Enhanced Photodegradation of Sulfamethoxazole Through Cutting-Edge Titania-Zirconia-Based Materials
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermogravimetric Analysis
2.2. Nitrogen Adsorption–Desorption
2.3. Scanning Electron Microscopy
2.4. X-Ray Diffraction
2.5. UV-Vis Spectroscopy
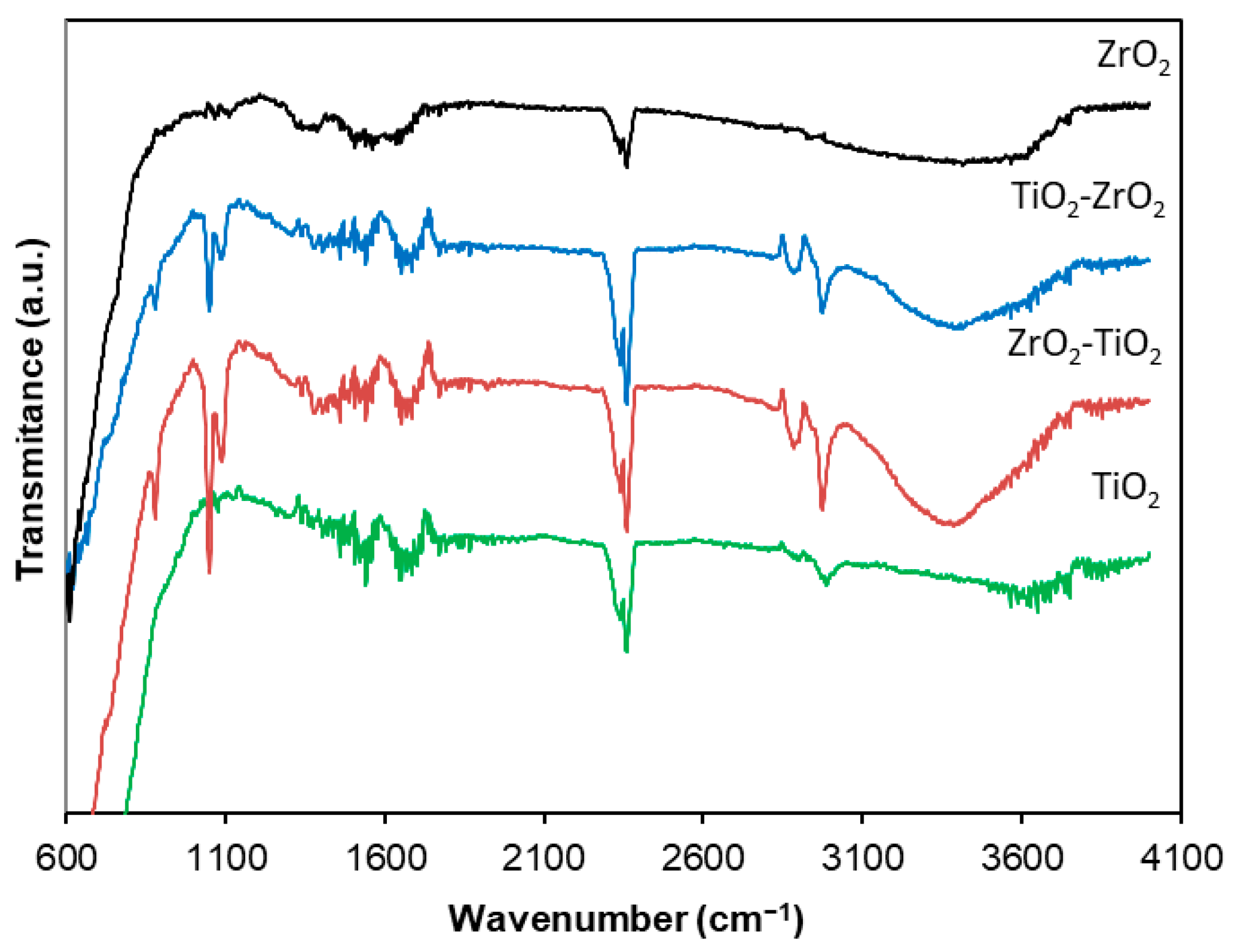
2.6. FTIR Spectroscopy
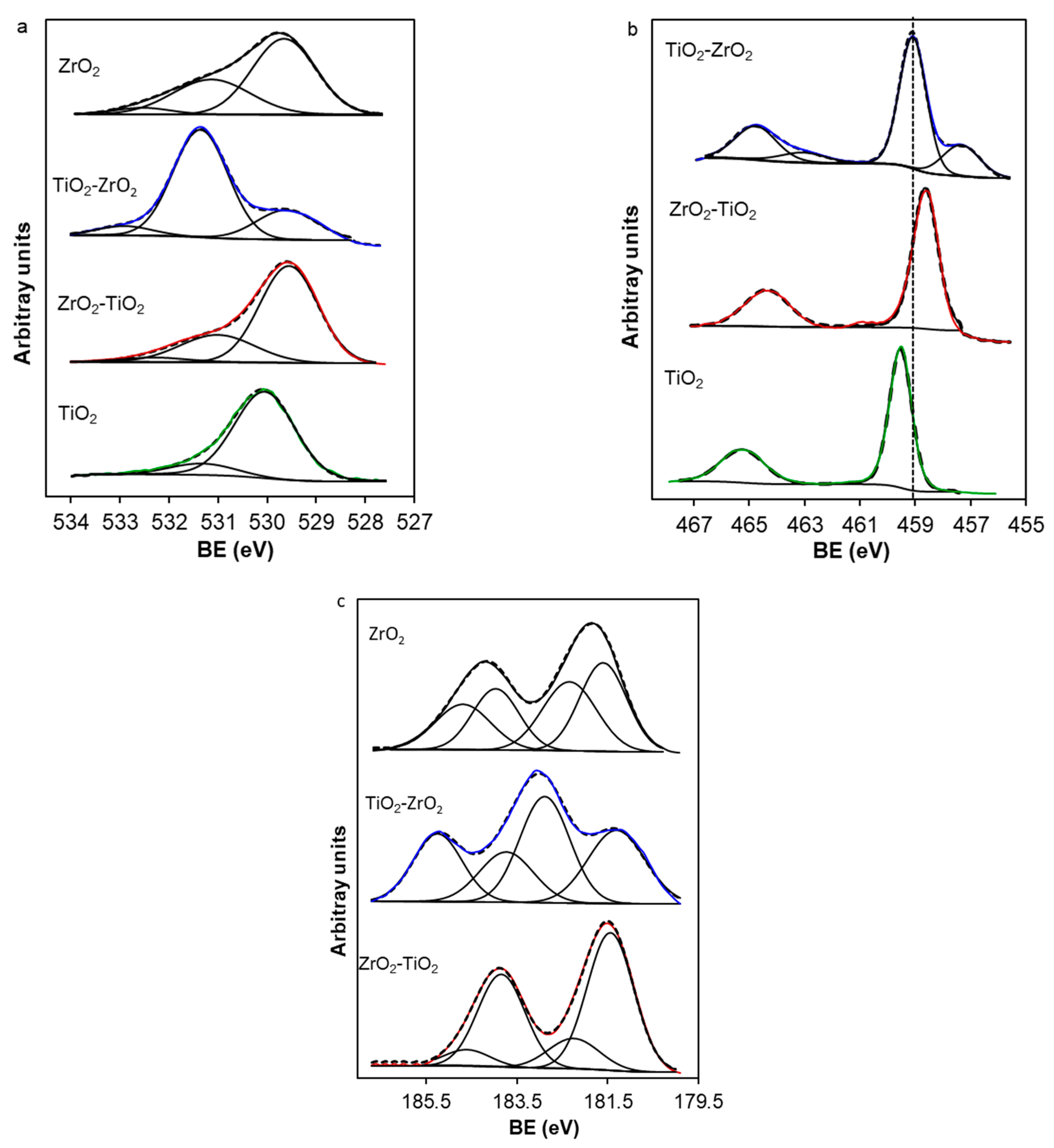
2.7. X-Ray Photoelectron Spectroscopy
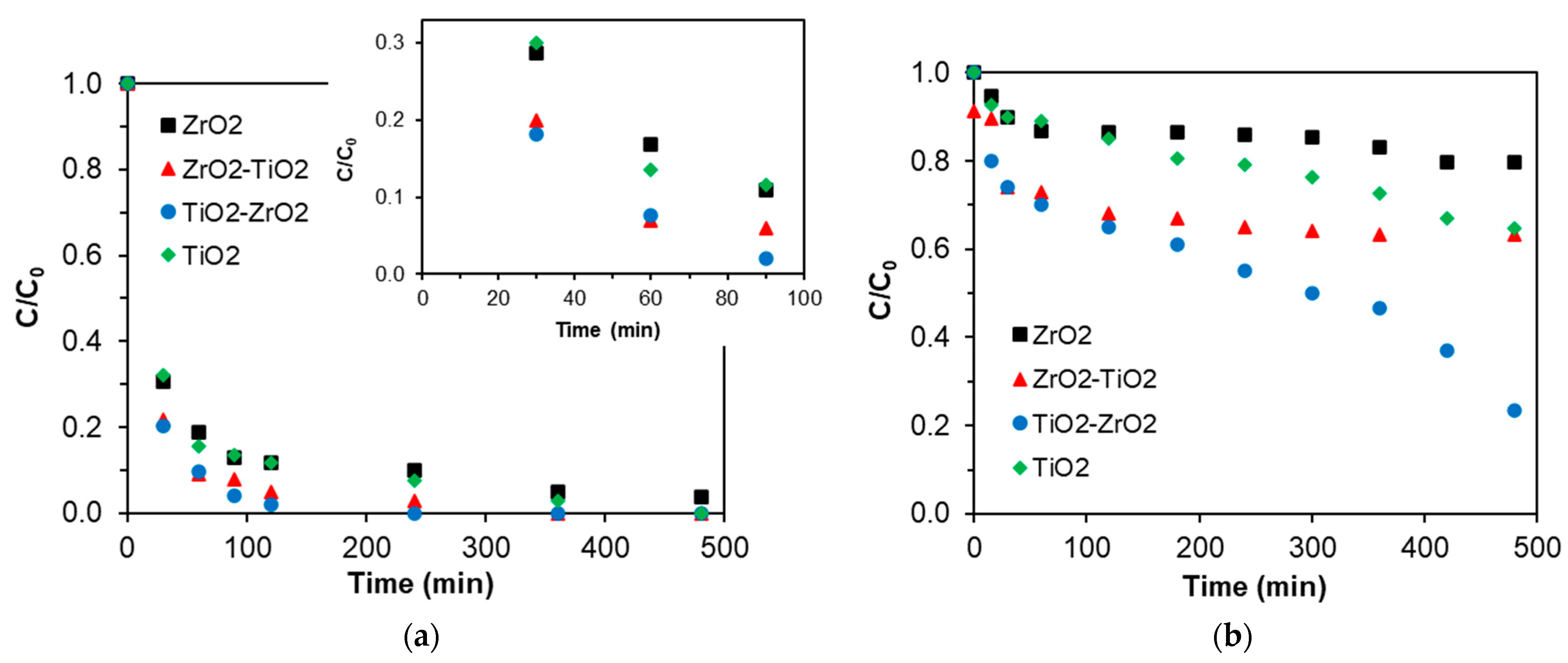
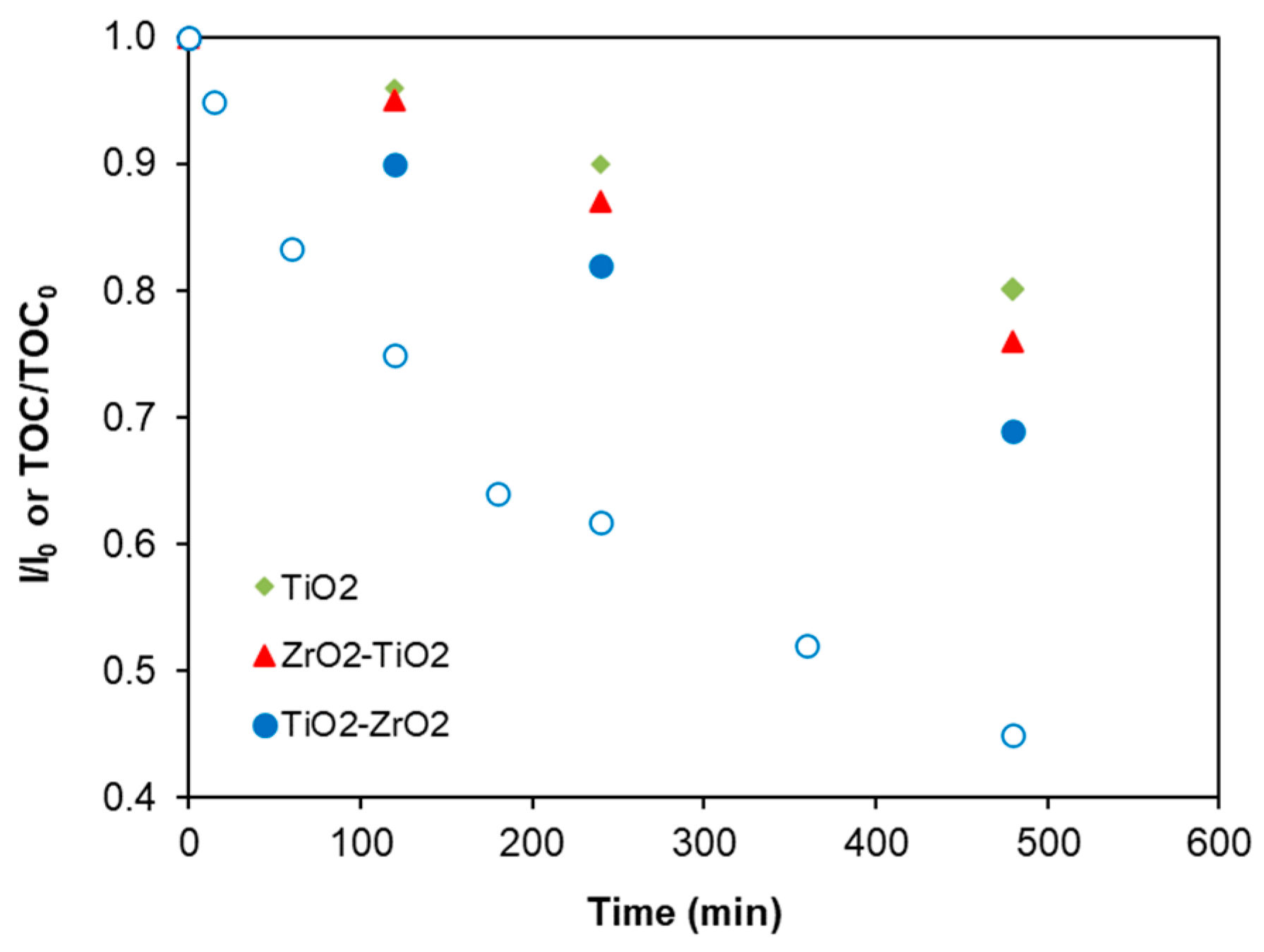
2.8. Photocatalytic Reaction
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.2.1. Simple Oxides
3.2.2. Mixed Oxides
3.2.3. Catalysts Characterization
3.2.4. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muralikrishna, I.V.; Manickam, V. Industrial wastewater treatment technologies, recycling, and reuse. In Environmental Management; Elsevier: Amsterdam, The Netherlands, 2017; pp. 295–336. [Google Scholar]
- Oller, I.; Malato, S.; Sánchez-Pérez, J. Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A. Analytical methods for the determination of pharmaceuticals in aqueous environmental samples. TrAC Trends Anal. Chem. 2001, 20, 419–434. [Google Scholar] [CrossRef]
- Kümmerer, K. Introduction: Pharmaceuticals in the environment. In Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–8. [Google Scholar]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2013, 12, 27–47. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Liu, Z.H.; Kanjo, Y.; Mizutani, S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—Physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.; Vakili, M.; Van Le, H. Removal performance and optimisation of pharmaceutical micropollutants from synthetic domestic wastewater by hybrid treatment. J. Contam. Hydrol. 2020, 235, 103736. [Google Scholar] [CrossRef]
- Biń, A.K.; Sobera-Madej, S. Comparison of the Advanced Oxidation Processes (UV, UV/H2O2 and O3) for the Removal of Antibiotic Substances during Wastewater Treatment. Ozone-Sci. Eng. 2012, 34, 136–139. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, R.; Tan, C.; Chen, P.; Yu, G.; Deng, S. Efficient degradation of typical pharmaceuticals in water using a novel TiO2/ONLH nano-photocatalyst under natural sunlight. J. Hazard. Mater. 2021, 403, 123582. [Google Scholar] [CrossRef]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Li, C.; Mei, Y.; Qi, G.; Xu, W.; Zhou, Y.; Shen, Y. Degradation characteristics of four major pollutants in chemical pharmaceutical wastewater by Fenton process. J. Environ. Chem. Eng. 2021, 9, 104564. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Barthe, L.; Gonzalez-Labrada, K.; Jauregui Haza, U.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Lacombe, S.; Tran-Thi, T.; Guillard, C.; Herrmann, J.; Keller-Spitzer, V.; Keller, N.; Maurette, M.; Pichat, P.; Pigot, T.; Pulgarin, C. La photocatalyse pour l’elimination des polluants. Actual. Chim. 2007, 308, 79. [Google Scholar]
- Agrios, A.G.; Pichat, P. State of the art and perspectives on materials and applications of photocatalysis over TiO2. J. Appl. Electrochem. 2005, 35, 655–663. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Photocatalytic removal of spiramycin from wastewater under visible light with N-doped TiO2 photocatalysts. Chem. Eng. J. 2015, 261, 3–8. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. The feasibility of using combined TiO2 photocatalysis-SBR process for antibiotic wastewater treatment. Desalination 2011, 272, 218–224. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Matos, J.; Montana, R.; Rivero, E.; Escudero, A.; Uzcategui, D. Influence of anatase and rutile phase in TiO2 upon the photocatalytic degradation of methylene blue under solar irradiation in presence of activated carbon. Water Sci. Technol. 