Manganese- and Nitrogen-Doped Biomass-Based Carbons as Catalysts for the Oxygen Reduction Reaction
Abstract
1. Introduction
2. Results
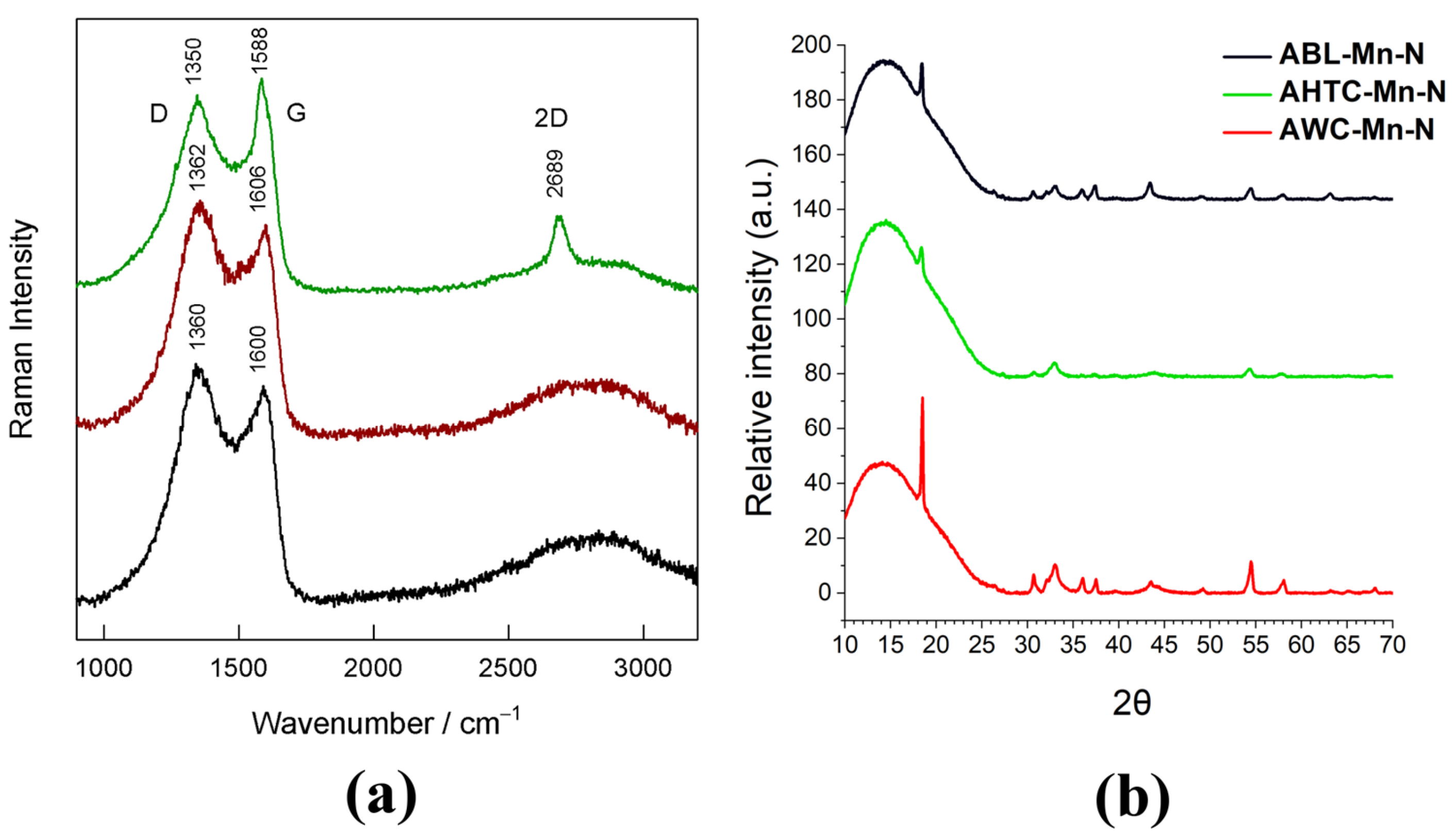
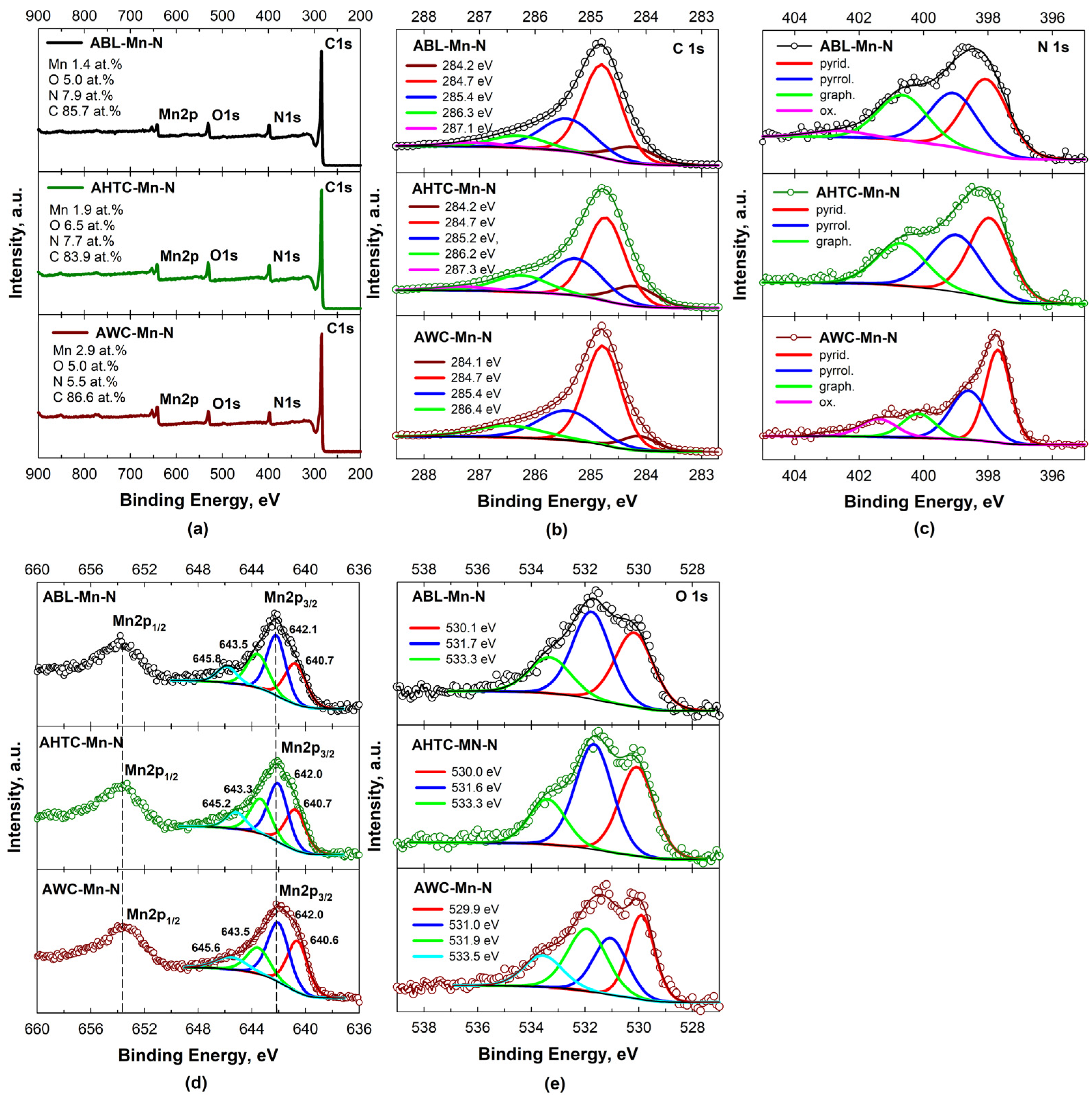
2.1. Physical Characterization
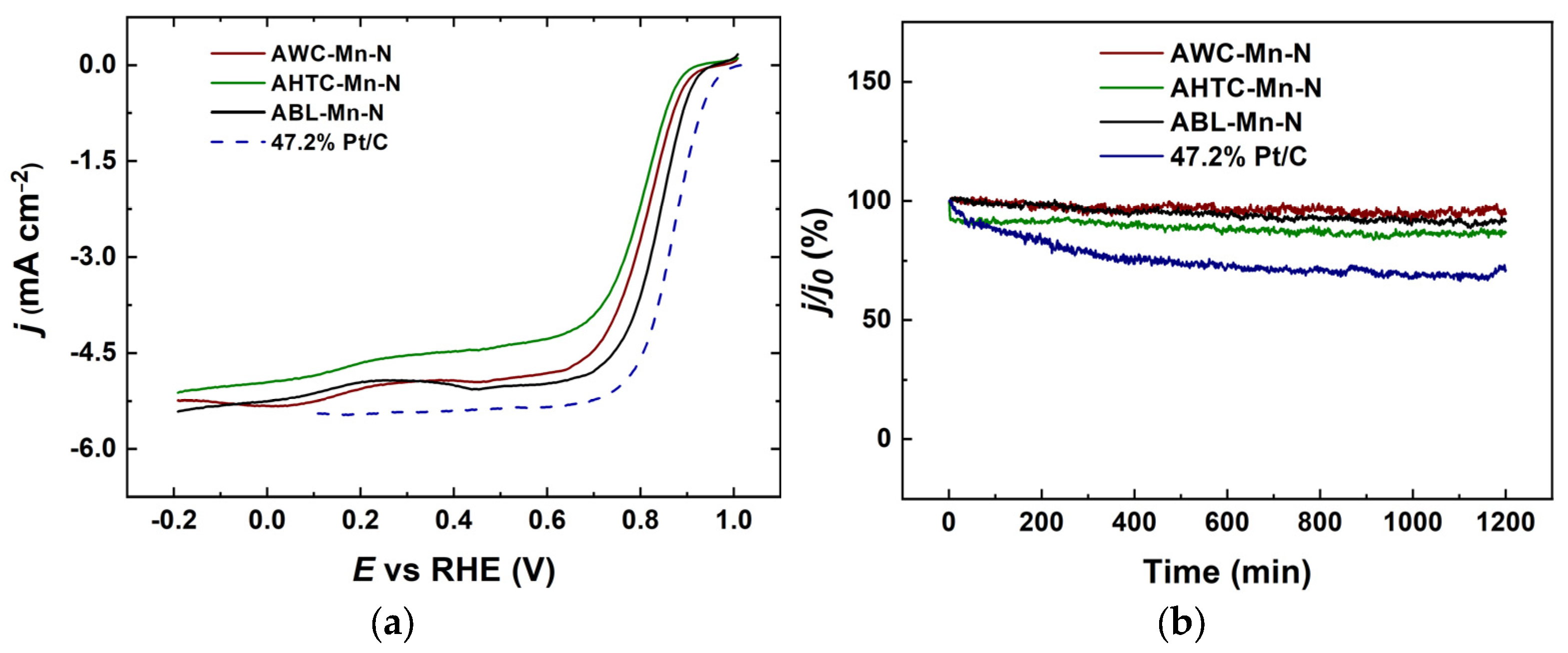
2.2. Electrochemical Characterization
3. Materials and Methods
3.1. Synthesis of Carbon Material
3.2. Characterization of Carbon Materials
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Li, X.; Wang, H.; Wu, J.; Ma, H.; Zhou, J. Hydrothermal, KOH-assisted synthesis of lignin-derived porous carbon for supercapacitors: Value-added of lignin and constructing texture properties/specific capacitance relationships. J. Mater. Res. Technol. 2022, 16, 570–580. [Google Scholar] [CrossRef]
- Rashidi, S.; Karimi, N.; Sunden, B.; Kim, K.C.; Olabi, A.G.; Mahian, O. Progress and challenges on the thermal management of electrochemical energy conversion and storage technologies: Fuel cells, electrolysers, and supercapacitors. Prog. Energy Combust. Sci. 2022, 88, 100966. [Google Scholar] [CrossRef]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Bhuvanendran, N.; Ravichandran, S.; Xu, Q.; Maiyalagan, T.; Su, H. A quick guide to the assessment of key electrochemical performance indicators for the oxygen reduction reaction: A comprehensive review. Int. J. Hydrogen Energy 2022, 47, 7113–7138. [Google Scholar] [CrossRef]
- Machan, C.W. Advances in the Molecular Catalysis of Dioxygen Reduction. ACS Catal. 2020, 10, 2640–2655. [Google Scholar] [CrossRef]
- Yi, S.; Jiang, H.; Bao, X.; Zou, S.; Liao, J.; Zhang, Z. Recent progress of Pt-based catalysts for oxygen reduction reaction in preparation strategies and catalytic mechanism. J. Electroanal. Chem. 2019, 848, 113279. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen reduction electrocatalysts toward practical fuel cells: Progress and perspectives. Angew. Chem. 2021, 133, 17832–17852. [Google Scholar] [CrossRef]
- Sandhiran, N.; Ganapathy, S.; Manoharan, Y.; Ganguly, D.; Kumar, M.; Ramanujam, K.; Balachandran, S. CuO–NiO binary transition metal oxide nanoparticle anchored on rGO nanosheets as high-performance electrocatalyst for the oxygen reduction reaction. Environ. Res. 2022, 211, 112992. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Doan, T.L.L.; Tran, D.T.; Kim, N.H.; Lee, J.H. Transition metal nanoparticles as electrocatalysts for ORR, OER, and HER. In Nanomaterials for Electrocatalysis; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–83. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, Y.; Yang, R.; Kakimov, A.; Li, X. Recent advances of layered double hydroxides–based bifunctional electrocatalysts for ORR and OER. Mater. Today Chem. 2021, 21, 100488. [Google Scholar] [CrossRef]
