Depolymerization of Lignosulfonate Catalyzed by Different Solid Base Oxides to Prepare Phenolic Compounds
Abstract
1. Introduction
2. Results and Discussion
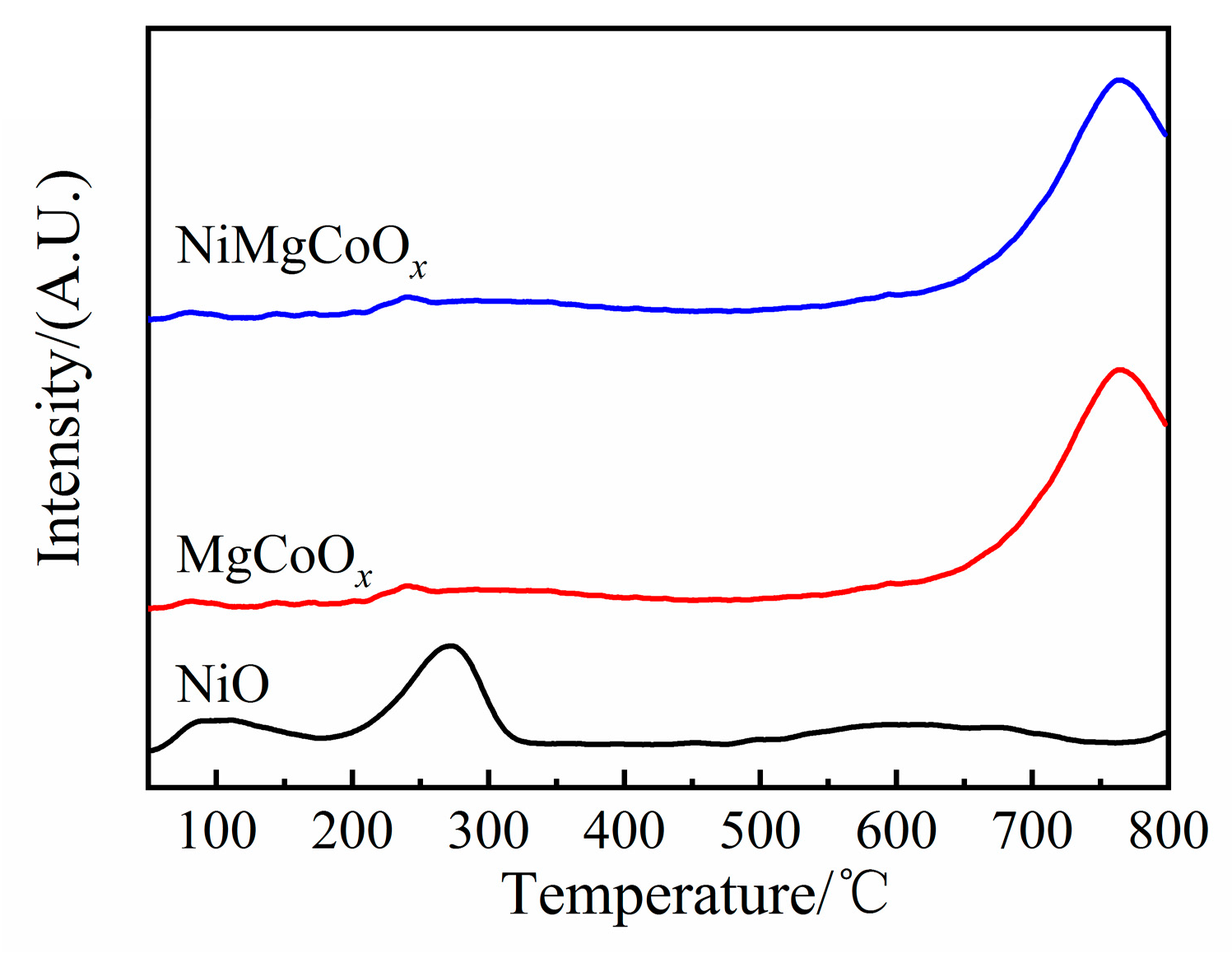
2.1. Characterization of Catalysts
2.1.1. Characterization of the Precursors
2.1.2. Characterization of the Solid Base Oxides
2.1.3. Basicity of the Solid Base Oxides
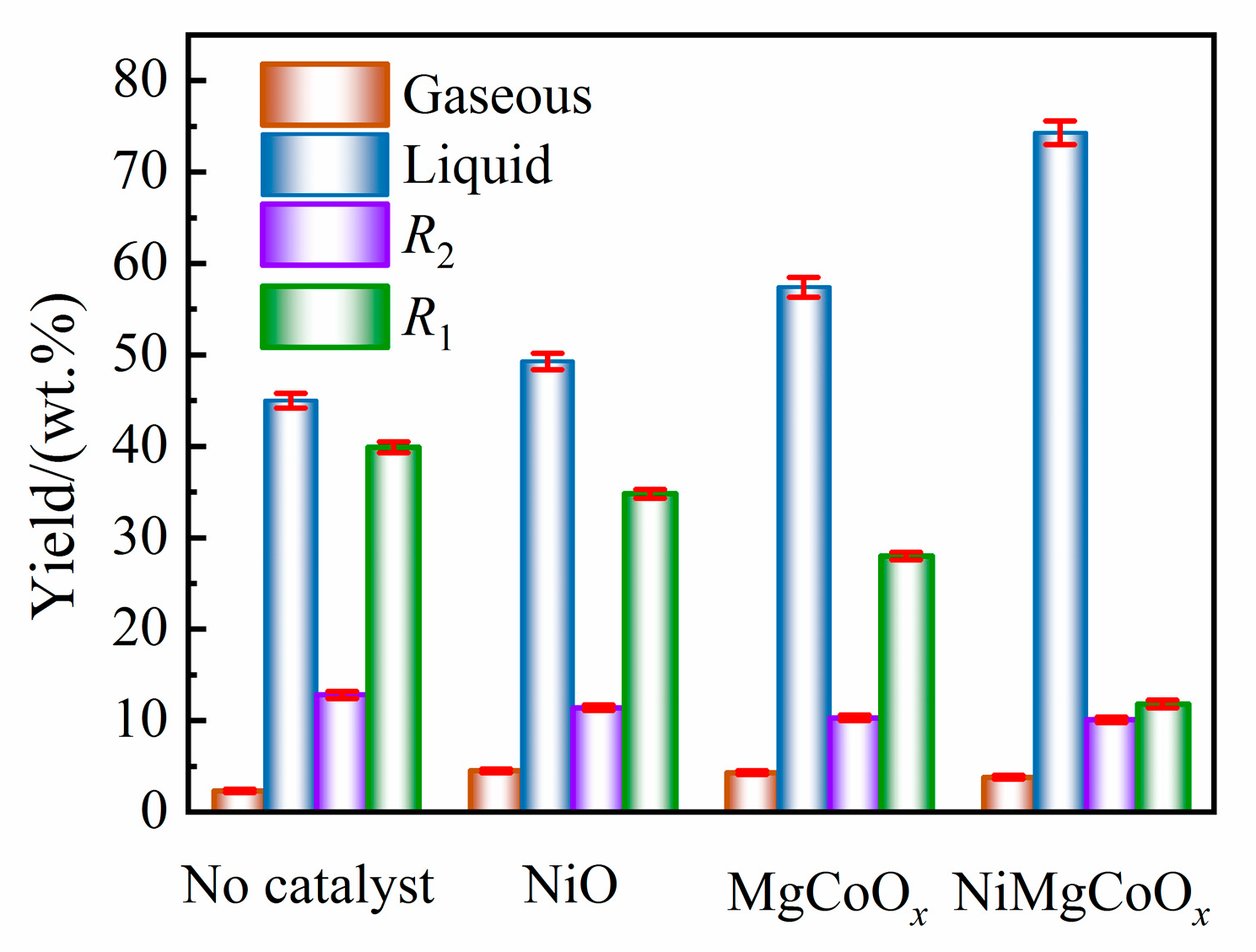
2.2. Base-Catalyzed Depolymerization of CSL
2.2.1. Product Yields
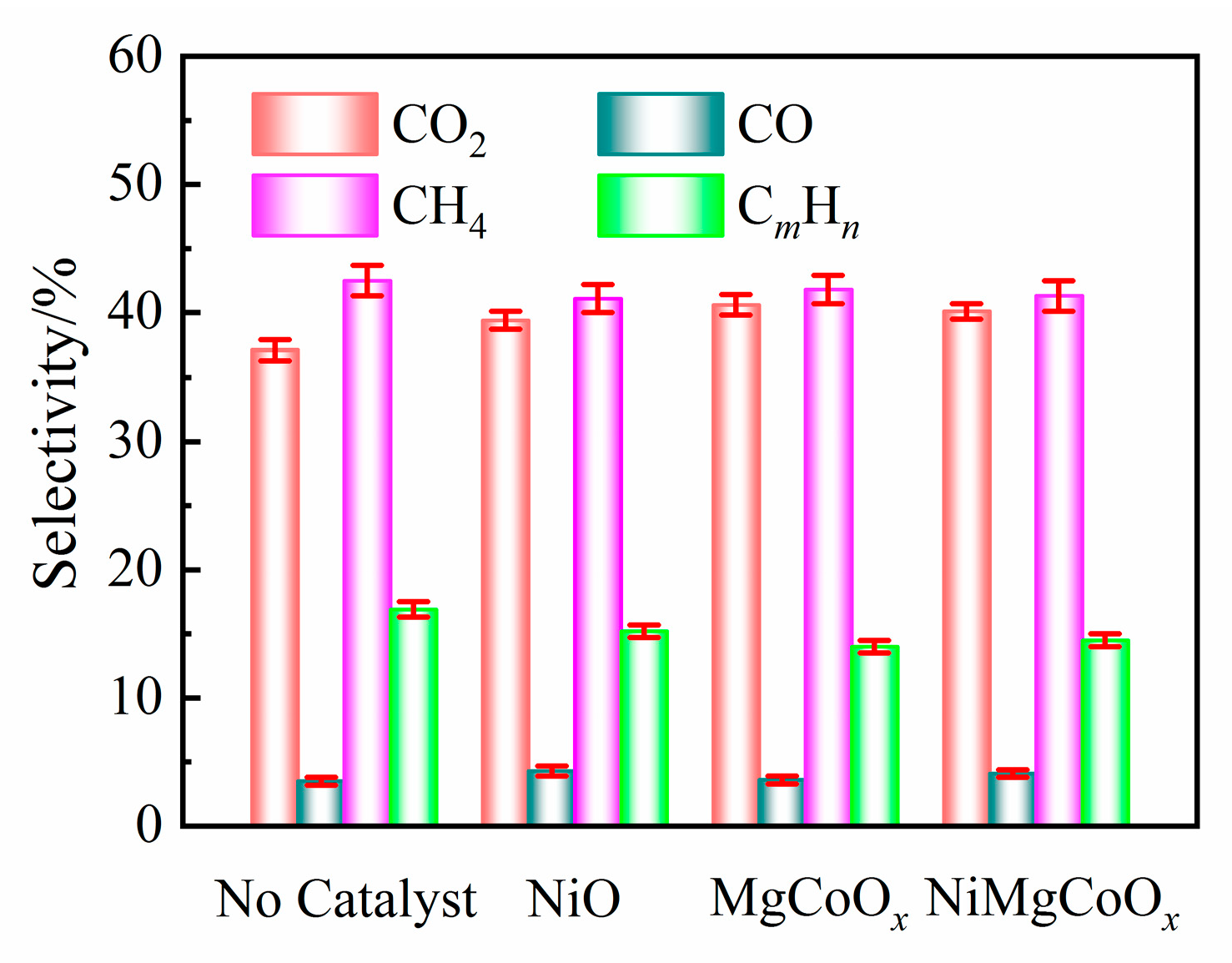
2.2.2. Composition of Gaseous and Liquid Products
2.2.3. Solid Residue Analyses
2.3. Regeneration Performance
3. Possible CLS Depolymerization Pathway Catalyzed by Solid Base Oxides
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of NiO, MgCoOx, and NiMgCoOx
4.3. Catalytic Depolymerization of CLS
4.4. Catalyst Regeneration
4.5. Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, X.; Lin, J.; Suopajarvi, T.; Mankinen, O.; Mikkelson, A.; Liu, R.; Huttunen, H.; Chen, L.; Xu, C.; Telkki, V.V.; et al. Conversion of highly polymerized lignin into monophenolic products via pyrolysis: A comparative study of acidic and alkaline depolymerization pretreatments using deep eutectic solvents. Chem. Eng. J. 2023, 478, 147368. [Google Scholar] [CrossRef]
- Sapouna, I.; Alexakis, A.E.; Malmstrom, E.; Mckee, L.S. Structure-property relationship of native-like lignin nanoparticles from softwood and hardwood. Ind. Crops Prod. 2023, 206, 117660. [Google Scholar] [CrossRef]
- Wang, H.; Han, H.; Sun, E.; Han, Y.; Zhang, Y.; Li, J.; Chen, Y.; Song, H.; Zhao, H.; Kang, Y. Production of aryl oxygen-containing compounds by the pyrolysis of bagasse alkali lignin catalyzed by LaM0.2Fe0.8O3 (M = Fe, Cu, Al, Ti). Energy Fuels 2019, 33, 8596–8605. [Google Scholar] [CrossRef]
- Rui, K.; Mittal, A.; Mckinney, K.; Chen, X.; Tucker, M.P.; Johnson, D.K.; Bechham, G.T. Base-catalyzed depolymerization of biorefinery lignins. ACS Sustain. Chem. Eng. 2016, 4, 1474–1486. [Google Scholar]
- Kong, L.; Dai, L.; Wang, Y. Enhancing aromatic hydrocarbon formation via catalytic depolymerization of lignin waste over Ru/WOx/N-C catalyst. Fuel 2023, 332, 126263. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, X.; Guo, Y.; Wang, Y. Self-hydrogen supplied catalytic fractionation of raw biomass into lignin-derived phenolic monomers and cellulose-rich pulps. J. Am. Chem. Soc. Au 2023, 3, 1911–1917. [Google Scholar] [CrossRef]