2014, 69, 2184–2190. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Botta, S.G.; Navio, J.A.; Hidalgo, M.C.; Restrepo, G.M.; Litter, M.I. Photocatalytic properties of ZrO2 and Fe/ZrO2 semiconductors prepared by a sol–gel technique. J. Photochem. Photobiol. A Chem. 1999, 129, 89–99. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Q.; Xie, C.; Yan, W.; Du, Y. Structure, luminescence properties and photocatalytic activity of europium doped-TiO2 nanoparticles. J. Mater. Sci. 2005, 40, 1539–1541. [Google Scholar] [CrossRef]
- Thabet, S.M.; Abdelhamid, H.N.; Ibrahim, S.A.; El-Bery, H.M. Boosting photocatalytic water splitting of TiO2 using metal (Ru, Co, or Ni) co-catalysts for hydrogen generation. Sci. Rep. 2024, 14, 10115. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Quan, X.; Qiu, F.; Zhang, X. Synthesis of CuOx/TiO2 Photocatalysts with Enhanced Photocatalytic Performance. ACS Omega 2023, 8, 2723–2732. [Google Scholar] [CrossRef]
- Karunakaran, C.; Senthilvelan, S. Photocatalysis with ZrO2: Oxidation of aniline. J. Mol. Catal. A Chem. 2005, 233, 1–8. [Google Scholar] [CrossRef]
- Polisetti, S.; Deshpande, P.A.; Madras, G. Photocatalytic Activity of Combustion Synthesized ZrO2 and ZrO2–TiO2 Mixed Oxides. Ind. Eng. Chem. Res. 2011, 50, 12915–12924. [Google Scholar] [CrossRef]
- Kralik, B.; Chang, E.K.; Louie, S.G. Structural properties and quasiparticle band structure of zirconia. Phys. Rev. B 1998, 57, 163–1829. [Google Scholar] [CrossRef]
- Binitha, N.; Yaakob, Z.; Resmi, R. Influence of synthesis methods on zirconium doped titania photocatalysts. Open Chem. 2010, 8, 182–187. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Roh, H.-S.; Kim, J.-W.; Jun, K.-W. Surface properties and catalytic activity of TiO2–ZrO2 mixed oxides in dehydration of methanol to dimethyl ether. Catal. Lett. 2004, 96, 23–28. [Google Scholar] [CrossRef]
- Bailón-García, E.; Elmouwahidi, A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Development of Carbon-ZrO2 composites with high performance as visible-light photocatalysts. Appl. Catal. B Environ. 2017, 217, 540–550. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, S.; Guo, X.; Jin, B.; Tian, Y. Preparation and photocatalytic activity of CeO2/TiO2 interface composite film. Appl. Surf. Sci. 2009, 255, 5975–5978. [Google Scholar] [CrossRef]
- Zheng, R.; Meng, X.; Tang, F. Synthesis, characterization and photodegradation study of mixed-phase titania hollow submicrospheres with rough surface. Appl. Surf. Sci. 2009, 255, 5989–5994. [Google Scholar] [CrossRef]
- McManamon, C.; Holmes, J.D.; Morris, M.A. Improved photocatalytic degradation rates of phenol achieved using novel porous ZrO2-doped TiO2 nanoparticulate powders. J. Hazard. Mater. 2011, 193, 120–127. [Google Scholar] [CrossRef]
- Sandoval, S.; Yang, J.; Alfaro, J.G.; Liberman, A.; Makale, M.; Chiang, C.E.; Schuller, I.K.; Kummel, A.C.; Trogler, W.C. Europium Doped TiO2 Hollow Nanoshells: Two-Photon Imaging of Cell Binding. Chem. Mater. 2012, 24, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, M.; Qiang, W.; Li, Z.; Wang, C.; Chen, N.; Wang, C.-C.; Wan, C.; Chen, S. Controllable synthesis of cerium zirconium oxide nanocomposites and their application for photocatalytic degradation of sulfonamides. Appl. Catal. B Environ. 2019, 259, 107–118. [Google Scholar] [CrossRef]
- Ding, P.; Ji, H.; Li, P.; Liu, Q.; Wu, Y.; Guo, M.; Zhou, Z.; Gao, S.; Xu, W.; Liu, W.; et al. Visible-light degradation of antibiotics catalyzed by titania/zirconia/graphitic carbon nitride ternary nanocomposites: A combined experimental and theoretical study. Appl. Catal. B Environ. 2022, 300, 120633. [Google Scholar] [CrossRef]
