Abstract
Lignin is the most abundant aromatic renewable polymer in nature. However, its very stable structure limits its widespread application. To achieve high-value utilization of lignin, this study used solid base oxides to depolymerize calcium lignosulfonate (CLS) for the synthesis of phenolic compounds. The catalyst precursors were prepared by hydrothermal synthesis, and the corresponding mesoporous metal oxides NiO, MgCoOx, and NiMgCoOx were obtained after calcination. MgCoOx and NiMgCoOx had similar but stronger basicity compared to NiO. While all oxides promoted the depolymerization of CLS, NiMgCoOx was identified as the best catalyst, achieving a maximum liquid product yield of 74.3 wt.% and a selectivity of phenolic compounds of 74.52% in the liquid product. In addition, NiMgCoOx showed satisfactory structural and catalytic stability. The experimental results indicated that solid base oxides can capture the active hydrogen in CLS, causing the hydrolysis reaction of ether bonds, and the resulting products continuously depolymerize or polymerize; Co present in the catalyst promotes the adsorption of hydrogen by Ni, while NiO in NiMgCoOx facilitates the adsorption of both reactants and hydrogen. The combination of Ni and Co improves hydrogenation performance.
1. Introduction
The energy crisis and environmental pollution caused by fossil fuel consumption have attracted special attention on a global level. Using renewable energy to replace traditional fossil-based energy is an effective way to solve these problems [1]. As one of the three major components of lignocellulosic biomass, lignin accounts for about 15–40% of the total biomass, depending on the species [2]. The oxygen content in lignin is high (approximately 30%), resulting in a low calorific value, for which reason the direct combustion of lignin not only wastes precious resources but also causes serious environmental pollution. Lignin is a complex phenolic polymer formed by three kinds of phenylpropane structural units (phenyl, syringyl, and guaiacyl) through C-O-C or C-C bonds; therefore, lignin is the only potential natural source for producing aromatic compounds [3]. Manufacturing phenolic chemicals from lignin can assist in reducing the waste produced by discarding or burning lignin and in solving the problem of fossil resource shortage.
In recent years, the hydrothermal conversion of lignin has become a hot research topic to realize its utilization [4]. Kong et al. [5] discovered that lignin can be rapidly hydrodeoxygenated on a Ru/WOx/N-C catalyst with a hierarchical pore structure. After the depolymerization reaction, the bio-oil and monomer yields reached 96.89 wt.% and 36.85 wt.%, respectively, while the aromatic hydrocarbon yield was 20.85 wt.%. Zhou et al. [6] used Pt/NiAl2O4 to convert lignocellulosic biomass into lignin oils without the need for exogenous hydrogen, but the reaction conditions were harsh. The yield of phenolic monomers was 46.6 wt.%. Other commonly used precious metal catalysts include Pd [7], Rh and Ir [8]. These catalysts exhibit satisfactory effects, but the costs are high.
Acid or base catalysts are normally used to improve the conversion of lignin and the selectivity of the target product. Mahmood et al. [9] used H2SO4 and NaOH as catalysts to depolymerize hydrolysis lignin at 250 °C for 1 h in a 50/50 (v/v) water–ethanol solvent, reaching maximum liquid product yields of 75.2 wt.% and 66.5 wt.%, respectively. For soluble acid or base catalysts, however, the separation of the catalyst from the products is a problem and a serious obstacle to the industrialization of lignin hydrolysis. Using solid acid or base oxides instead of soluble catalysts is an effective method to solve the separation problem [10,11]. Long et al. [12] adopted MgO as a base catalyst to depolymerize lignin in tetrahydrofuran (THF), and the yield of monophenolic compounds was approximately 13.2 wt.%. Zhang et al. [13] found that Co/MgAl2O4 had a better catalytic depolymerization effect than Ni/MgAl2O4, and the yield of liquid products reached 72 wt.%. The bonds connecting structural units are quite complex in lignin; thus, simple catalysts cannot meet the demands of high-efficiency lignin depolymerization. The development of multifunctional base catalysts has become a focus point to improve the conversion rate of lignin. Li et al. [14] used CuNi/MgO to catalyze lignin depolymerization, and obtained high-quality bio-oil whose heating value was 31.4 MJ·kg−1. It was found that the alkaline MgO can restrain the recondensation of phenolic oligomers effectively. When depolymerizing wheat stalk in supercritical ethanol [15], the conversion increased from 12.0% to 29.0% and 42.4% using CaO/Al2O3 or CaO/Al2O3/Fe3O4 as a catalyst, respectively. Based on these results, the combination of appropriate active metals and alkali can effectively catalyze the depolymerization of lignin. Although traditional supported solid base catalysts have satisfactory basicity, the reduction in specific surface area and pore size caused by loading results in unsatisfactory lignin depolymerization. Composite metal oxides are alkaline and have good specific surface areas and pore sizes, so they are promising catalysts for lignin depolymerization.
Calcium lignosulfonate (CLS) is the main component of sulfite pulping waste liquid, and its discharge causes water pollution and resource wastage. To realize resource recovery and high-value utilization of CLS, how to depolymerize it to produce high-value-added fine chemicals has attracted wide attention. Our work aims to provide a theoretical basis for the conversion of CLS to phenols by studying the synergistic catalysis of Ni and MgCoOx.
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. Characterization of the Precursors
The X-ray diffraction (XRD) patterns of the precursors are presented in Figure 1a. According to Figure 1, hexagonal Ni(OH)2 (JCPDS No. 73-1520) was formed, and the characteristic diffraction peaks at 19.2°, 33.2°, 38.6°, 52.2°, 59.2°, 62.9°, 69.7, 70.3°, 73.0° and 73.2° corresponded to the crystal planes (001), (100), (011), (012), (110), (111), (200), (103), (201), and (112), respectively. The MgCo layered double hydroxide (MgCo-LDH) precursor showed characteristic diffraction peaks at 11.3°, 22.6°, 34.3°, 37.2°, 46.1°, 60.0°, and 61.3° [16], corresponding to the crystal planes (003), (006), (009), (015) (018), (110), and (113), respectively [17], and no other phases were detected. Weak diffraction peaks of Ni(OH)2 were observed at 19.2° and 38.6° in the XRD pattern of the NiMgCo-LDH precursor, indicating that the layered hydrotalcite MgCo-LDH was synthesized next to a small amount of Ni(OH)2.
