notably, Cu-TiO2 appears to be rougher than the pure polycrystalline TiO2, supporting the existence of defects or coordination sites. The considerable change in surface morphology suggests the formation of the product in the form of Cu-TiO2. The AFM images provide valuable insights on the surface characteristics of both the pure polycrystalline TiO2 and Cu-TiO2. The observed differences in roughness and grain shape demonstrate that the successful modification of Cu(II) ions into the TiO2 surface lattice took place, leading to the formation of Cu-TiO2 with distinct surface properties.
2.2.6. Optical and Emission Spectral Analyses
The introduction of copper(II) ions into the polycrystalline TiO2 induced significant modifications in the semiconductor’s optical absorption properties. To scrutinize the light absorption characteristics of both the pristine polycrystalline TiO2 and Cu-TiO2, we employed diffuse reflectance spectroscopy, continuously monitoring spectral changes at various time intervals to elucidate the Cu(II) complex’s binding kinetics. The absorption spectra of Cu-TiO2 polycrystals in the range of 200–850 nm at room temperature in Figure S1b unveil distinct spectral features reflective of unique energy levels associated with visible light excitation in copper-grafting anatase titania.
A discernible shift in the spectra of the surface complex compared to pristine TiO2 is evident, showcasing a band with a λmax at 319–327 nm (Table 1). This band is attributed to the O2− → Ti4+ charge transfer transition. Moreover, an intriguing wide absorption band emerges in the 400–800 nm region (λmax at 781–789 nm) in Figure S1b. With an increase in Cu(II) content in the anatase lattice, a systematic low-energy shift in the absorption edge is observed. This minimum red shift is associated with the band gap narrowing effect or the induction of impurity-induced states within the band gap.
The identified band gap shift of 0.07 eV in the Cu-grafted (0.14 at.%) polycrystalline TiO2 is attributed to the charge-transfer transition from the d-orbitals of Cu(II) to the conduction band of polycrystalline TiO2. These alterations in the optical properties of TiO2, induced by the integration of Cu(II) ions, hold significant implications for the material’s photocatalytic behavior and light-harvesting efficiency [59,60]. These findings pave the way for informed advancements in harnessing the unique optical properties of the material for tailored applications in various scientific and technological domains.
The effect of copper(II) grafting into the anatase surface matrix was also assessed by photoluminescence spectroscopy (PL), and the results are shown in Figure S1. As is depicted in Figure S1c, a luminescence signal within the range from ~350 nm to 600 nm is observed. The line shapes in the photoluminescence spectra for all samples closely resemble each other, suggesting similar electronic state distributions within the band gap. Both pure anatase and the isolated samples from various time intervals of the adsorption experiments exhibited emission signals (excitation at 312 nm) composed of three bands at 558 nm (2.22 eV), 467 nm (2.65 eV), and 398 nm (3.12 eV), respectively.
Notably, Cu-TiO2 revealed additional fluorescence emission bands (λexc ~531) at λemi ~724 nm (1.71 eV), ~822 nm (1.51 eV), and 873 nm (1.42 eV) (Figure S1d). These luminescence bands are likely attributed to radiative processes occurring in three deep energy levels within the band gap, serving as potential carrier recombination centers [51,61,62]. The introduction of these deep energy levels in the anatase band gap, induced by the presence of copper, may act as traps for carriers or recombination centers.
Furthermore, the emission intensity of the anatase matrix at λemi ~467 nm decreased with the increase in the copper content in the lattice. This reduction in emission intensity suggests that electrons and holes in the polycrystalline TiO2 matrix could recombine more easily due to the grafting of copper(II) ions into the surface matrix. The changes in photoluminescence characteristics offer valuable insights into the modification of electronic properties brought about by the presence of copper(II) ions, which can have significant implications for the material’s photocatalytic performance and electronic applications.
The observed alterations in the photoluminescence characteristics hint at a complex interplay between carrier dynamics and recombination processes in Cu-TiO2. Further investigations into these aspects could lead to a deeper understanding of its photoactive behavior, paving the way for potential applications in optoelectronic devices. The photoluminescence analysis serves as a crucial tool for unraveling the electronic intricacies of Cu-TiO2, offering valuable insights for optimizing its performance in various applications.
2.2.7. EPR Spectral Analysis of Cu-TiO2
EPR spectroscopy, a powerful technique for paramagnetic ions, has proven to be a reliable method for assessing the successful incorporation of Cu(II) grafting in polycrystalline anatase [62]. Figure 5 displays the X-band EPR spectra measured at both room temperature (RT) and 77 K, revealing a characteristic peak with g ≈ 2.0 at approximately 308 mT. At RT, the peak is faint, whereas at the lower temperature (77 K), a more prominent and broader peak is observed. The EPR spectra exhibit an asymmetric shape, indicating the presence of Cu(II) in the distorted octahedral coordination of anatase. A distinct and intense peak centered at g⊥ = 2.13 (B⊥ = 308.5 mT and ∆Hpp = 29.6 mT) is followed by weaker signals at g1|| = 1.98 and g2|| = 1.94. While the hyperfine spectra have not been fully resolved in these materials, the characteristic spectrum can be explained by the spin-Hamiltonian: = gβ(B·S) + A(I·S), employing the usual notations. The presence of g⊥ > ge = 2.0023 (free electron) points to the existence of copper(II) ions (3d9 configuration; S = 1/2, and I = 3/2) in a regular static octahedral stereochemistry or within a tetragonal host containing misaligned axes.
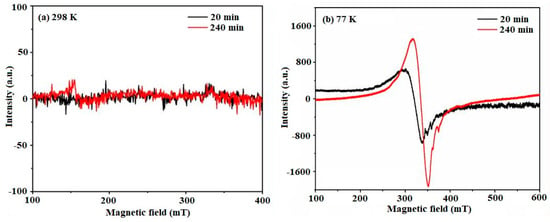
Figure 5.
EPR spectra of Cu-TiO2 at room temperature and low temperature: (a) 20 and 240 min at 298 K and (b) 20 and 240 min at 77 K.