- Aguirre-Cortés, J.M.; Munguía-Ubierna, A.; Moral-Rodríguez, A.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Bailón-García, E. Size-miniaturization of TiO2-ZrO2 coupled semiconductors to develop highly efficient visible- driven photocatalysts for the degradation of drugs in wastewater. Appl. Surf. Sci. 2024, 670, 160609. [Google Scholar] [CrossRef]
- Hammami, R.; Aïssa, S.B.; Batis, H. Effects of thermal treatment on physico-chemical and catalytic properties of lanthanum manganite LaMnO3+y. Appl. Catal. A Gen. 2009, 353, 145–153. [Google Scholar] [CrossRef]
- Kite, S.V.; Sathe, D.J.; Kadam, A.N.; Chavan, S.S.; Garadkar, K.M. Highly efcient photodegradation of 4-nitrophenol over the nano-TiO2 obtained from chemical bath deposition technique. Res. Chem. Intermed. 2020, 46, 1255–1282. [Google Scholar] [CrossRef]
- Prime, R.B.; Bair, H.E.; Vyazovkin, S.; Gallagher, P.K.; Riga, A. 3. Thermogravimetric analysis (TGA). In Thermal Analysis of Polymers. Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2009; pp. 241–314. [Google Scholar]
- Vahid, B.R.; Haghighi, M. Biodiesel production from sunflower oil over MgO/MgAl2O4 nanocatalyst: Effect of fuel type on catalyst nanostructure and performance. Energy Convers. Manag. 2017, 134, 290–300. [Google Scholar] [CrossRef]
- Kambur, A.; Pozan, G.S.; Boz, I. Preparation, characterization and photocatalytic activity of TiO2–ZrO2 binary oxide nanoparticles. Appl. Catal. B Environ. 2012, 115–116, 149–158. [Google Scholar] [CrossRef]
- Zare, M.H.; Mehrabani-Zeinabad, A. Photocatalytic activity of ZrO2/TiO2/Fe3O4 ternary nanocomposite for the degradation of naproxen: Characterization and optimization using response surface methodology. Sci. Rep. 2022, 12, 10388. [Google Scholar] [CrossRef] [PubMed]
- Ökte, A.N.; Sayınsöz, E. Characterization and photocatalytic activity of TiO2 supported sepiolite catalysts. Sep. Purif. Technol. 2008, 62, 535–543. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Wang, W.; Zhang, Y.; Wang, X.; Su, H. The preparation of vanadium-doped TiO2–montmorillonite nanocomposites and the photodegradation of sulforhodamine B under visible light irradiation. Appl. Surf. Sci. 2011, 257, 7276–7285. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, X.; Ren, Y.; Yue, Y.; Hua, W.; Cao, Y.; Tang, Y.; Gao, Z. Gas-phase photo-oxidations of organic compounds over different forms of zirconia. J. Mol. Catal. A Chem. 2005, 229, 233–239. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Behbahani, A.; Rowshanzamir, S.; Esmaeilifar, A. Hydrothermal Synthesis of Zirconia Nanoparticles from Commercial Zirconia. Procedia Eng. 2012, 42, 908–917. [Google Scholar] [CrossRef]
- Jayakumar, S.; Ananthapadmanabhan, P.V.; Thiyagarajan, T.K.; Perumal, K.; Mishra, S.C.; Suresh, G.; Su, L.T.; Tok, A.I.Y. Nanosize stabilization of cubic and tetragonal phases in reactive plasma synthesized zirconia powders. Mater. Chem. Phys. 2013, 140, 176–182. [Google Scholar] [CrossRef]
- George, A.; Solomon, S.; Thomas, J.K.; John, A. Characterizations and electrical properties of ZrTiO4 ceramic. Mater. Res. Bull. 2012, 47, 3141–3147. [Google Scholar] [CrossRef]
- Srinivasan, R.; De Angelis, R.J.; Ice, G.; Davis, B.H. Identification of tetragonal and cubic structures of zirconia using synchrotron x-radiation source. Mater. Res. Soc. 1991, 6, 1287–1292. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.; Wang, D. Monoclinic zirconium oxide nanostructures synthesized by a hydrothermal route. Nanotechnology 2008, 19, 195602. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yan, X.; Chen, X.; Tian, L.; Xia, Q.; Chen, X. Mesoporous TiO2 nanoparticles terminated with carbonate-like groups: Amorphous/crystalline structure and visible-light photocatalytic activity. Catal. Today 2016, 264, 243–249. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Huang, Z.; Xu, H.; Shen, W. The Effects of the Crystalline Phase of Zirconia on C–O Activation and C–C Coupling in Converting Syngas into Aromatics. Catalysts 2020, 10, 262. [Google Scholar] [CrossRef]