- Gnanaprakasam, P.; Mangalaraja, R.V.; Salvo, C. Microwave driven synthesis of tungsten sulfide nanosheets: An efficient electrocatalyst for oxygen reduction reaction. Mater. Sci. Semicond. Process. 2022, 137, 106213. [Google Scholar] [CrossRef]
- Wan, K.; Luo, J.; Zhang, X.; Zhou, C.; Seo, J.W.; Subramanian, P.; Yan, J.W.; Fransaer, J. A template-directed bifunctional NiSx/nitrogen-doped mesoporous carbon electrocatalyst for rechargeable Zn–air batteries. J. Mater. Chem. A 2019, 7, 19889–19897. [Google Scholar] [CrossRef]
- Gu, S.; Chandra Mallick, B.; Hsieh, C.T.; Gandomi, Y.A.; Zhang, R.S. Hexagonal boron nitride nanosheets as metal-free electrochemical catalysts for oxygen reduction reactions. Ceram. Int. 2022, 48, 9506–9517. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Y.; Huang, S. Doping engineering on carbons as electrocatalysts for oxygen reduction reaction. Fundam. Res. 2021, 1, 807–823. [Google Scholar] [CrossRef]
- Song-lin, Z.; Shang-yu, G.; Xi-gen, Y.; Bo-sen, X. Carbonization mechanism of bamboo (phyllostachys) by means of Fourier Transform Infrared and elemental analysis. J. For. Res. 2003, 14, 75–79. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Rawat, S.; Mishra, R.K.; Bhaskar, T. Biomass derived functional carbon materials for supercapacitor applications. Chemosphere 2022, 286, 131961. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Lee, J.; Park, J.S. Biomass-derived bifunctional electrocatalysts for oxygen reduction and evolution reaction: A review. J. Energy Chem. 2022, 65, 149–172. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, G.; Sekhon, S.S. Biomass derived hierarchical porous carbon materials as oxygen reduction reaction electrocatalysts in fuel cells. Prog. Mater. Sci. 2019, 102, 1–71. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z. The rational design of biomass-derived carbon materials towards next-generation energy storage: A review. Renew. Sustain. Energy Rev. 2020, 134, 110308. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Z.; Hoang, T.K.A.; Liu, Y.; Sun, A. The intriguing ORR performance of iron and nitrogen co-doped biomass carbon composites incorporating surface-modified polyaniline-derived carbon. Fuel 2022, 317, 123496. [Google Scholar] [CrossRef]
