Abstract
Methane is an abundant and relatively clean fossil fuel resource; therefore, its utilization as a chemical feedstock has a major impact on the chemical industry. However, its inert nature makes direct conversion into value-added products difficult under mild conditions. Compared to the gas-phase selective oxidation of methane, there have been several recent advances in the liquid-phase conversion of methane. This review categorizes the reports on the liquid-phase selective oxidation of methane according to the solvent and oxidant used. The advantages and disadvantages of each approach are discussed. High yields of methyl bisulfate as a methanol precursor can be achieved using SO3 in sulfuric acid; however, more attention should be paid to the separation process and overall economic analysis. However, the aqueous-phase selective oxidation of methane with in situ generated H2O2 is quite promising from an environmental point of view, provided that an economical reducing agent can be used. Based on the current state-of-the-art on this topic, directions for future research are proposed.
1. Introduction
Methane is abundant in nature and is the main component of natural gas, shale gas, coal bed methane, associated gases, biogas, and gas hydrates [1]. As these resources are relatively clean compared to other fossil resources, including oil and coal, methane is considered a promising feedstock for the chemical industry. However, because methane exists as a gas in nature, its utilization is limited as it must either be utilized on-site or transported to consumers in the form of liquefied or pipelined natural gas. In addition, many natural gas resources are not sufficiently large to be economically transported by conventional means; therefore, they are flared away or left unutilized [2].
Methane is primarily used as a fuel due to having the highest calorific value of combustion per carbon of any hydrocarbon and emitting less carbon dioxide from any fossil fuel while producing the same amount of heat. However, the demand for methane as a fuel is expected to decrease as renewable energy becomes more prevalent. It is more economical to convert methane into high-value chemicals than to use it as a fuel. However, its utilization as a chemical feedstock is limited because of its inertness in chemical reactions.
In general, methane activation is difficult because of its very high C-H bond dissociation energy (BDE) of 439 kJ/mol (Figure 1) [3,4]. Additionally, the target product (e.g., methanol) is more reactive than methane itself, as the C-H BDE of methanol is ~402 kJ/mol (Figure 1) [4]. Therefore, it is difficult to achieve high selectivity for the target product with a high methane conversion. Furthermore, when comparing the ionization potential, proton affinity, electron affinity, highest occupied molecular orbital, and water solubility (applicable to liquid-phase reactions), which are measures of reaction activity, methane is expected to have a lower reactivity than methanol (Figure 1) [4]. Accordingly, even if methane is activated and oxidized, over-oxidation or complete oxidation to CO2 of the reaction intermediate occurs easily, making it difficult to select a catalyst and set the reaction conditions for the partial oxidation of methane (POM) to achieve high yields of reaction intermediates. Consequently, the yield of the target product is low, making it costly to separate the product and recover the unreacted methane.
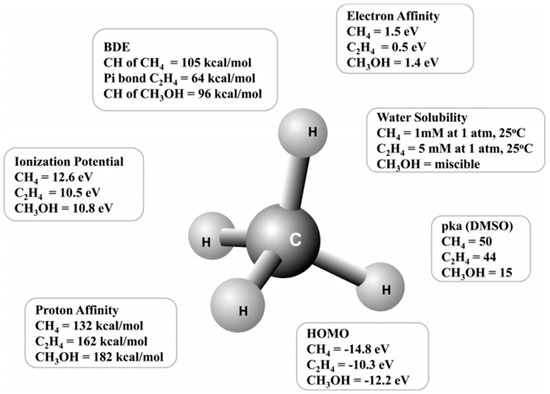
Figure 1.
Various quantitative measures of the reactivity of methane, ethylene, and methanol [4]. Copyright 2017, American Chemical Society.
The current commercial routes for methane conversion rely on an indirect methane conversion pathway involving the initial synthesis of syngas, a mixture of H2 and CO, through processes such as methane steam reforming (CH4 + H2O ⇄ CO + 3H2, ∆G0298K = 142 kJ/mol, ∆H0298K = 206 kJ/mol), methane autothermal reforming (CH4 + 1/3O2 + 1/3H2O ⇄ CO + 7/3H2, ∆G0298K = −10.5 kJ/mol, ∆H0298K = 45 kJ/mol), or methane dry reforming (CH4 + CO2 ⇄ 2CO + 2H2, ∆G0298K = 171 kJ/mol, ∆H0298K = 247 kJ/mol). The syngas produced is further processed using well-established C1 chemical processes, including methanol synthesis [5] and Fischer–Tropsch synthesis [6], to produce various chemicals, including methanol, olefins, and synthetic fuels. Because this indirect methane conversion process includes an energy-intensive syngas synthesis step, it is only economically viable at a large scale [7]. It has several drawbacks, such as high production costs, significant energy consumption, and substantial capital investments. As a result, there has been growing interest in exploring direct methane conversion methods as alternatives to current indirect routes. The direct conversion of methane has the potential for more cost-effective and energy-efficient processes, making it an attractive option for producing value-added products from methane.
Direct methane conversion can be broadly categorized into gas- and liquid-phase pathways (Figure 2). The gas-phase routes encompass the POM (CH4 + 1/2O2 → CH3OH, ∆G0298K = −112 kJ/mol, ∆H0298K = −126 kJ/mol), selective halogenation (CH4 + 1/2X2 → CH3X, X = Cl, Br, and I) with subsequent hydrolysis (CH3X + H2O ⇄ CH3OH + HX, X = Cl, Br, and I), oxidative coupling of methane (OCM) (CH4 + 1/2O2 → 1/2C2H4 + H2O, ∆G0298K = −144 kJ/mol, ∆H0298K = −141 kJ/mol), dehydroaromatization (DHA) (CH4 → 1/6C6H6 + 3/2H2, ∆G0298K = 72.1 kJ/mol, ∆H0298K = 89 kJ/mol), and non-oxidative coupling of methane (NOCM) (CH4 → 1/2C2H4 + H2, ∆G0298K = 84.7 kJ/mol, ∆H0298K = 101 kJ/mol). In contrast, the liquid-phase reactions involve two representative routes: the direct oxidation of methane to methane oxygenates (e.g., methanol, formaldehyde, and formic acid) and methanol synthesis via a stable methanol precursor (e.g., methyl bisulfate (MBS) and methyl trifluoroacetate (MeTFA)) in strong acids (e.g., sulfuric acid (H2SO4) and trifluoroacetic acid (HTFA)). The POM has a typical characteristic in which the selectivity to the value-added methane oxygenates decreases with increasing methane conversion [8,9]. However, recent noticeable progress has been made in the liquid-phase direct conversion of methane [9,10]. Therefore, this review focuses on liquid-phase selective oxidation of methane to methane oxygenates using various oxidants in different solvents.
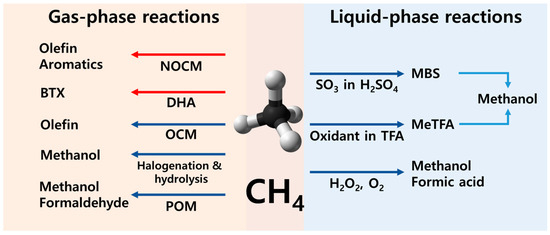
Figure 2.
Direct conversion of methane to various chemicals. The red and blue arrows mean endothermic and exothermic reactions, respectively.
2. Liquid-Phase Partial Oxidation of Methane in Strong Acids
Over the last few decades, homogeneous organometallic catalysts have been investigated for the selective oxidation of methane in strong acids [4,10]. Because methane has a strong C-H bond, the choice of the central metal and ligand is important for the activation of methane. In addition, an appropriate choice of oxidizing agent is important to activate C-H bonds, oxidize low-valent central metals to high-valent ones, and avoid the overoxidation of methane oxygenates and ligand degradation. The use of strong acids (e.g., H2SO4 and HTFA) is beneficial for stabilizing the reaction intermediates (MBS and MeTFA) because these methanol precursors are more resistant to electrophilic attack than the methanol itself.
The Shilov system can be introduced as a homogeneous organometallic catalyst for the selective functionalization of methane. The reaction follows the Shilov cycle (Figure 3), which is composed of three major steps: electrophilic activation of the C-H bond, oxidation of the complex, and nucleophilic oxidation of the alkane substrate [11]. Therefore, an alkane (RH) is selectively oxidized to an alcohol (ROH) or alcohol precursor (RCl) catalyzed by PtIICl2 with an oxidant ([PtIVCl6]2−). Considerable research has been conducted to increase the productivity of methanol precursors and make the entire process more economical through changes in catalysts, oxidants, and solvents.
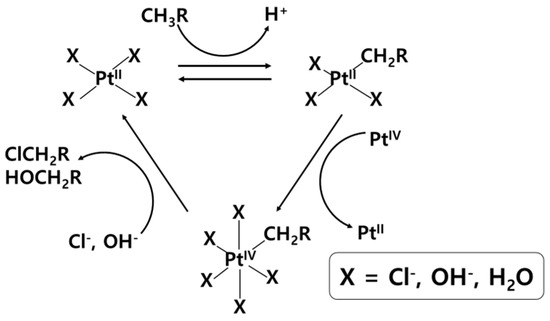
Figure 3.
Shilov cycle for functionalization of C−H bond in alkanes [11].
2.1. HTFA
2.1.1. Potassium Persulfate (K2S2O8)
K2S2O8 is a radical initiator and strong oxidizing agent. It has been frequently used for methane oxidation in the presence of metal catalysts, and the reduced catalysts can be reoxidized with K2S2O8. Electrophilic transition-metal compounds have been reported for the C-H bond activation of alkanes, including methane, in HTFA [12]. In particular, the Pd(II) complex is an attractive choice because of its strong electrophilic properties and ease of reoxidation to Pd(II) ions using K2S2O8 as the oxidant (entry 1, Table 1). N-Heterocyclic carbene (NHC)-Pd complexes were examined for the POM using K2S2O8 (entry 2, Table 1) [13,14,15]. Despite the high activity of the catalysts with bromide ligands, the reaction of Pd-NHC with iodide ligands did not produce any product [13]. On the other hand, when Pd was substituted with Pt, which is already known as an active metal for the POM, decomposition of the Pt complex and aggregation to form Pt black were observed. PdCl42−-HTFA systems with large amine-based cations, such as tetramethylammonium ([Me4N]+), 1,2,3-trimethylimidazolium ([TMIm]+), and 1,1,3,3-tetramethylguanidine ([TMG]+), have also been used for the POM with K2S2O8 [16]. Among the various PdCl42− catalysts, [Me4N]2[PdCl4] showed the best catalytic activity. During the reaction (entry 3, Table 1), PdCl42− is first converted to PdTFA42−, which can activate the C-H bond in methane, and Pd(II) is oxidized to Pd(IV) with H2S2O8. Finally, reductive elimination produces MeTFA (Figure 4). Generally, metals are easily leached, and most conventional supports degrade in HTFA, making it difficult to heterogenize homogeneous metal complexes for this reaction. Recently, Zhang et al. [17] immobilized Pd species in the porous organic polymer Pyr-POPs(pyridine-based porous organic polymers), which can predominantly capture methane, and reported high catalytic activity for this reaction (entry 4, Table 1).
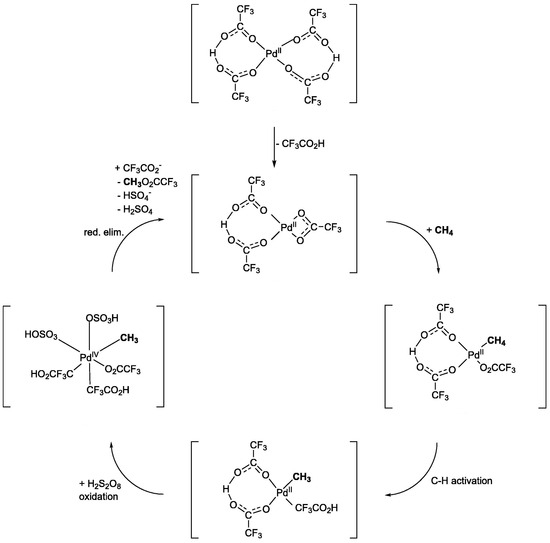
Figure 4.
Plausible reaction mechanism for methane oxidation using PdTFA42− [16]. Adapted with permission. Copyright 2022, Elsevier.