- Das, I.; Chattopadhyay, S.; Mahato, A.; Kundu, B.; De, G. Fabrication of a cubic zirconia nanocoating on a titanium dental implant with excellent adhesion, hardness and biocompatibility. RSC Adv. 2016, 6, 59030–59038. [Google Scholar] [CrossRef]
- Kelly, A.; Knowles, K.M. Appendix 7: Crystal Structure Data. In Crystallography and Crystal Defects; Wiley: Hoboken, NJ, USA, 2012; pp. 491–498. [Google Scholar]
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration curve for quantitative analysis of the monoclinic-tetragonal ZrO2 system by X-ray diffraction. J. Am. Ceram. Soc. 1984, 67, 119–121. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Aghakhanpour, R.B. Synthesis and characterization of nanocrystalline zirconia powder by simple sol–gel method with glucose and fructose as organic additives. Powder Technol. 2011, 205, 193–200. [Google Scholar] [CrossRef]
- Tahmasebpour, M.; Babaluo, A.A.; Aghjeh, M.K.R. Synthesis of zirconia nanopowders from various zirconium salts via polyacrylamide gel method. J. Eur. Ceram. Soc. 2008, 28, 773–778. [Google Scholar] [CrossRef]
- Bhaskar, S.; Awin, E.W.; Kumar, K.H.; Lale, A.; Bernard, S.; Kumar, R. Design of nanoscaled heterojunctions in precursor-derived t-ZrO2/SiOC (N) nanocomposites: Transgressing the boundaries of catalytic activity from UV to visible light. Sci. Rep. 2020, 10, 430. [Google Scholar] [CrossRef]
- Khattab, E.-S.R.; Abd El Rehim, S.S.; Hassan, W.M.; El-Shazly, T.S. Band structure engineering and optical properties of pristine and doped monoclinic zirconia (m-ZrO2): Density functional theory theoretical prospective. ACS Omega 2021, 6, 30061–30068. [Google Scholar] [CrossRef]
- Honda, M.; Ochiai, T.; Listiani, P.; Yamaguchi, Y.; Ichikawa, Y. Low-Temperature Synthesis of Cu-Doped Anatase TiO2 Nanostructures via Liquid Phase Deposition Method for Enhanced Photocatalysis. Materials 2023, 16, 639. [Google Scholar] [CrossRef]
- Chang, S.-M.; Doong, R.-A. Interband Transitions in Sol-Gel-Derived ZrO2 Films under Different Calcination Conditions. Chem. Mater. 2007, 19, 4804–4810. [Google Scholar] [CrossRef]
- Katsumata, H.; Makita, Y.; Kobayashi, N.; Shibata, H.; Hasegawa, M.; Aksenov, I.; Kimura, S.; Obara, A.; Uekusa, S.-I. Optical absorption and photoluminescence studies of β-FeSi2 prepared by heavy implantation of Fe+ ions into Si. J. Appl. Phys. 1996, 80, 5955–5962. [Google Scholar] [CrossRef]
- Hidalgo, N.; Colon, G.; Botta, S.; Litter, M. Preparation and physicochemical properties of ZrO2 and Fe/ZrO2 prepared by a sol–gel technique. Langmuir 2001, 17, 202–210. [Google Scholar]
- Simon, S.M.; George, G.; Chandran, A.; Prakashan, V.P.; Sajna, M.S.; Saritha, A.C.; Biju, P.R.; Joseph, C.; Unnikrishnan, N.V. Morphological and thermal studies of mesoporous TiO2-ZrO2 and TiO2-ZrO2-polymer composites as potential self cleaning surface. Mater. Today Proc. 2020, 33, 1327–1332. [Google Scholar] [CrossRef]
- Gao, N.; Zhou, Y.; Fan, M.; Xu, H.; Chen, Y.; Shen, S. Promoting effect and role of alkaline earth metal added to ZrO2-TiO2-supported CeO2 for dichloromethane oxidation. Chem. Eng. J. 2020, 396, 125193. [Google Scholar] [CrossRef]
- Fang, D.; Luo, Z.; Liu, S.; Zeng, T.; Liu, L.; Xu, J.; Bai, Z.; Xu, W. Photoluminescence properties and photocatalytic activities of zirconia nanotube arrays fabricated by anodization. Opt. Mater. 2013, 35, 1461–1466. [Google Scholar] [CrossRef]