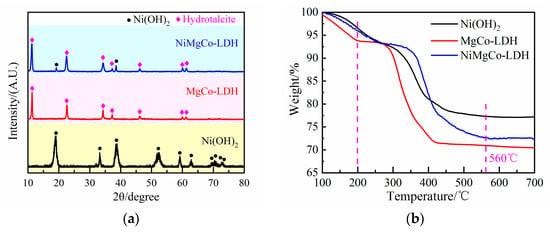
Figure 1.
(a) XRD patterns and (b) TG curves of the precursors.
The thermogravimetry (TG) curves of the precursors are shown in Figure 1b. As the temperature increased, the precursor degradation process followed three stages [18,19]: (1) below 200 °C, the materials lost absorbed water and interlayer water; (2) in the temperature range of 200–560 °C, brucite-like octahedral layers in NO3·Ni-Mg-Al LDHs dehydroxylated and CO2 was lost from the intermediate layers; (3) above 560 °C, the decomposition process was finished, and no further weight loss occurred. Therefore, the calcination temperature of the precursors is 600 °C.
2.1.2. Characterization of the Solid Base Oxides
The solid base oxides were obtained after calcination of the precursors, and the XRD patterns are shown in Figure 2a. Cubic NiO (JCPDS No. 78-0423) was formed by Ni(OH)2 calcination, and no impurity peaks were present in the XRD spectrum; the characteristic diffraction peaks at 37.3°, 43.3°, 62.9°, 75.5°, and 79.4° corresponded to the crystal planes (111), (200), (220), (311), and (222), respectively [20]. The calcination of MgCo-LDH resulted in cubic MgCo2O4 (JCPDS No. 81-0667) [21], and the peaks at 19.0°, 31.2°, 36.8°, 38.5°, 44.8°, 55.5°, 59.3°, 65.1°, 74.0°, 77.2°, and 78.3° belonged to the (111), (220), (311), (200), (400), (422), (511), (440), (600), (533), and (622) crystal planes of this spinel, respectively. Cubic MgO (JCPDS No. 45-0946) also formed, and the peaks observed at 36.9° (very small peak intensity), 42.9°, 62.3°, 74.7°, and 78.6° correspond to the (111), (200), (220), (311), and (222) crystal planes. Furthermore, the XRD results revealed that the calcination of NiMgCo-LDHs led to the formation of cubic MgCo2O4, MgO, and NiO.

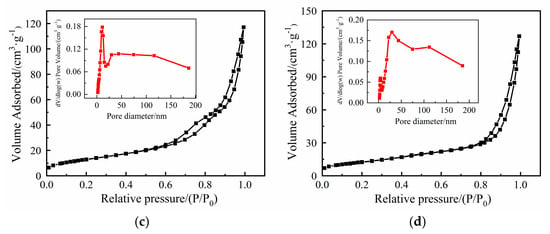
Figure 2.
(a) XRD patterns of NiO, MgCoOx, and NiMgCoOx; N2 adsorption–desorption curves of (b) NiO, (c) MgCoOx, and (d) NiMgCoOx.
According to the IUPAC classification, NiO, MgCoOx, and MgCoOx exhibited type IV N2 adsorption–desorption isotherms, typical of mesoporous materials [22]. The isotherms had H3 hysteresis loops, illustrating the existence of slit-like pores associated with nanosheet aggregates [23]. The sharply increasing hysteresis loops close to P/P0 = 1 indicated the existence of macropores (>50 nm) [24], which was consistent with the pore size distribution curves. According to Table 1, the difference in specific surface area (SBET) of the samples was not significant. SBET decreased after Mg in MgCoOx was partially replaced by Ni, which due to a small amount of NiO was generated after calcination, blocking the pores of the hydrotalcite-based oxides.

Table 1.
Specific surface area and pore structure parameters determined by N2 adsorption–desorption.
The scanning electron microscopy (SEM) images of the precursors and the oxides are shown in Figure 3a–f. The morphology of Ni(OH)2 was irregular hexagonal, and granular NiO was obtained after calcination of Ni(OH)2. The layer structures of the hydrotalcite precursors, MgCo-LDH and NiMgCo-LDH, were clear and uniform, and after calcination, the formed oxides, MgCoOx and NiMgCoOx, had structures similar to those of the precursors [25]. Maintaining the precursor structures was attributed to the “memory effect” and “size effect” of the hydrotalcite [26], and some studies reported that hydrotalcite-based solid base oxides have stronger adsorption capacity than the hydrotalcites because of the synergy of the two effects [27]. The morphology of the samples presented in the TEM images in Figure 3g–i was consistent with that observed in the SEM images, and their lattice stripe arrangement exhibited polycrystalline characteristics. The NiO particles were tens of nanometers in size, which was close to the size of NiO particles reported in previous studies [28,29]. The crystal planes of the samples corresponding to the calculated lattice spacing are labeled in the HRTEM images as shown in Figure 3j–l.
