Progress in the Synthesis and Applications of C3N5-Based Catalysts in the Piezoelectric Catalytic Degradation of Organics
Abstract
1. Introduction
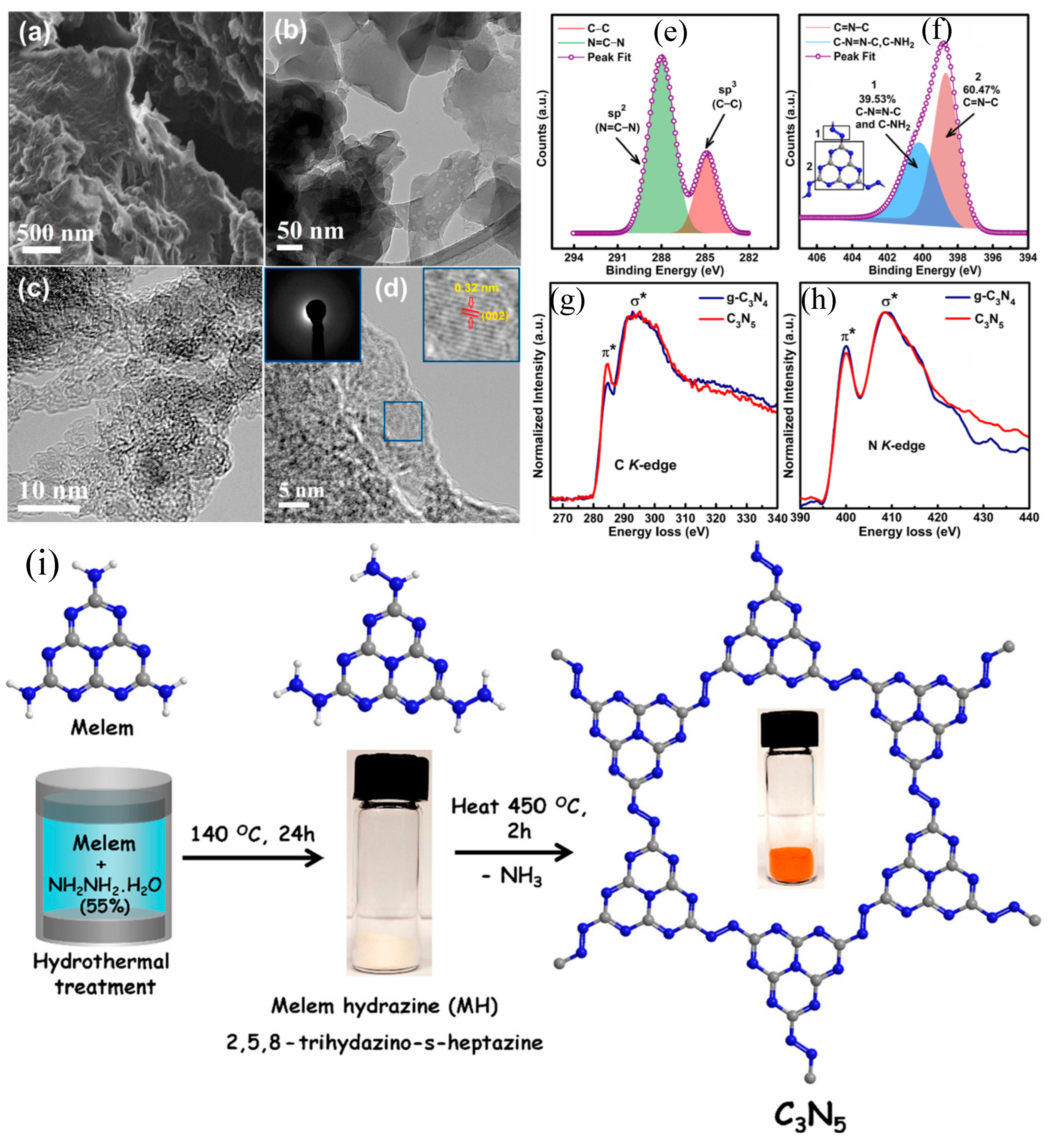
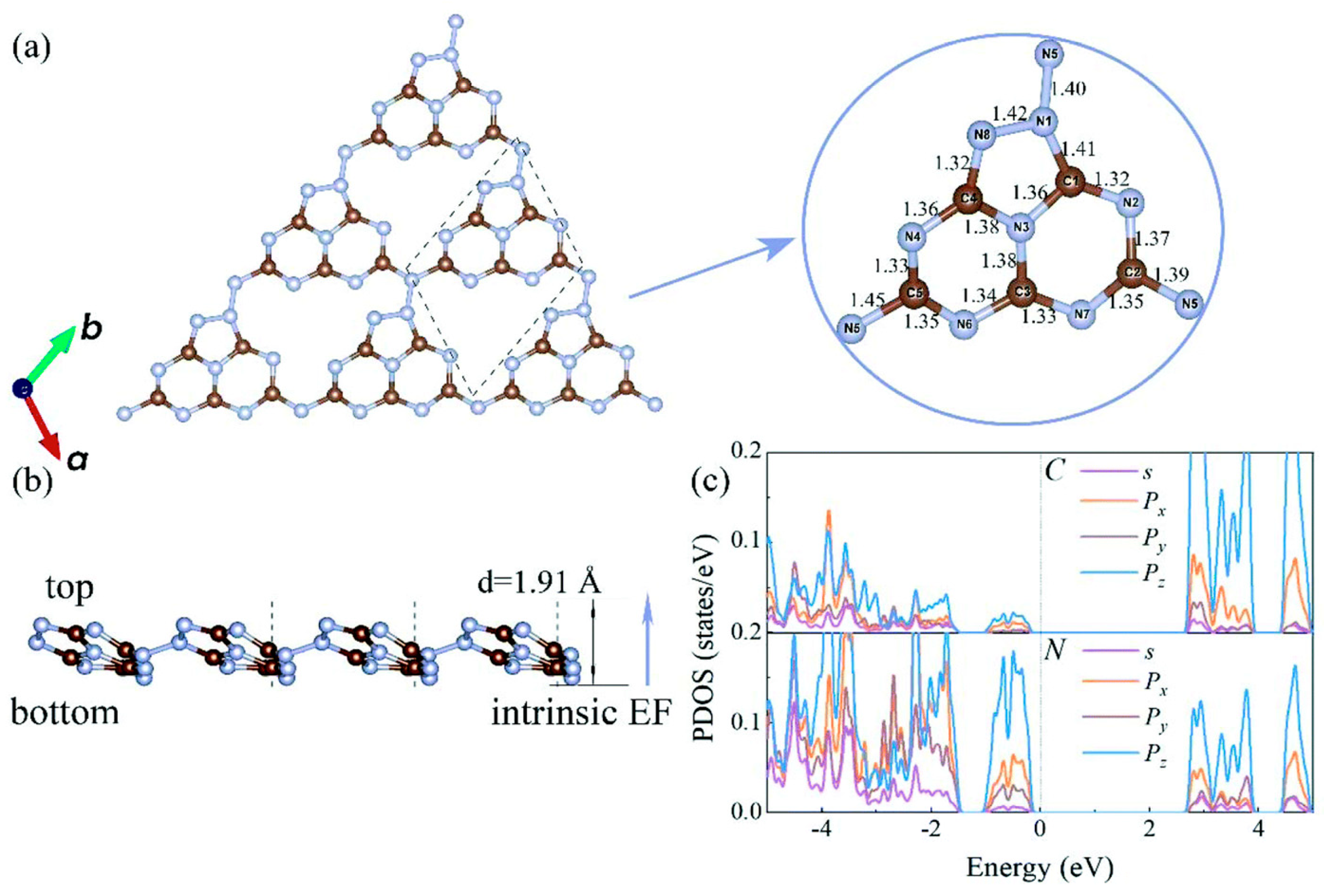
2. Synthesis and Characterization of C3N5-Based Catalysts
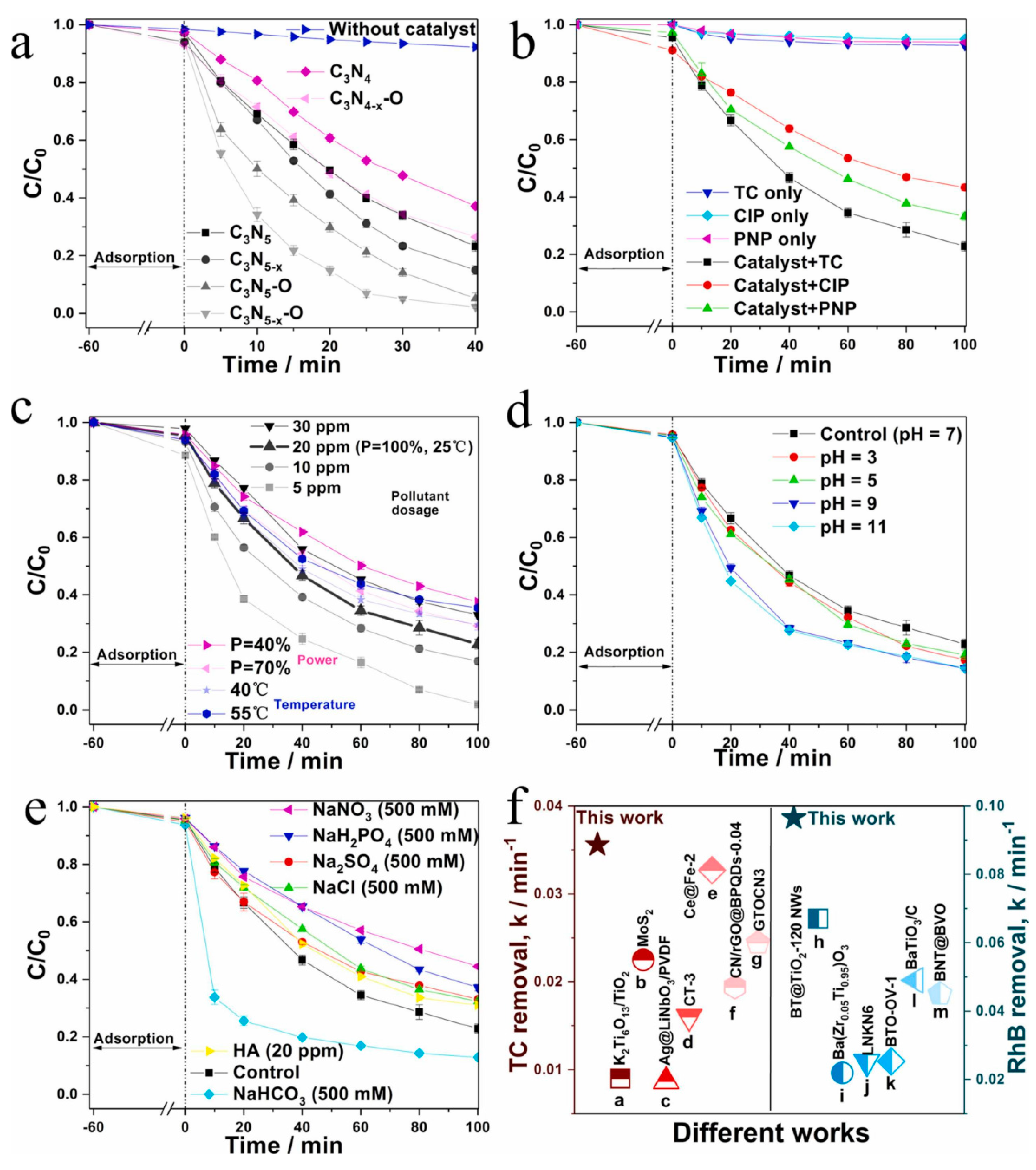
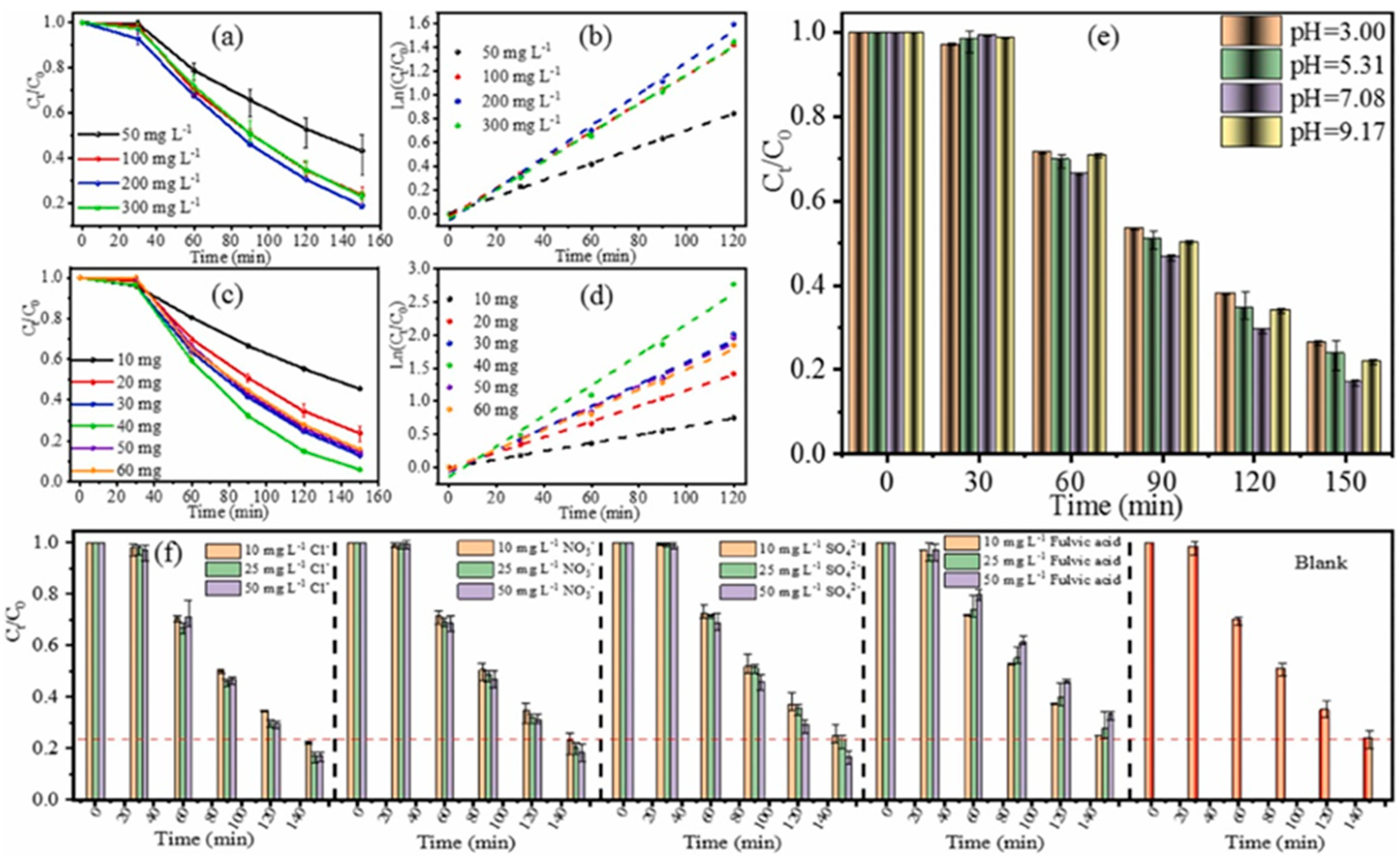
3. Synthesis of C3N5-Based Catalysts and Application of Piezoelectric Catalytic Degradation of Organic Matter
4. Conclusions and Prospects
- (1)
- With the rapid development of society and growing environmental awareness, the need for enhanced prevention and control of organic pollutants is increasing, emphasizing the need for greater piezoelectric catalytic efficiency. Improvements in morphology modulation, elemental doping, and piezoelectric material innovation are necessary to advance the piezoelectric degradation capabilities of organic pollutants.
- (2)
- In environmental applications, research has predominantly focused on degrading antibiotics and dyes in wastewater. However, limited attention has been given to removing nitrogen oxides (NOx) and volatile organic compounds (VOCs) from the air. Future research should expand the applications of C3N5-based catalysts to include air purification and investigate their performance in addressing gaseous pollutants.
- (3)
- In the future, noise, micro-vibrations, and other ambient energy sources could be utilized to activate piezoelectric catalytic effects. This approach could not only address environmental pollution but also harness renewable energy, minimizing energy losses and improving overall system efficiency.