- Saleem, A.M.; Gnanasaravanan, S.; Saravanakkumar, D.; Rajasekar, S.; Ayeshamariam, A.; Jayachandran, M. Preparation and characterization studies of TiO2 doped ZrO2 on ITO nanocomposites for optoelectronic applications. Mater. Today Proc. 2021, 36, 408–415. [Google Scholar] [CrossRef]
- Pipornpong, W.; Wanbayor, R.; Ruangpornvisuti, V. Adsorption CO2 on the perfect and oxygen vacancy defect surfaces of anatase TiO2 and its photocatalytic mechanism of conversion to CO. Appl. Surf. Sci. 2011, 257, 10322–10328. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on TiO2 surfaces: Quantum chemical modeling of CO2 adsorption on oxygen vacancies. Fuel Process. Technol. 2011, 92, 805–811. [Google Scholar] [CrossRef]
- Adam, F.; Chew, T.-S.; Andas, J. Liquid Phase Oxidation of Acetophenone over Rice Husk Silica Vanadium Catalyst. Chin. J. Catal. 2012, 33, 518–522. [Google Scholar] [CrossRef]
- Bailón-García, E.; Elmouwahidi, A.; Álvarez, M.A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. New carbon xerogel-TiO2 composites with high performance as visible-light photocatalysts for dye mineralization. Appl. Catal. B Environ. 2017, 201, 29–40. [Google Scholar] [CrossRef]
- Gao, L.S.; Zhang, S.N.; Zou, X.; Wang, J.; Su, J.; Chen, J.S. Oxygen Vacancy Engineering of Titania-Induced by Sr2+ Dopants for Visible-Light-Driven Hydrogen Evolution. Inorg. Chem. 2021, 60, 32–36. [Google Scholar] [CrossRef]
- Hamad, H.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. ZrO2-TiO2/Carbon core-shell composites as highly efficient solar-driven photo-catalysts: An approach for removal of hazardous water pollutants. J. Environ. Chem. Eng. 2020, 8, 104350. [Google Scholar] [CrossRef]
- Reddy, C.V.; Babu, B.; Reddy, I.N.; Shim, J. Synthesis and characterization of pure tetragonal ZrO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2018, 44, 6940–6948. [Google Scholar] [CrossRef]
- Mishra, S.; Debnath, A.K.; Muthe, K.P.; Das, N.; Parhi, P. Rapid synthesis of tetragonal zirconia nanoparticles by microwave-solvothermal route and its photocatalytic activity towards organic dyes and hexavalent chromium in single and binary component systems. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125551. [Google Scholar] [CrossRef]
- Ahmed, O.; Pons, M.-N.; Lachheb, H.; Houas, A.; Zahraa, O. Degradation of sulfamethoxazole by photocatalysis using supported TiO2. Sustain. Environ. Res. 2014, 24, 381–387. [Google Scholar]
- Kim, J.R.; Kan, E. Heterogeneous Photocatalytic Degradation of Sulfamethoxazole in Water Using a Biochar-Supported TiO2 Photocatalyst. J. Environ. Manag. 2016, 180, 94–101. [Google Scholar] [CrossRef]
- Hamad, H.; Bailón-García, E.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Morales-Torres, S. Synthesis of TixOy nanocrystals in mild synthesis conditions for the degradation of pollutants under solar light. Appl. Catal. B Environ. 2019, 241, 385–392. [Google Scholar] [CrossRef]
- Jahdi, M.; Mishra, S.B.; Nxumalo, E.N.; Mhlanga, S.D.; Mishra, A.K. Smart pathways for the photocatalytic degradation of sulfamethoxazole drug using F-Pd co-doped TiO2 nanocomposites. Appl. Catal. B Environ. 2020, 267, 118716. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Szepesi, C.J.; Adair, J.H.; Chan, H. High Yield Hydrothermal Synthesis of Nano-Scale Zirconia and YTZP. J. Am. Ceram. Soc. 2011, 94, 4239–4246. [Google Scholar] [CrossRef]
- Balarak, D.; Mostafapour, F.K. Batch equilibrium, kinetics and thermodynamics study of sulfamethoxazole antibiotics onto Azolla filiculoides as a novel biosorbent. Br. J. Pharm. Res. 2016, 13, 1–14. [Google Scholar] [CrossRef]
- ISO 11348-2:2007/Amd 1:2018; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test)—Part 2: Method Using Liquid-Dried Bacteria—Amendment 1. ISO: Geneva, Switzerland, 2018. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0062815 (accessed on 31 October 2024).