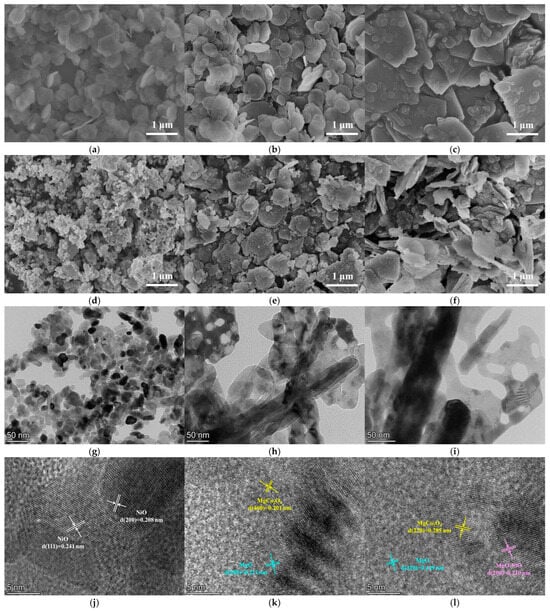
Figure 3.
SEM images of (a) Ni(OH)2, (b) MgCo-LDH, and (c) NiMgCo-LDH; SEM images of (d) NiO, (e) MgCoOx, and (f) NiMgCoOx; TEM images of (g) NiO, (h) MgCoOx, and (i) NiMgCoOx; HRTEM images of (j) NiO, (k) MgCoOx, and (l) NiMgCoOx.
The valence of the elements present in the oxides was determined by XPS, as shown in Figure 4. The Ni 2p XPS spectrum of prepared NiO in Figure 4a was completely consistent with that reported in the literature [30,31]. The peaks at 853.9 eV, 855.1 eV, and 872.80 eV were assigned to Ni 2p3/2 and Ni 2p1/2, respectively, and the peaks at 860.9 eV and 878.9 eV were satellite peaks [32], suggesting that Ni2+ ions were dominant in the product [33].

Figure 4.
(a) Ni 2p XPS analysis of NiO and NiMgCoOx; (b) Co 2p XPS analysis of MgCoOx and NiMgCoOx.
The Co 2p XPS spectra in Figure 4b included two strong peaks corresponding to Co 2p1/2 and Co 2p3/2. The fitted peaks at binding energies of 796.7 eV and 781.1 eV belonged to Co2+, and the peaks at 794.8 eV and 779.7 eV were attributed to Co3+. The remaining two weak peaks centered at 786.2 eV and 803.0 eV were identified as satellites. These XPS results proved the spinel structure of cobalt [34], which was consistent with the results of the XRD investigation, presented in Figure 2a. Comparing the areas of corresponding sub-peaks revealed that the ratio of Co3+ to Co2+, and therefore the average valence of Co, was higher in NiMgCoOx than MgCoOx. The interaction between Ni and Co leads to charge redistribution [35], resulting in an increase in the valence of Co [36].
2.1.3. Basicity of the Solid Base Oxides
The basicities of solid base oxides were analyzed by the temperature-programmed desorption of CO2 (CO2-TPD), as shown in Figure 5. The desorption peaks below 300 °C were attributed to weak basic sites, the peaks between 300 °C and 600 °C belonged to medium–strong basic sites, and those above 600 °C were attributed to strong basic sites. Only one weak desorption peak at about 280 °C was observed for NiO. The type and content of base sites in MgCoOx and NiMgCoOx were similar, indicating that the addition of Ni had no significant effect on the basicity of the material. The acidity of the oxides determined by NH3-TPD was shown in Figure S1.
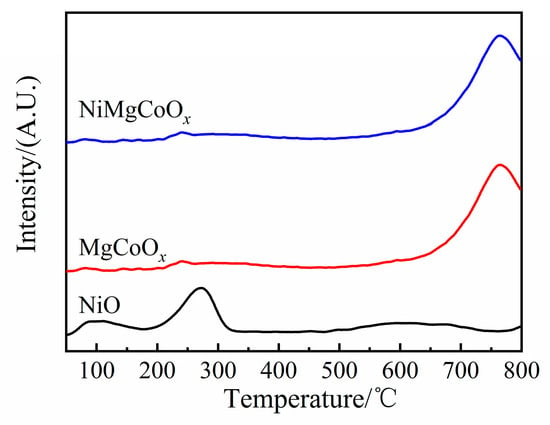
Figure 5.
CO2-TPD of NiO, MgCoOx, and NiMgCoOx.
2.2. Base-Catalyzed Depolymerization of CSL
2.2.1. Product Yields
The yields of gaseous, liquid, and solid (R1 and R2) products obtained from the depolymerization of CSL catalyzed by NiO, MgCoOx, and NiMgCoOx are shown in Figure 6. With the addition of solid base oxides, the yield of liquid products increased from no catalyst (45.0 wt.%) in the order NiO (49.3 wt.%), MgCoOx (57.4 wt.%), and NiMgCoOx (74.3 wt.%), while the yield of the solid residue R1 decreased in the same order. There was no significant difference in the yield of gaseous products between the catalysts.

Figure 6.
Distribution of gaseous, liquid, and solid products obtained from CSL depolymerization without catalyst or catalyzed by NiO, MgCoOx, and NiMgCoOx.
2.2.2. Composition of Gaseous and Liquid Products
The composition of the gaseous products is shown in Figure 7, indicating CO2, CO, CH4, and CmHn (m = 2–4, n = 2m or 2m + 2) as the main gaseous products. The release of CO2 and CO mainly originated from the cracking of oxygen-containing functional groups, i.e., carboxyl, carbonyl, and ether groups, in the phenylpropane side chains. The hydrocarbons were mainly generated by the fragmentation of alkyl side chains present on aromatic rings [3]. The composition of gaseous products obtained with the various catalysts was not significantly different.

Figure 7.
Composition of the gaseous products obtained from uncatalyzed or catalytic CLS depolymerization.