- (4)
- Photocatalytic technology can be used to purify volatile organic compounds in the air. This is because these small molecules can react with photo-generated electrons/holes with redox ability. The original C3N5 has been proven to be an excellent candidate for the adsorption of some small molecules due to its high nitrogen content as an active center. Therefore, further research on applying C3N5 photocatalytic technology to air pollution control is both necessary and meaningful.
- (5)
- Future efforts should prioritize sustainable synthesis methods, such as employing green chemistry techniques or novel catalysts, to improve synthesis efficiency and reduce environmental impact. Identifying and overcoming potential technical challenges in the synthesis process, such as optimizing reaction conditions, improving yield, and controlling material purity, are important steps in driving research. Efforts should be made to thoroughly explore the application of C3N5 in fields such as environmental protection, energy conversion, and materials science, to design specific experimental and research questions, and to verify its actual effectiveness. Interdisciplinary collaboration and promotion of in-depth research and widespread application of C3N5 by combining knowledge from fields such as chemistry, materials science, and engineering should be encouraged.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Water is life, water is food. Nat. Food 2023, 4, 823. [CrossRef] [PubMed]
- Kumar, A.; Thakur, A.; Panesar, P.S. A review on the industrial wastewater with the efficient treatment techniques. Chem. Pap. 2023, 77, 4131–4163. [Google Scholar] [CrossRef]
- Nassiri Koopaei, N.; Abdollahi, M. Health risks associated with the pharmaceuticals in wastewater. DARU J. Pharm. Sci. 2017, 25, 9. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater Treatment and Reuse: A Review of its Applications and Health Implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Amara, N.I.; Chukwuemeka, E.S.; Obiajulu, N.O.; Chukwuma, O.J. Yeast-driven valorization of agro-industrial wastewater: An overview. Environ. Monit. Assess. 2023, 195, 1252. [Google Scholar] [CrossRef]
- Htira, T.; Cogné, C.; Gagnière, E.; Mangin, D. Experimental study of industrial wastewater treatment by freezing. J. Water Process Eng. 2018, 23, 292–298. [Google Scholar] [CrossRef]
- Gkika, D.A.; Tolkou, A.K.; Evgenidou, E.; Bikiaris, D.N.; Lambropoulou, D.A.; Mitropoulos, A.C.; Kalavrouziotis, I.K.; Kyzas, G.Z. Fate and Removal of Microplastics from Industrial Wastewaters. Sustainability 2023, 15, 6969. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater treatment: A short assessment on available techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Poornima, S.; Manikandan, S.; Karthik, V.; Balachandar, R.; Subbaiya, R.; Saravanan, M.; Lan Chi, N.T.; Pugazhendhi, A. Emerging nanotechnology based advanced techniques for wastewater treatment. Chemosphere 2022, 303, 135050. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Chen, L. A green composite hydrogel based on cellulose and clay as efficient absorbent of colored organic effluent. Carbohydr. Polym. 2019, 210, 314–321. [Google Scholar] [CrossRef]
- Liu, J.; Ghanizadeh, H.; Li, X.; An, L.; Qiu, Y.; Zhang, Y.; Chen, X.; Wang, A. Facile synthesis of core\shell Fe3O4@mSiO2(Hb) and its application for organic wastewater treatment. Environ. Res. 2022, 203, 111796. [Google Scholar] [CrossRef]