- Guo, X.; Hong, P.; Yao, L.; Liu, X.; Jiang, Z.; Shi, B. Catalytic hydrogenation of lignin-derived aldehydes over bimetallic PdNi/hydrotalcite catalyst under mild conditions. Fuel 2023, 353, 129231. [Google Scholar] [CrossRef]
- Sreenavya, A.; Aswin, P.; Ganesh, V.; Venkatesha, N.; Sakthivel, A. Facile water-free synthesis of noble metal-containing hydrotalcitesderived materials and their application for hydrotreatment of anisole. Mater. Today Sustain. 2022, 18, 100153. [Google Scholar]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef]
- Chaudhary, R.; Dhepe, P.L. Solid base catalyzed depolymerization of lignin into low molecular weight products. Green Chem. 2016, 19, 778–788. [Google Scholar] [CrossRef]
- Limarta, S.O.; Ha, J.M.; Park, Y.K.; Li, H.; Suh, D.J.; Jae, J. Efficient depolymerization of lignin in supercritical ethanol by a combination of metal and base catalysts. J. Ind. Eng. Chem. 2018, 57, 45–54. [Google Scholar] [CrossRef]
- Long, J.; Zhang, Q.; Wang, T.; Zhang, X.; Xu, Y.; Ma, L. An efficient and economical process for lignin depolymerization in biomass-derived solvent tetrahydrofuran. Bioresour. Technol. 2014, 154, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yue, H.; Zou, J.; Yao, R.; Duan, W.; Ma, H.; Zhao, Y.; He, Z. Oxidative lignin depolymerization using metal supported hydrotalcite catalysts: Effects of process parameters on phenolic compounds distribution. Fuel 2023, 331, 125805. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Shu, F.; Gao, Y.; Long, J. Hydrodeoxygenation of heavy lignin bio-oil to oxygenated fuel catalyzed by CuxNiy/MgO. Fuel 2024, 357, 129805. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Liu, H.; Ao, L.; Liu, K.; Guan, Y.; Zai, S.; Chen, S.; Zong, Z.; Wei, X. Supercritical ethanolysis of wheat stalk over calcium oxide. Renew. Energy 2018, 120, 300–305. [Google Scholar] [CrossRef]
- Fasolini, A.; Spennati, E.; Atakoohi, S.E.; Percivale, M.; Busca, G.; Basile, F.; Garbarino, G. A study of CO2 hydrogenation over Ni-MgAlOx catalysts derived from hydrotalcite precursors. Catal. Today 2023, 423, 114271. [Google Scholar] [CrossRef]
- Rodríguez, A.; Fernandez, L.; Domínguez, J.R.; González, G.; Martínez, O.; Espinoza-Montero, P. Mg and Ni nano-hydrotalcites modified with gold nanoparticles as platform of enzymatic electrochemical sensors for H2O2 detection. Sens. Bio-Sens. Res. 2021, 33, 100446. [Google Scholar] [CrossRef]