The prominent signal observed at g = 2.13, characterized by its broad profile, signifies the presence of a dipolar interaction among adjacent Cu2+ ions. Furthermore, the identification of weaker peaks with g-values g1|| = 1.98 (B1 = 329.5 mT and ∆Hpp = 3.6 mT) and g2|| = 1.94 (B2 = 338.1 mT and ∆Hpp = 2.0 mT) points towards the existence of Ti3+ species, acting as electron traps within the system.
The comprehensive EPR analysis not only confirms the successful incorporation of Cu(II) into the polycrystalline-TiO2 lattice, but also reveals the generation of point defects such as Ti3+ and oxygen vacancies. This intricate insight into the induced modifications in the TiO2 matrix enhances our understanding of the material’s electronic and magnetic properties.
Undoubtedly, the discerned changes resulting from the grafting process hold great significance. They not only contribute to the fundamental knowledge of the material, but also play a pivotal role in optimizing its photocatalytic performance. This newfound understanding opens up avenues to tailor the material’s functionalities to meet diverse application requirements, marking a crucial step towards harnessing its full potential across various domains. The EPR analysis provides valuable information that can guide further advancements in the utilization of Cu(II)-grafting polycrystalline-TiO2 for customized applications.
2.2.8. Ferromagnetism in Cu-TiO2
The magnetization hysteresis loops of Cu-TiO2 particles obtained from VSM measurements at 298 K are presented in Figure 6. The magnetization, coercivity, and retentivity of Cu-TiO2 are listed in Table 3 with the diamagnetic contribution subtracted [63,64]. It is evident that Cu-TiO2 exhibits ferromagnetic behavior with a weak magnetic moment and feeble coercivity. The reason behind the differences in the saturation moment (Ms) values observed for the different Cu-TiO2 samples lies in their varying compositions and microstructures. The Cu-TiO2 (0 min) sample had a relatively lower Ms value compared to Cu-TiO2 (240 min), likely due to the lower concentration of Cu(II) ions incorporated into the TiO2 lattice (0.48 at.% from XRF results). Cu-TiO2 (240 min) had a higher concentration of Cu(II) ions (0.73 at.% from XRF results), and Cu-TiO2 (240 min) exhibited a higher Ms value compared to Cu-TiO2 (0 min). Interestingly, Cu-TiO2 (20 min) displayed a significantly higher Ms value compared to the other samples (0.67 at.% from XRF results). This could be attributed to factors such as the formation of specific Cu-TiO2 microstructures or the presence of Cu(II) ions in particular configurations, resulting in enhanced magnetic properties. The saturation moment values are influenced by the concentration of Cu(II) ions incorporated into the TiO2 lattice, as well as other factors such as microstructure and configuration, which can affect the magnetic properties of the material. These results are in good agreement with other reports [65]. The appearance of the magnetic moment is attributed to the generation of oxygen vacancies and distortion in the local structure around the impurity (CuII) ion [66]. The VSM measurements reveal the ferromagnetic behavior of the Cu-TiO2 samples, and the observed magnetic moments are consistent with the Cu(II) concentrations in the material. The presence of oxygen vacancies and local structural distortions induced by Cu(II) ions contributed to the magnetic properties observed in the Cu-TiO2 particles.
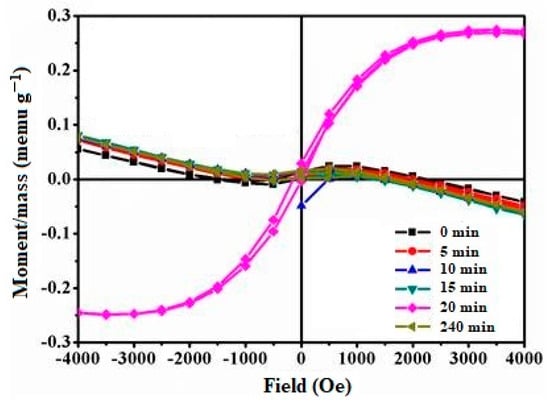
Figure 6.
Hysteresis loop of Cu-TiO2 (0 to 240 min) at 298 K.

Table 3.
Magnetic measurements of Cu-TiO2 obtained from VSM analysis at 298 K.
Consequently, the expected enhancements in the photocatalytic properties of the material are attributed to the well-engineered surface grafting process. This improvement in the distribution of Cu(II) ions contributes to the creation of a well-defined and highly efficient photocatalytic surface, enabling improved charge separation and transfer. The overall photocatalytic activity of this novel material is proved in the following section.
2.3. Photocatalytic Activity of Cu-TiO2 Materials
Prior to the photocatalytic study, our prepared samples showed minimal activity for RhB degradation in dark conditions, as depicted in Figure S7. Among these samples, Cu-TiO2 (240 min) exhibited an adsorption efficiency of 34%. This suggests that RhB readily interacted with the surface of the Cu-TiO2 composite. Such high adsorption capacity is likely to impact the degradation efficiency in a light-irradiating system. Consequently, a high photocatalytic efficiency was expected to occur for the Cu-TiO2 (240 min) sample.
In Figure 7a, the photocatalytic degradation of rhodamine B (RhB) using Cu-TiO2 under visible-light irradiation was investigated at different time intervals by monitoring changes in the UV–vis spectra. The degradation of RhB was manifested through alterations in the UV–vis absorption bands of the dye. As the photocatalytic reaction progressed, the characteristic absorption peaks of RhB gradually decreased in intensity, signifying the degradation of the dye molecule. Concurrently, the emergence of new peaks or shifts in the absorbance spectrum hinted at the formation of oxidation by-products. Analyzing the changes in the UV–vis spectrum over time allowed us to determine the degradation kinetics of RhB, providing insights into the efficiency and performance of the Cu(II)-complex grafted TiO2 as a visible-light photocatalyst for RhB degradation. The spectral data facilitated an assessment of the photocatalytic activity and the extent of RhB degradation under visible-light irradiation, shedding light on the effectiveness of the photocatalytic process for environmental remediation and water treatment applications.

Figure 7.