- Xia, S.; Guo, W.; Cai, N.; Sun, L.; Zhou, H.; Lu, W.; Chen, X.; Zhang, J.; Chen, Y.; Yang, H.; et al. Synthesis and application in oxygen reduction reaction of N-doping porous graphitic carbon from biomass waste. Fuel Process. Technol. 2021, 224, 107028. [Google Scholar] [CrossRef]
- Kuo, H.C.; Lin, Y.G.; Chiang, C.L.; Liu, S.H. FeN@N-doped graphitic biochars derived from hydrothermal-microwave pyrolysis of cellulose biomass for fuel cell catalysts. J. Anal. Appl. Pyrolysis 2021, 153, 104991. [Google Scholar] [CrossRef]
- Li, Z.; Gao, C.; Zhao, H.; Meng, A.; Ding, S.; Wang, X.; Li, S. Porous biomass-derived carbon modified by Cu, N co-doping and Cu nanoparticles as high-efficient electrocatalyst for oxygen reduction reaction and zinc-air battery. J. Alloys Compd. 2022, 897, 163175. [Google Scholar] [CrossRef]
- Zhao, J.R.; Hu, J.; Li, J.F.; Chen, P. N-doped carbon nanotubes derived from waste biomass and its electrochemical performance. Mater. Lett. 2020, 261, 127146. [Google Scholar] [CrossRef]
- Maliutina, K.; Huang, J.; Su, T.; Yu, J.; Fan, L. Biomass-derived Ta,N,S co-doped CNTs enriched carbon catalyst for efficient electrochemical oxygen reduction. J. Alloys Compd. 2021, 888, 161479. [Google Scholar] [CrossRef]
- Hildago-Oporto, P.; Navia, R.; Hunter, R.; Coronado, G.; Gonzalez, M.E. Synthesis of carbon nanotubes using biochar as precursor material under microwave irradiation. J. Environ. Manag. 2019, 244, 83–91. [Google Scholar] [CrossRef]
- Wan, W.; Zhao, W.; Wu, Y.; Dai, C.; Zhu, X.; Wang, Y.; Qin, J.; Chen, T.; Lü, Z. A highly efficient biomass based electrocatalyst for cathodic performance of lithium–oxygen batteries: Yeast derived hydrothermal carbon. Electrochim. Acta 2020, 349, 136411. [Google Scholar] [CrossRef]
- Ma, L.L.; Liu, W.J.; Hu, X.; Lam, P.K.S.; Zeng, J.R.; Yu, H.Q. Ionothermal carbonization of biomass to construct sp2/sp3 carbon interface in N-doped biochar as efficient oxygen reduction electrocatalysts. Chem. Eng. J. 2020, 400, 125969. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Kaur, P.; Park, J.S. From coconut shell biomass to oxygen reduction reaction catalyst: Tuning porosity and nitrogen doping. Renew. Sustain. Energy Rev. 2021, 147, 111173. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Mestre, A.S.; Martins, A.; Nunes, N.; Carvalho, A.P.; Freire, C. Biomass-derived nanoporous carbons as electrocatalysts for oxygen reduction reaction. Catal. Today 2020, 357, 269–278. [Google Scholar] [CrossRef]