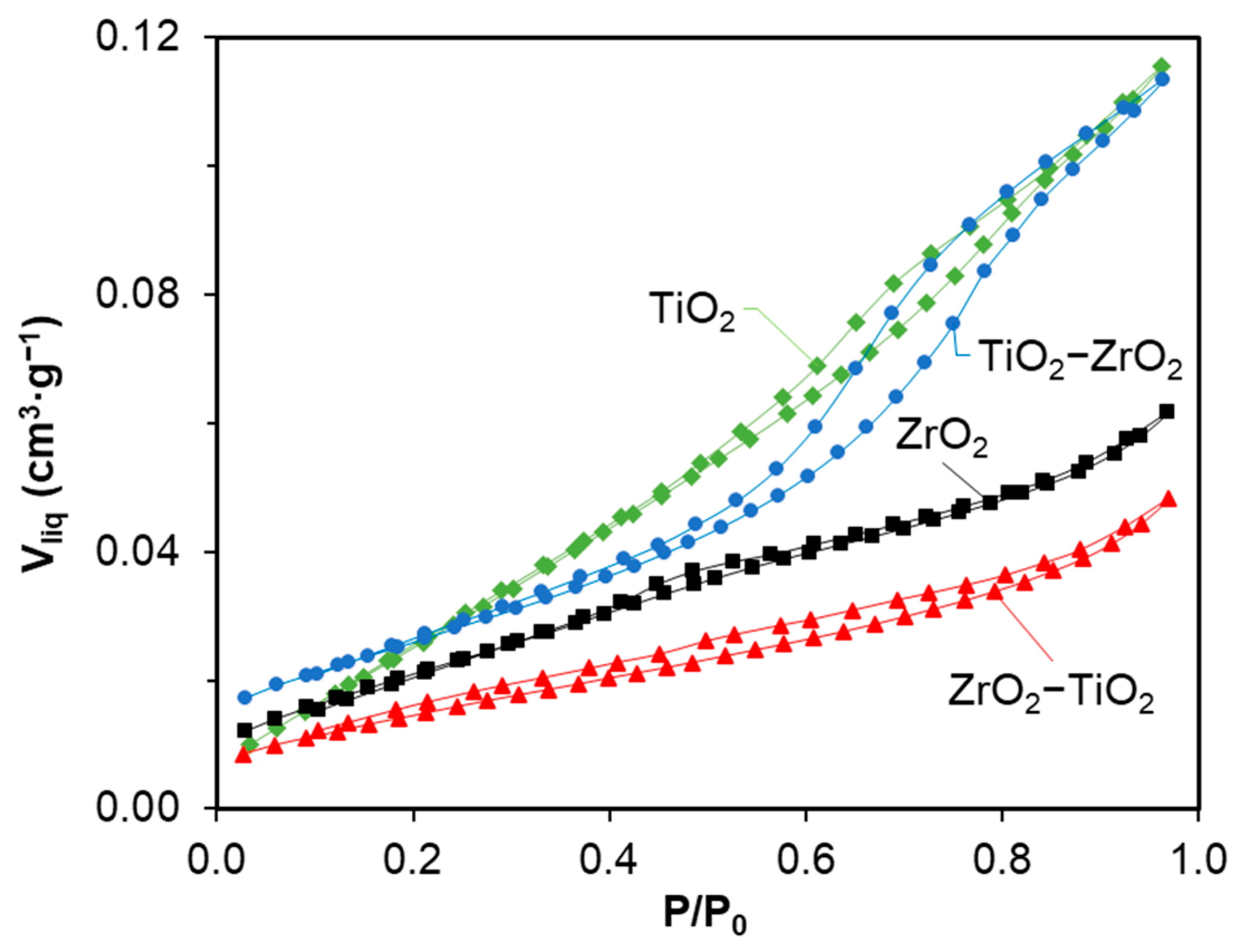
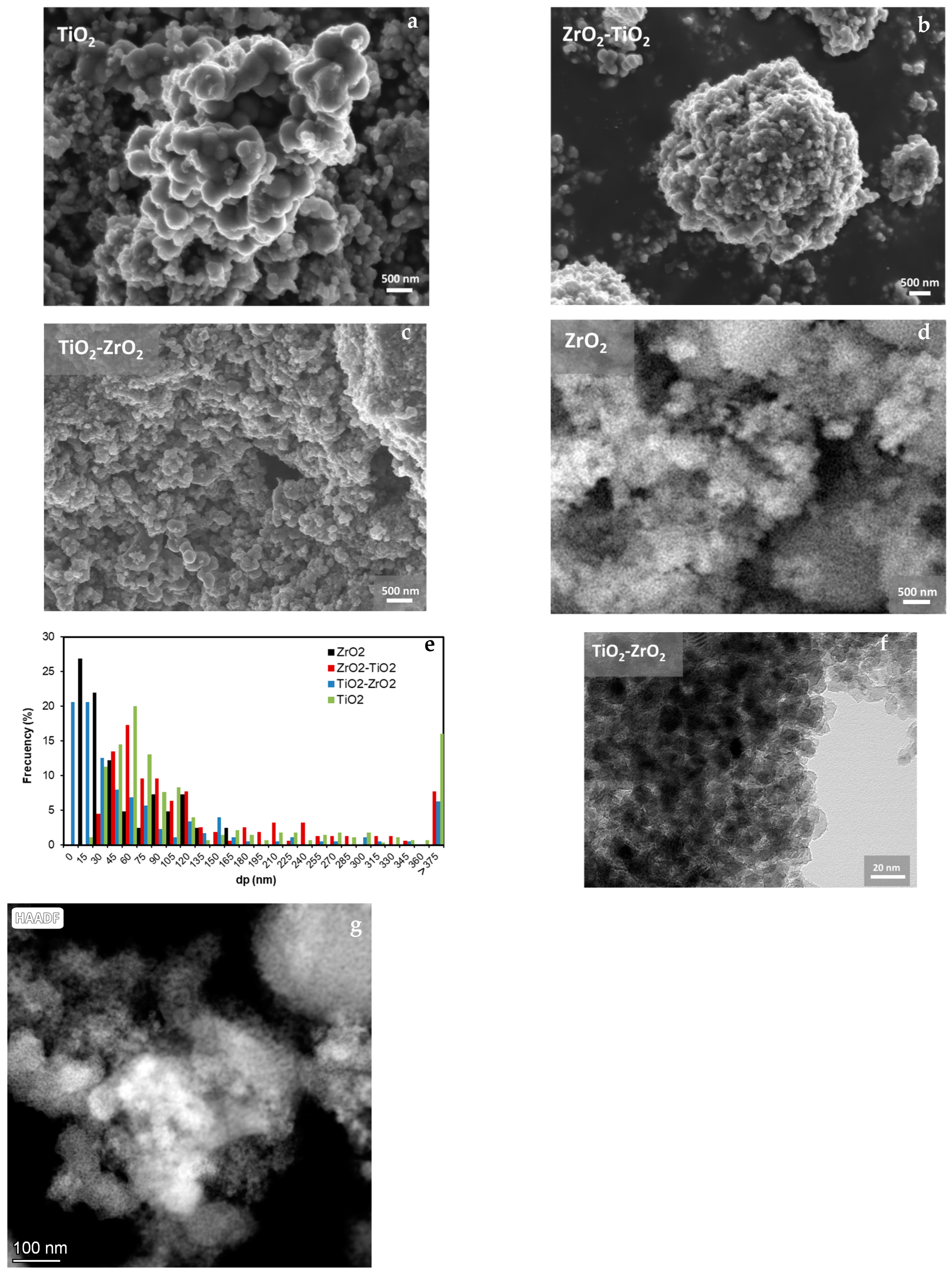

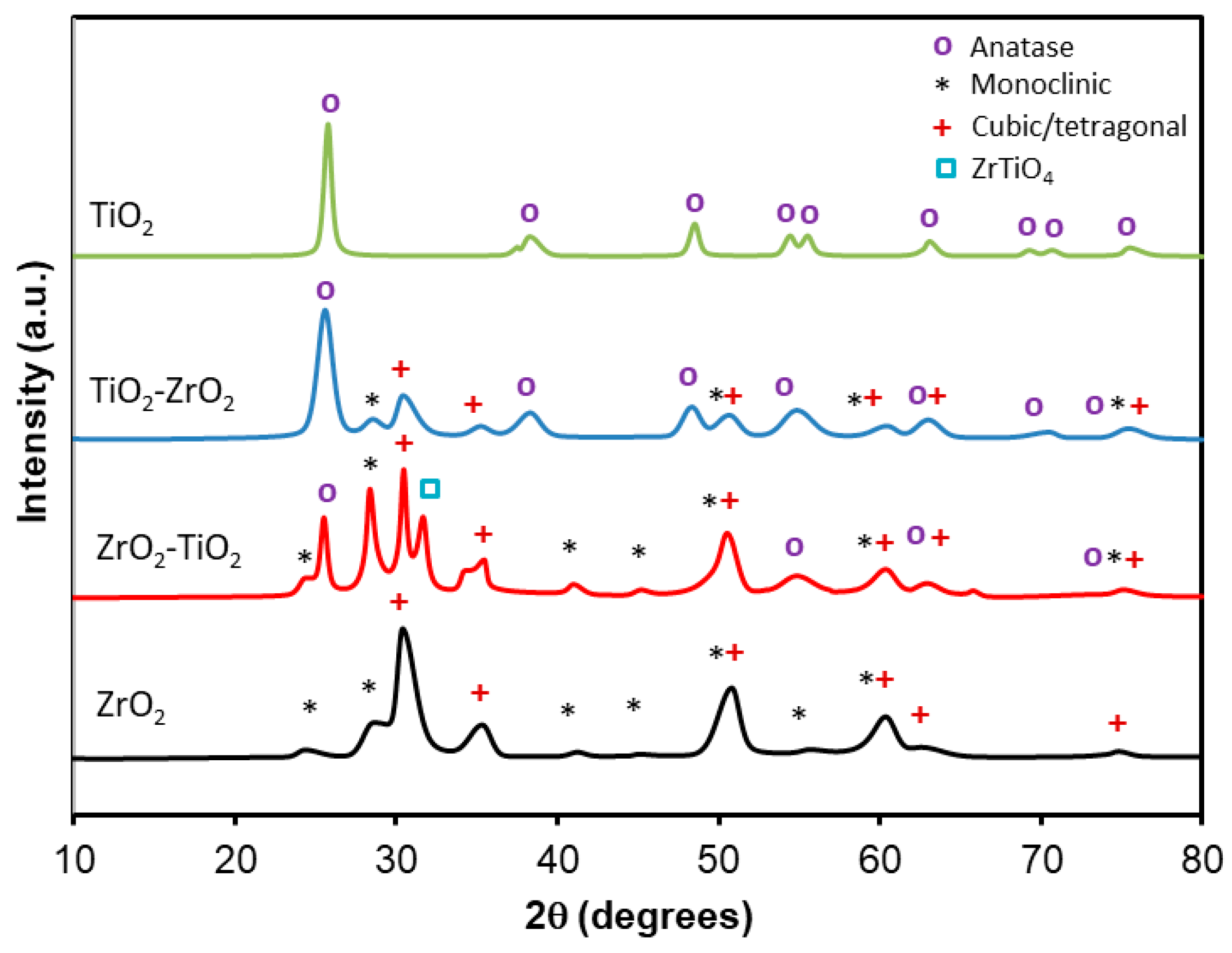

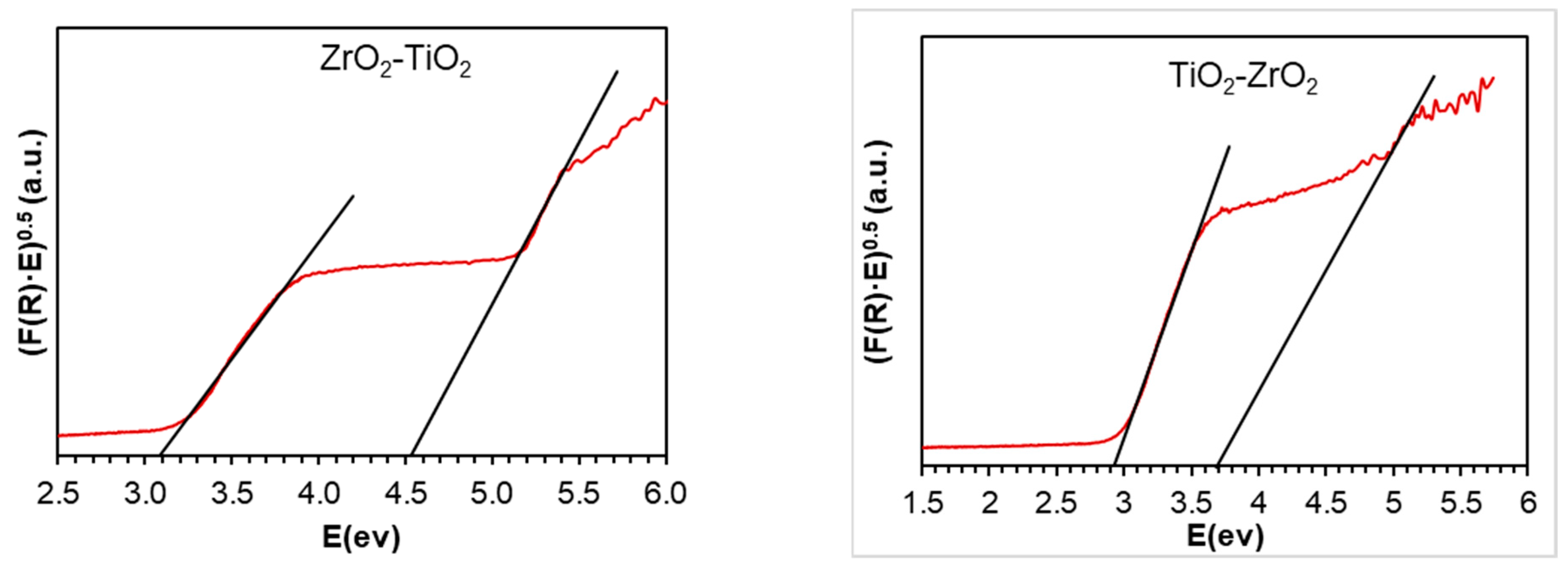
| Materials | SBET (m2g−1) | Vmicro (cm3g−1) | Vmeso (cm3g−1) | L0 (nm) |
|---|---|---|---|---|
| TiO2 | 90 | 0.029 | 0.087 | 3.86 |
| ZrO2 | 54 | 0.022 | 0.040 | 2.81 |
| ZrO2-TiO2 | 37 | 0.015 | 0.033 | 2.68 |
| TiO2-ZrO2 | 64 | 0.027 | 0.087 | 2.40 |
| Sample | Crystal Phase | Molar Fraction (%) | dp (nm) | d-Spacing (nm) |
|---|---|---|---|---|
| TiO2 | Anatase | 100 | 14 | 0.345 |
| ZrO2 | Tetragonal/cubic | 78 | 6 | 0.294 |
| Monoclinic | 22 | 6 | 0.312 | |
| TiO2-ZrO2 | Anatase | 100 | 7 | 0.349 |
| Tetragonal/cubic | 51 | 6 | 0.294 | |
| Monoclinic | 49 | 6 | 0.312 | |
| ZrO2-TiO2 | Anatase | 100 | 14 | 0.349 |
| Tetragonal/cubic | 51 | 11 | 0.293 | |
| Monoclinic | 49 | 12 | 0.313 |
| Catalyst | Catalyst Preparation | Pollutant | Conditions | % Degradation | Ref |
|---|---|---|---|---|---|
| Monoclinic ZrO2 Tetragonal ZrO2 Cubic ZrO2 | Sol–gel Precipitation-colloidal Hydrothermal | MO | UV; 110 min; Cat. 100 mg/100 mL; Dye 10 ppm | 99% 90% 80% | [49] |