The high-valued compounds obtained from the depolymerization of CLS were mainly concentrated in liquid products [37]; thus, a high liquid product yield was targeted. The composition of the liquid products is provided in Table 2. According to the gas chromatography–coupled mass spectrometry (GC-MS) characterization results (the curves are shown in Supplementary Materials, Figure S2), the liquid products contained many types of compounds, which can be classified as esters, alcohols, furans, ethers, ketones, aromatic hydrocarbons, aromatic ethers, aromatic esters, aromatic alcohols, phenolics, guaiacols, and syringols. The structure of lignin is quite complex, and although the weaker bonds (such as β-O-4) are more likely to break [13], many complex chemical reactions occur simultaneously during the depolymerization process.

Table 2.
The composition of the liquid products obtained from uncatalyzed and catalytic CLS depolymerization.
The used CLS was extracted from grass. Previous studies have shown that p-hydroxyphenyl units (H), guaiacyl units (G), and syringyl units (S) account for about 10–25%, 25–50%, and 25–50% in grass, respectively [38]. The total selectivity of all phenolic compounds (H + G + S) decreased in the order NiMgCoOx > NiO > MgCoOx > no catalyst and was 21.7% higher for NiMgCoOx compared to the uncatalyzed reaction. Under catalysis, the selectivity of H-type phenolic compounds was significantly improved because some side chains attached to the aromatic rings in the G-type and S-type compounds were removed. In the presence of solid base oxides, the contents of aromatic hydrocarbons increased and the total selectivity of esters, alcohols, furans, ethers, and ketones decreased, indicating that the catalysts promote the cracking of the connecting bonds between the structural units in CLS, as well as hydrogenation.
2.2.3. Solid Residue Analyses
The molecular weight distribution of the solid residues was characterized by gel permeation chromatography (GPC), and the average molecular weights are listed in Table 3. The degree of CLS depolymerization was measured by Mn (number-average molecular weight) and Mw (weight-average molecular weight) of THF-soluble residues. The heavy molecules originated from unreacted CLS or condensation reactions between CLS fragments. According to the data in Table 3, Mn (or Mw) of the solid residues (either R1 or R2) decreased in the order no catalyst > NiO > MgCoOx > NiMgCoOx, and the molecular weight of R1 was greater than that of R2, indicating that compared to the uncatalyzed reaction, solid base oxides promoted the depolymerization of CLS, and NiMgCoOx demonstrated the best catalytic performance, which could be ascribed to the existence of basic centers and metal active centers that promote CLS degradation and prevent the recombination of fragments in the hydrothermal reaction system.

Table 3.
Molecular weight of the solid residues obtained by GPC.
2.3. Regeneration Performance
According to the above experimental results, NiMgCox exhibited the best catalytic performance. Therefore, its regeneration ability was studied. Figure 8a shows that as the number of catalytic cycles increased, the liquid product yield first slightly decreased because of partial deactivation and then remained at around 70 wt.% from the fourth to the tenth cycle. According to Figure 8b, although the XRD peak intensity of the spent NiMgCox slightly decreased due to coverage with coke, the characteristic peak positions of fresh, spent, and regenerated NiMgCoOx were the same, indicating that the crystal structure did not change after reaction and regeneration. Based on the Ni 2p XPS spectra in Figure 8c, the chemical environment of Ni did not change after catalysis or regeneration since the peak positions of the samples were the same. However, in the Co 2p XPS spectra in Figure 8d, the binding energies of the Co sub-peaks were higher in spent NiMgCoOx than in fresh and regenerated NiMgCoOx. The difference indicated a change in the chemical environment after catalysis, and the specific reasons required further investigation.

Figure 8.
(a) Liquid yields obtained over different catalysis-regeneration cycles; (b) XRD patterns and (c) Ni 2p and (d) Co 2p XPS spectra of fresh, spent, and regenerated NiMgCoOx.
3. Possible CLS Depolymerization Pathway Catalyzed by Solid Base Oxides
The structure of lignin is quite complex, and even lignins derived from the same plant may have different structures due to differences in growth location and stage [39]. Therefore, various complex primary and secondary reactions occur during the depolymerization process of lignin and its derivatives [40], and many different compounds are present in the liquid products. Based on previous research and experimental results, this study briefly speculated on the mechanism of the catalytic depolymerization process. The active hydrogen in CLS can be captured by solid base oxides (like soluble bases), which triggers the hydrolysis reaction of ether bonds [41]. Figure 9 shows the possible depolymerization pathways of CLS. As shown in route I, the phenolic hydroxyl groups in CLS are converted into phenolic anions under solid base oxide catalysis [42]. The alkene–ketone conversion occurs after electron transfer, and phenolic compounds are formed after the scission of the weak ether bonds (mainly β-O-4) [43]. Repolymerization occurs simultaneously with depolymerization, and the products obtained from depolymerization/repolymerization can again depolymerize or repolymerize. Ultimately, monomers, char, or other compounds can be obtained as shown in route II [44].
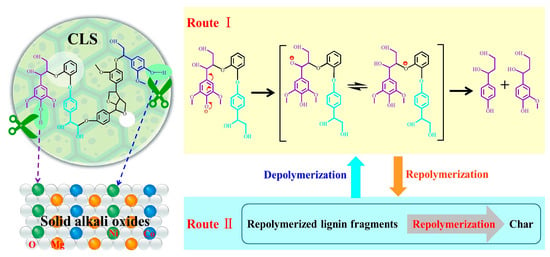
Figure 9.
Possible CLS depolymerization pathways catalyzed by solid base oxides.