In addition to Pd complexes, various transition metal salts (e.g., Ti, Fe, Cr, Mn, and Cu) have been tested for this reaction. Among these, Cu(OAc)2 appeared to be the most effective catalyst for the production of MeTFA and methyl acetate from a mixture of HTFA and trifluoroacetic anhydride (TFAA) (entry 5, Table 1) [18]. A redox cycle between Cu(I) and Cu(II) and the participation of a methyl radical are proposed in the reaction mechanism (Figure 5).
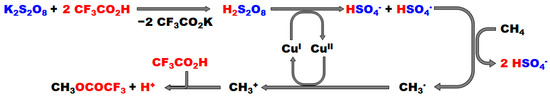
Figure 5.
Possible mechanism for the partial oxidation of methane catalyzed by Cu(II) cation [18]. The red and blue letters mean chemical species related to HTFA and K2S2O8, respectively. Adapted with permission. Copyright 2000, Wiley.
Recently, a simple CuO catalyst was reported for the POM with K2S2O8 in HTFA/TFAA (entry 6, Table 1) [19]. The copper species dissolved in the solvent and generated KSO4 radicals from K2S2O8. This radical abstracts H from methane to form a methyl radical, which further reacts to produce MeTFA. Ultraviolet–visible spectra showed the reoxidation of reduced copper oxide (Cu2O) after the reaction with persulfate. Table 1 compares the activities of some active catalyst systems based on Pd and Cu complexes for POM with K2S2O8 in HTFA. Even though relatively high turnover frequencies (TOFs) can be obtained at low temperatures (≤100 °C), there are some critical problems in this system. K2S2O8 is not regenerative and HTFA is decomposed in the presence of K2S2O8 [16].

Table 1.
Comparison of catalytic systems for the partial oxidation of methane using K2S2O8 in HTFA.
Table 1.
Comparison of catalytic systems for the partial oxidation of methane using K2S2O8 in HTFA.
| Entry | Catalyst | Temp. (°C) | K2S2O8 (mmol) | Gas Composition (bar) | TON | TOF (h−1) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Pd(CH3COO)2 | 80 | 21 | CH4 = 20 | 3.8 | 0.2 | [13] |
| 2 | Pd-NHC * | 90 | 21 | CH4 = 30 | 30 | 2.1 | [13] |
| 3 | [Me4N]2[PdCl4] | 80 | 10 | CH4 = 20 | 330 | 22.0 | [16] |
| 4 | Pyr-POPs-Pd * | 80 | 20 | CH4 = 1 | 664 | 33.2 | [17] |
| 5 | Cu(CH3COO)2 | 100 | 5 | CH4:N2 = 5:25 | 30.4 | 1.5 | [18] |
| 6 | CuO | 90 | 2.8 | CH4 = 5.2 | 33 | 1.9 | [19] |
* NHC: N-doped heterocyclic carbene. Pyr-POPs: pyridine-based porous organic polymers.
2.1.2. Hydrogen Peroxide (H2O2)
Hydrogen peroxide (H2O2) is an environmentally friendly oxidant because it emits only water after oxidation. The conventional commercial process for H2O2 synthesis is based on the use of anthraquinone (AQ) [20]. Recently, the synthesis of H2O2 directly from H2 and dioxygen (O2) has been actively investigated [21,22,23,24]; however, it is not yet competitive.
H2O2 was used instead of K2S2O8 for the POM in a Pd/TFA catalytic system with excess TFAA to remove the H2O formed from H2O2 [18,19,25,26,27,28]. In the absence of TFAA, MeTFA was further hydrolyzed to CH3OH, which was readily oxidized to CO2. H2O2 can be added directly to the reaction medium or synthesized in situ from H2 and O2. Lin et al. [29] used a CO/O2/H2O system instead of an H2/O2 system to synthesize H2O2 in situ at relatively high temperatures (70–100 °C) to oxidize methane to MeTFA (entry 1, Table 2). In the CO/O2/H2O system, the water–gas shift reaction (CO + H2O ⇄ CO2 + H2) occurs over Pd/C, and H2 and O2 can be combined to produce H2O2 over Pd/C. The addition of CuCl2 to the CO/O2/H2O system resulted in the formation of methanol and its derivative (MeTFA) as the main products [29]. The presence of Cl− ion is essential for the conversion of methane to methanol and its ester. The yield of MeTFA was affected by the halide ions and decreased in the order Cl− > Br− > I−. The POM was further examined over Pd/C with various metal ions (Cu, V, etc.) in the CO/O2/H2O system (entries 2–5, Table 2) [27,28,30]. It was found that the nature of the co-catalyst (mainly Cu and V species), the presence of Cl−, and the composition of the solvent significantly impacted the structure of Pd species and consequently influenced the yield of MeTFA.

Table 2.
Comparison of catalytic systems for the partial oxidation of methane using H2O2 and H2O2 generated in situ from a CO/O2/H2O or an H2/O2 system in HTFA/TFAA.
TFAA must be used in conjunction with TFA when using H2O2 directly or when generated in situ. Otherwise, the produced MeTFA hydrolyzes to methanol, which can easily be further oxidized to HCOOH and CO2, resulting in lower yields of methanol and its derivatives. Therefore, an additional unit for the synthesis of TFAA from TFA via dehydration was required for the synthesis of methanol from methane using H2O2 as an oxidant.
2.1.3. O2
O2 is an ideal oxidant for selective oxidation of hydrocarbons. However, the POM with O2 is a spin-forbidden reaction because methane and O2 exist in the singlet and triplet states, respectively. Therefore, this reaction is generally performed at relatively high temperatures, even in the presence of a catalyst.
The POM using O2 was examined in the presence of various metal-trifluoroacetate salts, including Pd, Mn, Fe, Co, Cu, and Pb, in HTFA at 180 °C [32]. Mn and Co salts showed 30 and 90% yields of MeTFA based on the amount of catalyst added, respectively. Furthermore, a 50% yield of MeTFA based on the amount of methane introduced was obtained at 180 °C in the presence of Co salts using O2 as an oxidant in TFA/TFAA solution [33].
Recently, Blankenship et al. [34] demonstrated the conversion of aerobic methane to methyl esters in the presence of dilute TFA in perfluorohexane over a Co/SiO2 catalyst. Among the catalytic systems using O2 as the sole oxidant, this catalyst exhibited the highest MeTFA productivity, and the spent catalyst could be easily reactivated by heat treatment. The catalytic activity of supported Mn catalysts was recently reported by the same group [35]. The higher methyl ester productivity (c.a. 1000 μmol/gcat./h) than Co/SiO2 has been reported for supported Mn catalysts. However, the leaching of the active metal from the catalyst surface and deactivation of the catalyst to MnF2 have also been reported.
There have only been a few reports on the aerobic oxidation of methane in HTFA; however, all reactions require relatively high temperatures, resulting in the decomposition of HTFA. The problem of corrosion caused by HTFA cannot be overlooked from a practical perspective and raises concerns from an economic perspective.
2.2. H2SO4
Oleum, also known as fuming H2SO4, has sulfur trioxide (SO3) in H2SO4. SO3 is commercially produced through the oxidation of SO2 with O2 and can be hydrolyzed to H2SO4. SO3 can act as an oxidizing agent for POM [36] during the conversion to SO2, which can then be converted back to SO3 via an oxidation reaction with O2. Throughout the entire process, oxygen was indirectly utilized for the POM with SO3. MBS was produced as a methanol precursor during the POM with SO3 in H2SO4 (Figure 6a). This MBS was more stable against further oxidation than methanol, similar to MeTFA in the HTFA system.
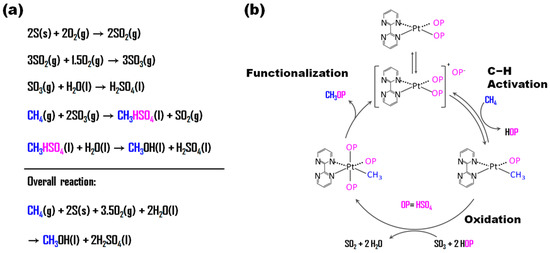
Figure 6.
(a) Overall scheme for methanol synthesis via MBS. The blue and pink letters mean chemical species related to methane and HSO4, respectively. (b) Proposed reaction mechanism for the oxidation of methane over Pt complex [36]. Adapted with permission. Copyright 1998, American Association for the Advancement of Science.
Periana et al. [37] reported a high yield (43%) of MBS during the POM using Hg(II) triflate in triflic acid and H2SO4 at 180 °C (entry 1, Table 3). They also observed that thallium (Tl) and gold (Au) salts converted methane to MBS but their reduced forms could not be reoxidized with SO3. In a subsequent study, they reported high yields (≥70%) of MBS based on the moles of methane over PtII(bpym)Cl2 (bpym = 2,2′-bipyrimidinyl) using SO3 as an oxidant in the oleum system [36] (Figure 6b). They claimed that the key role of the ligand was to prevent the aggregation and reduction of the active Pt species to inactive Pt(0).

Table 3.
Comparison of catalytic systems for the partial oxidation of methane using SO3 in H2SO4.
Zimmermann et al. [38,42] compared the catalytic activity for the POM over (bpym)PtCl2, PtCl2, Pt(acac)2, and K2PtCl4 and found that simple platinum salts were stable, selective, and unprecedently active for the POM in oleum. The extremely high TOF exceeding 20,000 h−1 was obtained with low concentrations of the catalyst (entry 2, Table 3) [38]. It was also proven that when the concentration of the catalysts was sufficiently high, higher MBS formation rates were achieved with (bpym)PtCl2, indicating that catalyst solubility is a key factor in this catalytic system. To enhance the stability of chloride-ligated Pt catalysts, Dang et al. [39] introduced the DMSO (dimethyl sulfoxide) ligand to the Pt catalysts and obtained an 84% yield of MBS at 180 °C (entry 3, Table 3). (DMSO)2PtCl2 was deactivated to PtCl2 although it could be reactivated by adding excess DMSO. They also investigated the POM over Pt black in the presence of 20 wt.% oleum at 180 °C and found that the dissolved Pt was active but the decomposition of MBS to CO2 occurred on Pt(0) (entry 4, Table 3) [40] (Figure 7).
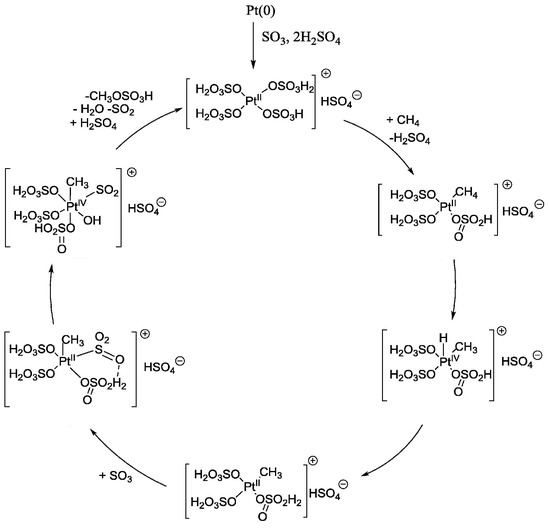
Figure 7.
Proposed mechanism of Pt black catalyzed methane oxidation to methyl bisulfate (MBS) [40]. Adapted with permission. Copyright 2019, Elsevier.
Compared with homogeneous catalysts, heterogenized homogeneous or heterogeneous catalysts have practical advantages [43]. They can be easily separated from the reaction medium to decrease separation costs. However, it is difficult to develop stable and highly active POM catalysts for the highly corrosive and oxidizing oleum. Palkovits et al. [41,44] and Soorholtz et al. [41,44] synthesized a covalent triazine-based framework (CTF) containing multiple bipyridyl structural units utilizing 2,6-dicyanopyridine as a monomer and succeeded in providing coordination sites similar to the platinum coordination sites found in (bpym)PtCl2 (entries 5 and 6, Table 3).
The separation of MBS from H2SO4 requires distillation at high temperatures or depressurization up to 100 mbar, which in turn decomposes the MBS to SO3, dimethyl ether, and dimethyl sulfate [40]. In the case of the direct hydrolysis of MBS in H2SO4, the addition of water wastes a large amount of diluted H2SO4. According to Ahlquist et al. [3], the methanol concentration cannot be higher than 10 µM in H2SO4 as methanol undergoes additional oxidation. Accordingly, the MBS produced should be separated from H2SO4 before it is converted to methanol [45]. Im et al. [46] proposed a modified reactive distillation process in which HTFA was used as a mediator to form MeTFA and H2SO4 from MBS to facilitate the separation of methanol from a mixture of MBS and H2SO4 (Figure 8).