- Sharma, Y.; Li, B. Optimizing hydrogen production from organic wastewater treatment in batch reactors through experimental and kinetic analysis. Int. J. Hydrogen Energy 2009, 34, 6171–6180. [Google Scholar] [CrossRef]
- Kong, Z.; Li, L.; Xue, Y.; Yang, M.; Li, Y.-Y. Challenges and prospects for the anaerobic treatment of chemical-industrial organic wastewater: A review. J. Clean. Prod. 2019, 231, 913–927. [Google Scholar] [CrossRef]
- Gao, F.; Fang, M.; Zhang, S.; Ni, M.; Cai, Y.; Zhang, Y.; Tan, X.; Kong, M.; Xu, W.; Wang, X. Symmetry-breaking induced piezocatalysis of Bi2S3 nanorods and boosted by alternating magnetic field. Appl. Catal. B Environ. 2022, 316, 121664. [Google Scholar] [CrossRef]
- Ruan, L.; Jia, Y.; Guan, J.; Xue, B.; Huang, S.; Wu, Z.; Li, G.; Cui, X. Highly piezocatalysis of metal-organic frameworks material ZIF-8 under vibration. Sep. Purif. Technol. 2022, 283, 120159. [Google Scholar] [CrossRef]
- Liu, B.; Sun, L.-Y.; Chen, Y.-X.; Su, S.-X.; Wang, X.-F.; Wang, X. A novel Ag2O/Bi2WO6 composite with enhanced piezocatalytic performance for ciprofloxacin removal. Inorg. Chem. Commun. 2024, 159, 111832. [Google Scholar] [CrossRef]
- Shen, Y.; Song, S.; Cui, Y.; Feng, X.; Huang, M.; Lu, B.; Huang, B.; Huang, F.; Chen, Y. Highly efficient piezocatalytic degradation of organic dye over few-layer MoS2/carbon sponge composite boosted by mild mechanical stimuli. J. Environ. Chem. Eng. 2024, 12, 112616. [Google Scholar] [CrossRef]
- Fu, C.; Wu, T.; Sun, G.; Yin, G.; Wang, C.; Ran, G.; Song, Q. Dual-defect enhanced piezocatalytic performance of C3N5 for multifunctional applications. Appl. Catal. B Environ. 2023, 323, 122196. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Liu, C.; Zhao, W.; Meng, F.; Wang, Y.; Zhu, Z. Energy-saving piezoelectric pipe functionalized by C3N5/polyvinylidene fluoride composites for enhanced degradation of ciprofloxacin. Chem. Eng. Sci. 2024, 287, 119696. [Google Scholar] [CrossRef]
- Wu, L.; Sun, C.; Jiao, S.; Hu, J.; Yang, J.; Jiao, F. Internal Electric-Field-Driven CoAl-LDH Coupled N-Rich Carbon Nitride of C3N5 for Improved Photocatalytic Performance. Ind. Eng. Chem. Res. 2023, 62, 2729–2740. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, X.; Li, Z.; Deshpande, S.; Fawaz, M.; Dharmarajan, N.P.; Lin, C.-H.; Lei, Z.; Hu, L.; Huang, J.-K.; et al. Sulfoxide-Functional Nanoarchitectonics of Mesoporous Sulfur-Doped C3N5 for Photocatalytic Hydrogen Evolution. Chem. Mater. 2024, 36, 4511–4520. [Google Scholar] [CrossRef]
- Li, T.; Cui, P.; Wang, X.; Liu, C.; Zeng, Y.; Fang, G.; Zhao, Y.; Gao, J.; Wang, Y.; Zhou, D. Efficient activation of peroxymonosulfate by C3N5 doped with cobalt for organic contaminant degradation. Environ. Sci. Nano 2022, 9, 2534–2547. [Google Scholar] [CrossRef]
- Kumar, P.; Vahidzadeh, E.; Thakur, U.K.; Kar, P.; Alam, K.M.; Goswami, A.; Mahdi, N.; Cui, K.; Bernard, G.M.; Michaelis, V.K.; et al. C3N5: A Low Bandgap Semiconductor Containing an Azo-Linked Carbon Nitride Framework for Photocatalytic, Photovoltaic and Adsorbent Applications. J. Am. Chem. Soc. 2019, 141, 5415–5436. [Google Scholar] [CrossRef]
- Qi, S.; Fan, Y.; Wang, J.; Song, X.; Li, W.; Zhao, M. Metal-free highly efficient photocatalysts for overall water splitting: C3N5 multilayers. Nanoscale 2020, 12, 306–315. [Google Scholar] [CrossRef]