During the depolymerization process, ethanol has multiple functions: (1) dissolving CLS, (2) providing hydrogen to the reaction system [45], and (3) serving as a capping agent to stabilize highly reactive phenolic intermediates through O-alkylation of hydroxyl groups and C-alkylation of aromatic rings, thereby inhibiting repolymerization and improving monomer yields [11]. According to the CO2-TPD characterization results, the types and contents of basic sites were similar in MgCoOx and NiMgCoOx. The highest liquid product yield is obtained under the catalytic action of NiMgFeOx, which indicates that the combination of Ni and Co is effective for improving hydrogenation performance in the presence of hydrogen provided by ethanol [46]. The electron-donating ability of Co is slightly stronger than that of Ni, meaning that Co atoms exhibit a more electron-rich state, which is favorable for nearby Ni atoms to adsorb hydrogen [47]. The existence of NiO in NiMgCoOx is beneficial for the adsorption of reactants and hydrogen [48,49] and generates a synergistic effect with other active components [50]. Thus, the catalyst demonstrates higher catalytic performance. Nevertheless, the specific catalytic mechanism still requires more detailed research.
4. Materials and Methods
4.1. Chemicals and Reagents
CLS (obtained from herbaceous plants after processing) was purchased from Tianjin Yeatschem Corp., Ltd., Tianjin, China. NaOH, Mg(NO3)2·6H2O, Ni(NO3)2·6H2O, Co(NO3)2·6H2O, anhydrous ethanol, THF, anhydrous magnesium sulfate, and ethyl acetate (EA) were purchased from Damao Chemical Ltd., Tianjin, China, and were of analytical grade. All chemicals were directly used without further purification.
4.2. Preparation of NiO, MgCoOx, and NiMgCoOx
The precursors of the oxides, i.e., Ni(OH)2, MgCo-LDHs, and NiMgCo-LDHs, were synthesized under the same conditions by the hydrothermal method. Stoichiometric amounts of Ni(NO3)2·6H2O, Mg(NO3)2·6H2O, and Co(NO3)2·6H2O were dissolved in ethanol–water solution (2.8 mol·L−1 ethanol), and 2 mol·L−1 NaOH solution was dropwise added under stirring at 100 r·min−1 until pH 10. Subsequently, the suspension was transferred to a hydrothermal reactor and heated at 180 °C for 24 h. The precipitate was washed with distilled water until the pH of the supernatant was 7, and then it was dried at 80 °C for 12 h. For the preparation of MgCo-LDHs and NiMgCo-LDHs, the ratios of n(Mg2+):n(Co2+) and n(Ni2+):n(Mg2+):n(Co2+) were 4:1 and 1:3:1, respectively. The precursors were calcined for 6 h in air atmosphere to obtain the target solid base oxides NiO, MgCoOx, and NiMgCoOx.
4.3. Catalytic Depolymerization of CLS
The CLS depolymerization performance catalyzed by NiO, MgCoOx, and NiMgCoOx was evaluated in a high-pressure autoclave. Typically, 1.0 g CLS and 0.5 g metal oxide were added to 60 mL ethanol–water solution (65 vol.% ethanol). The mixture was stirred at 100 r·min−1 and was placed inside the 100 mL high-pressure autoclave. Before sealing, the pressure vessel was flushed with N2 using a flow rate of 50 mL·min−1 for 30 min. Then, the autoclave was heated to 270 °C and kept at this temperature for 4 h under stirring. After the reaction was completed, the vessel was cooled to room temperature with flowing cold water, and the gaseous products were collected by depressurizing the autoclave into gas bags before opening. The inner wall of the vessel was rinsed with anhydrous ethanol to wash down any products to ensure accurate calculation of the product yields. The solid residue (R1) was separated by suction filtration under reduced pressure and dried at 80 °C for 12 h. The collected liquid was heated in a rotary evaporator at 45 °C under a vacuum of 50 mbar to remove ethanol. The liquid was then acidified to pH 1 using 1 mol·L−1 HCl solution and extracted three times by 20 mL EA. The EA-insoluble residue (R2) was dried at 80 °C for 12 h, while the EA-soluble product was dried by anhydrous magnesium sulfate before removing the solvent EA in a rotary evaporator at 45 °C under a vacuum of 50 mbar to recover the liquid product (LP). The liquid product was diluted to a certain multiple with EA for characterization. The yields of gaseous, liquid, and solid products were calculated by Equations (1)–(3). Further details have been reported in our previous studies [37].
where ySP, yLP, and yGP are the yield of the solid, liquid, and gaseous products, respectively; mR1, mR2, mOxide, mLP, and mCSL are the mass of R1, R2, oxide (NiO or MgCoOx or NiMgCoOx), liquid product, and CSL, respectively.
ySP = (mR1 + mR2 − mOxide)/mCSL × 100%
yLP = mLP/mCSL × 100%
yGP = 100% − ySP − yLP
4.4. Catalyst Regeneration
To regenerate the catalyst, the residue obtained after the depolymerization reaction was calcined at 600 °C for 6 h in a tube furnace under an air atmosphere.
4.5. Characterization
XRD patterns of samples were recorded with a D8 VENTURE powder X-ray diffractometer (Bruker, Bremen, Germany) using Cu Kα as the radiation source at 40 kV and 40 mA. Diffraction angles were measured in the 2θ range of 10–80° at a rate of 5°·min−1. The XRD data was analyzed by MDI Jade5. Crystalline phases were identified by comparison with reference patterns published in the JCPDS database. BET specific surface areas and pore structure data were determined by N2 adsorption–desorption at −196 °C on an ASAP2400 instrument (Micromeritics, Norcross, GA, USA). The morphologies of the samples were observed with a Sigma 300 scanning electron microscope (Zeiss, Oberkochen, Germany), using an accelerating voltage of 10 kV for image capture. The microstructure was observed using a Talos F200X transmission electron microscope (Thermofisher, Waltham, MA, USA) with an accelerating voltage of 200 kV. TPD experiments of oxides were performed on a U-shaped Pyrex tube furnace with helium as the carrier gas and CO2 (or NH3) as adsorption gas using a ChemBET 3000 instrument (Quantachrome, Boynton Beach, FL, USA). The composition of the gaseous products was analyzed by a GC-2014 gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector. The liquid products were analyzed by a 7890-5975 GC-MS system (Agilent, Santa Clara, CA, USA), equipped with a flame ionization detector, employing an HP-5MS UI column (30 m × 0.25 mm × 0.25 μm; helium was used as the carrier gas; the mass spectra were obtained from m/z 40 to 550). The area normalization method was used to calculate the selectivity of certain compounds in the liquid product. Before injecting the samples, the GC-MS was calibrated using a standard substance, octafluoronaphthalene in isooctane, with a concentration of 100 pg·μL−1. The specific calibration process was conducted according to the National Metrology Technical Specification of the People’s Republic of China, JF 1164-2018 [51]. The gel permeation chromatography (GPC) analyses of the THF-soluble residues were performed on a 1525 high-performance liquid chromatograph with a 1424 detector (Waters, Milford, MA, USA). THF was used as an eluent with a flow rate of 1.0 mL·min−1, and polystyrene was the standard substance used for calibration. X-ray photoelectron spectroscopy (XPS) characterization was performed on a PHI 1600 spectrometer (PerkinElmer, Shelton, CT, USA) with Mg Kα X-ray radiation operated at 15 kV to measure the binding energies of elements. The XPS data was analyzed by Thermo Avantage 5.9931.