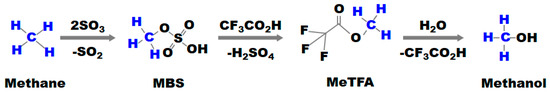
Figure 8.
Reaction scheme for the direct synthesis of methanol from methane with SO3 using HTFA as a mediator in the separation. The blue letters mean chemical species related to methane [46].
3. Liquid-Phase Partial Oxidation of Methane in Water
From an environmental perspective, water is an ideal solvent for organic synthesis. Moreover, the use of O2 as an oxidant for the partial oxidation of substrates in water is more desirable than the direct use of H2O2 as an oxidant. Therefore, the direct oxidation of methane to methanol using O2 is called the chemist’s dream reaction. In nature, methanotrophic bacteria, using enzymes called methane monooxygenases (MMOs), can directly and selectively convert methane to methanol using O2 under mild conditions. MMOs utilize two reducing equivalents to split the O-O bonds of O2 [47].
CH4 + O2 + 2H+ + 2e− → CH3OH + H2O
There are two types of MMOs: particulate MMO (pMMO) and soluble MMO (sMMO). While most methanotrophs rely solely on pMMO for methane oxidation, a few express sMMO under Cu-starved conditions [48]. sMMO consists of three main components: the hydroxylase component, MMOH, with non-heme diiron active sites; the reductase, MMOR, which reduces the diiron site using the nicotinamide adenine dinucleotide cofactor; and the regulatory protein, MMOB. The active site of sMMO, termed compound Q, contains a dinuclear FeIV cluster (Figure 9) [49]. The reaction mechanism of MMO in Figure 9 was proposed by Lippard et al. [50]. Electron transfer to the iron species initiates the diiron center to activate O2 and hydrocarbon hydroxylase.
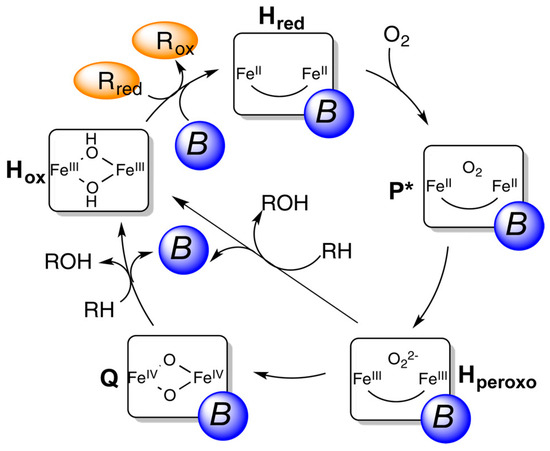
Figure 9.
Catalytic cycle of sMMO. Rred: reduced, ROX: oxidized reductase MMOR, respectively, P*: intermediate before Hperoxo, and B: the regulatory component MMOB [50]. Copyright 2015, American Chemical Society.
On the contrary, the membrane-bound, copper-dependent pMMO enzyme consists of three subunits encoded by PmoA (α), PmoB (β), and PmoC (γ) to form an αβγ protomer [51]. The crystal structures of pMMO from multiple methanotrophic species revealed the presence of three Cu-binding sites: bis-His, CuB, and CuC [52]. Computational studies have suggested that both dinuclear and mononuclear copper sites located at this specific location can catalyze methane oxidation [53,54,55]. Therefore, Fe-based and Cu-based heterogeneous catalyst systems have been actively investigated for the POM in liquid and gas phases.
3.1. H2O2
3.1.1. Fe-Zeolite
Inspired by sMMO, Fe zeolites have been investigated for the POM with H2O2 in water. Rahman et al. [56] reported the synthesis of methane oxygenates (mainly formic acid) over H-ZSM-5 using H2O2 at 100 °C (entry 1, Table 4). Soon after, Hammond et al. [57] reported that very small amounts of Fe species incorporated unintentionally into the zeolite framework were responsible for this reaction (entry 2, Table 4). Regarding the active Fe species, their research group observed that hydrothermally prepared Fe-silicalite-1 with an MFI structure possessed catalytic activity for the POM after heat treatment (entry 3, Table 4) and that the migration of Fe species from isolated framework sites to isolated or oligonuclear extra-framework sites occurred after calcination of the catalysts at high temperatures [58,59,60]. They proposed a dihydroxodiiron center as the active Fe species [61,62,63,64,65]. A good correlation was also reported between the catalytic activity of Fe/ZSM-5 and the peak intensity of the band (corresponding to the extra-framework Fe2+ species) at ~1880 cm−1 in the Fourier-transform infrared spectrum after NO adsorption [66,67].
In contrast, Zhu et al. [68] suggested the presence of a single Fe active site for the Fe/ZSM-5 catalyst (entry 4, Table 4). They observed only atomically dispersed Fe species in 0.03% Fe/ZSM-5 using high-angle annular dark-field scanning transmission electron microscopy (HAADF-TEM) images and claimed that mono- and diiron species were active species. Theoretical calculations of the reaction mechanism over mono- and binuclear Fe-O species showed that both iron species are possible active centers for the C-H bond dissociation of methane, with a moderate energy barrier. Oda et al. [69] also proposed mono-iron species with four coordination numbers of Fe-O as the active Fe species based on an extended X-ray absorption fine structure (EXAFS) study (entry 5, Table 4). Furthermore, Yu et al. [70] also suggested the monomeric Fe complex [(OH)2-FeIII-(H2O)2]+. The single Fe = O species can activate the C-H bond of methane, and the activated CH3• radical reacts with OH• radicals to produce methanol (Figure 10). Al-Shihri et al. [71] proposed another reaction mechanism. They reported the formation of diols (hydrated HCHO) and polyoxomethylene along with CH3OOH, CH3OH, and HCOOH over H-ZSM-5 (entry 6, Table 4). They also reported H2 formation owing to the oxidation of HCHO to HCOOH [72].
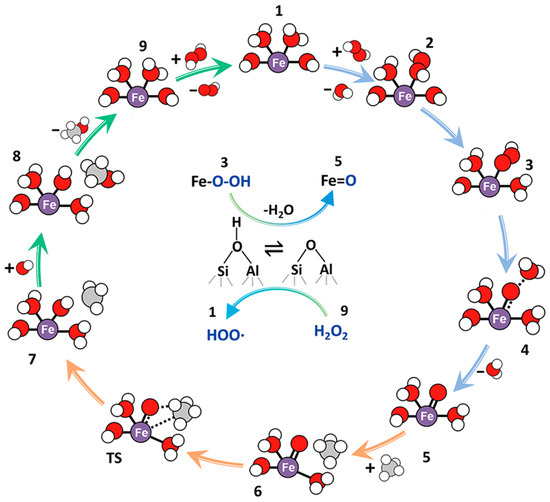
Figure 10.
Proposed reaction scheme of the reaction pathway for direct methane oxidation to methanol over Cu−Fe(2/0.1)/ZSM-5 using H2O2 as the oxidant. Red, purple, gray, and white balls represent O, Fe, C, and H atoms, respectively [70]. Adapted with permission. Copyright 2021, American Chemical Society.
The substitution of Si4+ with Al3+ or Ga3+ in MFI zeolites (ZSM-5 and silicalite-1) increased the number of cation exchange sites, resulting in higher POM activity over the [Fe,Al]- and [Fe,Ga]-MFI catalysts than over [Fe]-MFI [73]. Shahami and Shantz also reported that the MFI zeolite with Ga3+ in the framework showed higher reaction activity than those with Al3+ and B3+ (entries 7 and 8, Table 4) [74]. They observed that MFI catalysts with Al3+ and Ga3+ possessed a higher acid density than those with B3+ and that the catalyst with lower acidity showed much lower oxygenate productivity. Furthermore, when H+ ions in the MFI catalysts were replaced with Na+ ions, the catalytic activity significantly decreased. This means the Brønsted acid sites are essential for the methane oxidation reaction.
In addition to MFI zeolites, other zeolites have been examined for this reaction. Kalamaras et al. [75] compared the catalytic activities of Fe/zeolites (MFI, BEA, and FAU) and found that Fe/ZSM-5 was the best (entry 9, Table 4). Fang et al. [76] prepared various catalysts, including supported Fe catalysts on MOR, Al2O3, SBA-15, and SiO2, and found that MOR showed the best catalytic performance for POM (entry 10, Table 4).
The inertness and low solubility of methane result in low methane conversion in aqueous-phase reactions. Xiao [77] found that the utilization of sulfolane, a very stable aprotic polar solvent, could enhance the catalytic activity of POM. When sulfolane was used as an admixture in the reaction media, the yield of methane oxygenates increased significantly (entries 11 and 12, Table 4).

Table 4.
Comparison of catalytic systems for the partial oxidation of methane over Fe-zeolites using H2O2 in water.
Table 4.
Comparison of catalytic systems for the partial oxidation of methane over Fe-zeolites using H2O2 in water.
| Entry | Catalyst | Temp. (°C) | H2O2 (mmol) | CH4 (bar) | Total Productivity (mmol/gcat./h) | Product Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | H-ZSM-5 | 100 | 122 | 26 | 2.3 | CH3OH: 0.1 HCOOH: 55 CO2: 45 | [56] |
| 2 | 2.5%Fe/ZSM-5 | 50 | 5 | 30.5 | 16.8 | CH3OH: 10 HCOOH: 72 CO2: 17 | [57] |
| 3 | Fe-silicalite-1 | 50 | 5 | 30.5 | 9.5 | CH3OH: 19 HCOOH: 67 CO2: 9 | [62] |
| 4 | 0.03%Fe/ZSM-5(66) | 80 | 5 | 30 | 54.1 | CH3OH: 1 HCOOH: 84 CO2: 5 | [68] |
| 5 | 0.45%Fe-ZSM-5 | 50 | 5 | 30 | 45.2 | CH3OH: 2 HCOOH: 92 CO2: 0 | [69] |
| 6 | ZSM-5(30) | 50 | 5 | 10 | 26.7 | CH3OH: 11 HCOOH: 54 CO2: 1 | [71] |
| 7 | Ga,Fe-MFI(50) | 55 | 5 | 30 | 51.2 | CH3OH: 5 HCOOH: 90 CO2: 3 | [74] |
| 8 | Al,Fe-MFI(50) | 55 | 5 | 30 | 44.0 | CH3OH: 5 HCOOH: 87 CO2: 7 | [74] |
| 9 | Fe/ZSM-5 | 50 | 5 | 30.5 | 3.5 | - | [75] |
| 10 b | Fe-MOR | 80 | 10 | 28.5 | 8.9 | CH3OH: 17 HCOOH: 37 CO2: 9 | [76] |
| 11 a,b | Fe-MFI | 50 | 27 | 30 | 11.3 | CH3OH: 84 HCOOH: 11 CO2: 0 | [77] |
| 12 b | Fe-MFI | 50 | 27 | 30 | 13.1 | CH3OH: 1 HCOOH: 35 CO2: 63 | [77] |
a The mixture of sulfolane and H2O (50:50) was used as the reaction medium. b The data were inferred from the figure.
3.1.2. Promoted Fe-Zeolites
Various promoters have been applied to Fe-zeolites to increase methane conversion and methanol selectivity. Among these, copper is the most frequently reported promoter. Hutchings et al. [57] reported an increase in methanol selectivity as formic acid selectivity decreased with the addition of copper species to Fe-ZSM-5 without any change in methane conversion (entry 1, Table 5). Yu et al. [70] observed that the OH radical signal was enhanced by adding Cu to Fe/ZSM-5 based on electron paramagnetic resonance (EPR) radical trapping studies, implying that Cu species facilitated the production of OH radicals from H2O2. However, there have been a few reports on the contrary. Al-Shihri et al. [72] reported that as the amount of Cu introduced increased from 0 to 2 μmol, the amount of total product decreased from 464 to 5 μmol. They claimed that the addition of Cu to the reaction liquid accelerated the decomposition of HCOOH to CO2 and H2 as well as the decomposition of H2O2. Leaching of Cu species from Cu-Fe/ZSM-5 was observed during this reaction, which increased the H2O2 decomposition and CO2 selectivity [78].