- Kameda, T.; Fubasami, Y.; Uchiyama, N.; Yoshioka, T. Elimination behavior of nitrogen oxides from a NO3−-intercalated Mg-Al layered double hydroxide during thermal decomposition. Thermochim. Acta 2010, 499, 106–110. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Yu, J. Influences of polyhydric alcohol co-solvents on the hydration and thermal stability of MgAl-LDH obtained via hydrothermal synthesis. Appl. Clay Sci. 2013, 72, 37–43. [Google Scholar] [CrossRef]
- Yang, F.; He, G. Preparation of granular NiO for the electrochemical performance and CO2 adsorption performance. Chem. Ind. Eng. Prog. 2023, 42, 907–916. [Google Scholar]
- Luo, X.; Guo, L.; Wei, Q.; Xu, J.; Wang, K.; Lu, B. Influence of crystallinity and binder on the energy delivery efficiency for porous magnesium cobaltate supercapacitor electrodes. Chin. J. Inorg. Chem. 2018, 34, 823–833. [Google Scholar]
- Sukpanish, P.; Lertpanyapornchai, B.; Yokoi, T.; Ngamcharussrivichai, C. Lanthanum-doped mesostructured strontium titanates synthesized via sol-gel combustion route using citric acid as complexing agent. Mater. Chem. Phys. 2016, 181, 422–431. [Google Scholar] [CrossRef]
- Chen, H.; Wageh, S.; Al-Ghamdi, A.A.; Wang, H.; Yu, J.; Jiang, C. Hierarchical C/NiO-ZnO nanocomposite fibers with enhanced adsorption capacity for Congo red. J. Colloid Interface Sci. 2019, 537, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Pi, M.; Xu, D.; Jiang, C.; Cheng, B. Fabrication of hierarchical porous ZnO-Al2O3 microspheres with enhanced adsorption performance. Appl. Surf. Sci. 2017, 426, 360–368. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Z.; Yuan, T. Recent advances in the catalytic upgrading of biomass platform chemicals via hydrotalcite-derived metal catalysts. Trans. Tianjin Univ. 2022, 2, 89–111. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Zhou, B.; Meng, F.; Zhao, Z.; Wei, C.; Zhou, L.; Wen, G.; Zhang, X. Layered double oxide (CoAl-LDO) catalysis for enhanced ozonation of methyl orange: Performance assessment and mechanistic insights. J. Mol. Liq. 2024, 404, 124995. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Mageste, A.B.; Dias, A.; Virtuoso, L.S.; Siqueira, K. Layered double hydroxides for remediation of industrial wastewater containing manganese and fluoride. J. Clean. Prod. 2018, 171, 275–284. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Wang, X.; Gao, W.; Wang, Z.; Zhang, X.; Wang, Y.; Liu, W. The influence of anionic surfactant (SDS) on the optical, magnetic and capacitive properties of NiO nanocrystals via gas-liquid diffusion synthesis. Mater. Sci. Eng. B 2024, 299, 116970. [Google Scholar] [CrossRef]