5. Conclusions
The precursors Ni(OH)2, MgCo-LDHs, and NiMgCo-LDHs were synthesized by hydrothermal synthesis, and the solid base oxides NiO, MgCoOx, and NiMgCoOx were obtained after calcination. NiO had only weak basic sites while MgCoOx and NiMgCoOx exhibited strong and nearly equal basicity. Solid base oxides can promote the depolymerization of CLS and significantly improve the yield of liquid products. When NiMgCoOx was used as catalyst, the liquid product yield reached a maximum of 74.3 wt.%, the selectivity of phenolic compounds in the liquid product was as high as 74.52%, and the average relative molecular weight of solid products was the lowest. The synergistic effect of basic sites and hydrogenation sites enables it to exhibit good catalytic performance in CLS depolymerization to prepare phenolic compounds. This catalyst demonstrated good structural and catalytic stability after multiple regeneration.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal14110781/s1. Figure S1. NH3-TPD curves of NiO, NiMgCoOx and NiMgCoOx. Figure S2. The GC-MS patterns of the liquid product obtained from CLS depolymerization catalyzed by (a) No catalyst, (b) NiO, (c) MgCoOx, (d) NiMgCoOx.
Author Contributions
Conceptualization, H.W., W.Z., H.H., J.Z. and Y.C.; Investigation, H.W., Y.W. (Yejing Wang), Y.W. (Yizhen Wang) and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CNPC Innovation Found (No. 2021DQ02-0701), supported by China National Petroleum Corporation, the recipient is Haiying Wang; Guiding Innovation Fund of Northeast Petroleum University (No. 2021YDL-03), supported by Northeast Petroleum University, the recipient is Haiying Wang; Scientific Research Launch Fund of Northeast Petroleum University (No. 2021KQ07), supported by Northeast Petroleum University, the recipient is Haiying Wang; National Natural Science Foundation of China (No. 21908021), supported by National Natural Science Foundation of China, the recipient is Hongjing Han; Chunhui Project of Education Ministry (202201685), supported by Ministry of Education of the People’s Republic of China, the recipient is Hongjing Han; Heilongjiang Province Excellent Youth Basic Research Support Project (YQJH2023072), supported by Heilongjiang Provincial Department of Education, the recipient is Hongjing Han.
Data Availability Statement
All relevant data are within the paper.
Acknowledgments
The authors would like to thank Analysis and Testing Center of NEPU for their characterization support.
Conflicts of Interest
Authors Haiying Wang, Wencheng Zhang and Jiaren Zhang were employed by the company PetroChina. Author Yizhen Wang was employed by the company COOEC-Fluor Heavy Industries Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yue, X.; Lin, J.; Suopajarvi, T.; Mankinen, O.; Mikkelson, A.; Liu, R.; Huttunen, H.; Chen, L.; Xu, C.; Telkki, V.V.; et al. Conversion of highly polymerized lignin into monophenolic products via pyrolysis: A comparative study of acidic and alkaline depolymerization pretreatments using deep eutectic solvents. Chem. Eng. J. 2023, 478, 147368. [Google Scholar] [CrossRef]
- Sapouna, I.; Alexakis, A.E.; Malmstrom, E.; Mckee, L.S. Structure-property relationship of native-like lignin nanoparticles from softwood and hardwood. Ind. Crops Prod. 2023, 206, 117660. [Google Scholar] [CrossRef]
- Wang, H.; Han, H.; Sun, E.; Han, Y.; Zhang, Y.; Li, J.; Chen, Y.; Song, H.; Zhao, H.; Kang, Y. Production of aryl oxygen-containing compounds by the pyrolysis of bagasse alkali lignin catalyzed by LaM0.2Fe0.8O3 (M = Fe, Cu, Al, Ti). Energy Fuels 2019, 33, 8596–8605. [Google Scholar] [CrossRef]
- Rui, K.; Mittal, A.; Mckinney, K.; Chen, X.; Tucker, M.P.; Johnson, D.K.; Bechham, G.T. Base-catalyzed depolymerization of biorefinery lignins. ACS Sustain. Chem. Eng. 2016, 4, 1474–1486. [Google Scholar]
- Kong, L.; Dai, L.; Wang, Y. Enhancing aromatic hydrocarbon formation via catalytic depolymerization of lignin waste over Ru/WOx/N-C catalyst. Fuel 2023, 332, 126263. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, X.; Guo, Y.; Wang, Y. Self-hydrogen supplied catalytic fractionation of raw biomass into lignin-derived phenolic monomers and cellulose-rich pulps. J. Am. Chem. Soc. Au 2023, 3, 1911–1917. [Google Scholar] [CrossRef]
- Guo, X.; Hong, P.; Yao, L.; Liu, X.; Jiang, Z.; Shi, B. Catalytic hydrogenation of lignin-derived aldehydes over bimetallic PdNi/hydrotalcite catalyst under mild conditions. Fuel 2023, 353, 129231. [Google Scholar] [CrossRef]
- Sreenavya, A.; Aswin, P.; Ganesh, V.; Venkatesha, N.; Sakthivel, A. Facile water-free synthesis of noble metal-containing hydrotalcitesderived materials and their application for hydrotreatment of anisole. Mater. Today Sustain. 2022, 18, 100153. [Google Scholar]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef]