(a) UV–vis spectra of RhB solution in water after visible-light irradiation, and (b) photocatalytic efficiency comparison of pristine TiO2 and all our prepared samples of Cu-TiO2 (0 to 240 min), (c) recycle tests for Cu-TiO2 (240 min) sample, and (d) photoactive species test for Cu-TiO2 (240 min).
In Figure 7b, the pristine TiO2 is used as a reference for comparison. The aim of this study was to evaluate the enhanced photocatalytic performance of the Cu(II)-complex grafted TiO2 in degrading RhB dye under visible light. Both TiO2 samples were subjected to visible-light irradiation, and the degradation of RhB was monitored at regular time intervals. The photocatalytic activities of all the prepared samples were evaluated, and notably, Cu-TiO2 (240 min) demonstrated the highest efficiency in degrading RhB. After 180 min of irradiation, the photoefficiency reached an impressive 96%. This outcome underscores the superior performance of Cu-TiO2 (240 min) in catalyzing the photo-oxidation process. It is worth mentioning that the extended contact time of 240 min likely contributed to the enhanced photocatalytic efficiency observed in this sample. Additionally, the optimized synthesis conditions for Cu-TiO2 (240 min) might have played a crucial role in its superior performance compared to other samples. These results clearly demonstrated that the Cu(II)-complex grafted TiO2 exhibited a significantly higher photocatalytic activity compared to the pristine TiO2. The incorporation of the Cu(II) complex into the TiO2 surface lattice facilitated the formation of Cu-TiO2, enhancing the material’s photoactive properties under the near-UV to violet-blue portion of visible-light illumination.
The photocatalytic degradation of RhB displayed a time-dependent trend, with the Cu(II)-complex grafted TiO2 exhibiting faster degradation kinetics compared to the pristine TiO2. This enhanced photocatalytic efficiency was attributed to the material’s ability to efficiently harness visible light, leading to the generation of reactive oxygen species (ROS) that effectively degraded the RhB dye [67,68].
As is depicted in Figure 7c, the photocatalytic efficacy of the Cu-TiO2 (240 min) composite remained fairly consistent across five cycles, exhibiting a slight decline in activity. Although the photoefficiency experienced a minor decrease in the first three cycles, a more significant drop took place in the last two. This trend could be attributed to a reduction in surface activity and the appearance of additional defective sites due to prolonged irradiation.
To delineate the reactive species involved in RhB removal from water, the photocatalytic efficiency was assessed using various radical scavenging agents. For this investigation, the Cu-TiO2 catalyst (240 min) was employed. Long-term studies on free radical scavenging were conducted to explore how Cu-TiO2 (240 min) degraded RhB. Benzoquinone (BQ), ethylene diamine tetraacetic acid disodium salt (EDTA-2Na), and isopropanol (IPA) were utilized as scavengers for •O2−, h+, and •OH radicals, respectively.
As is illustrated in Figure 7d, the photocatalytic degradation of RhB markedly diminished when different scavengers were introduced into the photocatalytic system. The extent of scavenger-mediated suppression correlated with the presumed involvement of different radical species in the photo-oxidation of RhB. Notably, the IPA scavenger profoundly diminished the photocatalytic degradation activity of Cu-TiO2 (from 96 to 33%). Furthermore, combining BQ and EDTA-2Na resulted in a reduced efficacy of photocatalytic removal (from 95.7 to 66.5% and 82.4%, respectively). In the photocatalytic degradation process of RhB, •OH radicals exhibited significant prominence, with the hierarchy of importance being •OH >> •O2− >> h+. Consequently, •OH emerged as the predominant energetic radical throughout the photocatalytic process, playing a pivotal role in the degradation of RhB.
Radical scavenger agent tests conducted here contribute to supporting the statement that active photocatalytic species are generated through two main reaction mechanisms that facilitate the utilization of near-UV to violet-blue light (via a 400 nm long-pass filter): (i) interfacial electron transfer between the grafted copper complex and TiO2, and (ii) the generation of electron–hole pairs in the conduction and valence bands of TiO2. The experimental findings obtained here support the photocatalytic reaction mechanism proposed in Scheme 2. Furthermore, the adsorption affinities of the substrates to the photocatalyst surface could significantly influence the photocatalytic performance observed in this heterogeneous system. Such evidence is in agreement with that obtained in a previous study, where interfacial electron transfer played a fundamental role enhancing the photocatalytic activity of a nickel-imidazole complex grafted onto anatase [69].

Scheme 2.
Proposed photocatalytic reaction mechanism for the formation of reactive species and the degradation of RhB over Cu-TiO2 composite under near-UV to violet-blue light irradiation.
These findings highlight the promising potential of Cu(II)-complex grafted TiO2 as a visible-light photocatalyst for environmental remediation and wastewater treatment applications. The improved photocatalytic performance of this material suggests its application to addressing environmental pollution and the growing demand for sustainable and energy-efficient photocatalytic processes. Further research into and optimization of this material could lead to the development of advanced and efficient photocatalysts for various practical applications.
3. Materials and Methods
3.1. Materials
Cu(ClO4)2·6H2O (99%), KBr (spectral grade), and polycrystalline TiO2 (99.8% trace metals basis, with a melting point of 1825 °C (lit.) and a density of 3.9 g cm−3 at 25 °C (lit.)) were procured from Sigma Aldrich (Bangalore, India) for use in the preparations. Cu(ClO4)2·6H2O was handled carefully and safely, since perchlorate salts are explosive in nature. Imidazole (AR), ethylene diamine, and all other chemicals were purchased from Himedia (Mumbai, India) and SD Fine Chemicals (Chennai, India). Solvents were distilled and water was collected over alkaline potassium permanganate in glass apparatus. Analytically pure crystals of [CuII(en)(Im)2](ClO4)2 adsorbate were synthesized by a reported procedure [46,47] and recrystallized.