- Panigrahi, U.K.; Das, P.K.; Biswal, R.; Sathe, V.; Babu, P.D.; Mitra, A.; Mallick, P. Zn doping induced enhancement of multifunctional properties in NiO nanoparticles. J. Alloys Compd. 2020, 833, 155050. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Lin, S.; Yan, L.; Cao, R.; Yang, R.; Liang, X.; Xiang, W. Sol-gel synthesis of NiO nanoparticles doped sodium borosilicate glass with third-order nonlinear optical properties. J. Alloys Compd. 2016, 686, 564–570. [Google Scholar] [CrossRef]
- Choi, S.; Balamurugan, M.; Lee, K.G.; Cho, K.H.; Park, S.; Seo, H.; Nam, K.T. Mechanistic investigation of biomass oxidation using nickel oxide nanoparticles in a CO2-saturated electrolyte for paired electrolysis. J. Phys. Chem. Lett. 2020, 11, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Y.; Cheng, J.; Zhang, M. Promotional Effect of Ni-Co/TiO2 Catalysts on CO2 Hydrogenation. Chem. J. Chin. Univ. 2023, 44, 20230308. [Google Scholar]
- Li, J.; Liu, H.; Liu, Z.; Yang, D.; Zhang, M.; Gao, L.; Zhou, Y.; Lu, C. Facile synthesis of Z-scheme NiO/a-MoO3 p-n heterojunction for improved photocatalytic activity towards degradation of methylene blue. Arab. J. Chem. 2022, 15, 103513. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Li, S.; Zhang, Y.; Xu, C.; Chen, H. Hydrothermal synthesis of flower-like MgCo2O4 porous microstructures as high-performance electrode material for asymmetric supercapacitors. J. Alloys Compd. 2020, 824, 153939. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Xie, Z.; Wang, Y.; Wang, Y.; Wei, B. Tracking the structural evolution and activity origin of Co-doped NiFe layered double hydroxide for enhanced oxygen evolution reaction. Chem. Eng. J. 2024, 488, 151086. [Google Scholar] [CrossRef]
- Qian, G.; Lyu, W.; Zhao, X.; Zhou, J.; Fang, R.; Wang, F.; Li, Y. Efficient photoreduction of diluted CO2 to tunable syngas by Ni-Co dual sites through d-band center manipulation. Angew. Chem. Int. Ed. 2022, 61, e202210576. [Google Scholar] [CrossRef]
- Han, H.; Li, J.; Wang, H.; Han, Y.; Chen, Y.; Li, J.; Zhang, Y.; Wang, Y.; Wang, B. One-step valorization of calcium lignosulfonate to produce phenolics with the addition of solid base oxides in the hydrothermal reaction system. Energy Fuels 2019, 33, 4302–4309. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- del Río, J.C.; Lino, A.G.; Colodette, J.L.; Lima, C.F.; Gutierrez, A. Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenergy 2015, 81, 322–338. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wan, P.; Han, Y.; Qin, T. Degradation behaviors of lignin catalyzed by CaO/MgO composite as solid base catalyst. Chem. Ind. For. Prod. 2012, 32, 81–86. [Google Scholar]