Table 5.
Comparison of catalytic systems for the partial oxidation of methane over promoted Fe-zeolites using H2O2 in water.
The introduction of La to H-ZSM-5 and CuFe/ZSM-5 catalysts was reported to lower the strong Brønsted acid sites, resulting in the reduction of H2O2 decomposition and an increase in the H2O2 utilization efficiency (entry 2, Table 5) [79]. The reductive pretreatment with 5% H2/Ar was more beneficial for the catalytic activity than that with air because of the higher fraction of extra-framework Fe species (entries 3 and 4, Table 5).
The promotional effect of Ir on Ir-Fe/ZSM-5 was also observed in terms of methane oxygenation productivity and H2O2 efficiency (entries 5 and 6, Table 5) [80]. This was ascribed to the formation of an Ir-O-Fe complex, which induced increased radical production from H2O2 decomposition.
Huang et al. [81] reported the unique catalytic performance of singly dispersed Pd/ZSM-5 catalysts (entry 7, Table 5). The Pd1O4 structure in 0.04 wt.% Pd/ZSM-5 was confirmed by an extended EXAFS study. Increasing the Pd content in the Pd/ZSM-5 catalysts did not significantly improve the production of methane oxygenates (entry 7, Table 5). This lack of enhancement can be attributed to the aggregation of Pd particles in the catalyst structure. However, there has been no discussion on Fe impurities, which have been previously reported as active sites for selective methane oxidation.
3.1.3. Metal–Organic Framework (MOF)-Based Catalysts
Metal–organic frameworks (MOFs) have recently been utilized for the POM [82]. Szécsényi et al. [83] reported MOF-mediated POM using H2O2 as an oxidant. The Mössbauer spectra and EXAFS studies showed that MIL-53 facilitates the formation of catalytically active Fe species in diiron complexes. MIL-53 (Al,Fe) catalysts showed high methane oxidation activity with TOFs of 90 h−1 and a methane oxygenate selectivity of ca. 80% (entry 1, Table 6). After further characterization of the catalysts and DFT calculations, they concluded that the isolated Fe sites in the MOFs catalyzed the direct conversion of methane to methanol.

Table 6.
Comparison of catalytic systems for the partial oxidation of methane over MOF-based catalyst using H2O2 in water.
Fe-O clusters anchored on the Zr6 nodes of UiO-66 and modulated with acetic acid (AA) or HTFA have also been investigated [84]. Among these non-modulated, AA-modulated, and HTFA-modulated Fe-UiO-66 catalysts, one catalyst with HTFA showed the highest methane oxygenates productivity of 4799 μmol/gcat./h (only 105 μmol/gcat./h of CO2 was obtained) (entry 2, Table 6). An EPR study showed that the addition of Fe-UiO-66(TFA) gave rise to •OH radical signals in the presence of H2O2. Theoretical calculations indicated that the introduction of HTFA to the Fe-UiO-66 catalysts lowered the energy for H2O2 activation compared to the higher activation energy for Fe-UiO-66 without HTFA.
Recently, regarding copper-doped zeolitic imidazolate framework-7 (Cu/ZIF-7), it was reported that the mononuclear cupric ion (Cu2+) coordinated to four nitrogen ligands (CuN4) displayed catalytic activity for methane oxidation to methanol, methyl hydroperoxide, and hydroxymethyl hydroperoxide, and formic acid using H2O2 (entry 3, Table 6) [85]. The facile synthesis of multiple mononuclear CuN4 active centers is highly appealing because of its simplicity and clear preparation process. However, it is imperative to address the ongoing challenge of oxidative degradation of benzimidazole to further enhance its application. The same research group reported a single-atom Cu catalyst with a Cu1N4 structure on N-doped carbon prepared by the carbonization of Cu/ZIF-8 (entry 4, Table 6) and a Cu1N3 structure on N-doped carbon (entry 5, Table 6) prepared by the carbonization of a polymeric copper–dibenzimidazole complex [86,87].
3.1.4. Other Catalysts
Hutchings et al. [88] observed that Au-Pd nanoparticles supported on TiO2 showed catalytic activity in the POM using H2O2 (entry 1, Table 7). From the EPR study, it was concluded that the reaction was catalyzed over the Au-Pd catalyst via radical pathways, generating CH3 radicals from methane. The catalytic activity of the Au-Pd nanoparticle catalysts without any support was further investigated (entry 2, Table 7) [89]. The moles of the total product reached 16.8 μmol after 30 min of the reaction at 50 °C with a methane oxygenates selectivity of 90% for the Au-Pd colloid, which surpassed that of Au-Pd/TiO2 (1.6 μmol, 26%). Upon the addition of 5 bar of O2 as an additional oxidant, the product yield further increased to 28.3 μmol while maintaining a high selectivity of 88%. Notably, using only 50 μmol of H2O2 and 5 bar of O2 as the oxidant, the Au-Pd colloid still achieved a substantial total product yield of 20 μmol with an oxygenates selectivity of 92%. Furthermore, experiments employing isotopically labeled oxygen (O2) as the oxidant in the presence of H2O2 demonstrated that a significant fraction (70%) of the resulting CH3OH originated from the gas-phase O2 (Figure 11). The effect of additional O2 as an oxidant was also demonstrated by Xu et al. [90] in their isotopically labeled O2 experimental study on the partial oxidation of methane using H2O2 over a Au-Pd@ZIF-8 catalyst (entry 3, Table 7).
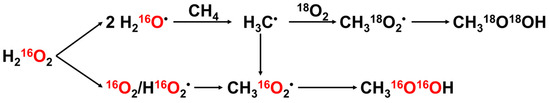
Figure 11.
Proposed reaction scheme for methane oxidation in the presence of H2O2 and molecular O2. The red letters mean oxygen related to 16O [89]. Adapted with permission. Copyright 2017, American Association for the Advancement of Science.
After optimization of the Au-Pd catalytic system, the methane oxidation reaction exhibited an impressive productivity value of 74.4 mmol/gcat./h [91]. This signifies the remarkable intrinsic activity of the unsupported Au-Pd nanoparticles specifically for this reaction. Furthermore, this productivity value significantly surpasses those of methane monooxygenase (MMO) and Fe-Cu/ZSM-5 catalysts, which demonstrate productivities of 5.1 mmol/gcat./h [92] and 16.5 mmol/gcat./h [57,61], respectively (entry 2, Table 7).
Yan et al. [93] investigated the effect of pH on the H2O2 efficiency of the POM over an AuPd colloid. They conducted the reaction in a pH range of 1–8 and found that not only the amount of primary oxygenates, such as CH3OH and CH3OOH, but also the efficiency of H2O2 (defined as [moles of H2O2 consumed]/[moles of total product]) were the highest at pH 3 (entry 3, Table 7).
Bao et al. [94] reported room-temperature methane conversion using Fe species confined in graphene nanosheets (GNs) (entry 4, Table 7). The unique FeN4 structure on the GNs was prepared by simple ball milling of graphite flakes with iron phthalocyanine, and the structure was confirmed by EXAFS. The single iron atom O-FeN4-O structure on the GNs could activate the C-H bond of methane to a methyl radical with a low energy barrier of 0.79 eV and produce CH3OH and CH3OOH. These primary products were further converted to HOCH2OOH and HCOOH, as confirmed by time-of-flight mass spectrometry (TOF-MS) and 13C−nuclear magnetic resonance spectroscopy (NMR).
A similar study using N-doped carbon-supported Fe species was reported by Lin et al. [95]. They prepared Fe/N-doped carbon by treating activated carbon under the flow of NH3 gas at 600 °C and impregnating iron precursor on it. The TOF which is defined as [moles of total products] [moles of Fe introduced]−1 [reaction time (h)]−1 was higher than 5 (entry 5, Table 7), and the recycling test was stably conducted.
A non-noble single-metal catalyst for the partial oxidation of methane was also reported by Shen et al. [96]. The well-dispersed Cr atoms supported on TiO2 nanoparticles showed methane oxygenates yield of 57.9 mol/molCr (8.8 mmol/gcat./h with a methane oxygenates selectivity of 92.8%) at 50 °C (entry 6, Table 7). The presence of CrIII and CrVI species was confirmed. However, CrVI species disappeared after the reaction, and the catalytic activity decreased severely in the reuse test. This implies that the CrVI species play a vital role in the POM.

Table 7.
Comparison of catalytic systems for the partial oxidation of methane over other catalysts using H2O2 in water.
Table 7.
Comparison of catalytic systems for the partial oxidation of methane over other catalysts using H2O2 in water.
| Entry | Catalyst | Temp. (°C) | H2O2 (mmol) | Feed Composition (bar) | Total Productivity (mmol/gcat./h) | Product Selectivity (%) a | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Au-Pd/TiO2 | 90 | 5 | CH4 = 30.5 | 1.9 | CH3OH: 88 HCOOH: 0 CO2: 12 | [88] |
| 2 | Au-Pd colloid | 50 | 1 | CH4:O2 = 30:5 | 53.6 | CH3OH: 88 HCOOH: 6 CO2: 5 | [89] |
| 3 | AuPd@ZIF-8 | 50 | 0.5 | CH4:O2 = 30:5 | 4.5 | CH3OH: 59 HCOOH: 26 CO2: 14 | [90] |
| 4 | 2.7%FeN4/GN | 25 | 49 | CH4:N2 = 18:2 | 0.2 | CH3OH: 39 HCOOH: 29 CO2: 6 | [94] |
| 5 | 2.5%Fe/NC-HH | 25 | 5 | CH4 = 40 | 1.6 | CH3OH: 29 HCOOH: 51 CO2: 20 | [95] |
| 6 | Cr/TiO2 | 50 | 5 | CH4 = 30 | 4.4 | CH3OH: 48 HCOOH: 5 CO2: 0 | [96] |
a CH3OH selectivity = (sum of concentrations of CH3OH and CH3OOH)/(sum of concentrations of total products) × 100.
3.2. In Situ Generated H2O2
Direct utilization of H2O2 as an oxidant is uneconomical because it is more expensive than methanol. This cost disparity poses a significant challenge to the practical implementation of H2O2-based processes on a large scale. The synergistic integration of in situ H2O2 generation and selective oxidation reactions in a single process greatly enhances the environmental and economic attractiveness of utilizing H2O2 as a green oxidant on an industrial scale. This tandem reaction not only improves safety by eliminating the need for H2O2 storage and transport but also reduces capital and operating costs. Therefore, the direct synthesis of hydrogen peroxide (DSHP) from H2 and O2 was investigated. Pd-based catalysts with various supports and secondary metals were tested for DSHP from H2 and O2 [22,23,24]. To enhance the yield of H2O2, acids and halide ions are frequently utilized as they increase the stability and selectivity of H2O2.
Because H2O2 is unstable and decomposes in the presence of a catalyst, a mineral acid is required to inhibit its decomposition of produced H2O2. Choudhary et al. [97,98] reported that oxidized Pd catalysts can facilitate the production of H2O2, even in a water-based environment, albeit with lower selectivity for H2O2. Conversely, the Pd0 catalysts exhibit minimal generation of H2O2 in non-acidic aqueous media. This was mainly due to the rapid decomposition of the H2O2 formed in the reaction. The incorporation of any of the mineral acids, including H2SO4, HCl, HNO3, H3PO4, and HClO4, resulted in improved H2O2 selectivity compared to a non-acidic aqueous medium [97]. However, the use of liquid acid can cause corrosion of the reactor and the leaching of active metals. For this reason, numerous studies have been conducted on the reaction over catalysts supported on acidic carriers, including sulfated SiO2 [99,100,101], zeolites [97,102,103,104], heteropoly acids [105,106], and acid-functionalized polymers [107].
The use of halide ions, including F−, Cl−, Br−, and I−, as promoters has also been investigated to enhance the conversion of H2 and selectivity to H2O2 in DSHP reactions. Halide ions have been utilized by directly adding them to the reaction liquid or depositing them onto solid catalysts. Choudhary and Samanta [108] investigated the effects of halide ions on the DSHP reaction using supported Pd catalysts. Bromide or chloride ions at optimum concentrations promote H2 to H2O2 oxidation, causing a drastic increase in H2O2 formation, but only in the presence of protons (protic acids). Regarding the H2O2 decomposition reaction, it seems that the presence of Br− or Cl− highly inhibits the decomposition rate of H2O2 to H2O. Too high concentrations of halide ions can cause the leaching of active metals and also deactivate the catalysts by adsorption on the active species [109]. The utilization of halide ions was also studied by incorporating them onto the catalyst surface. The Pd-based catalysts supported on SO42−-, Cl−-, F−-, and Br−-doped ZrO2 were tested for DSHP [110]. The best overall H2O2 selectivity was observed with F- and Br-dopants, followed by SO42−. Subsequently, Cl− and the non-doped sample exhibited lower H2O2 selectivity.
