Abstract
The swift exhaustion of natural oil reserves and worsening environmental issues have prompted the quest for an economical method to produce biofuels. The superiority of heterogeneous catalysis promotes the development of bio-based catalysts. Carbon materials prepared from agricultural and forestry biomass waste have good application prospects in catalysis. In the present study, Xanthoceras sorbifolia shell waste was used as the raw material, Xanthoceras sorbifolia Bunge Carbon (XC) was used as the catalyst carrier, and K2CO3 was used as the activator to prepare a heterogeneous catalyst (KXC). The heterogeneous catalyst was characterized by scanning electron microscopy-energy dispersion X-ray spectroscopy (SEM-EDS), Fourier-transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), X-ray diffraction (XRD), and Brunauer–Emmett–Teller (BET) and X-ray photoelectron spectroscopy (XPS) analysis techniques to evaluate its chemical composition, structure, and physical morphology. EDS and XPS revealed the presence of K metal, which provided an alkaline site for the transesterification reaction to produce biodiesel. The biodiesel yield was observed by gas chromatography−mass spectrometry (GCMS). Under the reaction conditions of a methanol-to-oil molar ratio of 12:1, a reaction time of 90 min, a temperature of 65 °C, and a catalyst loading of 4 wt.% using 25KXC-600-4, the yield of biodiesel can reach 95.13 ± 0.82%. After being repeated five times, the yield was still 58.11 ± 3.80%. The catalyst has no waste generation, and has the characteristics of simple preparation and environmental friendliness, which make it a green heterogeneous catalyst for biodiesel production.
1. Introduction
Currently, the globe is encountering various issues, including a swift population increase, intensified industrialization, and environmental pollution, resulting in an increasing global fossil fuel shortage dilemma. Over the past few decades, biodiesel has emerged as a crucial energy to address fossil fuel. This process focuses on the preparation of catalysts to produce biodiesel with low cost, high yield, and high selectivity. Biodiesel, made up of fatty acid methyl esters (FAMEs), offers various benefits compared to traditional fossil fuels. Primarily, it lowers greenhouse gas emissions and has a smaller carbon footprint []. Additionally, it generates reduced amounts of harmful pollutants including sulfur oxides, benzene, carbon monoxide, carbon dioxide, nitrogen oxides, and particulate matter []. Catalysts include homogeneous catalysts, heterogeneous catalysts, and enzyme catalysts. However, enzyme catalysts show a good catalytic effect and stability in high acid value and non-aqueous media, but their production costs are high and they are difficult to be applied to industrial production on a large scale. It is difficult to separate homogeneous catalysts from reaction solutions, which affect the quality of biodiesel products. Thus, it is essential to investigate new, affordable, and eco-friendly catalysts.
Consequently, the increasing focus on heterogeneous catalysts for biodiesel production in recent years is largely due to their simplicity in handling and separation from reaction mixtures. Materials such as metal oxides [], supported metal hydroxides [], and zeolites [] have been widely reported. Waste materials derived from rice husk, Musa paradisiaca plant [], Mangifera indica peel [], and Cocos nucifera [] have been extensively researched as potential sources for catalysts or catalyst carriers in biodiesel production.
Recent research has emphasized creating eco-friendly heterogeneous catalysts and their use in biodiesel production, taking advantage of the abundant natural minerals found in waste biomass that are ideal for producing these catalysts. The solid base catalysts employed are not depleted in the reaction, allowing for an easy separation from the biodiesel product. These catalysts remain effective at the boiling point of methanol (MeOH) throughout the transesterification process []. To support this assertion, researchers have explored the catalytic properties of different heterogeneous catalysts. Olugbenga et al. used radish leaves as raw materials to synthesize an effective green catalyst to replace the chemically mature basic catalysts. The synthesized catalyst showed a high conversion percentage of 98% for waste cooking oil and algal oil using 6 wt.% catalyst loading and 12:1 methanol-to-oil molar ratio for 150 min at 60 °C, respectively []. Asuquo et al. investigated various treatment methods for creating green biocatalysts from fermented kola nut pod husk. The resulting calcined catalysts demonstrated significant catalytic activity due to their high levels of K, Ca, and Mg, larger surface area, and the presence of mixed mineral oxides like CaO, MgO, and K2O []. Based on the findings from response surface methodology (RSM), Andi et al. identified the ideal conditions for the synthesis process as a molar ratio of oil to methanol of 1:9, a catalyst weight of 12% (w/w), a reaction duration of 105 min, and a constant temperature of 65 °C, which results in a methyl ester content of 98.88%. The solid catalyst made from jackfruit peel waste (JPW) shows outstanding catalytic efficiency during biodiesel synthesis []. Collins et al. emphasized optimizing and predicting the fuel yield and characteristics of FAME produced from BSO (inedible seed oil) using KOH-activated waste banana bunch stalk biochar through deep learning techniques. The multi-input–multi-output model on the logsig transfer function accurately predicted the biodiesel yield and properties more than the MISO and response surface methodology models did. The optimum yield (96 wt.%), cetane number (48), kinematic viscosity (3.3 mm2/s), and purity (98.3%) were concurrently accomplished at a temperature of 56 °C for 115 min, using a molar ratio of CH3OH to BSO of 15:1, a catalyst amount of 6 wt.%, and a stirring speed of 400 rpm, achieving an optimal result of 98% validation accuracy. They view nonedible baobab seeds as feedstocks with considerable potential to generate high-quality FAME products with significant yields as an alternative to fossil fuels [].