- Li, J.; Gao, S.; Li, B.; Li, Y.; Cheng, C.; Maouche, C.; Wu, Y.; Rahman, N.; Zhou, Y.; Yang, J. Biomass-derived nitrogen-doped porous carbons with ultra-high surface area for electrocatalytic oxygen reduction reaction. J. Electroanal. Chem. 2020, 878, 114542. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Oyola-Rivera, O.; He, J.; Huber, G.W.; Dumesic, J.A.; Cardona-Martínez, N. Catalytic dehydration of levoglucosan to levoglucosenone using Brønsted solid acid catalysts in tetrahydrofuran. Green Chem. 2019, 21, 4988–4999. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Liu, Y.; Chen, Y.; Gao, S. Favorable pore size distribution of biomass-derived N, S dual-doped carbon materials for advanced oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 12964–12974. [Google Scholar] [CrossRef]
- Lv, X.; Xiao, Z.; Wang, H.; Wang, X.; Shan, L.; Wang, F.; Wei, C.; Tang, X.; Chen, Y. In situ construction of Co/N/C-based heterojunction on biomass-derived hierarchical porous carbon with stable active sites using a Co-N protective strategy for high-efficiency ORR, OER and HER trifunctional electrocatalysts. J. Energy Chem. 2021, 54, 626–638. [Google Scholar] [CrossRef]
- Charles, V.; Zhang, X.; Yuan, M.; Zhang, K.; Cui, K.; Zhang, J.; Zhao, T.; Li, Y.; Liu, Z.; Li, B.; et al. CoNi nano-alloy anchored on biomass-derived N-doped carbon frameworks for enhanced oxygen reduction and evolution reactions. Electrochim. Acta 2022, 402, 139555. [Google Scholar] [CrossRef]
- Saianand, G.; Gopalan, A.I.; Lee, J.C.; Sathish, C.I.; Gopalakrishnan, K.; Unni, G.E.; Shanbhag, D.; Dasireddy, V.D.B.C.; Yi, J.; Xi, S.; et al. Mixed Copper/Copper-Oxide Anchored Mesoporous Fullerene Nanohybrids as Superior Electrocatalysts toward Oxygen Reduction Reaction. Small 2020, 16, 1903937. [Google Scholar] [CrossRef]
- Sudarsono, W.; Wong, W.Y.; Loh, K.S.; Majlan, E.H.; Syarif, N.; Kok, K.Y.; Yunus, R.M.; Lim, K.L.; Hamada, I. Sengon wood-derived RGO supported Fe-based electrocatalyst with stabilized graphitic N-bond for oxygen reduction reaction in acidic medium. Int. J. Hydrogen Energy 2020, 45, 23237–23253. [Google Scholar] [CrossRef]
- Kisand, K.; Sarapuu, A.; Douglin, J.C.; Kikas, A.; Treshchalov, A.; Käärik, M.; Piirsoo, H.M.; Paiste, P.; Aruväli, J.; Leis, J.; et al. Templated Nitrogen-, Iron-, and Cobalt-Doped Mesoporous Nanocarbon Derived from an Alkylresorcinol Mixture for Anion-Exchange Membrane Fuel Cell Application. ACS Catal. 2022, 12, 14050–14061. [Google Scholar] [CrossRef]