- Quan, C.; Xiao, S.; Yi, Y.; Sun, D.; Ji, S.; Zhou, S.; Yang, J.; Niu, X.; Li, X.a. Explore the underlying mechanism of graphitic C3N5-hosted single-atom catalyst for electrocatalytic nitrogen fixation. Int. J. Hydrogen Energy 2022, 47, 22035–22044. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, M.; Chen, X.; Sun, G.; Wang, C.; Song, Q. Unraveling the dual defect effects in C3N5 for piezo-photocatalytic degradation and H2O2 generation. Appl. Catal. B Environ. 2023, 332, 122752. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Hu, X.; Zhang, S.; Dai, Q.; Chai, H.; Gu, L. Activation of sodium percarbonate by vanadium for the degradation of aniline in water: Mechanism and identification of reactive species. Chemosphere 2019, 215, 647–656. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Lu, Q.; Cen, Y.; Zhang, Y.; Yao, S. A Mesoporous Rod-like g-C3N5 Synthesized by Salt-Guided Strategy: As a Superior Photocatalyst for Degradation of Organic Pollutant. ACS Sustain. Chem. Eng. 2018, 7, 625–631. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.; Wang, C.; Gan, L.; Chen, X.; Zhang, P.; Wang, Y.; Li, H.; Wang, L.; Zhou, X.; et al. Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci. 2022, 15, 830–842. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Geng, W.; Su, H.; Long, M. Efficient bifunctional piezocatalysis of Au/BiVO4 for simultaneous removal of 4-chlorophenol and Cr(VI) in water. Appl. Catal. B Environ. 2019, 259, 118084. [Google Scholar] [CrossRef]
- Ma, C.; Yu, Z.; Wei, J.; Tan, C.; Yang, X.; Wang, T.; Yu, G.; Zhang, C.; Li, X. Metal-free ultrathin C3N5 photocatalyst coupling sodium percarbonate for efficient sulfamethoxazole degradation. Appl. Catal. B Environ. 2022, 319, 121951. [Google Scholar] [CrossRef]
- Farooq, U.; Zhuang, J.; Wang, X.; Lyu, S. A recyclable polydopamine-functionalized reduced graphene oxide/Fe nanocomposite (PDA@Fe/rGO) for the enhanced degradation of 1,1,1-trichloroethane. Chem. Eng. J. 2021, 403, 126405. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, X.; Huang, Y.; Kang, W.; Wang, Z.; Zheng, H. Roles of hydroxyl and carbonate radicals in bisphenol a degradation via a nanoscale zero-valent iron/percarbonate system: Influencing factors and mechanisms. RSC Adv. 2021, 11, 3636–3644. [Google Scholar] [CrossRef]
- Fu, X.; Gu, X.; Lu, S.; Miao, Z.; Xu, M.; Zhang, X.; Qiu, Z.; Sui, Q. Benzene depletion by Fe2+-catalyzed sodium percarbonate in aqueous solution. Chem. Eng. J. 2015, 267, 25–33. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, J. Iron-Based Dual Active Site-Mediated Peroxymonosulfate Activation for the Degradation of Emerging Organic Pollutants. Environ. Sci. Technol. 2021, 55, 15412–15422. [Google Scholar] [CrossRef]
- Solis, R.R.; Dinc, O.; Fang, G.; Nadagouda, M.N.; Dionysiou, D.D. Activation of inorganic peroxides with magnetic graphene for the removal of antibiotics from wastewater. Environ Sci. Nano. 2021, 8, 960–977. [Google Scholar] [CrossRef]
- Vadivel, S.; Hariganesh, S.; Paul, B.; Rajendran, S.; Habibi-Yangjeh, A.; Maruthamani, D.; Kumaravel, M. Synthesis of novel AgCl loaded g-C3N5 with ultrahigh activity as visible light photocatalyst for pollutants degradation. Chem. Phys. Lett. 2020, 738, 136862. [Google Scholar] [CrossRef]
- Vadivel, S.; Hariganesh, S.; Paul, B.; Mamba, G.; Puviarasu, P. Highly active novel CeTi2O6/g-C3N5 photocatalyst with extended spectral response towards removal of endocrine disruptor 2, 4-dichlorophenol in aqueous medium. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124583. [Google Scholar] [CrossRef]