Although numerous studies exist on the development of green catalysts for biodiesel production, challenges remain regarding conversion efficiency, catalyst recovery, and reusability, as well as availability and cost. These challenges have driven the quest for innovative catalysts to satisfy the increasing demand for green fuels. The goal of this research is to prepare a heterogeneous catalyst for biodiesel production from Xanthoceras sorbifolia bunge oil using Xanthoceras sorbifolia bunge carbon (XC) with different loadings of K2CO3 (KXC). At a fixed reaction temperature (65 °C), the parameters related to biodiesel production were assessed and analyzed.
2. Results and Discussion
2.1. Influence of Potassium Percentage
The catalyst used in the transesterification reaction affects the physical and chemical properties of biodiesel. Before determining the final catalyst, the incorporation of potassium content was evaluated for the preparation of biodiesel. The conditions included a 25KXC-600-4 catalyst loading of 2 wt.%, a methanol–oil molar ratio of 12:1, a reaction time of 120 min, and a temperature of 65 °C; the influence of potassium percentages 5, 10, 15, 25, and 35% on the final catalyst composition in the biodiesel production process was assessed.
In Figure 1a, the effect of the potassium content in the catalyst on the yield of biodiesel is shown; with the increase of potassium content in the XC support, the biodiesel yield reached the maximum value with the doping of 25% potassium content, indicating that when the potassium content was doped into the XC support as the active phase, the doping amount was not always proportional to the biodiesel yield []. This phenomenon indicates that a too great amount of the active phase will hinder the mass transfer relationship between methanol, Xanthoceras sorbifolia bunge oil, and the catalyst. Therefore, 25% potassium content was selected as catalyst in subsequent experiments. Next, the 25KXC catalyst was activated for 1–5 h at 400–800 °C, and the catalyst was tested under fixed transesterification reaction conditions [].
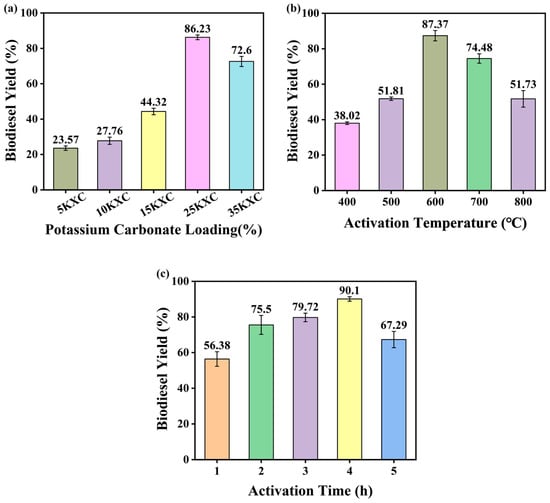
Figure 1.
Biodiesel yield under the (a) influence of the potassium percentage present in the catalysts, (b) the influence of the activation temperature, and (c) the influence of the activation time.
Figure 1b,c show the effect of different activation temperatures and activation times on the biodiesel yield of 25KXC. Whether it is the activation temperature or the activation time, the biodiesel yield will increase first and then decrease, indicating that the two are not completely proportional to the biodiesel yield. Therefore, 25KXC-600-4 was selected as the catalyst in subsequent experiments.
2.2. XRD Analysis
The XRD patterns of the KXC catalysts are shown in Figure 2. The XRD patterns of the catalysts synthesized at varying K2CO3 loadings, activation temperatures, and activation times are available in Figure 2a–c. These patterns show a broad band between 2θ = 20–30°, corresponding to a (002) plane, indicating the amorphous structure. K2CO3 shows well-established crystalline peaks at 2θ = 32.6°and 42.8°, which are convenient with the JCPDs card: (01-070-0292). This confirms the successful coverage of K2CO3 on KXC [,,].
