Zn-Layered Double Hydroxide Intercalated with Graphene Oxide for Methylene Blue Photodegradation and Acid Red Adsorption Studies
Abstract
1. Introduction
2. Results and Discussion
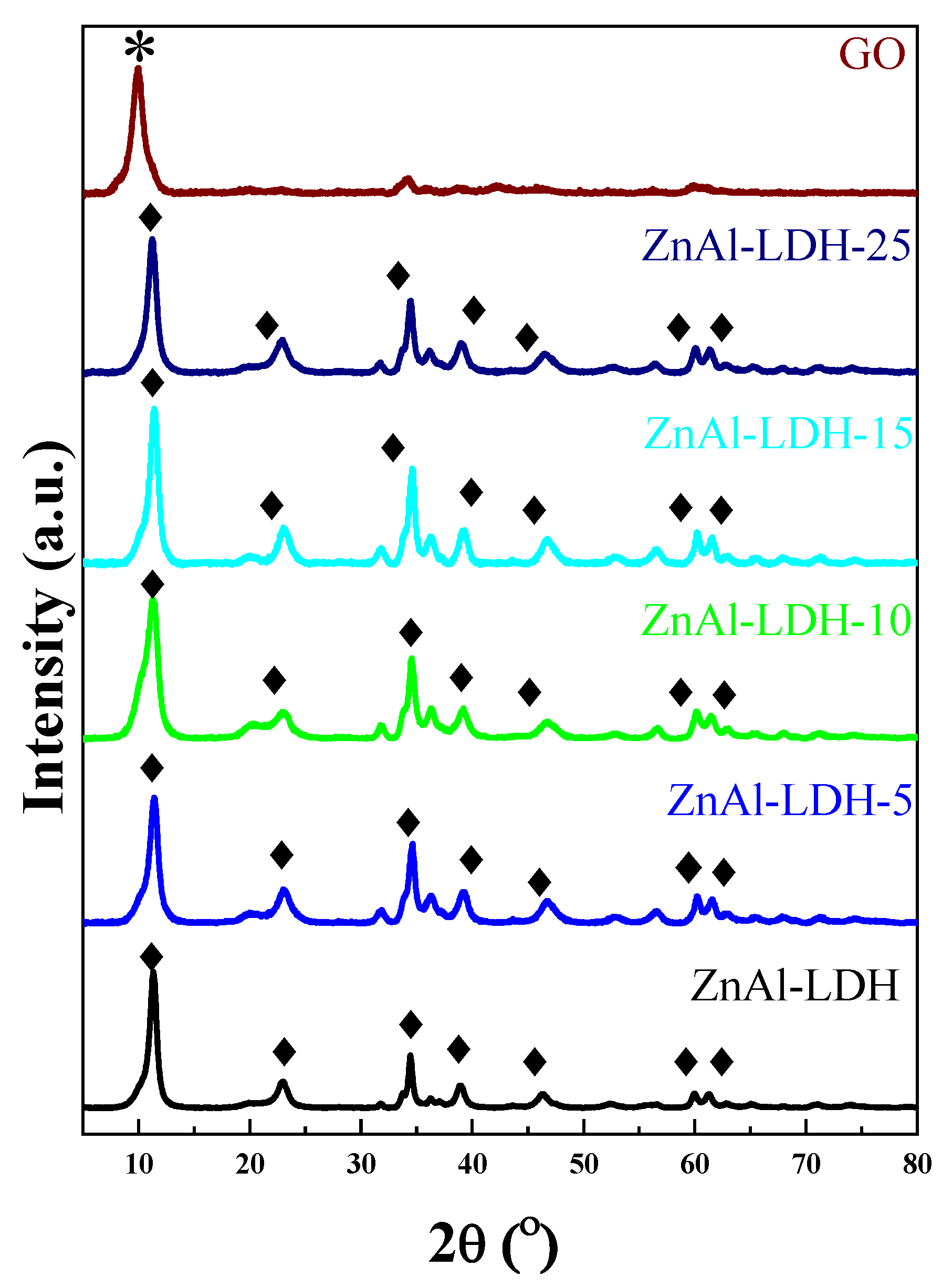
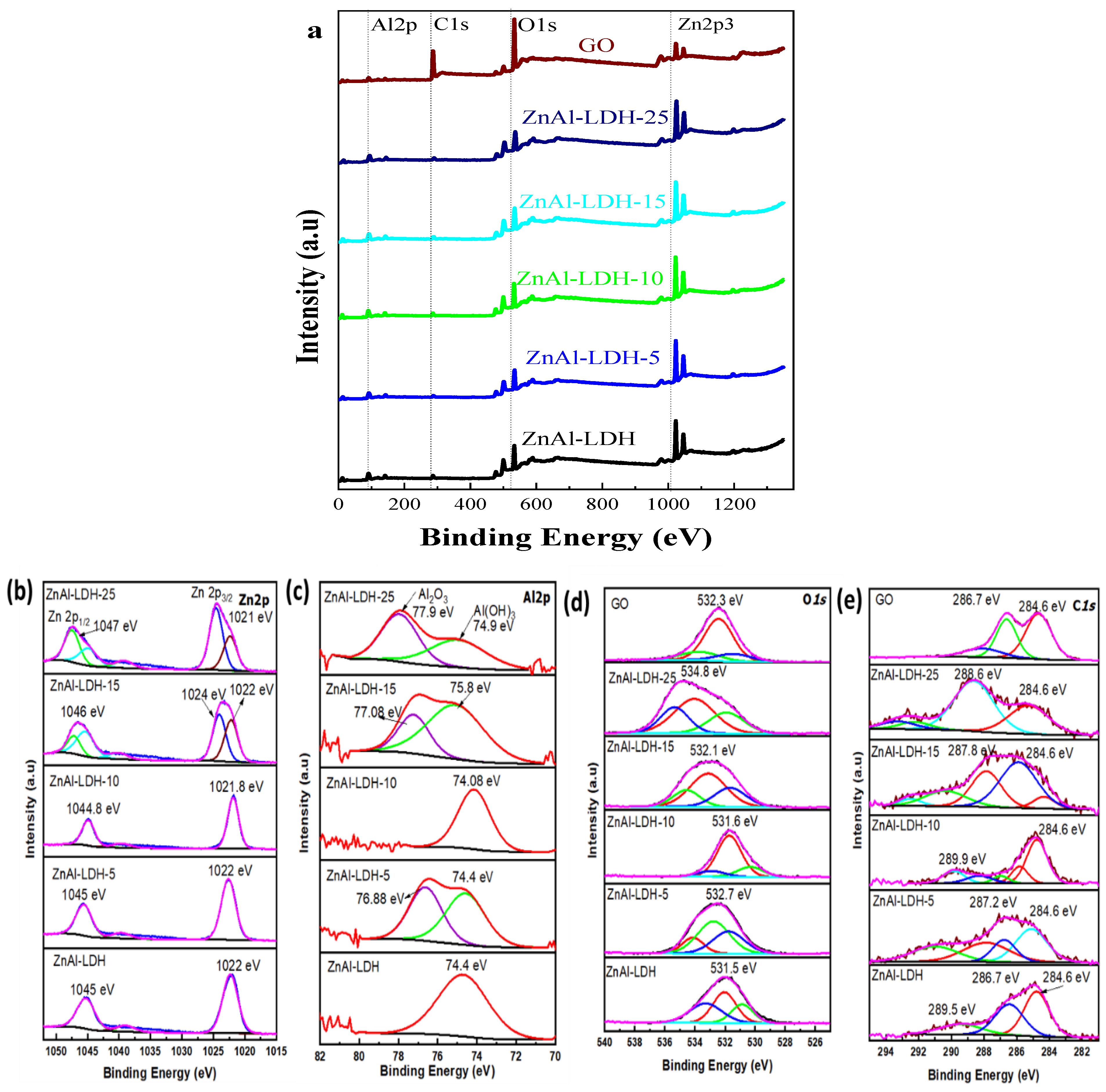
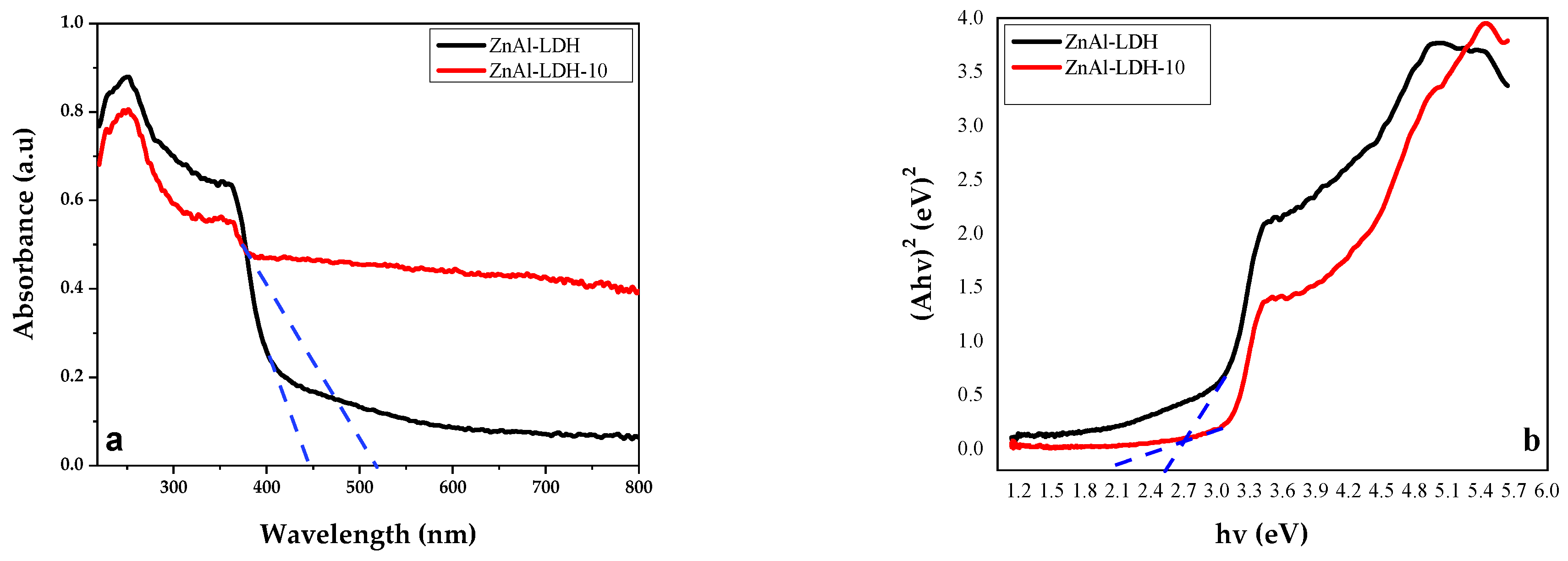
2.1. Physicochemical and Structural Characterization
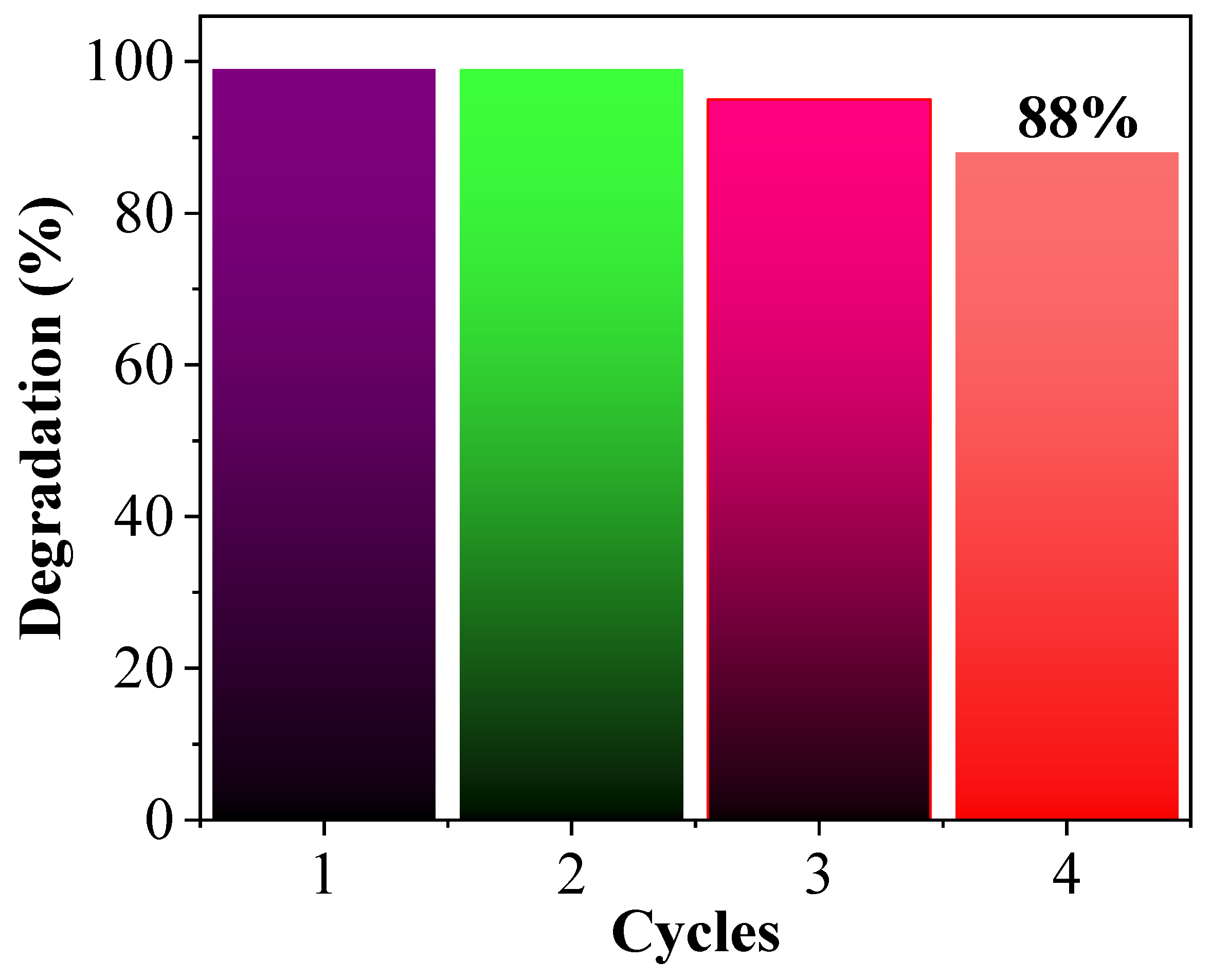
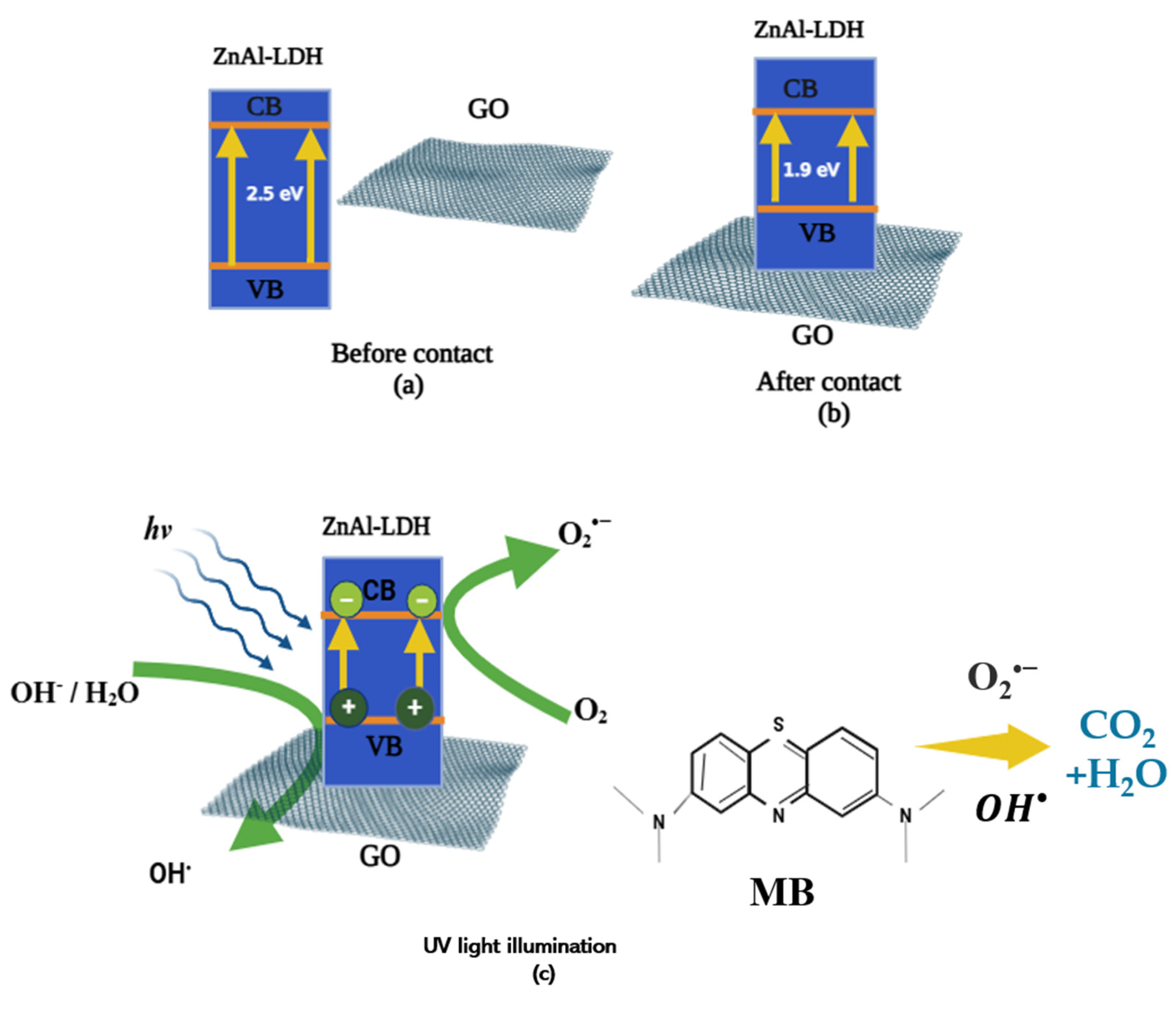
2.2. Photocatalytic Degradation of Methylene Blue
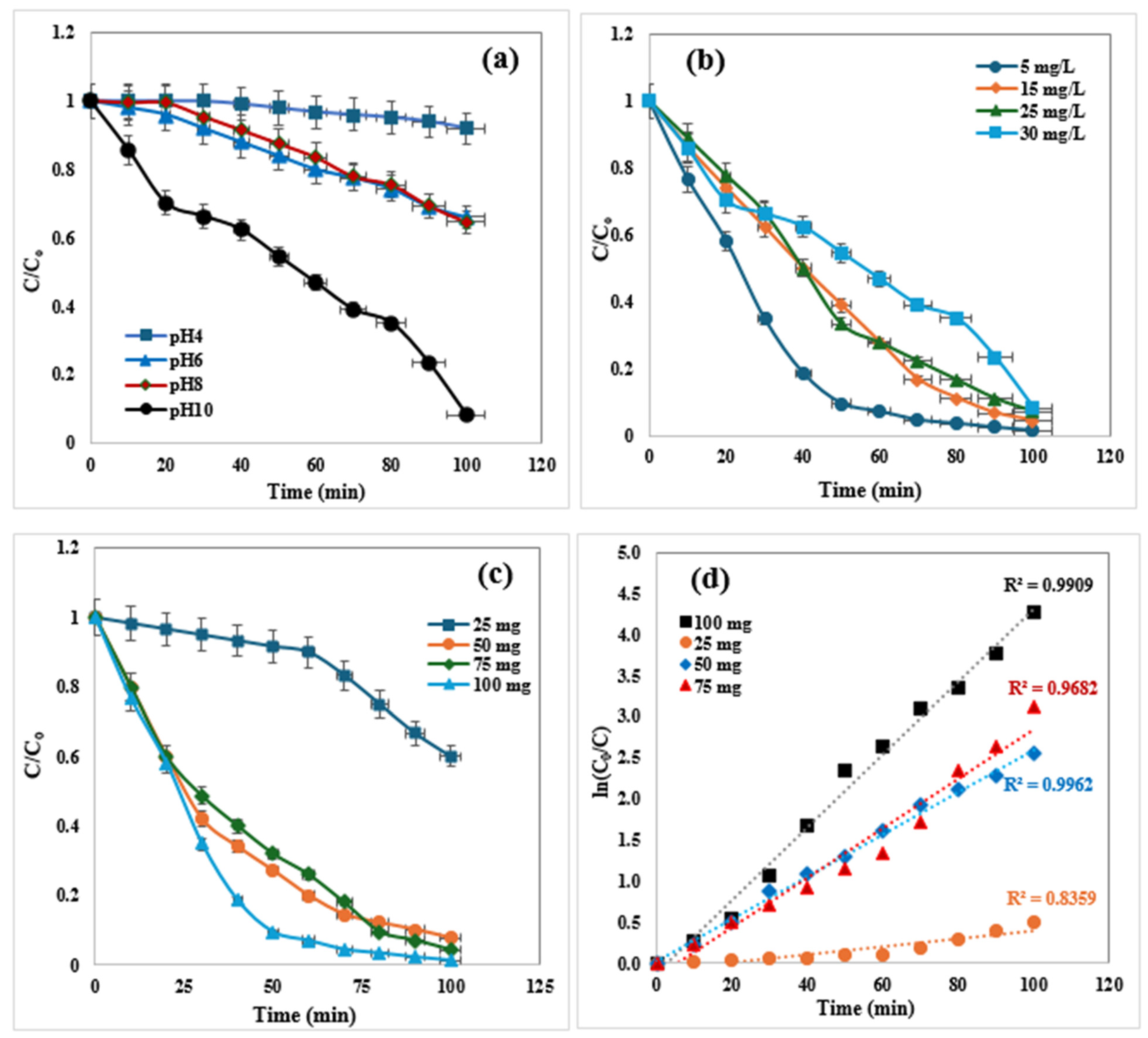
2.3. Adsorption Study
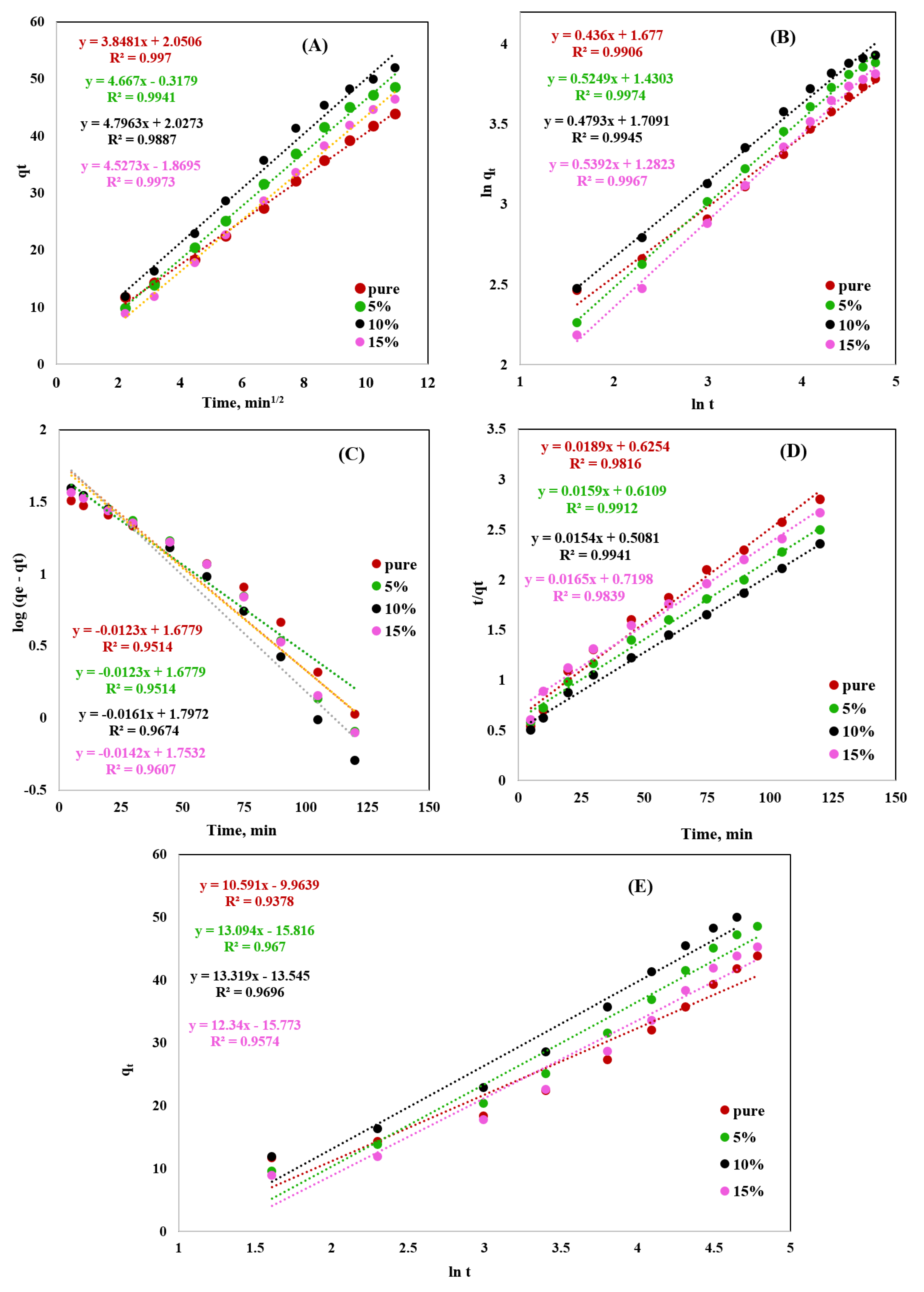
2.4. Kinetic Study
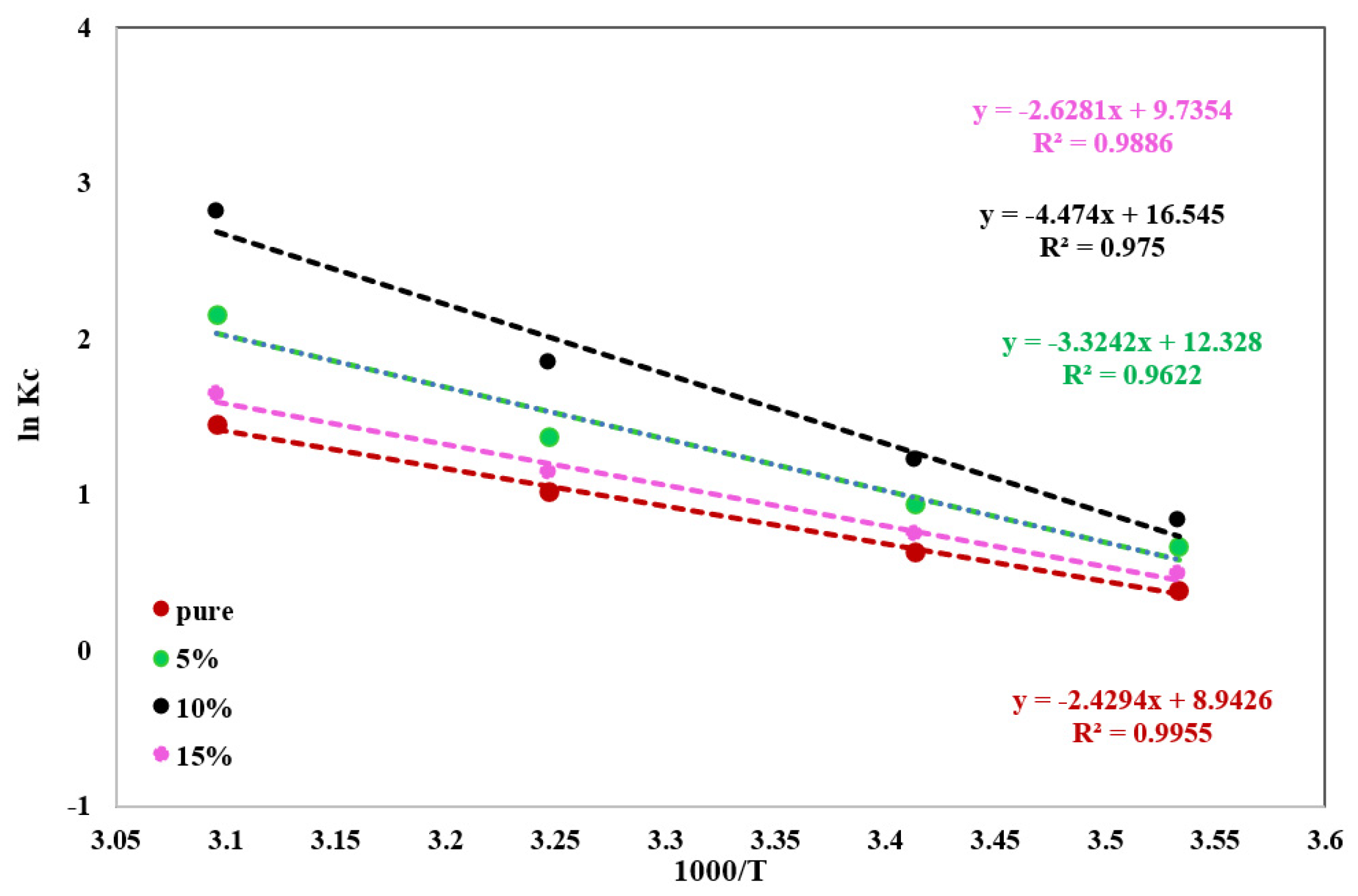
2.5. Thermodynamic Characteristics for A.R Dye Uptake by Pure and Modified ZnAl-LDH
2.6. Applications Study
3. Materials and Methods
3.1. Materials
3.2. Zinc Aluminum–Layered Double Hydroxide/Graphene Oxide (ZnAl-LDH/GO) Synthesis
3.3. Characterization Techniques
3.4. Photocatalytic Degradation of Methylene Blue (MB) Dye
3.5. Removal of Acid Red Dye by Adsorption Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, Y.; Chen, M.; Huang, P.; Li, T.; Zhang, X.; Jiang, K. Efficient removal and fouling-resistant of anionic dyes by nanofiltration membrane with phosphorylated chitosan modified graphene oxide nanosheets incorporated selective layer. J. Water Process Eng. 2020, 34, 101086. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, M.; Liu, H.; Zhu, Y.; Wang, D.; Yan, M. Adsorptive removal of dye and antibiotic from water with functionalized zirconium-based metal organic framework and graphene oxide composite nanomaterial Uio-66-(OH) 2/GO. Appl. Surf. Sci. 2020, 525, 146614. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- ISO 14001; Environmental Management Systems—Requirements with Guidance for Use. ISO: Geneva, Switzerland, 2015.
- Al-Soihi, A.S.; Alsulami, Q.A.; Mostafa, M.M.M. Amalgamated titanium oxide-carbon hollow sphere/nickel-layered double hydroxide as an efficient photocatalyst for the degradation of methyl orange. Catalysts 2022, 12, 1200. [Google Scholar] [CrossRef]
- Al-Soihi, A.S.; Bajafar, W.; Abdel-Fadeel, M.A.; Alsulami, Q.A.; Saleh, T.S.; Mostafa, M.M.M. Titania-carbon amalgamated nickel and copper layered double hydroxide for dye removal and catalytic reduction of p-nitrophenol. J. Water Process Eng. 2024, 61, 105361. [Google Scholar] [CrossRef]