3.2.1. Pd-Based Catalyst and Transition Metal-Based Catalyst
Kang and Park [111] demonstrated the POM using iron salts and Pd/C for methane oxidation and DSHP of H2 and O2, respectively. H2O2 was either added directly to the reaction liquid or synthesized in situ from H2 and O2, and the pH of the reaction liquid was adjusted using H2SO4 to increase the stability of H2O2. In this process, Fe2+ ions act as catalysts. When combined with H2O2, they can generate hydroxyl radicals (OH·). These hydroxyl radicals play a crucial role in the oxidation of methane. Methane reacts with hydroxyl radicals, resulting in the formation of methane oxygenates, such as CH3OOH, CH3OH, and HCOOH (entry 1, Table 8). The reduction of Fe3+ to Fe2+ was accelerated by the dissociation of atomic hydrogen from molecular hydrogen on Pd. Fe-ZSM-5 catalysts have been used as substitutes for iron salts under acidic conditions [112]. However, severe leaching of Fe species was observed with high yields of methane oxygenates at low pH values, indicating that homogeneous Fe species were responsible for this reaction. The same group also performed the POM over Fe-ZSM-5 using H2O2 generated in situ over acid-functionalized porous polymer-supported Pd catalysts in the absence of liquid acid [113]. Metal leaching from Fe-ZSM-5 was not observed because of the absence of liquid acids in the reaction system. The cooperation between Pd/c-s-HCPP and Fe-ZSM-5 resulted in a total productivity of 3.7 mmol/gcat./h and selectivity to methane oxygenates of 89% at 50 °C (entry 2, Table 8). Moreover, a one-body Pd-Fe/ZSM-5 catalyst was used for aqueous-phase POM in the presence of H2 and O2 [114]. The Pd-Fe/ZSM-5 catalysts exhibited significantly better catalytic performance than the physical mixture of the Pd/ZSM-5 and Fe/ZSM-5 catalysts (entry 3, Table 8). This implies that the intimate contact between Pd and Fe is important for methane oxidation with in situ generated H2O2 from H2 and O2. The effect of halide ions on the catalytic activity of aqueous-phase POM using H2O2 generated in situ over Pd/C was also examined [115]. Among various halide ions, including F−, Cl−, Br−, and I−, Br− and I− were effective for the synthesis of H2O2 from H2 and O2, resulting in the synthesis of methane oxygenates from methane over Fe/ZSM-5. Compared with Br−, the higher product yield was obtained with a much lower concentration of I−, guaranteeing that no detectable leaching of metal from Fe/ZSM-5 and Pd/C was found (entry 4, Table 8).
Zhong et al. [116] investigated various M-Pd/ZSM-5 catalysts (M = Cu, Fe, Co, and Ni) for the partial oxidation of methane in tandem with the direct synthesis of H2O2 in the presence of H2 and O2. Among those bicomponent Pd-M catalysts, PdCu/ZSM-5 showed the highest productivity of 1178 mmol/gmetal/h with a methane oxygenates selectivity of 95% at 120 °C (entry 5, Table 8). Based on the control experiments and EPR study, it was proven that PdO nanoparticles facilitated the generation of H2O2, whereas Cu single atoms accelerated the generation of OH• radicals and the consequent homolytic cleavage of methane by OH• to produce CH3• radicals.
Pd-containing phosphomolybdates, which are activated by molecular hydrogen (H2), have been reported to convert methane and O2 to methanol at room temperature [117]. The highest activity reached 67.4 μmol/gcat./h (entry 6, Table 8). In this catalytic system, Pd enables rapid H2 dissociation and H spillover to the phosphomolybdate for Mo reduction, whereas facile O2 activation and subsequent methane activation occur at the reduced phosphomolybdate sites. The continuous production of methanol from methane was also achieved by concurrently introducing H2, O2, and methane to the system.
3.2.2. Pd-Au-Based Catalyst
Hutchings et al. [88] observed a lower overall productivity of the tandem system (0.12 mmol/gcat./h) (entry 7, Table 8) compared with the direct H2O2 injection system (0.28 mmol/gcat./h). He et al. [118,119] tested the catalytic activity of Pd-Au nanoparticles on carbonaceous materials for the POM using H2O2 generated in situ. Among supports such as activated carbon (AC), reduced graphene oxide (rGO), and carbon nanotubes (CNTs), Pd-Au supported on CNTs exhibited the highest catalytic activity (entry 8, Table 8). They proposed that the strong interaction between the Pd and Au nanoparticles and AC and rGO suppressed methane activation. In addition, methane oxygenates, including CH3OH, CH3OOH, and HCOOH, were obtained over Pd/CNT and Au/CNT.
An impressive methane oxygenate yield of 91.6 mmol/gAuPd/h was achieved by Xiao et al. [120] over AuPd catalyst encapsulated in hydrophobic sheath-modified ZSM-5 at 70 °C (entry 9, Table 8). The AuPd catalysts were prepared by modifying AuPd@ZSM-5 with an organosilane. These organic chains appear to allow the diffusion of H2, O2, and CH4 to the catalyst active sites while trapping the H2O2 generated inside the catalyst pores to enhance the reaction probability.
The direct utilization of O2 in the absence of any reducing agent was initially reported. Qi et al. [121] reported that Au nanoparticles supported on ZSM-5 could oxidize methane to CH3OH and CH3COOH with a small amount of CO2 while using O2 as the sole oxidant in an aqueous solution. The relatively low product yields and high operating temperatures are still problems to be solved in this case.

Table 8.
Comparison of catalytic systems for the partial oxidation of methane using H2O2 generated in situ from H2 and O2 in water.
Table 8.
Comparison of catalytic systems for the partial oxidation of methane using H2O2 generated in situ from H2 and O2 in water.
| Entry | Catalyst | Temp. (°C) | Feed Composition (bar) | Total Productivity (mmol/gcat./h) | Product Selectivity (%) a | Ref. |
|---|---|---|---|---|---|---|
| 1 | FeSO4 + Pd/C | 20 | CH4:H2:Air = 15:3:10 | 64.2 b | CH3OH: 5 HCOOH: 61 CO2: 34 | [111] |
| 2 | Fe/ZSM-5 + Pd/c-s-HCPP c | 50 | CH4:H2:Air = 15:3:10 | 3.4 | CH3OH: 28 HCOOH: 61 CO2: 11 | [112] |
| 3 | Pd-Fe/ZSM-5 | 50 | CH4:H2:Air = 15:3:10 | 0.5 | CH3OH: 52 HCOOH: 37 CO2: 11 | [114] |
| 4 | Fe/ZSM-5 + Pd/AC | 50 | CH4:H2:Air = 15:3:10 | 3.5 | CH3OH: 34 HCOOH: 45 CO2: 20 | [115] |
| 5 | Pd-Cu/ZSM-5 d | 120 | CH4:H2:O2 = 73:24:9 | 2.2 | CH3OH: 55 HCOOH: 40 CO2: 5 | [116] |
| 6 | Pd/CsPMA-H e | 25 | CH4:O2 = 20:0.3 | 0.067 | CH3OH: 100 | [117] |
| 7 | AuPd/TiO2 | 50 | CH4:H2:O2:N2 = 30.5:0.3:0.7:8.7 | 0.14 | CH3OH: 83 HCOOH: 0 CO2: 17 | [88] |
| 8 | Pd-Au/CNTs | 50 | CH4:H2:O2:Ar = 15.5:1.3:2.6:13.5 | 0.4 | CH3OH: 78 HCOOH: 22 CO2: 0 | [118] |
| 9 | AuPd@ZSM-5-C16 | 70 | CH4:H2:O2:Ar = 0.5:0.9:1.8:27 | 5.0 | CH3OH: 95 HCOOH: 5 CO2: 0 | [120] |
a CH3OH selectivity = (sum of concentrations of CH3OH and CH3OOH)/(sum of concentrations of total products) × 100. b The total productivity is the turnover frequency based on moles of Fe species. c HCPP: hyper cross-linked porous polymer. d The data were inferred from the figure. e CsPMA: Cs-exchanged phosphomolybdate catalyst.
4. Summary and Outlook
The current indirect route for utilizing methane as a chemical feedstock via syngas is economically applicable only to large methane gas fields. To exploit the many small- and medium-sized methane resources, highly efficient direct methane conversion technologies must be developed. Among the various direct methane conversion pathways, the POM is the most attractive because it is thermodynamically feasible and has successful examples in nature, such as MMOs.
This review discussed liquid-phase POM in strong acids and water according to the oxidant and catalyst used. As summarized in Table 9, higher yields of methanol precursors (MeTFA or MBS) were generally obtained in strong acids (HTFA or H2SO4) than in water. However, the instability of HTFA (entry 1, Table 9) and additional requirements of TFAA (entries 2 and 4, Table 9) are significant drawbacks of using K2S2O8 and H2O2 as oxidants in the HTFA system. The direct synthesis of MBS from methane in oleum, subsequent esterification with HTFA, and hydrolysis of MeTFA could compete with the current indirect method for methanol synthesis from methane. However, the co-production of methanol and H2SO4 is inevitable (entry 6, Table 9), which would need to be market acceptable.

Table 9.
Comparison of different catalytic systems for the liquid-phase partial oxidation of methane.
Compared with the POM in strong acids, the methanol yields for POM in water are generally much lower. Similar to MMO, which requires a reducing agent, methane can be oxidized by H2O2 generated in situ from H2 and O2. In particular, well-designed AuPd nanoalloys encapsulated in nanocages with controlled surface hydrophobicity provided excellent methanol yields under mild conditions [120]. The synergistic and cooperative effect between active centers and supports should be considered and sought in future studies. This approach paves the way for the development of a direct methanol synthesis process that is cost-competitive with conventional methanol processes based on indirect methane conversion. The hydrogen required for this process can be supplied by water electrolysis powered by renewable energy sources.
However, the direct conversion of methane to methane oxygenates without a reducing agent cannot be sufficiently stressed. Since the direct oxidation of methane and O2 is a spin-forbidden reaction, relatively high reaction temperatures are required for thermal catalysis. In view of this, additional energy sources for the activation of methane and O2, as well as well-designed thermal catalysts, must be continuously sought.
Recently, (photo)electrochemical [122,123] and photocatalytic [124,125,126] direct methane conversions have been actively investigated. Although these are still in the early stages of research and development, their performance should be monitored as thermal methane conversion technologies develop.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14030167/s1, Table S1: The catalytic performance for selective oxidation of methane with H2O2 over 0.38 wt.% Fe-ZSM-5 under different conditions. Figure S1: UV–Vis spectra of 0.38 wt.% Fe-ZSM-5 [57,63,127,128].