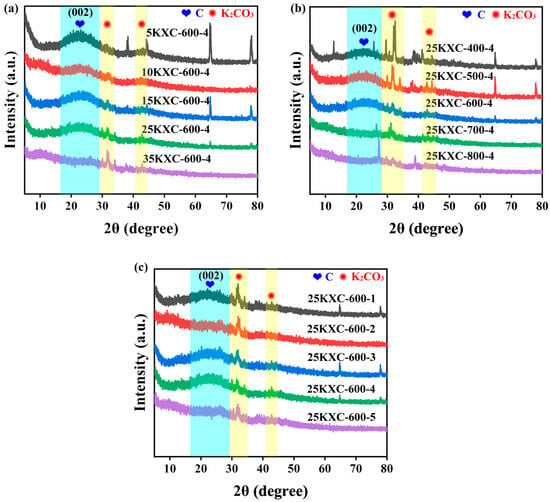
Figure 2.
XRD patterns under the (a) influence of the potassium percentage present in the catalysts, (b) the influence of the activation temperature, and (c) the influence of the activation time.
2.3. SEM-EDS Analysis
SEM-EDS was performed on the KXC and 25KXC-600-4 catalyst samples to provide their surface morphology and elementary analysis, as shown in Figure 3. The KXC surface shows a regularly arranged pore structure; it provides energetic conditions for the loading of K2CO3 and transport of reactants []. The surface of the 25KXC-600-4 catalyst was covered by K2CO3. Due to aggregation caused by the volatilization of organic matter during calcination activation, H2O (g) and CO2 were released from the catalyst surface, leaving K2O as the main active material for catalytic performance []. The EDS analysis of the KXC and 25KXC-600-4 catalyst showed that the corresponding composition of the O, C, and K elements were 50.00%, 43.68%, 6.32%, and 47.83%, 15.47%, 36.71%, respectively, which indicated that K2CO3 was well demonstrated on the surface of KXC; the mapping configuration is utilized to assess how effectively the elements are dispersed on the catalyst profile’s surface, which was consistent with the interpretation of the SEM data [,,].
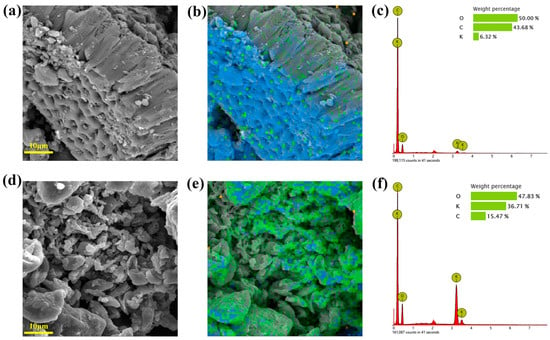
Figure 3.
The SEM-EDS of the (a) KXC (b) Mapping of the KXC (c)EDS of the KXC (d) 25KXC-600-4 catalyst (e) Mapping of the 25KXC-600-4 catalyst and (f) EDS of the 25KXC-600-4 catalyst.
2.4. FTIR Analysis of Catalysts
The infrared spectra of the KXC and 25KXC-600-4 catalysts are shown in Figure 4 and reveal the catalysts’ functional groups. The FT-IR spectrum of KXC displays a wide band, as observed at 3650 cm−1 []. The assignment corresponds to the O-H stretching of hydroxyl groups found in cellulose and hemicellulose. The peaks at 2960 cm−1 and 2856 cm−1 result from changes in the methyl and methylene groups []. The absorption peak at 2960 cm−1 is caused by the symmetric stretching vibration of CO2. The CO2 adsorbed on the catalyst reacts with the basic substances on the surface. The presence of CO32− at 854 cm−1 indicates the formation of metal oxides or carbonates and this result agreed well with that of XRD [,,,].
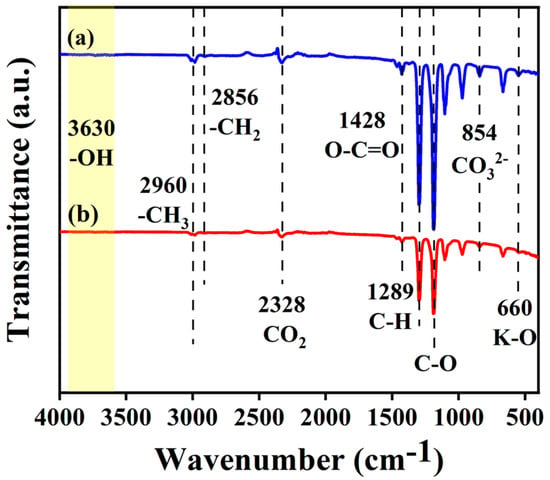
Figure 4.