- Javier, F.-R.; Xabier, E.; Fabio, H.-R.; Oihana, G.; Maria, G.A.; Jalel, L. Direct lignin depolymerization process from sulfur-free black liquors. Fuel Process. Technol. 2020, 197, 106201. [Google Scholar]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kornáyi, T.I.; Boot, M.D.; Hensen, E. Catalytic depolymerization of lignin in supercritical ethanol. Chemsuschem 2014, 7, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Lui, M.Y.; Masters, A.F.; Maschmeyer, T.; Yuen, A.K.L. Molybdenum carbide, supercritical ethanol and base: Keys for unlocking renewable BTEX from lignin. Appl. Catal. B Environ. 2023, 325, 122351. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Jiang, W.; Yu, T.; Tan, K.; Yin, S. Strong electronic coupling of CoNi and N-doped-carbon for efficient urea-assisted H2 production at a large current density. Carbon Energy 2023, 5, e368. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, D.; Wang, M.; Wang, Y. Study on catalytic performance of Ni-Co-P amorphous alloy for HDO of vanillin. J. Fuel Chem. Technol. 2019, 47, 1205–1213. [Google Scholar] [CrossRef]
- Fu, Q.; Yan, L.; Liu, D.; Zhang, S.; Jiang, H.; Xie, W.; Yang, L.; Wang, Y.; Wang, H.; Zhao, X. Highly-dispersed surface NiO species and exposed Ni (200) facets facilitating activation of furan ring for high-efficiency total hydrogenation of furfural. Appl. Catal. B Environ. 2024, 343, 123501. [Google Scholar] [CrossRef]
- Campisi, S.; Chan-Thaw, C.E.; Chinchilla, L.E.; Chutia, A.; Botton, G.A.; Mohammed, K.M.H.; Dimitratos, N.; Wells, P.P.; Villa, A. Dual-site-mediated hydrogenation catalysis on Pd/NiO: Selective biomass transformation and maintenance of catalytic activity at low Pd loading. ACS Catal. 2020, 10, 5483–5492. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, X.; Yang, K.; Xu, Y.; Lu, J.; Li, W.; Li, B.; Xue, C.; Liu, Y. Efficient and low-cost Ni-NiO/Al2O3 catalysts with dual-active-sites for selective catalytic conversion of phthalic anhydride to phthalide. Chem. Eng. J. 2024, 497, 154753. [Google Scholar] [CrossRef]
- JF 1164–2018; Calibration Specificationfor Gas Chromatography-MassSpectrometries. National Metrology Technical Specification of China. National Institute of Metrology (NIM) of China: Beijing, China, 2018.