3.2. Preparation of Copper Grafted TiO2 by Adsorption Method
The adsorption procedure was slightly modified based on the Fuerstenau method [70]. Approximately 0.1 g of the [Cu(en)(Im)2]2+ complex (1.65 mM) was dissolved in distilled water inside a 125 mL stopper bottle. To maintain the ionic strength, a 1 M NaClO4 aqueous solution was utilized. Subsequently, 200 mg of polycrystalline TiO2 was added to the solution. The entire mixture was placed in a Technico cooling water bath shaker and subjected to different intervals of contact time (0, 5, 10, 15, 20, and 240 min) at 298 K. After specific time intervals, the resulting solutions were centrifuged to separate the solid and solution phases. The solid samples were then dried at room temperature. Both the final solid and solution samples were subjected to spectral and instrumental analyses to understand the surface affinity of the complex ion for the polycrystalline TiO2. The solid samples were labeled as 0, 5, 10, 20, and 240 min, corresponding to the intervals of contact time. It is crucial to note that the designation “0 min” indicates that TiO2 was promptly added to the concentrated precursor copper complex solution, and the solid sample was immediately isolated and dried. This process involved very brief contact time, likely less than 30 s, hence why these samples are denoted as “0 min”.
3.3. Physico-Chemical Characterization
Electron paramagnetic resonance (EPR) spectra were recorded at room temperature on a JEOL JES-FA200 ESR spectrometer (JEOL, Tokyo, Japan), operating with X-band frequencies at 298 K, −8.75−9.65 GHz, and a sensitivity of −7 × 109 spins/0.1 mT. Field modulation was employed to obtain the first derivative EPR spectrum within the temperature range from +200 °C to liquid nitrogen temperature. DPPH served as the standard for magnetic field correction in the g-factor calculations. Fourier transform infrared (FTIR) spectra were recorded using a Thermo Nicolet 6700 FTIR spectrophotometer (Waltham, MA, USA). Raman spectra were obtained on a Renishaw inVia™ confocal Raman Microscope (Renishaw, Bengaluru, India), utilizing Argon ion lasers at 488 and 514 nm (30 mV) and a diode source solid-state laser at 785 nm (100 mV). UV–Vis absorption spectra were obtained on a double-beam Shimadzu model 2450 spectrophotometer (Shimadzu Corp., Kyoto, Japan) with an integrating sphere attachment (ISR-2200) for measuring diffuse reflectance spectra (DRS). Photoluminescence (PL) spectroscopy measurements were conducted (with excitation wavelength λexc ~531 nm) using a Horiba-Jobin Yvon, SPEX-SF13-11 spectrofluorimeter (HORIBA, Ltd., Kyoto, Japan) equipped with a xenon lamp source. X-ray diffraction (XRD) measurements were conducted on an 800 W PANANALYTICAL Diffractometer (PANALYTICAL, Almelo, The Netherlands) with Cu Ka radiation and a scanning step of 0.02°. Scanning electron microscope (SEM) and Energy dispersive X-ray analysis (EDX) spectra and mapping were recorded on a Hitachi S-3400N microscope (Hitachi High-Technologies Corporation, Tokyo, Japan). X-ray Fluorescence (XRF) studies were performed using a wavelength-dispersive XRF spectrometer (Wd-XRF, Bruker S4 PIONEER) (Bruker, Bengaluru, India). High-resolution transmission electron microscope (HR-TEM) images were obtained using a JEOL model JEM 3010 EX instrument (JEOL, Tokyo, Japan) at an accelerating voltage of 200 kV. Atomic force microscopic features (AFM) of Cu-TiO2 at 298 K were collected on a Universal Scanning Probe Microscope (Innova SPM, Bengaluru, India) in tapping mode imaging, using a silicon nitride cantilever probe with a nominal tip radius of 5–10 nm. Room temperature magnetic studies were carried out using a vibrating sample magnetometer (VSM) in powder form on a Lake Shore-7404 instrument (Lake Shore Cryotronics, Woburn, MA, USA), with a vibration frequency of 82.5 Hz, dynamic range from 1 × 10−7 to 103 emu, and a maximum field at 1″ gap: 15 kOe.
3.4. Experimental Procedures to Evaluate the Photocatalytic Performance of Synthesized Materials
The photocatalytic activities of the samples were assessed by following the photodegradation of Rhodamine B (RhB, 10−4 M), using a PE.ILC CERMAX xenon illumination system (Model LX 175/300, Peccell Technologies, Inc., Yokohama, Japan). This system, equipped with a CX-04E power supply (10 A), utilized a 400 nm long-pass filter as its light source. The purpose of this filter was to permit wavelengths longer than 400 nm, typically within the visible light spectrum and occasionally extending into the near-UV range, to pass through while obstructing shorter wavelengths. A wavelength of 400 nm lies within the violet-blue portion of the visible spectrum and is categorized as near-ultraviolet light, marking the boundary between visible light and UV light. The energy of a photon with a wavelength of 400 nm is approximately 3.11 electron volts (eV) or E ≈ 4.97 × 10−19 Joules (J). The band gap energy of the synthesized samples was calculated, ranging from 3.16 to 3.23 eV. Given this range, our observations are particularly suited to explore the near-UV to violet-blue portion of the visible spectrum. All experiments were conducted within a light-sealed reactor.
Rhodamine B dye was selected here as a target contaminant, since its molecular structure is representative of widely used organic dye pollutants and many other toxic organic contaminants found in wastewater. Moreover, rhodamine B is a well-established model compound for studying photocatalytic degradation due to its stability, solubility in water, and easy detection. In future works, priority pollutants will be included to provide a more comprehensive assessment of novel materials as potential candidates for environmental remediation applications.