- Chaudhary, R.; Dhepe, P.L. Solid base catalyzed depolymerization of lignin into low molecular weight products. Green Chem. 2016, 19, 778–788. [Google Scholar] [CrossRef]
- Limarta, S.O.; Ha, J.M.; Park, Y.K.; Li, H.; Suh, D.J.; Jae, J. Efficient depolymerization of lignin in supercritical ethanol by a combination of metal and base catalysts. J. Ind. Eng. Chem. 2018, 57, 45–54. [Google Scholar] [CrossRef]
- Long, J.; Zhang, Q.; Wang, T.; Zhang, X.; Xu, Y.; Ma, L. An efficient and economical process for lignin depolymerization in biomass-derived solvent tetrahydrofuran. Bioresour. Technol. 2014, 154, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yue, H.; Zou, J.; Yao, R.; Duan, W.; Ma, H.; Zhao, Y.; He, Z. Oxidative lignin depolymerization using metal supported hydrotalcite catalysts: Effects of process parameters on phenolic compounds distribution. Fuel 2023, 331, 125805. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Shu, F.; Gao, Y.; Long, J. Hydrodeoxygenation of heavy lignin bio-oil to oxygenated fuel catalyzed by CuxNiy/MgO. Fuel 2024, 357, 129805. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Liu, H.; Ao, L.; Liu, K.; Guan, Y.; Zai, S.; Chen, S.; Zong, Z.; Wei, X. Supercritical ethanolysis of wheat stalk over calcium oxide. Renew. Energy 2018, 120, 300–305. [Google Scholar] [CrossRef]
- Fasolini, A.; Spennati, E.; Atakoohi, S.E.; Percivale, M.; Busca, G.; Basile, F.; Garbarino, G. A study of CO2 hydrogenation over Ni-MgAlOx catalysts derived from hydrotalcite precursors. Catal. Today 2023, 423, 114271. [Google Scholar] [CrossRef]
- Rodríguez, A.; Fernandez, L.; Domínguez, J.R.; González, G.; Martínez, O.; Espinoza-Montero, P. Mg and Ni nano-hydrotalcites modified with gold nanoparticles as platform of enzymatic electrochemical sensors for H2O2 detection. Sens. Bio-Sens. Res. 2021, 33, 100446. [Google Scholar] [CrossRef]
- Kameda, T.; Fubasami, Y.; Uchiyama, N.; Yoshioka, T. Elimination behavior of nitrogen oxides from a NO3−-intercalated Mg-Al layered double hydroxide during thermal decomposition. Thermochim. Acta 2010, 499, 106–110. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Yu, J. Influences of polyhydric alcohol co-solvents on the hydration and thermal stability of MgAl-LDH obtained via hydrothermal synthesis. Appl. Clay Sci. 2013, 72, 37–43. [Google Scholar] [CrossRef]
- Yang, F.; He, G. Preparation of granular NiO for the electrochemical performance and CO2 adsorption performance. Chem. Ind. Eng. Prog. 2023, 42, 907–916. [Google Scholar]
- Luo, X.; Guo, L.; Wei, Q.; Xu, J.; Wang, K.; Lu, B. Influence of crystallinity and binder on the energy delivery efficiency for porous magnesium cobaltate supercapacitor electrodes. Chin. J. Inorg. Chem. 2018, 34, 823–833. [Google Scholar]
- Sukpanish, P.; Lertpanyapornchai, B.; Yokoi, T.; Ngamcharussrivichai, C. Lanthanum-doped mesostructured strontium titanates synthesized via sol-gel combustion route using citric acid as complexing agent. Mater. Chem. Phys. 2016, 181, 422–431. [Google Scholar] [CrossRef]
- Chen, H.; Wageh, S.; Al-Ghamdi, A.A.; Wang, H.; Yu, J.; Jiang, C. Hierarchical C/NiO-ZnO nanocomposite fibers with enhanced adsorption capacity for Congo red. J. Colloid Interface Sci. 2019, 537, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Pi, M.; Xu, D.; Jiang, C.; Cheng, B. Fabrication of hierarchical porous ZnO-Al2O3 microspheres with enhanced adsorption performance. Appl. Surf. Sci. 2017, 426, 360–368. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Z.; Yuan, T. Recent advances in the catalytic upgrading of biomass platform chemicals via hydrotalcite-derived metal catalysts. Trans. Tianjin Univ. 2022, 2, 89–111. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Zhou, B.; Meng, F.; Zhao, Z.; Wei, C.; Zhou, L.; Wen, G.; Zhang, X. Layered double oxide (CoAl-LDO) catalysis for enhanced ozonation of methyl orange: Performance assessment and mechanistic insights. J. Mol. Liq. 2024, 404, 124995. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Mageste, A.B.; Dias, A.; Virtuoso, L.S.; Siqueira, K. Layered double hydroxides for remediation of industrial wastewater containing manganese and fluoride. J. Clean. Prod. 2018, 171, 275–284. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, L.; Wang, X.; Gao, W.; Wang, Z.; Zhang, X.; Wang, Y.; Liu, W. The influence of anionic surfactant (SDS) on the optical, magnetic and capacitive properties of NiO nanocrystals via gas-liquid diffusion synthesis. Mater. Sci. Eng. B 2024, 299, 116970. [Google Scholar] [CrossRef]