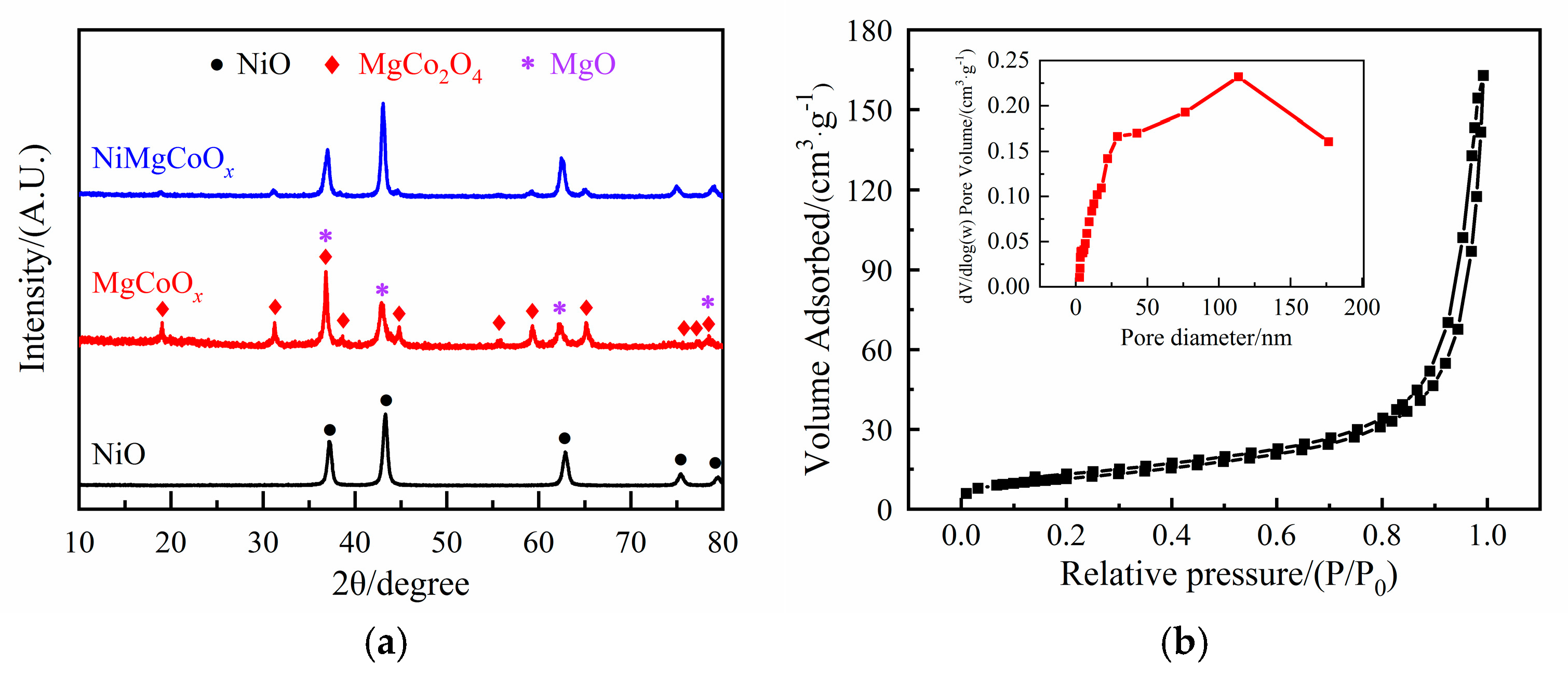
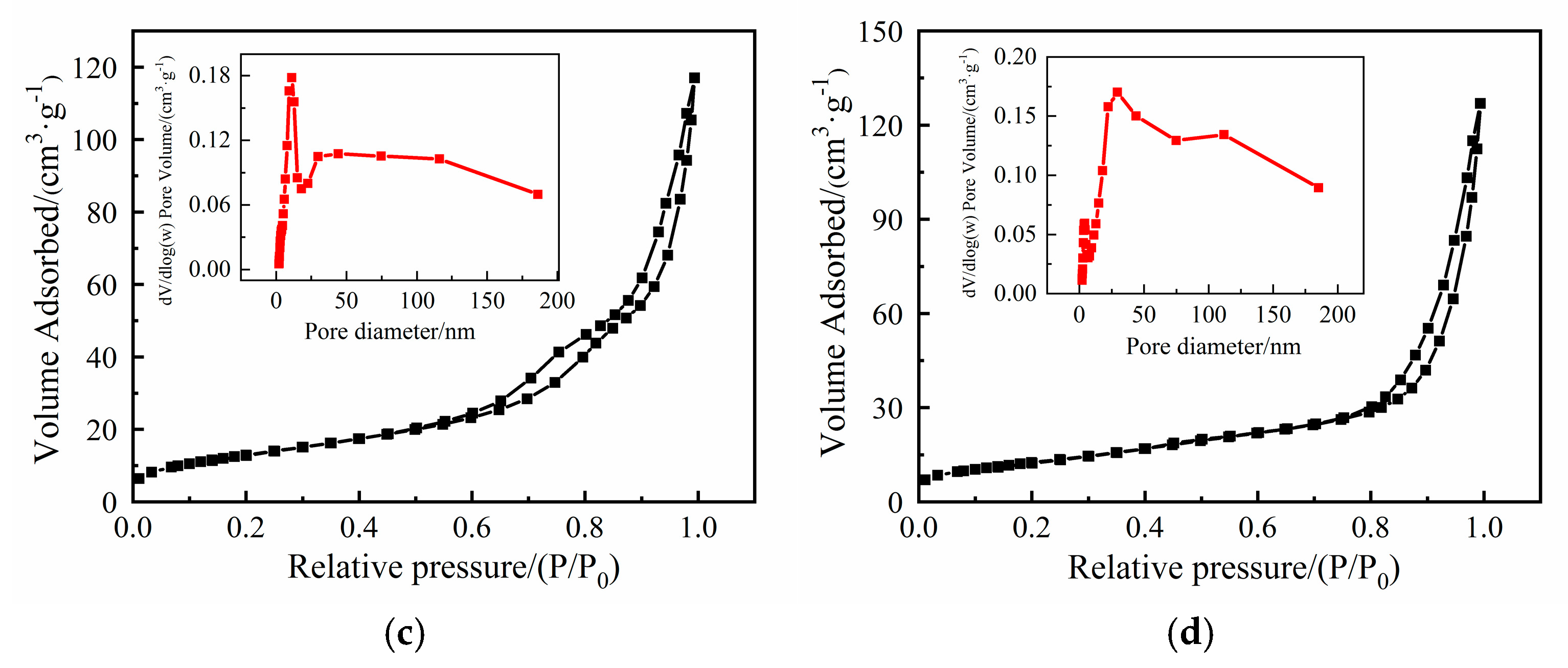




| Sample | Specific Surface Area SBET/(m2·g−1) | Pore Volume V/(cm3·g−1) | Pore Size d/nm |
|---|---|---|---|
| NiO | 41 | 0.252 | 24.3 |
| MgCoOx | 48 | 0.181 | 15.0 |
| NiMgCoOx | 46 | 0.196 | 17.1 |
| Residence Time/Min | Component | Type | Percent/% | |||
|---|---|---|---|---|---|---|
| No Catalyst | NiO | MgCox | NiMgCoOx | |||
| 3.93 | Cyclopentanol | Alcohol | 0 | 0.11 | 0.08 | 0.12 |
| 5.11 | Cyclopentanol, 2-methyl-, trans | Alcohol | 0 | 0.16 | 0 | 0 |
| 5.47 | Benzene, ethyl- | Aromatic hydrocarbon | 0.11 | 0.12 | 0.26 | 0.21 |
| 5.64 | Benzene, 1,4-dimethyl | Aromatic hydrocarbon | 0.89 | 1.00 | 1.34 | 3.17 |
| 6.03 | Benzene, 1,3-dimethyl | Aromatic hydrocarbon | 0.25 | 0.29 | 0.65 | 0.50 |
| 6.19 | Butanoic acid, 2-hydroxy-, ethyl ester | Ester | 1.03 | 0.24 | 0.00 | 0.00 |
| 6.30 | Furan, 2-ethyl | Furan | 0 | 0.22 | 0.10 | 0.21 |
| 6.87 | Furan, tetrahydro-2,5-dimethyl-, trans- | Furan | 0.26 | 0.18 | 0 | 0.23 |
| 7.11 | 3-Pentanol | Alcohol | 1.06 | 0 | 0 | 0 |
| 7.22 | phenol | Phenolic | 9.31 | 12.06 | 14.23 | 14.40 |
| 7.33 | Cyclohexanone, 4-hydroxy | Phenolic | 0 | 0.65 | 1.07 | 1.10 |
| 7.87 | benzyl alcohol | Aromatic alcohol | 0.63 | 0.37 | 0.33 | 0.40 |
| 8.04 | Phenol, 4-methyl | Phenolic | 0.67 | 0.27 | 0.28 | 0.26 |
| 8.24 | Phenol, 3-methyl | Phenolic | 0.77 | 0.91 | 0.92 | 0.70 |
| 8.36 | Phenol, 2-methoxy | Phenolic | 7.65 | 13.07 | 14.04 | 16.14 |
| 8.51 | 2(3H)-Furanone, dihydro-3,3-dimethyl | Furan | 1.68 | 0 | 0 | 0 |
| 8.77 | Phenol, 2-ethyl | Phenolic | 0 | 0.93 | 0.81 | 0.12 |
| 8.79 | Benzenemethanol, 4-methyl | Aromatic alcohol | 0.75 | 0 | 0 | 0 |
| 8.85 | Benzenemethanol, 2-methyl | Phenolic | 0.75 | 0.80 | 0.45 | 0.64 |
| 9.00 | Phenol, 4-ethyl | Phenolic | 3.64 | 3.91 | 3.12 | 3.14 |
| 9.1 | Phenol, 2-methoxy-4-methyl | Guaiacol | 0 | 0.56 | 0.27 | 0.32 |
| 9.2 | 2-Methoxy-5-methylphenol | Guaiacol | 4.24 | 4.64 | 5.01 | 6.05 |
| 9.28 | 3-Hexenoic acid, ethyl ester | Ester | 1.58 | 0 | 0 | 0 |
| 9.43 | Phenol, 4-(1-methylethyl)- | Phenolic | 0 | 0.29 | 0.35 | 0 |
| 9.67 | Phenol, 4-ethyl-2-methoxy | Guaiacol | 1.92 | 1.27 | 1.79 | 1.57 |