- Mokhtar, M.; Inayat, A.; Ofili, J.; Schwieger, W. Thermal decomposition, gas phase hydration and liquid phase reconstruction in the system Mg/Al hydrotalcite/mixed oxide: A comparative study. Appl. Clay Sci. 2010, 50, 176–181. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, N.; Qiu, G.; Ma, R. Cobalt-doped Ni–Mn layered double hydroxide nanoplates as high-performance electrocatalyst for oxygen evolution reaction. Appl. Clay Sci. 2018, 165, 277–283. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Jing, M.; Tang, S.; Wu, Y.; Liu, W. Synthesis of CuNiSn LDHs as highly efficient Fenton catalysts for degradation of phenol. Appl. Clay Sci. 2020, 186, 105433. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Zhou, X.; Pan, G.; Ni, Z. The photocatalytic property for water splitting and the structural stability of CuMgM layered double hydroxides (M = Al, Cr, Fe, Ce). Appl. Clay Sci. 2015, 114, 577–585. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Xiao, T.; Tang, Z.; Shen, J.; Li, H.; Zhou, Y.; Zou, Z. Urchin-like hierarchical CoZnAl-LDH/RGO/g-C3N4 hybrid as a Z-scheme photocatalyst for efficient and selective CO2 reduction. Appl. Catal. B Environ. 2019, 255, 117771. [Google Scholar] [CrossRef]
- Lan, M.; Fan, G.; Yang, L.; Li, F. Significantly enhanced visible-light-induced photocatalytic performance of hybrid Zn–Cr layered double hydroxide/graphene nanocomposite and the mechanism study. Ind. Eng. Chem. Res. 2014, 53, 12943–12952. [Google Scholar] [CrossRef]
- Dinari, M.; Neamati, S. Surface modified layered double hydroxide/polyaniline nanocomposites: Synthesis, characterization and Pb2+ removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124438. [Google Scholar] [CrossRef]
- Gao, B.; Feng, X.; Zhang, Y.; Zhou, Z.; Wei, J.; Qiao, R.; Bi, F.; Liu, N.; Zhang, X. Graphene-based aerogels in water and air treatment: A review. Chem. Eng. J. 2024, 484, 149604. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Mariska, S.; Van, H.T.; Hai, N.D.; Chao, H.-P. Synthesis of titanate nanotubes/layered double hydroxides/graphene oxide composites and applications for the removal of methylene blue, methylene green 5, and acid red 1 from aqueous solutions. Inorg. Chem. Commun. 2023, 152, 110723. [Google Scholar] [CrossRef]
- Wambu, E.W.; Huang, J. Chemistry of Graphene: Synthesis, Reactivity, Applications and Toxicities: BoD–Books on Demand; IntechOpen: London, UK, 2024. [Google Scholar]
- Liu, X.; Pan, L.; Lv, T.; Zhu, G.; Sun, Z.; Sun, C. Microwave-assisted synthesis of CdS–reduced graphene oxide composites for photocatalytic reduction of Cr (VI). Chem. Commun. 2011, 47, 11984–11986. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Wang, Y.; Wang, Z. ZnO/graphene-oxide nanocomposite with remarkably enhanced visible-light-driven photocatalytic performance. J. Colloid Interface Sci. 2012, 377, 114–121. [Google Scholar] [CrossRef]
- Ni, J.; Xue, J.; Shen, J.; He, G.; Chen, H. Fabrication of ZnAl mixed metal-oxides/RGO nanohybrid composites with enhanced photocatalytic activity under visible light. Appl. Surf. Sci. 2018, 441, 599–606. [Google Scholar] [CrossRef]