- Alam, K.M.; Jensen, C.E.; Kumar, P.; Hooper, R.W.; Bernard, G.M.; Patidar, A.; Manuel, A.P.; Amer, N.; Palmgren, A.; Purschke, D.N.; et al. Photocatalytic Mechanism Control and Study of Carrier Dynamics in CdS@C3N5 Core–Shell Nanowires. ACS Appl. Mater. Interfaces 2021, 13, 47418–47439. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lin, Y.-H.; Yoshida, M.; Ashimura, S. Influence of Phosphorus Doping on Triazole-Based g-C3N5 Nanosheets for Enhanced Photoelectrochemical and Photocatalytic Performance. ACS Appl. Mater. Interfaces 2021, 13, 24907–24915. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Cao, Y.; Fan, T.; Zhang, M.; Yao, J.; Li, P.; Chen, S.; Liu, X. In situ synthesis of Ag3PO4/C3N5 Z-scheme heterojunctions with enhanced visible-light-responsive photocatalytic performance for antibiotics removal. Sci. Total Environ. 2021, 754, 141926. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, S.; Fujii, M.; Rajendran, S. Facile synthesis of broom stick like FeOCl/g-C3N5 nanocomposite as novel Z-scheme photocatalysts for rapid degradation of pollutants. Chemosphere 2022, 307, 135716. [Google Scholar] [CrossRef]
- Teng, M.; Shi, J.; Qi, H.; Shi, C.; Wang, W.; Kang, F.; Eqi, M.; Huang, Z. Effective enhancement of electron migration and photocatalytic performance of nitrogen-rich carbon nitride by constructing fungal carbon dot/molybdenum disulfide cocatalytic system. J. Colloid Interface Sci. 2022, 609, 592–605. [Google Scholar] [CrossRef]

| Catalytic System | Reaction Conditions | Target Pollutants | Removal Efficiency | Ref |
|---|---|---|---|---|
| RN-g-C3N5 | 0.02 g RN-g-C3N5 20 ML 20 mg/L MB | Methylene Blue (MB) | 98% (120 min) | [32] |
| AgCl/g-C3N5 | 50 mg AgCl/g-C3N5 50 mL 10 mg/L RhB | Rhodamine B (RhB) | 96% (30 min) | [39] |
| CeTiO6/g-C3N5 | 1.6 g/L CeTi2O6/g-C3N5 75 mL 10 ppm (λ > 420 nm) | 2,4-dichlorophenol (2,4-DCP) | 96% (120 min) | [40] |
| CdS/g-C3N5 | 0.1 g/L CdS-MHP 50 mL 0.01 mM RhB1 sun AM1.5 G | Rhodamine B (RhB) | 90% (80 min) | [41] |
| Xp-/g-C3N5 | 1 sun AM1.5 G 6P-g-C3N5 5 ppm RhB, 20 ppm | Rhodamine B (RhB) Tetracycline (TC) | 100% (180 min) | [42] |
| Ag3PO4/g-C3N5 | 1.0 g/L Ag3PO4/C3N5 50 mL 20 mg/L TCH 300 W Xe lamp (λ > 400 nm) | Tetracycline hydrochloride (TCH) | 90.5% (60 min) | [43] |
| FeOCl/g-C3N5 | 1.0 mg/mL Catalyst 75 mL 10 mg/L TC30% 200 μL H2O2 | Tetracycline (TC) | 95% (40 min) | [44] |
| CDs/MoS2/g-C3N5 | 0.02 g/L Catalyst 50 mL 30 mg/L (λ > 420 nm) | Methylene blue (MB) | 94% (120 min) | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Yu, H.; Fu, H.; Wang, Y.; Meng, F. Progress in the Synthesis and Applications of C3N5-Based Catalysts in the Piezoelectric Catalytic Degradation of Organics. Catalysts 2024, 14, 854. https://doi.org/10.3390/catal14120854
Yin S, Yu H, Fu H, Wang Y, Meng F. Progress in the Synthesis and Applications of C3N5-Based Catalysts in the Piezoelectric Catalytic Degradation of Organics. Catalysts. 2024; 14(12):854. https://doi.org/10.3390/catal14120854
Chicago/Turabian StyleYin, Shupeng, Huiguo Yu, Haifeng Fu, Yinglong Wang, and Fanqing Meng. 2024. "Progress in the Synthesis and Applications of C3N5-Based Catalysts in the Piezoelectric Catalytic Degradation of Organics" Catalysts 14, no. 12: 854. https://doi.org/10.3390/catal14120854
APA StyleYin, S., Yu, H., Fu, H., Wang, Y., & Meng, F. (2024). Progress in the Synthesis and Applications of C3N5-Based Catalysts in the Piezoelectric Catalytic Degradation of Organics. Catalysts, 14(12), 854. https://doi.org/10.3390/catal14120854