| TiO2 thin film | Commercial TiO2 | SMX | UV; 420 min; Cat. 400 mg/100 mL; SMX 10 ppm; | 97% | [80] |
| Biochar supported TiO2 | Sol–gel | SMX | UV; 360 min; Cat. 500 mg/100 mL; SMX 10 ppm; | 91% | [81] |
| F-Pd co-doped TiO2 | microwave-assisted hydrothermal | SMX | Solar; 70 min; Cat.50/50 mL; SMX 30 ppm; | 98% | [83] |
| ZrO2-TiO2 | Solution combustion technique | OG | UV; 120 min; Cat. 1 g/L; OG 100 ppm | 22% | [28] |
| AB | UV; 90 min; Cat. 1 g/L; AB 100 ppm | 50% | |||
| RBBR | UV; 120 min; Cat. 1 g/L; RBBR 100 ppm | 60% | |||
| ACG | UV; 120 min; Cat. 1 g/L; ACG 100 ppm | 52% | |||
| ZrO2 TiO2 ZrO2-TiO2 TiO2-ZrO2 | Sol–gel | SMX | Blue LED; 480 min; Cat. 1 g/L; SMX 12 ppm | 18% 30% 38% 76% | current work |
| ZrO2 TiO2 ZrO2-TiO2 TiO2-ZrO2 | Sol–gel | SMX | UV; 480 min; Cat. 1 g/L; SMX 12 ppm | 100% 100% (360 min) 100% (240 min) 100% (120 min) | current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensmaine, Z.; El Korso, S.; Moral-Rodríguez, A.I.; Bedrane, S.; Ziani-Cherif, C.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Bailón-García, E. Enhanced Photodegradation of Sulfamethoxazole Through Cutting-Edge Titania-Zirconia-Based Materials. Catalysts 2024, 14, 784. https://doi.org/10.3390/catal14110784