- Tabasum, A.; Alghuthaymi, M.; Qazi, U.Y.; Shahid, I.; Abbas, Q.; Javaid, R.; Nadeem, N.; Zahid, M. UV-accelerated photocatalytic degradation of pesticide over magnetite and cobalt ferrite decorated graphene oxide composite. Plants 2020, 10, 6. [Google Scholar] [CrossRef]
- Spilarewicz-Stanek, K.; Jakimińska, A.; Kisielewska, A.; Dudek, M.; Piwoński, I. Graphene oxide photochemical transformations induced by UV irradiation during photocatalytic processes. Mater. Sci. Semicond. Process. 2021, 123, 105525. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, P.; Yao, W.; Wang, X.; Liu, Y.; Yang, D.; Wang, L.; Hou, J.; Alsaedi, A.; Hayat, T.; et al. Synergistic immobilization of UO22+ by novel graphitic carbon nitride@ layered double hydroxide nanocomposites from wastewater. Chem. Eng. J. 2017, 330, 573–584. [Google Scholar] [CrossRef]
- Yin, P.; Wu, G.; Wang, X.; Liu, S.; Zhou, F.; Dai, L.; Wang, X.; Yang, B.; Yu, Z.-Q. NiCo-LDH nanosheets strongly coupled with GO-CNTs as a hybrid electrocatalyst for oxygen evolution reaction. Nano Res. 2021, 14, 4783–4788. [Google Scholar] [CrossRef]
- Mokhtar, M.; Alhashedi, B.F.; Kashmery, H.A.; Ahmed, N.S.; Saleh, T.S.; Narasimharao, K. Highly efficient nanosized mesoporous cumgal ternary oxide catalyst for nitro-alcohol synthesis: Ultrasound-assisted sustainable green perspective for the Henry reaction. ACS Omega 2020, 5, 6532–6544. [Google Scholar] [CrossRef] [PubMed]
- Dhar, L.; Hossain, S.; Rahman, M.S.; Quraishi, S.B.; Saha, K.; Rahman, F.; Rahman, M.T. Adsorption mechanism of methylene blue by graphene oxide-shielded Mg–Al-layered double hydroxide from synthetic wastewater. J. Phys. Chem. A 2021, 125, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Al-Salihi, K.J.J. Evaluation of Dye Loading on Photoanodes of Dye-Sensitized Solar Cells Utilizing a Mixture of TiO2 and Magnesium/Aluminum Layered Double Hydroxide (LDH). J. Electron. Mater. 2024, 53, 6012–6022. [Google Scholar] [CrossRef]
- Mokhtar, M.; Saleh, T.S.; Ahmed, N.; Al-Thabaiti, S.; Al-Shareef, R. An eco-friendly N-sulfonylation of amines using stable and reusable Zn–Al–hydrotalcite solid base catalyst under ultrasound irradiation. Ultrason. Sonochemistry 2011, 18, 172–176. [Google Scholar] [CrossRef]
- Peng, D.; Jing, Q.; Feng, Z.; Niu, J.; Cheng, X.; Wu, X.; Zheng, X.; Yuan, X. Facile preparation of AB-stacking graphene oxide/ZnAl-layered double hydroxide composites and enhanced visible-light photocatalytic performance of the calcined product. J. Phys. Chem. Solids 2020, 136, 109199. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, J.; Lv, Y.; Jing, Q.; Li, L. Ultrahigh-capacity and fast-rate removal of graphene oxide by calcined MgAl layered double hydroxide. Appl. Clay Sci. 2018, 156, 61–68. [Google Scholar] [CrossRef]