Author Contributions
Conceptualization, E.D.P.; writing—original draft preparation, J.K.; writing—review and editing, E.D.P.; supervision, project administration, and funding acquisition, E.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the C1 Gas Refinery Program of the National Research Foundation (NRF) of Korea and funded by the Ministry of Science, ICT, and Future Planning (2015M3D3A1A01064899).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We acknowledge Gun Sik Yang for his assistance in obtaining the data in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schwach, P.; Pan, X.; Bao, X. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef] [PubMed]
- Alola, A.A.; Onifade, S.T.; Magazzino, C.; Obekpa, H.O. The Effects of Gas Flaring as Moderated by Government Quality in Leading Natural Gas Flaring Economies. Sci. Rep. 2023, 13, 14394. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, M.; Nielsen, R.J.; Periana, R.A.; Goddard, W.A. Product Protection, the Key to Developing High Performance Methane Selective Oxidation Catalysts. J. Am. Chem. Soc. 2009, 131, 17110–17115. [Google Scholar] [CrossRef] [PubMed]
- Gunsalus, N.J.; Koppaka, A.; Park, S.H.; Bischof, S.M.; Hashiguchi, B.G.; Periana, R.A. Homogeneous Functionalization of Methane. Chem. Rev. 2017, 117, 8521–8573. [Google Scholar] [CrossRef]
- Biswal, T.; Shadangi, K.P.; Sarangi, P.K.; Srivastava, R.K. Conversion of Carbon Dioxide to Methanol: A Comprehensive Review. Chemosphere 2022, 298, 134299. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-Liquid via Synthesis of Methanol, DME or Fischer–Tropsch-Fuels: A Review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Xu, Z.C.; Park, E.D. Gas-Phase Selective Oxidation of Methane into Methane Oxygenates. Catalysts 2022, 12, 314. [Google Scholar] [CrossRef]
- Ravi, M.; Ranocchiari, M.; van Bokhoven, J.A. The Direct Catalytic Oxidation of Methane to Methanol—A Critical Assessment. Angew. Chem.—Int. Ed. 2017, 56, 16464–16483. [Google Scholar] [CrossRef] [PubMed]
- Dummer, N.F.; Willock, D.J.; He, Q.; Howard, M.J.; Lewis, R.J.; Qi, G.; Taylor, S.H.; Xu, J.; Bethell, D.; Kiely, C.J.; et al. Methane Oxidation to Methanol. Chem. Rev. 2023, 123, 6359–6411. [Google Scholar] [CrossRef] [PubMed]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 21, ISBN 1402004206. [Google Scholar]
- Gretz, E.; Oliver, T.F.; Sen, A. Carbon-Hydrogen Bond Activation by Electrophilic Transition-Metal Compounds. Palladium (II)-Mediated Oxidation of Arenes and Alkanes Including Methane. J. Am. Chem. Soc. 1987, 109, 8109–8111. [Google Scholar] [CrossRef]
- Muehlhofer, M.; Strassner, T.; Herrmann, W.A. New Catalyst Systems for the Catalytic Conversion of Methane into Methanol. Angew. Chem.—Int. Ed. 2002, 41, 1745–1747. [Google Scholar] [CrossRef]
- Strassner, T.; Muehlhofer, M.; Zeller, A.; Herdtweck, E.; Herrmann, W.A. The Counterion Influence on the CH-Activation of Methane by Palladium(II) Biscarbene Complexes—Structures, Reactivity and DFT Calculations. J. Organomet. Chem. 2004, 689, 1418–1424. [Google Scholar] [CrossRef]
- Ahrens, S.; Strassner, T. Detour-Free Synthesis of Platinum-Bis-NHC Chloride Complexes, Their Structure and Catalytic Activity in the CH Activation of Methane. Inorganica Chim. Acta 2006, 359, 4789–4796. [Google Scholar] [CrossRef]
- Cheong, S.H.; Kim, D.; Dang, H.T.; Kim, D.; Seo, B.; Cheong, M.; Hong, S.H.; Lee, H. Methane Oxidation to Methyl Trifluoroacetate by Simple Anionic Palladium Catalyst: Comprehensive Understanding of K2S2O8-Based Methane Oxidation in CF3CO2H. J. Catal. 2022, 413, 803–811. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Han, Z.; Huang, S.; Yuan, D.; Su, W. Atmosphere-Pressure Methane Oxidation to Methyl Trifluoroacetate Enabled by a Porous Organic Polymer-Supported Single-Site Palladium Catalyst. ACS Catal. 2021, 11, 1008–1013. [Google Scholar] [CrossRef]
- Yin, G.; Piao, D.-G.; Kitamura, T.; Fujiwara, Y. Cu(OAc)2-Catalyzed Partial Oxidation of Methane to Methyl Trifuoroacetate in the Liquid Phase. Appl. Organomet. Chem. 2000, 14, 438–442. [Google Scholar] [CrossRef]
- Ravi, M.; van Bokhoven, J.A. Homogeneous Copper-Catalyzed Conversion of Methane to Methyl Trifluoroacetate in High Yield at Low Pressure. ChemCatChem 2018, 10, 2383–2386. [Google Scholar] [CrossRef]
- Goyal, R.; Singh, O.; Agrawal, A.; Samanta, C.; Sarkar, B. Advantages and Limitations of Catalytic Oxidation with Hydrogen Peroxide: From Bulk Chemicals to Lab Scale Process. Catal. Rev. 2022, 64, 229–285. [Google Scholar] [CrossRef]
- Puértolas, B.; Hill, A.K.; García, T.; Solsona, B.; Torrente-Murciano, L. In-Situ Synthesis of Hydrogen Peroxide in Tandem with Selective Oxidation Reactions: A Mini-Review. Catal. Today 2015, 248, 115–127. [Google Scholar] [CrossRef]
- Samanta, C. Direct Synthesis of Hydrogen Peroxide from Hydrogen and Oxygen: An Overview of Recent Developments in the Process. Appl. Catal. A Gen. 2008, 350, 133–149. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Grunwaldt, J.D.; Pashkova, A. A Review of Catalyst Performance and Novel Reaction Engineering Concepts in Direct Synthesis of Hydrogen Peroxide. Catal. Today 2015, 248, 149–159. [Google Scholar] [CrossRef]
- Ranganathan, S.; Sieber, V. Recent Advances in the Direct Synthesis of Hydrogen Peroxide Using Chemical Catalysis—A Review. Catalysts 2018, 8, 379. [Google Scholar] [CrossRef]
- Piao, D.-G.; Inoue, K.; Shibasaki, H.; Taniguchi, Y.; Kitamura, T.; Fujiwara, Y. An Efficient Partial Oxidation of Methane in Trifluoroacetic Acid Using Vanadium-Containing Heteropolyacid Catalysts. J. Organomet. Chem. 1999, 574, 116–120. [Google Scholar] [CrossRef]
- Ingrosso, G.; Midollini, N. Palladium(II)- or Copper(II)-Catalysed Solution-Phase Oxyfunctionalisation of Methane and Other Light Alkanes by Hydrogen Peroxide in Trifluoroacetic Anhydride. J. Mol. Catal. A Chem. 2003, 204–205, 425–431. [Google Scholar] [CrossRef]
- Park, E.D.; Hwang, Y.-S.; Lee, J.S. Direct Conversion of Methane into Oxygenates by H2O2 Generated in Situ from Dihydrogen and Dioxygen. Catal. Commun. 2001, 2, 187–190. [Google Scholar] [CrossRef]
- Park, E.D.; Hwang, Y.S.; Lee, C.W.; Lee, J.S. Copper- and Vanadium-Catalyzed Methane Oxidation into Oxygenates with in Situ Generated H2O2 over Pd/C. Appl. Catal. A Gen. 2003, 247, 269–281. [Google Scholar] [CrossRef]
- Lin, M.; Hogan, T.; Sen, A. A Highly Catalytic Bimetallic System for the Low-TemperatureSelective Oxidation of Methane and Lower Alkanes with Dioxygen as the Oxidant. J. Chem. Soc. Chem. Commun. 1997, 119, 6048–6053. [Google Scholar] [CrossRef]
- Park, E.D.; Choi, S.H.; Lee, J.S. Characterization of Pd/C and Cu Catalysts for the Oxidation of Methane to a Methanol Derivative. J. Catal. 2000, 194, 33–44. [Google Scholar] [CrossRef]
- Seki, Y.; Mizuno, N.; Misono, M. High-Yield Liquid-Phase Oxygenation of Methane with Hydrogen Peroxide Catalyzed by 12-Molybdovanadophosphoric Acid Catalyst Precursor. Appl. Catal. A Gen. 1997, 158, 47–51. [Google Scholar] [CrossRef]
- Vargaftik, M.N.; Stolarov, I.P.; Moiseev, I.I. Highly Selective Partial Oxidation of Methane to Methyl Trifluoroacetate. J. Chem. Soc. Chem. Commun. 1990, 1049–1050. [Google Scholar] [CrossRef]
- Strassner, T.; Ahrens, S.; Muehlhofer, M.; Munz, D.; Zeller, A. Cobalt-Catalyzed Oxidation of Methane to Methyl Trifluoroacetate by Dioxygen. Eur. J. Inorg. Chem. 2013, 2013, 3659–3663. [Google Scholar] [CrossRef]
- Blankenship, A.N.; Ravi, M.; Newton, M.A.; van Bokhoven, J.A. Heterogeneously Catalyzed Aerobic Oxidation of Methane to a Methyl Derivative. Angew. Chem.—Int. Ed. 2021, 60, 18138–18143. [Google Scholar] [CrossRef]
- Ji, Y.; Blankenship, A.N.; van Bokhoven, J.A. Heterogeneous Mn-Based Catalysts for the Aerobic Conversion of Methane-to-Methyl Trifluoroacetate. ACS Catal. 2023, 13, 3896–3901. [Google Scholar] [CrossRef]
- Periana, R.A.; Taube, D.J.; Gamble, S.; Taube, H.; Satoh, T.; Fujii, H. Platinum Catalysts for the High-Yield Oxidationof Methane to a Methanol Derivative. Science 1998, 280, 560–564. [Google Scholar] [CrossRef]
- Periana, R.A.; Taube, D.J.; Evitt, E.R.; Loffler, D.G.; Wentrcek, P.R.; Voss, G.; Masuda, T. A Mercury-Catalyzed, High-Yield System for the Oxidation of Methane to Methanol. Science 1993, 259, 340–343. [Google Scholar] [CrossRef]
- Zimmermann, T.; Bilke, M.; Soorholtz, M.; Schüth, F. Influence of Catalyst Concentration on Activity and Selectivity in Selective Methane Oxidation with Platinum Compounds in Sulfuric Acid and Oleum. ACS Catal. 2018, 8, 9262–9268. [Google Scholar] [CrossRef]
- Dang, H.T.; Lee, H.W.; Lee, J.; Choo, H.; Hong, S.H.; Cheong, M.; Lee, H. Enhanced Catalytic Activity of (DMSO)2PtCl2 for the Methane Oxidation in the SO3-H2SO4 System. ACS Catal. 2018, 8, 11854–11862. [Google Scholar] [CrossRef]
- Lee, H.W.; Dang, H.T.; Kim, H.; Lee, U.; Ha, J.M.; Jae, J.; Cheong, M.; Lee, H. Pt Black Catalyzed Methane Oxidation to Methyl Bisulfate in H2SO4-SO3. J. Catal. 2019, 374, 230–236. [Google Scholar] [CrossRef]
- Palkovits, R.; Antonietti, M.; Kuhn, P.; Thomas, A.; Schüth, F. Solid Catalysts for the Selective Low-Temperature Oxidation of Methane to Methanol. Angew. Chem.—Int. Ed. 2009, 48, 6909–6912. [Google Scholar] [CrossRef]
- Zimmermann, T.; Soorholtz, M.; Bilke, M.; Schüth, F. Selective Methane Oxidation Catalyzed by Platinum Salts in Oleum at Turnover Frequencies of Large-Scale Industrial Processes. J. Am. Chem. Soc. 2016, 138, 12395–12400. [Google Scholar] [CrossRef]
- Park, E.D.; Lee, K.H.; Lee, J.S. Easily Separable Molecular Catalysis. Catal. Today 2000, 63, 147–157. [Google Scholar] [CrossRef]
- Soorholtz, M.; White, R.J.; Zimmermann, T.; Titirici, M.M.; Antonietti, M.; Palkovits, R.; Schüth, F. Direct Methane Oxidation over Pt-Modified Nitrogen-Doped Carbons. Chem. Commun. 2013, 49, 240–242. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Zerella, M.; Bell, A.T. A High-Yield, Liquid-Phase Approach for the Partial Oxidation of Methane to Methanol Using SO3 as the Oxidant. Adv. Synth. Catal. 2005, 347, 1203–1206. [Google Scholar] [CrossRef]