- Xiong, C.; Li, B.; Dang, W.; Zhao, W.; Duan, C.; Dai, L.; Ni, Y. Co/CoS nanofibers with flower-like structure immobilized in carbonated porous wood as bifunctional material for high-performance supercapacitors and catalysts. Mater. Des. 2020, 195, 108942. [Google Scholar] [CrossRef]
- Yaddanapudi, H.S.; Tian, K.; Teng, S.; Tiwari, A. Facile preparation of nickel/carbonized wood nanocomposite for environmentally friendly supercapacitor electrodes. Sci. Rep. 2016, 6, 33659. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Chen, X.; Gong, T.; Liu, H.; Liu, Y.; Li, H.; Zhang, Y. N, P, S/Fe-codoped Carbon Derived from Feculae Bombycis as an Efficient Electrocatalyst for Oxygen Reduction Reaction. ChemCatChem 2019, 11, 6015–6021. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Soares, J.; Oliveros, M.E.; Garin, C.; David, M.V.; Martins, L.G.P.; Almeida, C.A.; Martins-Ferreira, E.H.; Takai, K.; Enoki, T.; Magalhães-Paniago, R.; et al. Structural analysis of polycrystalline graphene systems by Raman spectroscopy. Carbon 2015, 95, 646–652. [Google Scholar] [CrossRef]
- Jorio, A.; Cançado, L.G. Perspectives on Raman spectroscopy of graphene-based systems: From the perfect two-dimensional surface to charcoal. Phys. Chem. Chem. Phys. 2012, 14, 15246–15256. [Google Scholar] [CrossRef] [PubMed]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The importance of interbands on the interpretation of the raman spectrum of graphene oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Trusovas, R.; Ratautas, K.; Račiukaitis, G.; Niaura, G. Graphene layer formation in pinewood by nanosecond and picosecond laser irradiation. Appl. Surf. Sci. 2019, 471, 154–161. [Google Scholar] [CrossRef]
- Jorio, A.; Souza Filho, A.G. Raman Studies of Carbon Nanostructures. Annu. Rev. Mater. Res. 2016, 46, 357–382. [Google Scholar] [CrossRef]
- Sannasi, V.; Subbian, K. Influence of Moringa oleifera gum on two polymorphs synthesis of MnO2 and evaluation of the pseudo-capacitance activity. J. Mater. Sci. Mater. Electron. 2020, 31, 17120–17132. [Google Scholar] [CrossRef]
- Özcan, S.; Güler, A.; Cetinkaya, T.; Guler, M.O.; Akbulut, H. Freestanding graphene/MnO2 cathodes for Li-ion batteries. Beilstein J. Nanotechnol. 2017, 8, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, C.M.; Grossman, J.H.; Bobko, A.A.; Bennewitz, M.F. Tuning the size and composition of manganese oxide nanoparticles through varying temperature ramp and aging time. PLoS ONE 2020, 15, e0239034. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Chen, T.; Huang, Z.; Liu, J.; Jiang, L.; Chu, J.; Song, C.; Kong, A. Mn-Pyridine N site-enriched Mn-N–C derived from covalent organic polymer for electrochemical oxygen reduction and capacitive storage. Lonics 2021, 27, 5229–5239. [Google Scholar] [CrossRef]