- Li, M.; Zhu, J.E.; Zhang, L.; Chen, X.; Zhang, H.; Zhang, F.; Xu, S.; Evans, D.G. Facile synthesis of NiAl-layered double hydroxide/graphene hybrid with enhanced electrochemical properties for detection of dopamine. Nanoscale 2011, 3, 4240–4246. [Google Scholar] [CrossRef]
- Qian, R.; Yu, J.; Wu, C.; Zhai, X.; Jiang, P. Alumina-coated graphene sheet hybrids for electrically insulating polymer composites with high thermal conductivity. Rsc Adv. 2013, 3, 17373–17379. [Google Scholar] [CrossRef]
- Soheili-Azad, P.; Yaftian, M.R.; Dorraji, M.S.S. Application of zinc/aluminum layered double hydroxide nanosorbent in a fixed-bed column for SPE-preconcentration followed by HPLC determination of diclofenac in biological and hospital wastewater samples. Microchem. J. 2019, 148, 270–276. [Google Scholar] [CrossRef]
- Torbati, S.; Motlagh, P.Y.; Khataee, A. Toxicity of ZnFe-SO4 layered double hydroxide in Tetradesmus obliquus and evaluation of some physiological responses of the microalgae for stress management. Sci. Rep. 2024, 14, 975. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.A.; Pungot, N.H.; Soh, S.K.C.; Tajuddin, N.A. Facile and green hydrothermal synthesis of MgAl/NiAl/ZnAl layered double hydroxide nanosheets: A physiochemical comparison. Pure Appl. Chem. 2024, 96, 1667–1682. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azimi, F. Spectroscopic characterization techniques for layered double hydroxide polymer nanocomposites. In Layered Double Hydroxide Polymer Nanocomposites; Elsevier: Amsterdam, Netherlands, 2020; pp. 231–280. [Google Scholar]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, characterization and high photocatalytic activity of Ag–ZnO heterojunctions under UV-visible light. RSC Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef]
- George, G.; Saravanakumar, M.P. Facile synthesis of carbon-coated layered double hydroxide and its comparative characterisation with Zn–Al LDH: Application on crystal violet and malachite green dye adsorption—Isotherm, kinetics and Box-Behnken design. Environ. Sci. Pollut. Res. 2018, 25, 30236–30254. [Google Scholar] [CrossRef]
- Barnabas, M.J.; Parambadath, S.; Mathew, A.; Park, S.S.; Vinu, A.; Ha, C.-S. Highly efficient and selective adsorption of In3+ on pristine Zn/Al layered double hydroxide (Zn/Al-LDH) from aqueous solutions. J. Solid State Chem. 2016, 233, 133–142. [Google Scholar] [CrossRef]