- Im, J.; Cheong, S.H.; Dang, H.T.; Kim, N.K.; Hwang, S.; Lee, K.B.; Kim, K.; Lee, H.; Lee, U. Economically Viable Co-Production of Methanol and Sulfuric Acid via Direct Methane Oxidation. Commun. Chem. 2023, 6, 282. [Google Scholar] [CrossRef]
- Koo, C.W.; Rosenzweig, A.C. Biochemistry of Aerobic Biological Methane Oxidation. Chem. Soc. Rev. 2021, 50, 3424–3436. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic Bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef]
- Banerjee, R.; Proshlyakov, Y.; Lipscomb, J.D.; Proshlyakov, D.A. Structure of the Key Species in the Enzymatic Oxidation of Methane to Methanol. Nature 2015, 518, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, A.D.; Lippard, S.J. Coupling Oxygen Consumption with Hydrocarbon Oxidation in Bacterial Multicomponent Monooxygenases. Acc. Chem. Res. 2015, 48, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, R.L.; Rosenzweig, A.C. Crystal Structure of a Membrane-Bound Metalloenzyme That Catalyses the Biological Oxidation of Methane. Nature 2005, 434, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lawton, T.J.; Ham, J.; Sun, T.; Rosenzweig, A.C. Structural conservation of the B subunit in the ammonia monooxygenase/particulate methane monooxygenase superfamily. Proteins 2014, 82, 2263–2267. [Google Scholar] [CrossRef]
- Cao, L.; Caldararu, O.; Rosenzweig, A.C.; Ryde, U. Quantum Refinement Does Not Support Dinuclear Copper Sites in Crystal Structures of Particulate Methane Monooxygenase. Angew. Chem.—Int. Ed. 2018, 57, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Shiota, Y.; Yoshizawa, K. Comparison of the Reactivity of Bis(μ-Oxo)CuIICuIII and CuIIICuIII Species to Methane. Inorg. Chem. 2009, 48, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Shiota, Y. Conversion of Methane to Methanol at the Mononuclear and Dinuclear Copper Sites of Particulate Methane Monooxygenase (PMMO): A DFT and QM/MM Study. J. Am. Chem. Soc. 2006, 128, 9873–9881. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.K.M.L.; Kumashiro, M.; Ishihara, T. Direct Synthesis of Formic Acid by Partial Oxidation of Methane on H-ZSM-5 Solid Acid Catalyst. Catal. Commun. 2011, 12, 1198–1200. [Google Scholar] [CrossRef]
- Hammond, C.; Forde, M.M.; Ab Rahim, M.H.; Thetford, A.; He, Q.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Dummer, N.F.; Murphy, D.M.; et al. Direct Catalytic Conversion of Methane to Methanol in an Aqueous Medium by Using Copper-Promoted Fe-ZSM-5. Angew. Chem.—Int. Ed. 2012, 51, 5129–5133. [Google Scholar] [CrossRef]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and Reactivity of Framework and Extraframework Iron in Fe-Silicalite as Investigated by Spectroscopic and Physicochemical Methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Voskoboinikov, T.V.; Chen, H.-Y.; Sachtler, W.M.H. On the Nature of Active Sites in Fe/ZSM-5 Catalysts for NOx Abatement. Appl. Catal. B 1998, 19, 279–287. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Sachtler, W.M.H. Activity and Durability of Fe/ZSM-5 Catalysts for Lean Burn NOx Reduction in the Presence of Water Vapor. Catal. Today 1998, 42, 73–83. [Google Scholar] [CrossRef]
- Hammond, C.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Ab Rahim, M.H.; Forde, M.M.; Thetford, A.; Murphy, D.M.; Hagen, H.; Stangland, E.E.; et al. Catalytic and Mechanistic Insights of the Low-Temperature Selective Oxidation of Methane over Cu-Promoted Fe-ZSM-5. Chem.—A Eur. J. 2012, 18, 15735–15745. [Google Scholar] [CrossRef]
- Hammond, C.; Dimitratos, N.; Jenkins, R.L.; Lopez-Sanchez, J.A.; Kondrat, S.A.; Hasbi Ab Rahim, M.; Forde, M.M.; Thetford, A.; Taylor, S.H.; Hagen, H.; et al. Elucidation and Evolution of the Active Component within Cu/Fe/ZSM-5 for Catalytic Methane Oxidation: From Synthesis to Catalysis. ACS Catal. 2013, 3, 689–699. [Google Scholar] [CrossRef]
- Forde, M.M.; Armstrong, R.D.; McVicker, R.; Wells, P.P.; Dimitratos, N.; He, Q.; Lu, L.; Jenkins, R.L.; Hammond, C.; Lopez-Sanchez, J.A.; et al. Light Alkane Oxidation Using Catalysts Prepared by Chemical Vapour Impregnation: Tuning Alcohol Selectivity through Catalyst Pre-Treatment. Chem. Sci. 2014, 5, 3603–3616. [Google Scholar] [CrossRef]
- Hammond, C.; Hermans, I.; Dimitratos, N. Biomimetic Oxidation with Fe-ZSM-5 and H2O2-Identification of an Active, Extra-Framework Binuclear Core and an FeIII-OOH Intermediate with Resonance-Enhanced Raman Spectroscopy. ChemCatChem 2015, 7, 434–440. [Google Scholar] [CrossRef]
- Xu, J.; Armstrong, R.D.; Shaw, G.; Dummer, N.F.; Freakley, S.J.; Taylor, S.H.; Hutchings, G.J. Continuous Selective Oxidation of Methane to Methanol over Cu- and Fe-Modified ZSM-5 Catalysts in a Flow Reactor. Catal. Today 2016, 270, 93–100. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, E.D. Aqueous-Phase Partial Oxidation of Methane with H2O2 over Fe-ZSM-5 Catalysts Prepared from Different Iron Precursors. Microporous Mesoporous Mater. 2021, 324, 111278. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, K.H.; Cho, S.J.; Park, E.D. Partial Oxidation of Methane with Hydrogen Peroxide over Fe-ZSM-5 Catalyst. Catal. Today 2021, 376, 113–118. [Google Scholar] [CrossRef]
- Zhu, K.; Liang, S.; Cui, X.; Huang, R.; Wan, N.; Hua, L.; Li, H.; Chen, H.; Zhao, Z.; Hou, G.; et al. Highly Efficient Conversion of Methane to Formic Acid under Mild Conditions at ZSM-5-Confined Fe-Sites. Nano Energy 2021, 82, 105718. [Google Scholar] [CrossRef]
- Oda, A.; Aono, K.; Murata, N.; Murata, K.; Yasumoto, M.; Tsunoji, N.; Sawabe, K.; Satsuma, A. Rational Design of ZSM-5 Zeolite Containing a High Concentration of Single Fe Sites Capable of Catalyzing the Partial Oxidation of Methane with High Turnover Frequency. Catal. Sci. Technol. 2022, 12, 542–550. [Google Scholar] [CrossRef]
- Yu, T.; Li, Z.; Lin, L.; Chu, S.; Su, Y.; Song, W.; Wang, A.; Weckhuysen, B.M.; Luo, W. Highly Selective Oxidation of Methane into Methanol over Cu-Promoted Monomeric Fe/ZSM-5. ACS Catal. 2021, 11, 6684–6691. [Google Scholar] [CrossRef]
- Al-Shihri, S.; Richard, C.J.; Chadwick, D. Selective Oxidation of Methane to Methanol over ZSM-5 Catalysts in Aqueous Hydrogen Peroxide: Role of Formaldehyde. ChemCatChem 2017, 9, 1276–1283. [Google Scholar] [CrossRef]
- Al-Shihri, S.; Richard, C.J.; Al-Megren, H.; Chadwick, D. Insights into the Direct Selective Oxidation of Methane to Methanol over ZSM-5 Zeolytes in Aqueous Hydrogen Peroxide. Catal. Today 2020, 353, 269–278. [Google Scholar] [CrossRef]
- Hammond, C.; Dimitratos, N.; Lopez-Sanchez, J.A.; Jenkins, R.L.; Whiting, G.; Kondrat, S.A.; Ab Rahim, M.H.; Forde, M.M.; Thetford, A.; Hagen, H.; et al. Aqueous-Phase Methane Oxidation over Fe-MFI Zeolites; Promotion through Isomorphous Framework Substitution. ACS Catal. 2013, 3, 1835–1844. [Google Scholar] [CrossRef]
- Shahami, M.; Shantz, D.F. Zeolite Acidity Strongly Influences Hydrogen Peroxide Activation and Oxygenate Selectivity in the Partial Oxidation of Methane over M,Fe-MFI (M: Ga, Al, B) Zeolites. Catal. Sci. Technol. 2019, 9, 2945–2951. [Google Scholar] [CrossRef]
- Kalamaras, C.; Palomas, D.; Bos, R.; Horton, A.; Crimmin, M.; Hellgardt, K. Selective Oxidation of Methane to Methanol over Cu- And Fe-Exchanged Zeolites: The Effect of Si/Al Molar Ratio. Catal. Lett. 2016, 146, 483–492. [Google Scholar] [CrossRef]
- Fang, Z.; Murayama, H.; Zhao, Q.; Liu, B.; Jiang, F.; Xu, Y.; Tokunaga, M.; Liu, X. Selective Mild Oxidation of Methane to Methanol or Formic Acid on Fe-MOR Catalysts. Catal. Sci. Technol. 2019, 9, 6946–6956. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, Y.; Nishitoba, T.; Kondo, J.N.; Yokoi, T. Selective Oxidation of Methane to Methanol with H2O2 over an Fe-MFI Zeolite Catalyst Using Sulfolane Solvent. Chem. Commun. 2019, 55, 2896–2899. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yang, G.S.; Park, E.D. Effects of Cu Species on Liquid-Phase Partial Oxidation of Methane with H2O2 over Cu-Fe/ZSM-5 Catalysts. Catalysts 2022, 12, 1224. [Google Scholar] [CrossRef]
- Sun, S.; Barnes, A.J.; Gong, X.; Lewis, R.J.; Dummer, N.F.; Bere, T.; Shaw, G.; Richards, N.; Morgan, D.J.; Hutchings, G.J. Lanthanum Modified Fe-ZSM-5 Zeolites for Selective Methane Oxidation with H2O2. Catal. Sci. Technol. 2021, 11, 8052–8064. [Google Scholar] [CrossRef]
- Yu, X.; Wu, B.; Huang, M.; Lu, Z.; Li, J.; Zhong, L.; Sun, Y. IrFe/ZSM-5 Synergistic Catalyst for Selective Oxidation of Methane to Formic Acid. Energy Fuels 2021, 35, 4418–4427. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, S.; Tang, Y.; Li, Y.; Nguyen, L.; Li, Y.; Shan, J.; Xiao, D.; Gagne, R.; Frenkel, A.I.; et al. Low-Temperature Transformation of Methane to Methanol on Pd1O4 Single Sites Anchored on the Internal Surface of Microporous Silicate. Angew. Chem.—Int. Ed. 2016, 55, 13441–13445. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Szécsényi, Á.; Li, G.; Nasalevich, M.A.; Dugulan, I.A.; Crespo, P.S.; Hensen, E.J.M.; Veber, S.L.; Fedin, M.V.; et al. Isolated Fe Sites in Metal Organic Frameworks Catalyze the Direct Conversion of Methane to Methanol. ACS Catal. 2018, 8, 5542–5548. [Google Scholar] [CrossRef]
- Szécsényi, A.; Li, G.; Gascon, J.; Pidko, E.A. Unraveling Reaction Networks behind the Catalytic Oxidation of Methane with H2O2 over a Mixed-Metal MIL-53(Al,Fe) MOF Catalyst. Chem. Sci. 2018, 9, 6765–6773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shi, Y.; Jiang, Y.; Zhang, X.; Long, C.; An, P.; Zhu, Y.; Shao, S.; Yan, Z.; Li, G.; et al. Fe-O Clusters Anchored on Nodes of Metal–Organic Frameworks for Direct Methane Oxidation. Angew. Chem.—Int. Ed. 2021, 60, 5811–5815. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, C.; Keum, C.; Kim, H.E.; Lee, H.; Han, B.; Lee, S.Y. Methane Partial Oxidation by Monomeric Cu Active Center Confined on ZIF-7. Chem. Eng. J. 2022, 450, 138472. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, C.; Vikneshvaran, S.; Lee, S.; Lee, S.Y. Partial Oxidation of Methane to Methyl Oxygenates with Enhanced Selectivity Using a Single-Atom Copper Catalyst on Amorphous Carbon Support. Appl. Surf. Sci. 2023, 639, 158289. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.Y. High Metal Loaded Cu(i)N3 Single-Atom Catalysts: Superior Methane Conversion Activity and Selectivity under Mild Conditions. J. Mater. Chem. A Mater. 2023, 11, 15691–15701. [Google Scholar] [CrossRef]