In a typical photocatalytic experiment, 50 mg of the catalyst was dispersed in an 80 mL aqueous solution containing Rhodamine B (RhB), with an additional 5 mL of 0.1 N sodium hydroxide (NaOH) solution, all contained within a 100 mL glass beaker. The experiment was conducted at room temperature under basic pH conditions. Prior to irradiation, the mixture was continuously stirred using a magnetic stirrer in the dark to establish adsorption–desorption equilibrium. After that, the lamp was turned on and the photocatalytic assessment was initiated. Periodically, aliquots (3 mL) were withdrawn from the suspension at 30-min intervals. These aliquots underwent centrifugation (at 15,000 rpm for 2 min) and the supernatants were analyzed using a UV–vis spectrometer. Measurements were conducted at 554 nm. Photo-efficiencies (PE) were calculated using the formula PE = (A0 − At/A0) × 100%, where A0 represents the absorbance of the RhB solution when the adsorption equilibrium is reached and At denotes the absorbance (at 554 nm) of the RhB solution measured at various irradiation times. This calculation provided a quantitative assessment of the photocatalytic performance of the samples in degrading RhB under the specified experimental conditions.
In radical trapping experiments aimed at discerning different reactive species, specific chemical reagents were used. Isopropyl alcohol (IPA) acted as an inhibitor of hydroxyl radicals (•OH), benzoquinone (BQ) was employed to capture photo-generated superoxide radicals (•O2−), and ethylene diamine tetraacetic acid disodium salt (EDTA-2Na) was used to react with photo-generated holes (h+).
4. Conclusions
The preferential adsorption of the [Cu(en)(Im)2]2+ complex ion on the solid surface led to the formation of a surface-bound adduct, denoted as ≡TiO2:[Cu(en)(Im)2]2+. This bound ion, characterized by an unstable structure, underwent bond breaking, facilitating the creation of a material represented as the host–graftant precursor, [Cu(en)(Im)2]2+:TiO2. Such distinctive species play crucial roles in generating active Cu(II) ions, serving as grafting species that efficiently penetrate the surface layer of the TiO2 lattice by exploiting favorable surface residual forces. However, it is important to note that there are ongoing debates surround grafting efficiency and its impact on polycrystalline shape, with potential variations observed across different systems. EDAX and XRF measurements validated the presence of copper in the polycrystalline TiO2, supporting the formation of Cu-TiO2 (x(at.%)Cu(II):TiO2, where x = 0⋅48 and 0⋅67 at.%). Spectroscopies and EPR analyses underscored that the grafting of these species was predominantly influenced by the surface affinity of the respective complex ions. The XRD results dispel the possibility of a phase transition from anatase to rutile during the surface reaction, confirming the formation of Cu-grafted polycrystalline anatase, a conclusion further supported by the Raman spectral analysis. Isolated samples exhibited weak ferromagnetic characteristics, consistent with observations in copper grafted polycrystalline TiO2 materials. This ferromagnetism was attributed to point defects such as Ti3+ and oxygen vacancies, as confirmed by photoluminescence and Raman spectroscopy measurements. SEM, HRTEM, and AFM images provide compelling evidence of restricted particle agglomeration due to Cu(II) grafting into the surface level. Our investigation significantly contributes to identifying viable methods for grafting polycrystalline TiO2 and expands surface analysis techniques for metal oxide semiconductors. The photocatalytic study reveals excellent visible light photocatalytic activity for the degradation of RhB in water, while magnetic studies indicate weak ferromagnetic characteristics suitable for spintronic applications. Additionally, optical and photoluminescence investigations suggest a more stable near-UV to violet-blue portion of visible-light excitation for an enhanced photocatalytic performance. This prepared material exhibits excellent photocatalytic activity under near-UV to violet-blue portion visible light irradiation, making it a promising candidate for environmentally friendly and energy-efficient applications. The remarkable photocatalytic efficiency demonstrated by this material opens up new avenues to explore its use to address pressing global challenges related to environmental pollution and clean energy generation. This study marks a pivotal advance in unlocking the potential of copper complex-grafted metal oxide surfaces for visible-light active photocatalysis. The successful integration of copper onto TiO2 polycrystalline materials not only amplifies catalytic activity, but also expands the scope of applications for these materials in environmentally significant processes, such as water purification. The results obtained here provide valuable insights into the surface interactions and grafting processes associated with Cu(II)-grafted polycrystalline TiO2 materials, significantly advancing our understanding of metal oxide semiconductor materials and enhancing their potential applications in various technological fields, including photocatalysis and beyond. These findings pave the way for the development of advanced materials with enhanced properties, addressing real-world challenges and contributing to the advancement of sustainable technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14030169/s1, Figure S1 (a) Fourier transform infrared spectra, (b) UV-Visible diffuse reflectance spectra, (c) Photoluminescence spectra at λexc ~312, and (d) Photoluminescence spectra at λexc ~531 of surface complex, TiO2:CuIIsc at various contact times. Medium: 1 M NaClO4 at 298 K; Figure S2 Energy dispersive X-ray spectra of the Cu-TiO2 at various contact time. (a) Polycrystalline TiO2, (b) 0 min, (b) 10 min, (c) 15 min, (d) 20 min, and (e) 240 min; Figure S3 X-ray fluorescence spectra of the Cu-TiO2 (x(at.%)Cu(II):TiO2, where x = 0⋅48 and 0⋅67 at.%)) at various contact times: (a) polycrystalline TiO2, (b) 0 min, (b) 10 min, (c) 15 min, (d) 20 min, and (e) 240 min; Figure S4 SEM images of the (a) Polycrystalline TiO2 and Cu-TiO2 at various contact times: (b) 0 min, (c) 5 min, (d) 15 min, (e) 20 min, and (f) 240 min; Figure S5 AFM images of the surface complex of x(at.%)Cu(II):poly-TiO2 particles isolated from the adsorption of CuII(en)(Im)22+ on the TiO2 surface at (a) 20 min and (b) 240 min adsorption periods; Figure S6 AFM images of surface complex isolated at (a) Polycrystalline TiO2, (b) 0 min, and (c) 10 min contact time in the adsorption of CuII(en)(Im)22+ on TiO2 surface; Figure S7 Adsorption efficiency comparison studies; Table S1 Raman spectral data of Cu-TiO2 at 298 K.