- Mokhtar, M.; Alzhrani, G.; Aazam, E.S.; Saleh, T.S.; Al-Faifi, S.; Panja, S.; Maiti, D. Synergistic effect of NiLDH@ YZ hybrid and mechanochemical agitation on glaser homocoupling reaction. Chem. A Eur. J. 2021, 27, 8875–8885. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. In-situ growth of ZIF-8 on layered double hydroxide: Effect of Zn/Al molar ratios on their structural, morphological and adsorption properties. J. Colloid Interface Sci. 2017, 505, 206–212. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Stathi, P.; Gournis, D.; Deligiannakis, Y.; Rudolf, P. Stabilization of phenolic radicals on graphene oxide: An XPS and EPR study. Langmuir 2015, 31, 10508–10516. [Google Scholar] [CrossRef] [PubMed]
- Shabib, F.; Fazaeli, R.; Aliyan, H.; Richeson, D. Hierarchical mesoporous plasmonic Pd-Fe3O4/NiFe-LDH composites: Characterization, and kinetic study of a photodegradation catalyst for aqueous metoclopramide. Environ. Technol. Innov. 2022, 27, 102515. [Google Scholar] [CrossRef]
- Phan, M.V.; Tran, T.K.T.; Pham, Q.N.; Do, M.H.; Nguyen, T.H.N.; Nguyen, M.T.; Phan, T.T.; To, T.X.H. Controllable synthesis of layered double hydroxide nanosheets to build organic inhibitor-loaded nanocontainers for enhanced corrosion protection of carbon steel. Nanoscale Adv. 2024, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jing, R.; Wang, P.; Liang, D.; Huang, H.; Xia, C.; Zhang, Q.; Liu, A.; Meng, Z.; Liu, Y. Black phosphorus nanosheets and ZnAl-LDH nanocomposite as environmental-friendly photocatalysts for the degradation of Methylene blue under visible light irradiation. Appl. Clay Sci. 2021, 200, 105902. [Google Scholar] [CrossRef]
- Cai, X.; Li, J.; Liu, Y.; Yan, Z.; Tan, X.; Liu, S.; Zeng, G.; Gu, Y.; Hu, X.; Jiang, L. Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. J. Chem. Technol. Biotechnol. 2018, 93, 783–791. [Google Scholar] [CrossRef]
- Khataee, A.; Rad, T.S.; Nikzat, S.; Hassani, A.; Aslan, M.H.; Kobya, M.; Demirbaş, E. Fabrication of NiFe layered double hydroxide/reduced graphene oxide (NiFe-LDH/rGO) nanocomposite with enhanced sonophotocatalytic activity for the degradation of moxifloxacin. Chem. Eng. J. 2019, 375, 122102. [Google Scholar] [CrossRef]
- Chen, S.; Yang, F.; Cao, Z.; Yu, C.; Wang, S.; Zhong, H. Enhanced photocatalytic activity of molybdenum disulfide by compositing ZnAl–LDH. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124140. [Google Scholar] [CrossRef]
- Islam, D.A.; Acharya, H. Pd-Nanoparticles@ layered double hydroxide/reduced graphene oxide (Pd NPs@ LDH/rGO) nanocomposite catalysts for highly efficient green reduction of aromatic nitro compounds. New J. Chem. 2022, 46, 5346–5354. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, M.; Pang, X.; Gao, K. One-step in situ synthesis of reduced graphene oxide/Zn–Al layered double hydroxide film for enhanced corrosion protection of magnesium alloys. Langmuir 2019, 35, 6312–6320. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Cuong, T.V.; Pham, V.H.; Chung, J.S.; Khoa, N.T.; Kim, E.J.; Hahn, S.H. Enhanced photocatalytic activity of graphene oxide decorated on TiO2 films under UV and visible irradiation. Curr. Appl. Phys. 2011, 11, 805–808. [Google Scholar] [CrossRef]