| 9.77 | Benzene, 1,4-dimethoxy-2-methyl | Aromatic ether | 6.67 | 9.52 | 9.61 | 7.89 |
| 9.88 | 5-Oxotetrahydrofuran-2-carboxylic acid, ethyl este | Ether | 0.73 | 0 | 0 | 0 |
| 9.94 | 1,2,3-Trimethoxybenzene | Aromatic ether | 0.36 | 0.50 | 0.44 | 0.48 |
| 10.12 | 2,4,6-Trimethylbenzyl alcohol | Alcohol | 0.46 | 0.62 | 0.26 | 0.39 |
| 10.19 | Phenol, 2,6-dimethoxy | Syringol | 10.98 | 11.43 | 12.64 | 11.61 |
| 10.30 | Phenol, 2-methoxy-4-propyl | Guaiacol | 1.25 | 0.86 | 1.08 | 0.93 |
| 10.39 | Propanoic acid, 2-methyl-, anhydride | Ether | 1.38 | 0 | 0 | 0 |
| 10.52 | Vanillin | Guaiacol | 0.79 | 0.92 | 1.03 | 0.99 |
| 10.63 | 3-tert-Butyl-4-hydroxyanisole | Phenolic | 0.13 | 0.43 | 0.27 | 0.30 |
| 10.71 | 1,2,4-Trimethoxybenzene | Aromatic ether | 6.04 | 4.22 | 3.01 | 2.31 |
| 10.77 | Phenol, 2-methoxy-4-(1-propenyl)- | Guaiacol | 0 | 0 | 0.48 | 0.40 |
| 10.97 | Ethanone, 1-(4-hydroxy-3-methoxyphenyl)- | Guaiacol | 4.91 | 3.35 | 2.69 | 3.56 |
| 11.1 | Benzene, 1,2,3-trimethoxy-5-methyl | Aromatic ether | 9.43 | 5.87 | 5.47 | 3.11 |
| 11.2 | Phenol, 2-methoxy-4-propyl | Guaiacol | 1.12 | 1.43 | 1.98 | 1.84 |
| 11.26 | Benzeneacetic acid, 4-hydroxy-3-methoxy | Guaiacol | 1.74 | 1.69 | 1.27 | 1.88 |
| 11.45 | Vanillic acid, methyl ester | Aromatic ether | 0 | 0.32 | 0.43 | 0.54 |
| 11.51 | Benzenemethanol, 2,4-dimethoxy | Aromatic ether | 0.93 | 0.88 | 1.20 | 1.34 |
| 11.67 | Benzeneacetic acid, 4-hydroxy-3-methoxy | Guaiacol | 0.13 | 0 | 0 | 0 |
| 11.79 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy | Syringol | 2.69 | 1.60 | 1.19 | 0.73 |
| 12.1 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | Syringol | 8.30 | 7.42 | 5.71 | 5.35 |
| 12.33 | 3,5-Dimethoxy-4-hydroxyphenylacetic acid | Syringol | 0 | 1.22 | 1.28 | 1.16 |
| 12.41 | 2-Cyclopenten-1-one, 4-hydroxy-3-methyl-2-phenyl | Ketone | 0 | 0.20 | 0.31 | 0.36 |
| 12.51 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | Syringol | 0.25 | 0.33 | 0.32 | 0.34 |
| 12.78 | Ethanone, 1-(2,5-dimethoxyphenyl)- | Aromatic ether | 0.81 | 0 | 0 | 0 |
| 13.11 | 1,2-Benzenedicarboxylic acid, dibutyl ester | Aromatic ester | 3.71 | 5.14 | 3.99 | 5.21 |
| 13.26 | 9H-Fluoren-9-one, 2,3-dimethyl | Ketone | 0 | 0 | 0.24 | 0 |
| Summary | Esters | 2.61 | 0.24 | 0 | 0 | |