- Grissa, R.; Martinez, H.; Cotte, S.; Galipaud, J.; Pecquenard, B.; Cras, F. Le Thorough XPS analyses on overlithiated manganese spinel cycled around the 3V plateau. Appl. Surf. Sci. 2017, 411, 449–456. [Google Scholar] [CrossRef]
- Briggs, D.; Fairley, N. Valence-band x-ray photoelectron spectroscopic studies of manganese and its oxides interpreted by cluster and band structure calculations. Surf. Interface Anal. 2002, 33, 274–282. [Google Scholar] [CrossRef]
- Zhou, D.; Lin, H.; Zhang, F.; Niu, H.; Cui, L.; Wang, Q.; Qu, F. Freestanding MnO2 nanoflakes/porous carbon nanofibers for high-performance flexible supercapacitor electrodes. Electrochim. Acta 2015, 161, 427–435. [Google Scholar] [CrossRef]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn 3O4 electrodeposited films for the oxygen evolution reaction of water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Baer, D.R.; Artyushkova, K.; Cohen, H.; Easton, C.D.; Engelhard, M.; Gengenbach, T.R.; Greczynski, G.; Mack, P.; Morgan, D.J.; Roberts, A. XPS guide: Charge neutralization and binding energy referencing for insulating samples. J. Vac. Sci. Technol. A Vac. Surf. Film. 2020, 38, 31204. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as catalyst and support for electrochemical energy conversion. Carbon 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Plavniece, A.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Kaare, K.; Kruusenberg, I.; Kaprans, K.; Knoks, A.; Kleperis, J. Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application. Sustainability 2021, 13, 9237. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Friedman, A.; Poli, F.; Petri, E.; Honig, H.; Basile, F.; Fasolini, A.; Lorenzi, R.; Berretti, E.; Bellini, M.; et al. Lignin-derived bimetallic platinum group metal-free oxygen reduction reaction electrocatalysts for acid and alkaline fuel cells. J. Power Sources 2023, 556, 232416. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, P.; Xu, W. Structure-activity relationship of doped-nitrogen (N)-based metal-free active sites on carbon for oxygen reduction reaction. Carbon 2017, 115, 763–772. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, G.; Zhang, X.; Chen, B.; Lu, Z.; Xu, H.; Gao, R.; Shi, C. Engineering the Local Coordination Environment and Density of FeN4 Sites by Mn Cooperation for Electrocatalytic Oxygen Reduction. Small 2022, 18, 2200911. [Google Scholar] [CrossRef]