- Das, A.; Adak, M.K. Kinetic and mechanistic way for photocatalytic degradation of pollutants from textile wastewater by graphene oxide supported nanocomposite. Next Mater. 2024, 3, 100153. [Google Scholar] [CrossRef]
- Prabhakarrao, N.; Rao, T.S.; Lakshmi, K.V.D.; Divya, G.; Jaishree, G.; Raju, I.M.; Alim, S.A. Enhanced photocatalytic performance of Nb doped TiO 2/reduced graphene oxide nanocomposites over rhodamine B dye under visible light illumination. Sustain. Environ. Res. 2021, 31, 1–13. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, Z.; Zhang, Y.; Guo, W.; Sun, X.; Duan, X. Sandwich layered double hydroxides with graphene oxide for enhanced water desalination. Sci. China Mater. 2022, 65, 803–810. [Google Scholar] [CrossRef]
- Puspitarum, D.L.; Istiqomah, N.I.; Larasati, D.A.; Asri, N.S.; Angel, J.; Kusumaatmaja, A.; Suharyadi, E. Novel Magnetically Recoverable MnFe2O4/TiO2 Nanocomposites Synthesized using Green Route for Photocatalytic Degradation of Methylene Blue. Water Cycle 2024, in press. [Google Scholar] [CrossRef]
- Narasimharao, K.; Lingamdinne, L.P.; Al-Thabaiti, S.; Mokhtar, M.; Alsheshri, A.; Alfaifi, S.Y.; Chang, Y.-Y.; Koduru, J.R. Synthesis and characterization of hexagonal MgFe layered double hydroxide/grapheme oxide nanocomposite for efficient adsorptive removal of cadmium ion from aqueous solutions: Isotherm, kinetic, thermodynamic and mechanism. J. Water Process Eng. 2022, 47, 102746. [Google Scholar] [CrossRef]
- Sherryna, A.; Tahir, M.; Nabgan, W. Recent advancements of layered double hydroxide heterojunction composites with engineering approach towards photocatalytic hydrogen production: A review. Int. J. Hydrog. Energy 2022, 47, 862–901. [Google Scholar] [CrossRef]
- Wang, K.; Miao, C.; Liu, Y.; Cai, L.; Jones, W.; Fan, J.; Li, D.; Feng, J. Vacancy enriched ultrathin TiMgAl-layered double hydroxide/graphene oxides composites as highly efficient visible-light catalysts for CO2 reduction. Appl. Catal. B Environ. 2020, 270, 118878. [Google Scholar] [CrossRef]
- Gupta, N.M. Factors affecting the efficiency of a water splitting photocatalyst: A perspective. Renew. Sustain. Energy Rev. 2017, 71, 585–601. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.; Yan, J.; Liu, Y.; Jiang, H.; Zhang, H.; Tang, J.; Liu, Q. Research progresses on the application of perovskite in adsorption and photocatalytic removal of water pollutants. J. Hazard. Mater. 2023, 442, 130024. [Google Scholar] [CrossRef]