Author Contributions
Conceptualization, G.A.S.; methodology, G.A.S. and K.A.; software, R.K. and K.K.; validation, G.A.S., K.A. and H.V.; formal analysis, R.K. and K.K.; investigation, G.A.S. and R.K.; resources, K.A. and H.V.; data curation, G.A.S., R.K. and K.K.; writing—original draft preparation, G.A.S., K.A. and R.K.; writing—review and editing, K.K., and H.V.; visualization, H.V.; supervision, K.A. and H.V.; funding acquisition, H.V. and K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad Católica de la Santísima Concepción, Proyectos: Ingeniería 2030 (ING222010004); InES Ciencia Abierta (INCA 210005).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We gratefully acknowledge the instrumentation support from the Central Instrumentation Facility, Pondicherry University, Pondicherry, India and UGC-DAE Consortium for Scientific Research, Indore, India.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.; Jaber, F.; Dera, A.A.; Awwadi, N.S.; Ibrahium, H.A. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 2023, 13, 23087. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, J.; Zhang, Y.; Li, L.; Zhou, C.; Zhou, T.; Li, J.; Zhu, H.; Zhou, B. Unconventional Substitution for BiVO4 to Enhance Photoelectrocatalytic Performance by Accelerating Polaron Hopping. ACS Appl. Mater. Interfaces 2023, 15, 14359. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Vianello, F. Bare Iron Oxide Nanoparticles: Surface Tunability for Biomedical, Sensing and Environmental Applications. Nanomaterials 2019, 9, 1608. [Google Scholar] [CrossRef]
- Tovar-Lopez, F.J. Recent Progress in Micro- and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef]
- Haque, F.; Daeneke, T.; Kalantar-Zadeh, K.; Ou, J.Z. Two-Dimensional Transition Metal Oxide and Chalcogenide-Based Photocatalysts. Nano-Micro Lett. 2023, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Maccato, C. Nanoarchitectonics of metal oxide materials for sustainable technologies and environmental applications. CrystEngComm 2023, 25, 3968. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Salahshoori, I.; Golriz, M.; Nobre, M.A.; Mahdavi, S.; Malekshah, R.E.; Javdani-Mallak, A.; Jorabchi, M.N.; Khonakdar, H.A.; Wang, Q.; Mohammadi, A.H.; et al. Simulation-based approaches for drug delivery systems: Navigating advancements, opportunities, and challenges. J. Mol. Liq. 2024, 395, 123888. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Hossain, N.; Mobarak, M.H.; Mimona, M.A.; Islam, M.A.; Hossain, A.; Zohura, F.T.; Chowdhury, M.A. Advances and significances of nanoparticles in semiconductor applications—A review. Results Eng. 2023, 19, 101347. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, A.J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Altarawneh, S.S.; El-Kaderi, H.M.; Richard, A.J.; Alakayleh, O.M.; Aljaafreh, I.Y.; Almatarneh, M.H.; Ababneh, T.S.; Al-Momani, L.A.; Aldalabeeh, R.H. Synthesis, Characterization, and Environmental Applications of Novel Per-Fluorinated Organic Polymers with Azo- and Azomethine-Based Linkers via Nucleophilic Aromatic Substitution. Polymers 2023, 15, 4191. [Google Scholar] [CrossRef]
- Nguyen, S.-T.; Nguyen, C.Q.; Hieu, N.N.; Phuc, H.V.; Nguyen, C.V. First-principles investigations of metal–semiconductor MoSH@MoS2 van der Waals heterostructures. Nanoscale Adv. 2023, 5, 4979. [Google Scholar] [CrossRef] [PubMed]
- Alshangiti, D.M.; El-damhougy, T.K.; Zaher, A.; Madani, M.; Ghobashy, M.M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023, 13, 35251. [Google Scholar] [CrossRef] [PubMed]
- Wecker, A. Advanced Optical Materials: Visible Growth and Shiny Research. Adv. Optical Mater. 2024, 12, 2303084. [Google Scholar] [CrossRef]
- Anbalagan, K.; Ganeshraja, A.S.; Mahalakshmi, C.M. Excited Nanoscale-TiO2 Induced Interfacial Electron Transfer Reaction of Redox Active Cobalt(III)-Alkyl Amine Complex and the Solid Surface. Mater. Chem. Phys. 2012, 134, 747. [Google Scholar] [CrossRef]
- Anbalagan, K. UV-Sensitized Generation of Phasepure Cobalt-Doped Anatase: CoxTi1−xO2−δ Nanocrystals with Ferromagnetic Behavior Using Nano-TiO2/cis-[CoIII(en)2(MeNH2)Cl]2+. J. Phys. Chem. C 2011, 115, 3821. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, S.; Yu, M.; Wang, T.; Huang, S.; Yan, S.; Xu, M. Manipulating the Charge State of Au Clusters on Rutile TiO2 (110) Single Crystal Surfaces through Molecular Reactions Probed by Infrared Spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 17660. [Google Scholar] [CrossRef]
- Wooh, S.; Kim, T.Y.; Song, D.; Lee, Y.G.; Lee, T.K.; Bergmann, V.W.; Weber, S.A.L.; Bisquert, J.; Kang, Y.S.; Char, K. Surface Modification of TiO2 Photoanodes with Fluorinated Self Assembled Monolayers for Highly Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 25741. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Advancements in Improving Selectivity of Metal Oxide Semiconductor Gas Sensors Opening New Perspectives for Their Application in Food Industry. Sensors 2023, 23, 9548. [Google Scholar] [CrossRef]
- Choudhary, K.; DeCost, B.; Chen, C.; Jain, A.; Tavazza, F.; Cohn, R.; Park, C.W.; Choudhary, A.; Agrawal, A.; Billinge, S.J.; et al. Recent advances and applications of deep learning methods in materials science. npj Comput. Mater. 2022, 8, 59. [Google Scholar] [CrossRef]