| Alcohols | 1.51 | 0.88 | 0.34 | 0.50 | ||
| Furans | 1.94 | 0.40 | 0.10 | 0.45 | ||
| Ethers | 2.11 | 0 | 0 | 0 | ||
| Ketones | 0 | 0.20 | 0.56 | 0.36 | ||
| Aromatic hydrocarbons (AH) | 1.24 | 1.41 | 2.23 | 3.88 | ||
| Aromatic ethers (AEthers) | 24.25 | 21.34 | 20.17 | 15.68 | ||
| Aromatic esters (AEsters) | 3.71 | 5.14 | 4.00 | 5.21 | ||
| Aromatic alcohols (AA) | 1.38 | 0.37 | 0.33 | 0.40 | ||
| Phenolics (H) | 22.92 | 33.30 | 35.53 | 36.77 | ||
| Guaiacols (G) | 16.10 | 14.71 | 15.59 | 17.55 | ||
| Syringols (S) | 22.23 | 22.01 | 21.15 | 19.19 | ||
| Total content of phenolic compounds (H + G + S) | 61.25 | 70.02 | 69.27 | 74.52 | ||
| Sample | Mn (g·mol−1) | Mw (g·mol−1) | PD | ||
|---|---|---|---|---|---|
| CLS | 1300 | 7917 | 6.08 | ||
| Solid products obtained after catalytic depolymerization of CLS | No catalyst | R1 | 982 | 1124 | 1.14 |
| R2 | 944 | 1051 | 1.11 | ||
| NiO | R1 | 795 | 1812 | 2.28 | |
| R2 | 735 | 1701 | 2.31 | ||
| MgCoOx | R1 | 761 | 875 | 1.15 | |
| R2 | 703 | 812 | 1.16 | ||
| NiMgCoOx | R1 | 670 | 706 | 1.05 | |
| R2 | 617 | 669 | 1.08 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, Y.; Zhang, W.; Wang, Y.; Han, H.; Chen, Y.; Zhang, J.; Zhang, Y. Depolymerization of Lignosulfonate Catalyzed by Different Solid Base Oxides to Prepare Phenolic Compounds. Catalysts 2024, 14, 781. https://doi.org/10.3390/catal14110781
Wang H, Wang Y, Zhang W, Wang Y, Han H, Chen Y, Zhang J, Zhang Y. Depolymerization of Lignosulfonate Catalyzed by Different Solid Base Oxides to Prepare Phenolic Compounds. Catalysts. 2024; 14(11):781. https://doi.org/10.3390/catal14110781
Chicago/Turabian StyleWang, Haiying, Yejing Wang, Wencheng Zhang, Yizhen Wang, Hongjing Han, Yanguang Chen, Jiaren Zhang, and Yanan Zhang. 2024. "Depolymerization of Lignosulfonate Catalyzed by Different Solid Base Oxides to Prepare Phenolic Compounds" Catalysts 14, no. 11: 781. https://doi.org/10.3390/catal14110781
APA StyleWang, H., Wang, Y., Zhang, W., Wang, Y., Han, H., Chen, Y., Zhang, J., & Zhang, Y. (2024). Depolymerization of Lignosulfonate Catalyzed by Different Solid Base Oxides to Prepare Phenolic Compounds. Catalysts, 14(11), 781. https://doi.org/10.3390/catal14110781