- Velo-Gala, I.; López-Peñalver, J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Role of activated carbon surface chemistry in its photocatalytic activity and the generation of oxidant radicals under UV or solar radiation. Appl. Catal. B Environ. 2017, 207, 412–423. [Google Scholar] [CrossRef]
- Pan, G.; Xu, M.; Zhou, K.; Meng, Y.; Chen, H.; Guo, Y.; Wu, T. Photocatalytic degradation of methylene blue over layered double hydroxides using various divalent metal ions. Clays Clay Miner. 2019, 67, 340–347. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Katowah, D.F.; Abdel-Fadeel, M.A. Ultrahigh adsorption capacity of a new metal sieve-like structure nanocomposite-based chitosan-graphene oxide nanosheet coated with poly-o-toluidine for the removal of Acid Red dye from the aquatic environment. Nanocomposites 2023, 9, 80–99. [Google Scholar] [CrossRef]
- Al-Saidi, H.M.; Abdel-Fadeel, M.A.; Alharthi, S.S. Preconcentration and ultrasensitive spectrophotometric estimation of tungsten in soils using polyurethane foam in the presence of rhodamine B: Kinetic and thermodynamic studies, and designing a simple automated preconcentration system. J. Saudi Chem. Soc. 2021, 25, 101301. [Google Scholar] [CrossRef]
- Nijagala, M.; Vallameti, U.K. Application of Preformed Floc Adsorbents in the Removal of Acid Dye from an Aqueous Solution. Chem. Eng. Technol. 2024, 47, 605–609. [Google Scholar] [CrossRef]
- Gabal, M.; Al-Zahrani, N.; Al Angari, Y.; Al-Juaid, A.; Abdel-Fadeel, M.; Alharbi, S.; El-Shishtawy, R.M. CoFe2O4/MWCNTs nano-composites structural, thermal, magnetic, electrical properties and dye removal capability. Mater. Res. Express 2019, 6, 105059. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, F.; Ye, L.; Yang, X.; Song, Y.; Wang, H. Methylene blue and acid red adsorption on biochar made from modified sugarcane bagasse: A dynamic, equilibrium, and thermodynamic investigation. Adsorpt. Sci. Technol. 2024, 42, 02636174241273522. [Google Scholar] [CrossRef]
- Abdel-Fadeel, M.A.; Aljohani, N.S.; Al-Mhyawi, S.R.; Halawani, R.F.; Aljuhani, E.H.; Salam, M.A. A simple method for removal of toxic dyes such as Brilliant Green and Acid Red from the aquatic environment using Halloysite nanoclay. J. Saudi Chem. Soc. 2022, 26, 101475. [Google Scholar] [CrossRef]




| Sample | SBET (m2/g) ±1 | Smeso (m2/g) ±1 | Vt CC/g | Vp CC/g | Vmeso CC/g | Vmic CC/g | Average Pore Size (nm) | HF |
|---|---|---|---|---|---|---|---|---|
| ZnAl-LDH | 76 | 38 | 0.064 | 0.046 | 0.056 | ~0.004 | 2.94 | 0.033 |
| ZnAl-LDH-5 | 83 | 12 | 0.045 | 0.008 | 0.008 | 0.038 | 1.05 | 0.122 |
| ZnAl-LDH-10 | 115 | 17 | 0.071 | 0.017 | 0.012 | 0.052 | 1.05 | 0.108 |
| ZnAl-LDH-15 | 89 | 13 | 0.055 | 0.012 | 0.005 | 0.040 | 1.05 | 0.108 |