- Ab Rahim, M.H.; Forde, M.M.; Jenkins, R.L.; Hammond, C.; He, Q.; Dimitratos, N.; Lopez-Sanchez, J.A.; Carley, A.F.; Taylor, S.H.; Willock, D.J.; et al. Oxidation of Methane to Methanol with Hydrogen Peroxide Using Supported Gold-Palladium Alloy Nanoparticles. Angew. Chem.—Int. Ed. 2013, 52, 1280–1284. [Google Scholar] [CrossRef]
- Agarwal, N.; Freakley, S.J.; McVicker, R.U.; Althahban, S.M.; Dimitratos, N.; He, Q.; Morgan, D.J.; Jenkins, R.L.; Willock, D.J.; Taylor, S.H.; et al. Aqueous Au-Pd Colloids Catalyze Selective CH4 Oxidation to CH3OH with O2 under Mild Conditions. Science 2017, 358, 223–227. [Google Scholar] [CrossRef]
- Xu, G.; Yu, A.; Xu, Y.; Sun, C. Selective Oxidation of Methane to Methanol Using AuPd@ZIF-8. Catal. Commun. 2021, 158, 106338. [Google Scholar] [CrossRef]
- McVicker, R.; Agarwal, N.; Freakley, S.J.; He, Q.; Althahban, S.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Low Temperature Selective Oxidation of Methane Using Gold-Palladium Colloids. Catal. Today 2020, 342, 32–38. [Google Scholar] [CrossRef]
- Colby, J.; Stirling, D.I.; Dalton, H. The Soluble Methane Mono-Oxygenase of Methylococcus Capsulatus (Bath). Its Ability to Oxygenate n-Alkanes, n-Alkenes, Ethers, and Alicyclic, Aromatic and Heterocyclic Compounds. Biochem. J. 1977, 165, 395–402. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, C.; Zou, S.; Liu, J.; Xiao, L.; Fan, J. High H2O2 Utilization Promotes Selective Oxidation of Methane to Methanol at Low Temperature. Front. Chem. 2020, 8, 252. [Google Scholar] [CrossRef]
- Cui, X.; Li, H.; Wang, Y.; Hu, Y.; Hua, L.; Li, H.; Han, X.; Liu, Q.; Yang, F.; He, L.; et al. Room-Temperature Methane Conversion by Graphene-Confined Single Iron Atoms. Chem 2018, 4, 1902–1910. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y. Facile Synthesis of N-Doped Carbon Supported Iron Species for Highly Efficient Methane Conversion with H2O2 at Ambient Temperature. Appl. Catal. A Gen. 2021, 615, 118052. [Google Scholar] [CrossRef]
- Shen, Q.; Cao, C.; Huang, R.; Zhu, L.; Zhou, X.; Zhang, Q.; Gu, L.; Song, W. Single Chromium Atoms Supported on Titanium Dioxide Nanoparticles for Synergic Catalytic Methane Conversion under Mild Conditions. Angew. Chem.—Int. Ed. 2020, 59, 1216–1219. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Sansare, S.D.; Gaikwad, A.G. Direct Oxidation of H2 to H2O2 and Decomposition of H2O2 over Oxidized and Reduced Pd-Containing Zeolite Catalysts in Acidic Medium. Catal. Lett. 2002, 84, 81–87. [Google Scholar] [CrossRef]
- Gaikwad, A.; Sansare, S.; Choudhary, V. Direct Oxidation of Hydrogen to Hydrogen Peroxide over Pd-Containing Fluorinated or Sulfated Al2O3, ZrO2, CeO2, ThO2, Y2O3 and Ga2O3 Catalysts in Stirred Slurry Reactor at Ambient Conditions. J. Mol. Catal. A Chem. 2002, 181, 143–149. [Google Scholar] [CrossRef]
- Park, S.; Kim, T.J.; Chung, Y.M.; Oh, S.H.; Song, I.K. Direct Synthesis of Hydrogen Peroxide from Hydrogen and Oxygen over Palladium Catalyst Supported on SO3H-Functionalized SBA-15. Catal. Lett. 2009, 130, 296–300. [Google Scholar] [CrossRef]
- Park, S.; Baeck, S.H.; Kim, T.J.; Chung, Y.M.; Oh, S.H.; Song, I.K. Direct Synthesis of Hydrogen Peroxide from Hydrogen and Oxygen over Palladium Catalyst Supported on SO3H-Functionalized Mesoporous Silica. J. Mol. Catal. A Chem. 2010, 319, 98–107. [Google Scholar] [CrossRef]
- Blanco-Brieva, G.; de Frutos Escrig, M.P.; Campos-Martin, J.M.; Fierro, J.L.G. Direct Synthesis of Hydrogen Peroxide on Palladium Catalyst Supported on Sulfonic Acid-Functionalized Silica. Green. Chem. 2010, 12, 1163–1166. [Google Scholar] [CrossRef]
- Park, S.-E.; Huang, L.; Lee, C.W.; Chang, J.-S. Generation of H2O2 from H2 and O2 over Zeolite Beta Containing Pd and Heterogenized Organic Compounds. Catal. Today 2000, 61, 117–122. [Google Scholar] [CrossRef]
- Li, G.; Edwards, J.; Carley, A.F.; Hutchings, G.J. Direct Synthesis of Hydrogen Peroxide from H2 and O2 Using Zeolite-Supported Au-Pd Catalysts. Catal. Today 2007, 122, 361–364. [Google Scholar] [CrossRef]
- Li, G.; Edwards, J.; Carley, A.F.; Hutchings, G.J. Direct Synthesis of Hydrogen Peroxide from H2 and O2 and in Situ Oxidation Using Zeolite-Supported Catalysts. Catal. Commun. 2007, 8, 247–250. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.H.; Song, S.H.; Park, D.R.; Baeck, S.H.; Kim, T.J.; Chung, Y.M.; Oh, S.H.; Song, I.K. Direct Synthesis of Hydrogen Peroxide from Hydrogen and Oxygen over Palladium-Exchanged Insoluble Heteropolyacid Catalysts. Catal. Commun. 2009, 10, 391–394. [Google Scholar] [CrossRef]
- Alotaibi, F.; Al-Mayman, S.; Alotaibi, M.; Edwards, J.K.; Lewis, R.J.; Alotaibi, R.; Hutchings, G.J. Direct Synthesis of Hydrogen Peroxide Using Cs-Containing Heteropolyacid-Supported Palladium–Copper Catalysts. Catal. Lett. 2019, 149, 998–1006. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Yu, K.; Ahn, W.S.; Chung, Y.M. Pd Nanoparticles on a Dual Acid-Functionalized Porous Polymer for Direct Synthesis of H2O2: Contribution by Enhanced H2 Storage Capacity. J. Ind. Eng. Chem. 2020, 81, 375–384. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Samanta, C. Role of Chloride or Bromide Anions and Protons for Promoting the Selective Oxidation of H2 by O2 to H2O2 over Supported Pd Catalysts in an Aqueous Medium. J. Catal. 2006, 238, 28–38. [Google Scholar] [CrossRef]
- Lee, M.W.; Jo, D.Y.; Han, G.H.; Lee, K.Y. DFT Calculations on Selectivity Enhancement by Br Addition on Pd Catalysts in the Direct Synthesis of Hydrogen Peroxide. Catal. Today 2022, 397–399, 232–239. [Google Scholar] [CrossRef]
- Melada, S.; Rioda, R.; Menegazzo, F.; Pinna, F.; Strukul, G. Direct Synthesis of Hydrogen Peroxide on Zirconia-Supported Catalysts under Mild Conditions. J. Catal. 2006, 239, 422–430. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.D. Aqueous-Phase Selective Oxidation of Methane with Oxygen over Iron Salts and Pd/C in the Presence of Hydrogen. ChemCatChem 2019, 11, 4247–4251. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.D. Selective Oxidation of Methane over Fe-Zeolites by In Situ Generated H2O2. Catalysts 2020, 10, 299. [Google Scholar] [CrossRef]
- Kang, J.; Puthiaraj, P.; Ahn, W.S.; Park, E.D. Direct Synthesis of Oxygenates via Partial Oxidation of Methane in the Presence of O2 and H2 over a Combination of Fe-ZSM-5 and Pd Supported on an Acid-Functionalized Porous Polymer. Appl. Catal. A Gen. 2020, 602, 117711. [Google Scholar] [CrossRef]
- Yang, G.S.; Kang, J.; Park, E.D. Aqueous-Phase Partial Oxidation of Methane over Pd−Fe/ZSM-5 with O2 in the Presence of H2. ChemCatChem 2023, 15, e202201630. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.D. Partial Oxidation of Methane over Fe/ZSM-5 with Hydrogen Peroxide Generated in Situ over Pd/C in the Presence of Halide Ions. Catal. Today 2024, 426, 114367. [Google Scholar] [CrossRef]
- Wu, B.; Lin, T.; Huang, M.; Li, S.; Li, J.; Yu, X.; Yang, R.; Sun, F.; Jiang, Z.; Sun, Y.; et al. Tandem Catalysis for Selective Oxidation of Methane to Oxygenates Using Oxygen over PdCu/Zeolite. Angew. Chem.—Int. Ed. 2022, 61, e2022041. [Google Scholar] [CrossRef]
- Wang, S.; Fung, V.; Hülsey, M.J.; Liang, X.; Yu, Z.; Chang, J.; Folli, A.; Lewis, R.J.; Hutchings, G.J.; He, Q.; et al. H2-Reduced Phosphomolybdate Promotes Room-Temperature Aerobic Oxidation of Methane to Methanol. Nat. Catal. 2023, 6, 895–905. [Google Scholar] [CrossRef]
- He, Y.; Luan, C.; Fang, Y.; Feng, X.; Peng, X.; Yang, G.; Tsubaki, N. Low-Temperature Direct Conversion of Methane to Methanol over Carbon Materials Supported Pd-Au Nanoparticles. Catal. Today 2020, 339, 48–53. [Google Scholar] [CrossRef]
- He, Y.; Liang, J.; Imai, Y.; Ueda, K.; Li, H.; Guo, X.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Highly Selective Synthesis of Methanol from Methane over Carbon Materials Supported Pd-Au Nanoparticles under Mild Conditions. Catal. Today 2020, 352, 104–110. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic Zeolite Modification for in Situ Peroxide Formation in Methane Oxidation to Methanol. Science 2020, 367, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Davies, T.E.; Nasrallah, A.; Sainna, M.A.; Howe, A.G.R.; Lewis, R.J.; Quesne, M.; Catlow, C.R.A.; Willock, D.J.; He, Q.; et al. Au-ZSM-5 Catalyses the Selective Oxidation of CH4 to CH3OH and CH3COOH Using O2. Nat. Catal. 2022, 5, 45–54. [Google Scholar] [CrossRef]
- Mehmood, A.; Chae, S.Y.; Park, E.D. Photoelectrochemical Conversion of Methane into Value-Added Products. Catalysts 2021, 11, 1387. [Google Scholar] [CrossRef]
- Mehmood, A.; Chae, S.Y.; Park, E.D. Low-Temperature Electrochemical Oxidation of Methane into Alcohols. Catalysts 2024, 14, 58. [Google Scholar] [CrossRef]
- Yuniar, G.; Saputera, W.H.; Sasongko, D.; Mukti, R.R.; Rizkiana, J.; Devianto, H. Recent Advances in Photocatalytic Oxidation of Methane to Methanol. Molecules 2022, 27, 5496. [Google Scholar] [CrossRef] [PubMed]
- Belousov, A.S.; Shafiq, I. Heterogeneous Photocatalysis for C–H Bond Activation. J. Environ. Chem. Eng. 2023, 11, 110970. [Google Scholar] [CrossRef]
- Jia, T.; Wang, W. Research Progress and Outlook on Photocatalytic Conversion of Methane to Methanol. ChemCatChem 2024, e202301279. [Google Scholar] [CrossRef]
- Liang, C.; He, B. A Titration Method for Determining Individual Oxidant Concentration in the Dual Sodium Persulfate and Hydrogen Peroxide Oxidation System. Chemosphere 2018, 198, 297–302. [Google Scholar] [CrossRef]
- Forde, M.M.; Armstrong, R.D.; Hammond, C.; He, Q.; Jenkins, R.L.; Kondrat, S.A.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Willock, D.; et al. Partial Oxidation of Ethane to Oxygenates Using Fe- and Cu-Containing ZSM-5. J. Am. Chem. Soc. 2013, 135, 11087–11099. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).