- Weber, H.; Salanne, M.; Kirchner, B. Toward an Accurate Modeling of Ionic Liquid—TiO2 Interfaces. J. Phys. Chem. C 2015, 119, 25260. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis Mechanisms and Materials. Chem. Rev. 2014, 114, 9919. [Google Scholar] [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for Sensitized Solar Cells. Chem. Soc. Rev. 2015, 44, 8326. [Google Scholar] [CrossRef] [PubMed]
- Ihara, T.; Miyoshi, M.; Ando, M.; Sugihara, S.; Iriyama, Y. Preparation of a Visible Light-Active TiO2 Photocatalyst by RF Plasma Treatment. J. Mater. Sci. 2001, 36, 4201. [Google Scholar] [CrossRef]
- Neubert, S.; Mitoraj, D.; Shevlin, S.A.; Pulisova, P.; Heimann, M.; Du, Y.; Goh, G.K.L.; Pacia, M.; Kruczała, K.; Turner, S.; et al. Highly Efficient Rutile TiO2 Photocatalysts with Single Cu(II) and Fe(III) Surface Catalytic Sites. J. Mater. Chem. A 2016, 4, 3127. [Google Scholar] [CrossRef]
- Fujishima, M.; Takatori, H.; Tada, H. Interfacial Chemical Bonding Effect on the Photocatalytic Activity of TiO2–SiO2 Nanocoupling Systems. J. Colloid Interface Sci. 2013, 361, 628. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zou, X.; Chen, J.S. Self-Modification of Titanium Dioxide Materials by Ti3+ and/or Oxygen Vacancies: New Insights into Defect Chemistry of Metal Oxides. RSC Adv. 2014, 4, 13979. [Google Scholar] [CrossRef]
- Hutchings, G.S.; Lu, Q.; Jiao, F. Synthesis and Electrochemistry of Nanocrystalline M-TiO2 (M = Mn, Fe, Co, Ni, Cu) Anatase. J. Electrochem. Soc. 2013, 160, A511. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Rozman, N.; Leoni, M.; Seabra, M.P.; Skapin, A.S.; Pullar, P.C.; Labrincha, J.A. Cu–TiO2 Hybrid Nanoparticles Exhibiting Tunable Photochromic Behavior. J. Phys. Chem. C 2015, 119, 23658. [Google Scholar] [CrossRef]
- Liu, M.; Sunada, K.; Hashimoto, K.; Miyauchi, M. Visible-Light Sensitive Cu(II)–TiO2 with Sustained Anti-Viral Activity for Efficient Indoor Environmental Remediation. J. Mater. Chem. A 2015, 3, 17312. [Google Scholar] [CrossRef]
- Bashiri, R.; Mohamed, N.M.; Kait, C.F.; Sufian, S. Hydrogen Production from Water Photosplitting using Cu/TiO2 Nanoparticles: Effect of Hydrolysis Rate and Reaction Medium. Int. J. Hydrogen Energy 2015, 40, 6021. [Google Scholar] [CrossRef]
- Olszowski, P.; Zajac, L.; Godlewski, S.; Such, B.; Johr, R.; Glatzel, T.; Meyer, E.; Szymonski, E. Role of a Carboxyl Group in the Adsorption of Zn Porphyrins on TiO2(101) Surface. J. Phys. Chem. C 2015, 119, 21561. [Google Scholar] [CrossRef]
- Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D. Recent Investigations on the Use of Copper Complexes as Molecular Materials for Dye-Sensitized Solar Cells. Molecules 2023, 29, 6. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.; Park, J.; Biswas, P. In Situ Charge Characterization of TiO2 and Cu-TiO2 Nanoparticles in a Flame Aerosol Reactor. J. Nanopart. Res. 2012, 14, 678. [Google Scholar] [CrossRef]
- Yoong, L.S.; Chong, F.K.; Dutta, B.K. Development of Copper-Doped TiO2 Photocatalyst for Hydrogen Production under Visible Light. Energy 2009, 34, 1652. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Bao, S.H.; Lv, Y.H.; Jin, P. Activation and Enhancement of Room-Temperature Ferromagnetism in Cu-Doped Anatase TiO2 Films by Bound Magnetic Polaron and Oxygen Defects. Appl. Matter Interfaces 2014, 6, 22243. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.B.; Rafique, M.; Sagir, M.; Ullah, S.; Kiran, H. Nanomaterials for Photocatalytic Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem Catalysis 2022, 2, 1315. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.U.H. Modification strategies of TiO2 based photocatalysts for enhanced visible light activity and energy storage ability: A review. J. Environ. Chem. Eng. 2023, 11, 111532. [Google Scholar] [CrossRef]
- Navarro, T.; Jaroslav, L.K.C. Enhancing Photocatalytic Properties of TiO2 Photocatalyst and Heterojunctions: A Comprehensive Review of the Impact of Biphasic Systems in Aerogels and Xerogels Synthesis, Methods, and Mechanisms for Environmental Applications. Gels 2023, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-D.; Chen, C.-W.; Kao, C.-M.; Hung, C.-M. Synthesis, characterization, and application of CuO-modified TiO2 electrode exemplified for ammonia electro-oxidation. Process Saf. Environ. Prot. 2017, 112, 243. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Quan, X.; Qiu, F.; Zhang, X. Synthesis of CuOx/TiO2 Photocatalysts with Enhanced Photocatalytic Performance. ACS Omega 2023, 8, 2723. [Google Scholar] [CrossRef]
- Kim, K. FT-IR Spectroscopic Characterization of Oxidized and Reduced Titania. Bull. Korean Chem. Soc. 1990, 11, 396. [Google Scholar]
- Lay, G.L.; Oughaddou, H. Structure, Electronics and Dynamics of Clean and Metal Adsorbed Semiconductor Surfaces: Recent Results and Perspectives. J. Phys. Condens. Matter 2001, 13, 11195. [Google Scholar] [CrossRef]