- Berber, J.S.; Rice, R.L. Carbon black preparation from low-temperature lignite pitch. Ind. Eng. Chem. Prod. Res. Dev. 1969, 8, 188–193. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, H.; Dasappa, S.; Sabado, K.D.; Camacho, J. Production of Carbon Black in Turbulent Spray Flames of Coal Tar Distillates. Appl. Sci. 2021, 11, 10001. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Kaare, K.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I. Influence of Chemical Activation Temperatures on Nitrogen-Doped Carbon Material Structure, Pore Size Distribution and Oxygen Reduction Reaction Activity. Catalysts 2021, 11, 1460. [Google Scholar] [CrossRef]
- Plavniece, A.; Dobele, G.; Volperts, A.; Zhurinsh, A. Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials. Sustainability 2022, 14, 15982. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I.; Kaare, K.; Locs, J.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Biomass based activated carbons for fuel cells. Renew. Energy 2019, 141, 40–45. [Google Scholar] [CrossRef]
- Nacys, A.; Šimkūnaitė, D.; Balčiūnaitė, A.; Zabielaitė, A.; Upskuvienė, D.; Šebeka, B.; Jasulaitienė, V.; Kovalevskij, V.; Norkus, E.; Tamašauskaitė-Tamašiūnaitė, L. An Enhanced Oxidation of Formate on PtNi/Ni Foam Catalyst in an Alkaline Medium. Crystals 2022, 12, 362. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 82nd ed.; CRC Press: Boca Ration, FL, USA, 2001; ISBN 0849304849. [Google Scholar]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]

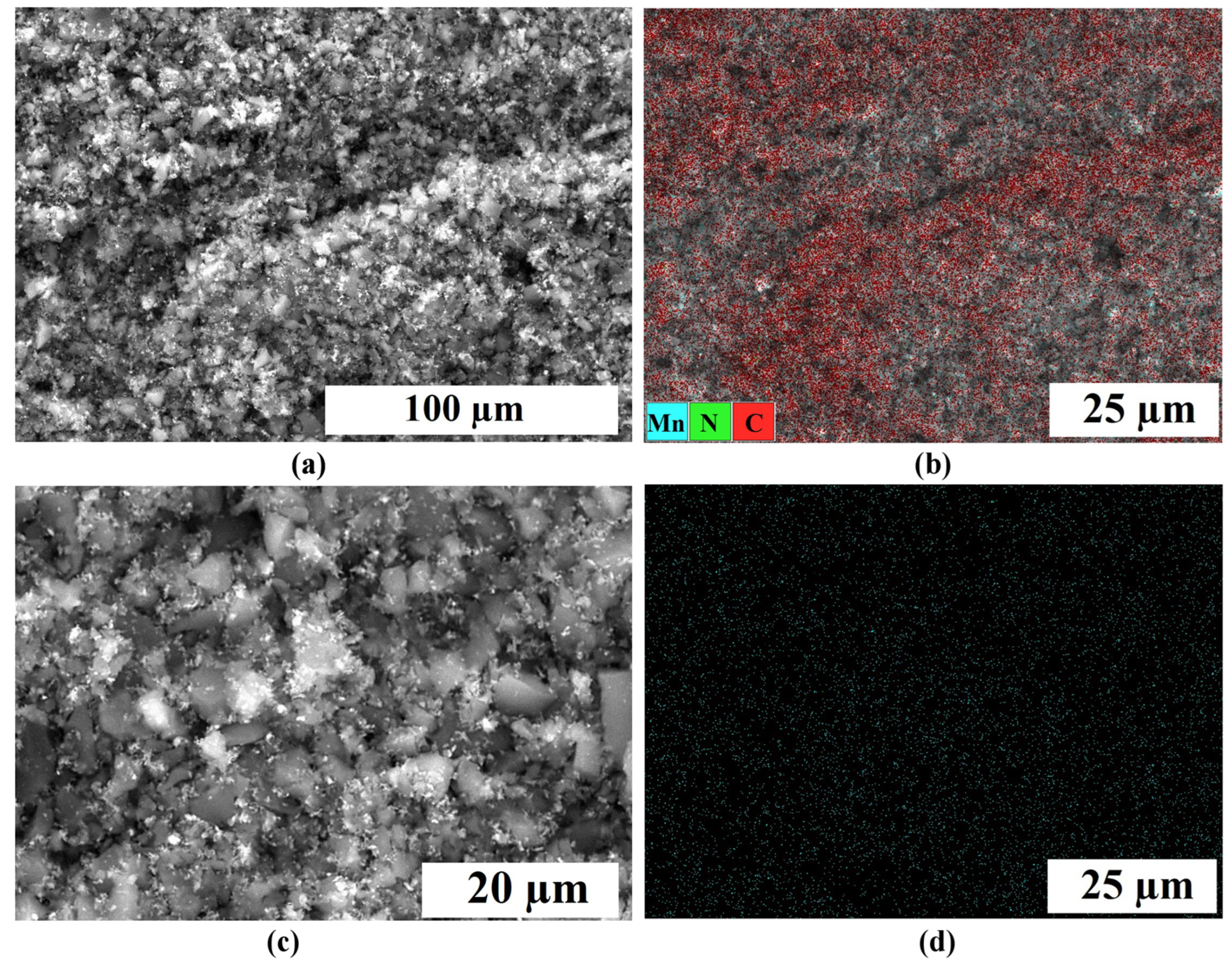
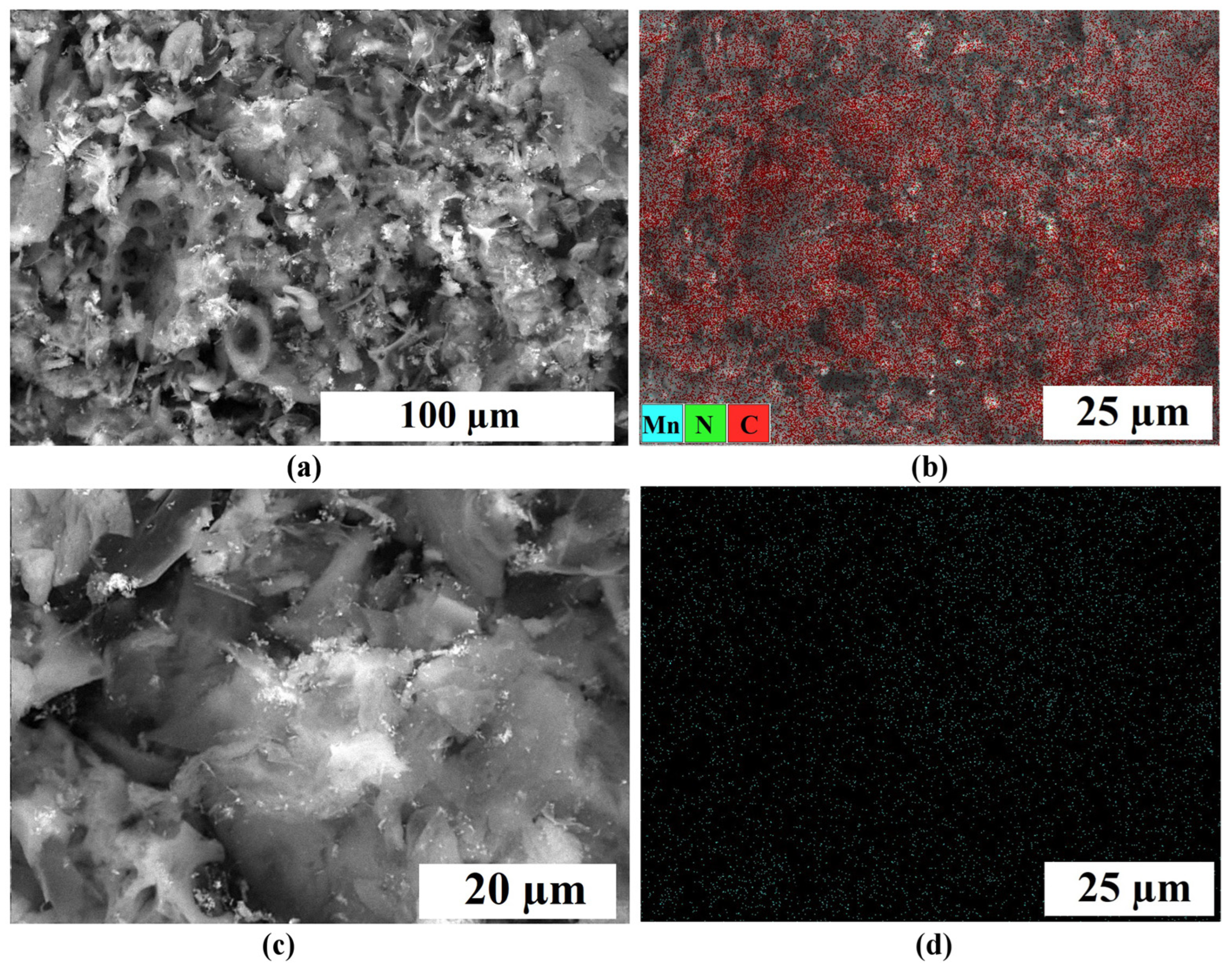
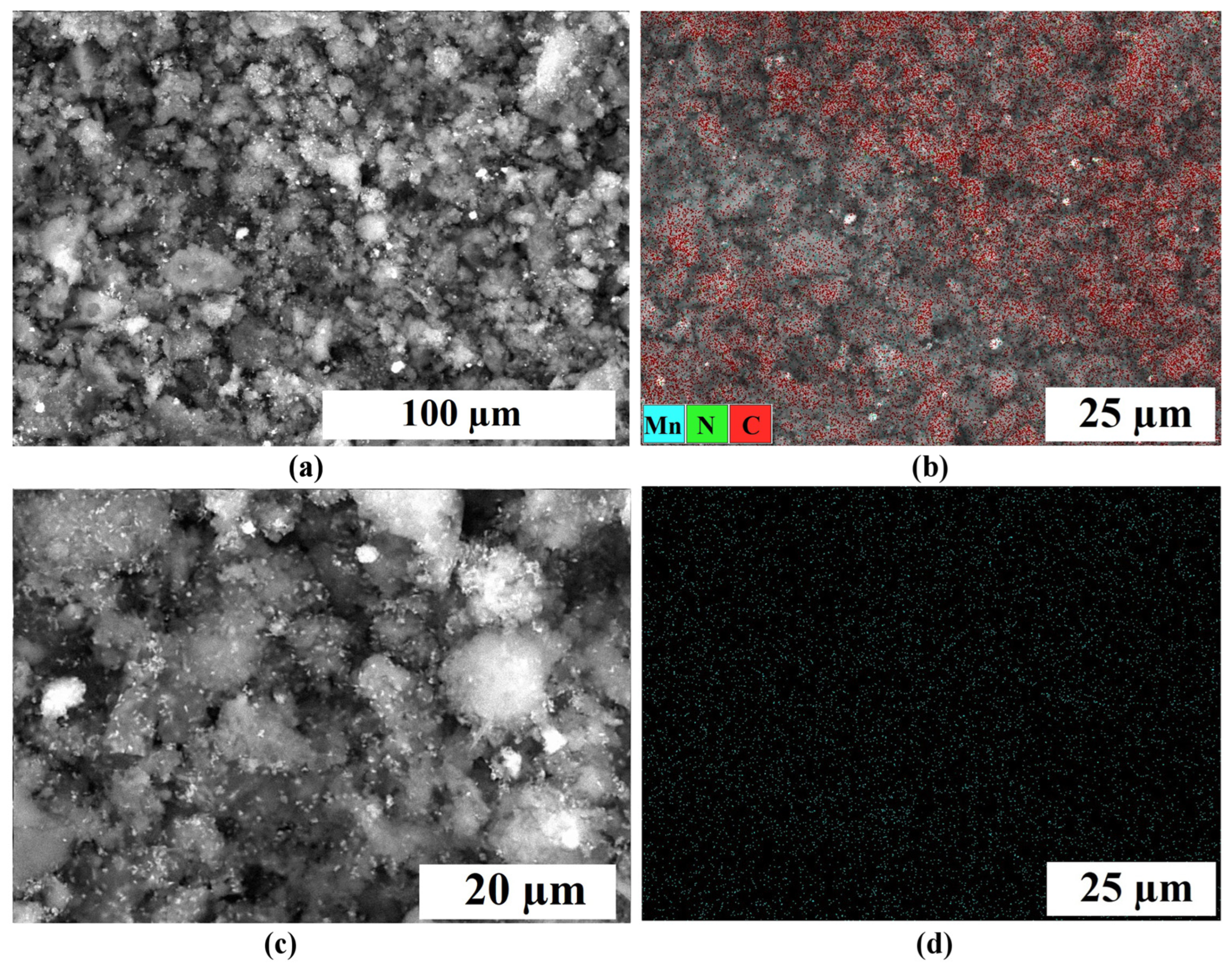
| Samples | Specific Surface Area, m2 g−1 | Pore Volume, cm3 g−1 | Average Pore Width, nm | Mesopores Volume from Total, % | ||||
|---|---|---|---|---|---|---|---|---|
| BET | DR * | DFT | Total | Micro | Meso | |||
| AWC-Mn-N | 2127 | 1905 | 1426 | 1.2 | 0.7 | 0.5 | 2.2 | 41.9 |
| AHTC-Mn-N | 2195 | 1932 | 1494 | 1.3 | 0.7 | 0.6 | 2.4 | 47.0 |
| ABL-Mn-N | 1808 | 1619 | 1276 | 1.7 | 0.6 | 1.1 | 3.8 | 66.8 |
| Sample | ν(G) (cm−1) | FWHM(G) (cm−1) | La (nm) |
|---|---|---|---|
| AWC-Mn-N | 1588 | 57.0 | 13.1 |
| AHTC-Mn-N | 1606 | 69.8 | 8.8 |
| ABL-Mn-N | 1600 | 74.9 | 7.4 |
| Element | ABL-Mn-N | AHTC-Mn-N | AWC-Mn-N |
|---|---|---|---|
| at. % | at. % | at. % | |
| N | 7.9 ± 0.1 | 7.7 ± 0.1 | 5.5 ± 0.1 |
| O | 5.0 ± 0.1 | 6.5 ± 0.1 | 3.5 ± 0.1 |