| ZnAl-LDH-25 | 71 | 55 | 0.094 | 0.066 | 0.088 | 0.006 | 2.44 | 0.059 |
| Weber–Morris Model | ||||
|---|---|---|---|---|
| Rd | R2 | |||
| Pure | 3.85 | 0.997 | ||
| 5% | 4.67 | 0.994 | ||
| 10% | 4.796 | 0.989 | ||
| 15% | 4.53 | 0.997 | ||
| Fractional Power Model | ||||
| A | B | Ab | R2 | |
| Pure | 5.349 | 0.436 | 2.332 | 0.991 |
| 5% | 4.178 | 0.525 | 2.194 | 0.997 |
| 10% | 5.529 | 0.479 | 2.648 | 0.995 |
| 15% | 3.603 | 0.539 | 1.942 | 0.997 |
| Pseudo-First-Order Model | ||||
| qe, exp, (mg·g−1) | qe, calc, (mg·g−1) | k1 | R2 | |
| Pure | 43.82 | 47.64 | 0.027 | 0.951 |
| 5% | 48.48 | 59.43 | 0.032 | 0.962 |
| 10% | 50.87 | 62.66 | 0.037 | 0.967 |
| 15% | 45.18 | 56.62 | 0.032 | 0.961 |
| Pseudo-Second-Order Model | ||||
| qe, exp, (mg·g−1) | qe, calc, (mg·g−1) | k2 | R2 | |
| Pure | 43.82 | 52.91 | 5.7 × 10−4 | 0.982 |
| 5% | 48.48 | 62.89 | 4.1 × 10−4 | 0.991 |
| 10% | 50.87 | 64.94 | 4.6 × 10−4 | 0.994 |
| 15% | 45.18 | 60.61 | 3.7 × 10−4 | 0.984 |
| Elovich Model | ||||
| α, [g·(mg·min)−1] | β, [mg·(g·min)−1] | R2 | ||
| Pure | 0.049 | 10.59 | 0.938 | |
| 5% | 0.023 | 13.09 | 0.967 | |
| 10% | 0.027 | 13.39 | 0.974 | |
| 15% | 0.022 | 12.34 | 0.957 | |
| Thermodynamic Equations | Solid Phase | Parameters | ||
|---|---|---|---|---|
ΔG = ΔH − TΔS | ΔH | ΔS | ΔG at 295K | |
| Pure | 20.20 ± 1.97 | 74.35 ± 2.11 | −1.58 ± 0.08 | |
| 5% | 27.63 ± 2.12 | 102.5 ± 2.43 | −2.39 ± 0.09 | |
| 10% | 37.19 ± 2.04 | 137.5 ± 2.82 | −3.11 ± 0.11 | |
| 15% | 21.85 ± 1.89 | 80.93 ± 1.93 | −1.86 ± 0.08 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ammari, R.H.; Al-Malwi, S.D.; Abdel-Fadeel, M.A.; Bawaked, S.M.; Mostafa, M.M.M. Zn-Layered Double Hydroxide Intercalated with Graphene Oxide for Methylene Blue Photodegradation and Acid Red Adsorption Studies. Catalysts 2024, 14, 897. https://doi.org/10.3390/catal14120897
Al-Ammari RH, Al-Malwi SD, Abdel-Fadeel MA, Bawaked SM, Mostafa MMM. Zn-Layered Double Hydroxide Intercalated with Graphene Oxide for Methylene Blue Photodegradation and Acid Red Adsorption Studies. Catalysts. 2024; 14(12):897. https://doi.org/10.3390/catal14120897
Chicago/Turabian StyleAl-Ammari, Rahmah H., Salwa D. Al-Malwi, Mohamed A. Abdel-Fadeel, Salem M. Bawaked, and Mohamed Mokhtar M. Mostafa. 2024. "Zn-Layered Double Hydroxide Intercalated with Graphene Oxide for Methylene Blue Photodegradation and Acid Red Adsorption Studies" Catalysts 14, no. 12: 897. https://doi.org/10.3390/catal14120897
APA StyleAl-Ammari, R. H., Al-Malwi, S. D., Abdel-Fadeel, M. A., Bawaked, S. M., & Mostafa, M. M. M. (2024). Zn-Layered Double Hydroxide Intercalated with Graphene Oxide for Methylene Blue Photodegradation and Acid Red Adsorption Studies. Catalysts, 14(12), 897. https://doi.org/10.3390/catal14120897