- Gebre, S.T.; Kiefer, L.M.; Guo, F.; Yang, K.R.; Miller, C.; Liu, Y.; Kubiak, C.P.; Batista, V.S.; Lian, T. Amine Hole Scavengers Facilitate Both Electron and Hole Transfer in a Nanocrystal/Molecular Hybrid Photocatalyst. J. Am. Chem. Soc. 2023, 145, 3238. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.; Amal, R.; Tran, T. Photocatalytic Degradation of Cyanide using Titanium Dioxide Modified with Copper Oxide. Adv. Environ. Res. 2002, 6, 471. [Google Scholar] [CrossRef]
- Walcarius, A.; Mercier, L. Mesoporous Organosilica Adsorbents: Nanoengineered Materials for Removal of Organic and Inorganic Pollutants. J. Mater. Chem. 2010, 20, 4478. [Google Scholar] [CrossRef]
- Kang, S.; Shin, K.; Prabakar, K.; Lee, C. Optical and Electrical Properties of ZnO Doped with Nitrogen. Phys. Status Solidi B 2004, 241, 2830. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Abu-Salah, K.M.; Dutta, J. Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward Efficient Visible Light Phenol Degradation. Catalysts 2017, 7, 133. [Google Scholar] [CrossRef]
- Lozano, H.; Devis, S.; Aliaga, J.; Alegría, M.; Guzmán, H.; Villarroel, R.; Benavente, E.; González, G. Two-Dimensional Titanium Dioxide–Surfactant Photoactive Supramolecular Networks: Synthesis, Properties, and Applications for the Conversion of Light Energy. Int. J. Mol. Sci. 2022, 23, 4006. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Dimitrijevic, N.M.; Chen, L.; Rajh, T.; Gray, K.A. Role of Surface/Interfacial Cu2+ Sites in the Photocatalytic Activity of Coupled CuO-TiO2 Nanocomposites. J. Phys. Chem. C 2008, 112, 19040. [Google Scholar] [CrossRef]
- Ganeshraja, A.S.; Anbalagan, K. Participatioin of Nanocrystalline TiO2 Surface in the Electron Transfer between Semiconductor Solid an Adsorbed Cobalt(III)-Rpy Complex. Nano Syst. Phys. Chem. Math. 2013, 4, 276. [Google Scholar]
- Zhang, J.Y.; Boyd, I.W.; O’Sullivan, B.J.; Hurley, P.K.; Kelly, P.V.; Senateur, J.P. Nanocrystalline TiO2 Films Studied by Optical, XRD and FTIR Spectroscopy. J. Non-Cryst. Solids 2002, 303, 134. [Google Scholar] [CrossRef]
- Francisco, M.S.P.; Mastelaro, V.R. Inhibition of the Anatase–Rutile Phase Transformation with Addition of CeO2 to CuO–TiO2 System: Raman Spectroscopy, X-ray Diffraction, and Textural Studies. Chem. Mater. 2002, 14, 2514. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Adsorption of Heavy Metals on Kaolinite and Montmorillonite: A Review. Phys. Chem. Chem. Phys. 2012, 14, 6698. [Google Scholar] [CrossRef] [PubMed]
- Hadjiivanov, K.; Klissurski, D.; Kantcheva, M. State and Localization of Cobalt, Nickel and Copper Ions Adsorbed on Titania (Anatase). J. Chem. Soc. Faraday Trans. 1991, 87, 907. [Google Scholar] [CrossRef]
- Navas, J.; Coronilla, A.S.; Aguilar, T.; Hernández, N.C.; De los Santos, D.M.; Márquez, J.S.; Zorrilla, D.; Lorenzo, C.F.; Alcántara, R.; Calleja, J.M. Experimental and Theoretical Study of the Electronic Properties of Cu-Doped Anatase TiO2. Phys. Chem. Chem. Phys. 2014, 16, 3835. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, W.; Liu, W.; Zhang, S. Preparation, Characterization and Activity Evaluation of p–n Junction Photocatalyst p-ZnO/n-TiO2. Appl. Surf. Sci. 2008, 255, 2478. [Google Scholar] [CrossRef]
- Rajkumar, K. UV Light Induced Formation of Co Ni Cu Zn Nanocrystaline SnO2 Semiconductor and Preparation of Coordination Compound Precursors. 2017. Available online: https://shodhganga.inflibnet.ac.in:8443/jspui/handle/10603/244246 (accessed on 21 January 2024).
- Hou, D.L.; Meng, H.J.; Jia, L.Y.; Ye, X.J.; Zhou, H.J.; Li, X.L. Impurity Concentration Study on Ferromagnetism in Cu-Doped TiO2 Thin Films. Europhys. Lett. 2007, 78, 67001. [Google Scholar] [CrossRef]
- Li, J.; Sow, C.H.; Rao, X.S.; Ong, C.K.; Zheng, D.N. Epitaxial Growth and Magnetic and Electric Properties of Co-Doped TiO2 Thin Films. Eur. Phys. J. B 2003, 32, 471. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gajbhiye, N.S. Room Temperature Magnetic Properties of Cu-Doped Titanate, TiO2(B) and Anatase Nanorods Synthesized by Hydrothermal Method. Mater. Chem. Phys. 2012, 132, 175. [Google Scholar] [CrossRef]
- Venkatesan, M.; Fitzgerald, C.B.; Coey, J.M.D. Thin films: Unexpected Magnetism in a Dielectric Oxide. Nature 2004, 430, 630. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Sang, D.; Zou, L.; Yao, Y.; Zhou, C.; Fu, H.; Xi, H.; Fan, J.; Meng, L.; Wang, C. A Review on the Progress of Optoelectronic Devices Based on TiO2 Thin Films and Nanomaterials. Nanomaterials 2023, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ju, Y.; Li, F.; Yan, S.; Gong, Y.; Deng, Z.; You, Y.; Luo, J.; Zhang, S. Simultaneous enhanced generation of reactive oxygen species and H2 over Pd-(Cu-s-FeO) trimetal for bifunctional catalytic degradation of refractory combined pollutants. J. Clean. Prod. 2024, 434, 139799. [Google Scholar] [CrossRef]
- Ganeshraja, A.S.; Thirumurugan, S.; Rajkumar, K.; Wang, J.; Anbalagan, K. Ferromagnetic nickel(II) imidazole-anatase framework: An enhanced photocatalytic performance. J. Alloys Comp. 2017, 706, 485–494. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Asare, K.O. Adsorption of Copper, Nickel, and Cobalt by Oxide Adsorbents from Aqueous Ammoniacal Solutions. J. Colloid Interface Sci. 1987, 118, 524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).