| C | 85.7 ± 0.1 | 83.9 ± 0.1 | 88.2 ± 0.1 |
| Mn | 1.4 ± 0.1 | 1.9 ± 0.1 | 2.8 ± 0.1 |
| Sample | C 1s | N 1s | O 1s | Mn 2p3/2 | ||||
|---|---|---|---|---|---|---|---|---|
| Eb, eV | at. % | Eb, eV | at. % | Eb, eV | at. % | Eb, eV | at. % | |
| ABL-Mn-N | 284.2 | 10.2 | 398.0 | 38.7 | 530.1 | 37.4 | 640.7 | 28.6 |
| 284.7 | 52.0 | 399.0 | 30.8 | 531.7 | 43.2 | 642.1 | 37.1 | |
| 285.4 | 25.0 | 400.6 | 26.2 | 533.3 | 19.4 | 643.5 | 23.8 | |
| 286.3 | 9.4 | 402.6 | 4.2 | 645.8 | 10.5 | |||
| 287.1 | 3.4 | |||||||
| AHTC-Mn-N | 284.2 | 12.0 | 397.9 | 39.7 | 530.0 | 36.2 | 640.7 | 27.3 |
| 284.7 | 44.7 | 398.9 | 34.9 | 531.7 | 44.8 | 642.0 | 37.9 | |
| 285.3 | 27.2 | 400.7 | 25.4 | 533.4 | 19.0 | 643.3 | 23.8 | |
| 286.2 | 12.7 | 645.2 | 11.0 | |||||
| 287.3 | 3.3 | |||||||
| AWC-Mn-N | 284.1 | 7.6 | 397.6 | 41.8 | 529.9 | 28.6 | 640.6 | 31.9 |
| 284.7 | 54.2 | 398.6 | 32.4 | 531.0 | 24.5 | 642.0 | 38.7 | |
| 285.4 | 26.0 | 400.1 | 14.3 | 531.9 | 31.1 | 643.5 | 18.2 | |
| 286.4 | 12.2 | 401.4 | 11.5 | 533.5 | 15.6 | 645.8 | 11.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plavniece, A.; Kaare, K.; Simkunaitė, D.; Balciunaite, A.; Jasulaitiene, V.; Niaura, G.; Volperts, A.; Dobele, G.; Colmenares-Rausseo, L.C.; Kruusenberg, I.; et al. Manganese- and Nitrogen-Doped Biomass-Based Carbons as Catalysts for the Oxygen Reduction Reaction. Catalysts 2024, 14, 92. https://doi.org/10.3390/catal14020092
Plavniece A, Kaare K, Simkunaitė D, Balciunaite A, Jasulaitiene V, Niaura G, Volperts A, Dobele G, Colmenares-Rausseo LC, Kruusenberg I, et al. Manganese- and Nitrogen-Doped Biomass-Based Carbons as Catalysts for the Oxygen Reduction Reaction. Catalysts. 2024; 14(2):92. https://doi.org/10.3390/catal14020092
Chicago/Turabian StylePlavniece, Ance, Kätlin Kaare, Dijana Simkunaitė, Aldona Balciunaite, Vitalija Jasulaitiene, Gediminas Niaura, Aleksandrs Volperts, Galina Dobele, Luis César Colmenares-Rausseo, Ivar Kruusenberg, and et al. 2024. "Manganese- and Nitrogen-Doped Biomass-Based Carbons as Catalysts for the Oxygen Reduction Reaction" Catalysts 14, no. 2: 92. https://doi.org/10.3390/catal14020092
APA StylePlavniece, A., Kaare, K., Simkunaitė, D., Balciunaite, A., Jasulaitiene, V., Niaura, G., Volperts, A., Dobele, G., Colmenares-Rausseo, L. C., Kruusenberg, I., Tamasauskaite-Tamasiunaite, L., & Norkus, E. (2024). Manganese- and Nitrogen-Doped Biomass-Based Carbons as Catalysts for the Oxygen Reduction Reaction. Catalysts, 14(2), 92. https://doi.org/10.3390/catal14020092