- Panigrahi, U.K.; Das, P.K.; Biswal, R.; Sathe, V.; Babu, P.D.; Mitra, A.; Mallick, P. Zn doping induced enhancement of multifunctional properties in NiO nanoparticles. J. Alloys Compd. 2020, 833, 155050. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Lin, S.; Yan, L.; Cao, R.; Yang, R.; Liang, X.; Xiang, W. Sol-gel synthesis of NiO nanoparticles doped sodium borosilicate glass with third-order nonlinear optical properties. J. Alloys Compd. 2016, 686, 564–570. [Google Scholar] [CrossRef]
- Choi, S.; Balamurugan, M.; Lee, K.G.; Cho, K.H.; Park, S.; Seo, H.; Nam, K.T. Mechanistic investigation of biomass oxidation using nickel oxide nanoparticles in a CO2-saturated electrolyte for paired electrolysis. J. Phys. Chem. Lett. 2020, 11, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Y.; Cheng, J.; Zhang, M. Promotional Effect of Ni-Co/TiO2 Catalysts on CO2 Hydrogenation. Chem. J. Chin. Univ. 2023, 44, 20230308. [Google Scholar]
- Li, J.; Liu, H.; Liu, Z.; Yang, D.; Zhang, M.; Gao, L.; Zhou, Y.; Lu, C. Facile synthesis of Z-scheme NiO/a-MoO3 p-n heterojunction for improved photocatalytic activity towards degradation of methylene blue. Arab. J. Chem. 2022, 15, 103513. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Li, S.; Zhang, Y.; Xu, C.; Chen, H. Hydrothermal synthesis of flower-like MgCo2O4 porous microstructures as high-performance electrode material for asymmetric supercapacitors. J. Alloys Compd. 2020, 824, 153939. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Xie, Z.; Wang, Y.; Wang, Y.; Wei, B. Tracking the structural evolution and activity origin of Co-doped NiFe layered double hydroxide for enhanced oxygen evolution reaction. Chem. Eng. J. 2024, 488, 151086. [Google Scholar] [CrossRef]
- Qian, G.; Lyu, W.; Zhao, X.; Zhou, J.; Fang, R.; Wang, F.; Li, Y. Efficient photoreduction of diluted CO2 to tunable syngas by Ni-Co dual sites through d-band center manipulation. Angew. Chem. Int. Ed. 2022, 61, e202210576. [Google Scholar] [CrossRef]
- Han, H.; Li, J.; Wang, H.; Han, Y.; Chen, Y.; Li, J.; Zhang, Y.; Wang, Y.; Wang, B. One-step valorization of calcium lignosulfonate to produce phenolics with the addition of solid base oxides in the hydrothermal reaction system. Energy Fuels 2019, 33, 4302–4309. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- del Río, J.C.; Lino, A.G.; Colodette, J.L.; Lima, C.F.; Gutierrez, A. Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass Bioenergy 2015, 81, 322–338. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wan, P.; Han, Y.; Qin, T. Degradation behaviors of lignin catalyzed by CaO/MgO composite as solid base catalyst. Chem. Ind. For. Prod. 2012, 32, 81–86. [Google Scholar]
- Javier, F.-R.; Xabier, E.; Fabio, H.-R.; Oihana, G.; Maria, G.A.; Jalel, L. Direct lignin depolymerization process from sulfur-free black liquors. Fuel Process. Technol. 2020, 197, 106201. [Google Scholar]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kornáyi, T.I.; Boot, M.D.; Hensen, E. Catalytic depolymerization of lignin in supercritical ethanol. Chemsuschem 2014, 7, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Lui, M.Y.; Masters, A.F.; Maschmeyer, T.; Yuen, A.K.L. Molybdenum carbide, supercritical ethanol and base: Keys for unlocking renewable BTEX from lignin. Appl. Catal. B Environ. 2023, 325, 122351. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Jiang, W.; Yu, T.; Tan, K.; Yin, S. Strong electronic coupling of CoNi and N-doped-carbon for efficient urea-assisted H2 production at a large current density. Carbon Energy 2023, 5, e368. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, D.; Wang, M.; Wang, Y. Study on catalytic performance of Ni-Co-P amorphous alloy for HDO of vanillin. J. Fuel Chem. Technol. 2019, 47, 1205–1213. [Google Scholar] [CrossRef]
- Fu, Q.; Yan, L.; Liu, D.; Zhang, S.; Jiang, H.; Xie, W.; Yang, L.; Wang, Y.; Wang, H.; Zhao, X. Highly-dispersed surface NiO species and exposed Ni (200) facets facilitating activation of furan ring for high-efficiency total hydrogenation of furfural. Appl. Catal. B Environ. 2024, 343, 123501. [Google Scholar] [CrossRef]
- Campisi, S.; Chan-Thaw, C.E.; Chinchilla, L.E.; Chutia, A.; Botton, G.A.; Mohammed, K.M.H.; Dimitratos, N.; Wells, P.P.; Villa, A. Dual-site-mediated hydrogenation catalysis on Pd/NiO: Selective biomass transformation and maintenance of catalytic activity at low Pd loading. ACS Catal. 2020, 10, 5483–5492. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, X.; Yang, K.; Xu, Y.; Lu, J.; Li, W.; Li, B.; Xue, C.; Liu, Y. Efficient and low-cost Ni-NiO/Al2O3 catalysts with dual-active-sites for selective catalytic conversion of phthalic anhydride to phthalide. Chem. Eng. J. 2024, 497, 154753. [Google Scholar] [CrossRef]
- JF 1164–2018; Calibration Specificationfor Gas Chromatography-MassSpectrometries. National Metrology Technical Specification of China. National Institute of Metrology (NIM) of China: Beijing, China, 2018.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).