Bensmaine Z, El Korso S, Moral-Rodríguez AI, Bedrane S, Ziani-Cherif C, Pérez-Cadenas AF, Carrasco-Marín F, Bailón-García E. Enhanced Photodegradation of Sulfamethoxazole Through Cutting-Edge Titania-Zirconia-Based Materials. Catalysts. 2024; 14(11):784. https://doi.org/10.3390/catal14110784
Chicago/Turabian StyleBensmaine, Zineb, Sanaa El Korso, Adriana Isabel Moral-Rodríguez, Sumeya Bedrane, Chewki Ziani-Cherif, Agustín Francisco Pérez-Cadenas, Francisco Carrasco-Marín, and Esther Bailón-García. 2024. "Enhanced Photodegradation of Sulfamethoxazole Through Cutting-Edge Titania-Zirconia-Based Materials" Catalysts 14, no. 11: 784. https://doi.org/10.3390/catal14110784
APA StyleBensmaine, Z., El Korso, S., Moral-Rodríguez, A. I., Bedrane, S., Ziani-Cherif, C., Pérez-Cadenas, A. F., Carrasco-Marín, F., & Bailón-García, E. (2024). Enhanced Photodegradation of Sulfamethoxazole Through Cutting-Edge Titania-Zirconia-Based Materials. Catalysts, 14(11), 784. https://doi.org/10.3390/catal14110784