FT-IR spectra of the (a) KXC and (b) 25KXC-600-4 catalyst.
2.5. BET Surface Area Analysis
The isotherms for N2 adsorption and desorption, along with the related pore size distribution of the KXC and 25KXC-600-4 catalysts, are shown in Figure 5. The specific surface area and average pore size of the catalyst modified by K2CO3 were 3.81 m2/g and 12.329 nm, while the specific surface area and average pore size of KXC were 324.38 m2/g and 2.12 nm. The Brunauer–Deming–Deming–Teller classification indicates that the 25KXC-600-4 catalysts possess a mesoporous structure [,]. The slit-shaped pores on the surface of the catalyst are opened by calcination, resulting in a wider pore size, which creates the necessary conditions for the transesterification reaction. It has been reported that when the dimension of triglyceride and glycerin molecules is 2.5 nm, the catalyst can realize the free transport of the product []. The decrease in the specific surface area of the 25KXC-600-4 catalyst may be due to the loading of K2CO3 covering the surface of the KXC support, and the previous SEM-EDS can also confirm this result [].
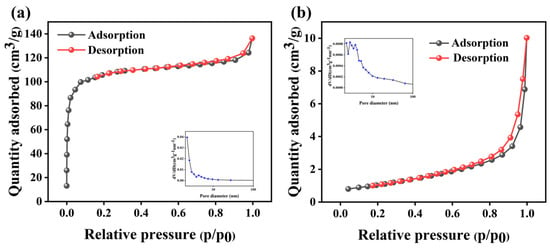
Figure 5.
N2 adsorption–desorption isotherm and adsorption pore size distribution of the (a) KXC and (b) 25KXC-600-4 catalyst.
2.6. TG Analysis
Figure 6 shows the TG analysis of the KXC and 25KXC-600-4 catalyst. The TGA for KXC showed a 8.24% weight loss from room temperature to 210 °C, which is due to the desorption of water and the dehydration of the surface hydroxyl groups []. Subsequently, the weight loss of KXC was 30.66% at 225 °C to 370 °C, which was caused by the decomposition of structural water and organic compounds []. From 400 to 600 °C, there was a breakdown of carbonaceous compounds into CO and CO2. In Figure 6b, the TGA profile of the 25KXC-600-4 catalyst illustrates two distinct phases of weight loss. During one period, between room temperature and 140 °C, water evaporation from the 25KXC-600-4 catalyst contributed to 7.29% weight loss. Another period, above 140 °C, which leads to the breakdown of metal carbonates into their oxides, contributed to a 19.72% weight loss []. Obviously, the 25KXC-600-4 catalyst exhibited its good thermal stability.
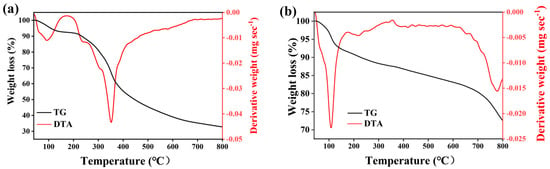
Figure 6.
TG-DTA thermogram of the (a) KXC and (b) 25KXC-600-4 catalyst.
2.7. XPS Analysis
Figure 7 illustrates the oxidation states and characterizes the surface chemical composition of the KXC and 25KXC-600-4 catalyst. The main elements of C, O, and K were investigated. The overall survey XPS spectra of the KXC and 25KXC-600-4 catalysts are shown in Figure 7a,e. According to the C 1s XPS spectrum in Figure 7b, there were two different peaks centered at 284.8 eV and 287.2 eV, corresponding to C-C and -C=O groups, respectively. In Figure 7f, two peaks at 292.9 eV and 295.7 eV in the XPS spectrum of C 1s were related to C-H groups and π-π*. In Figure 7c,g, the peaks at 293.1 eV and 295.9 eV are shifted to 292.8 eV and 295.6 eV. This positive shift indicates the electronic effect between K2CO3 and KXC, resulting in an increase in the basicity of the 25KXC-600-4 catalyst []. The XPS of the O 1s of the 25KXC-600-4 catalyst is shown in Figure 7h. The spectra of O 1s of the 25KXC-600-4 catalyst display two distinct peaks at 530.9 eV and 531.9 eV. The peak at 531.9 eV is referred to as the carbonate group [], while the peak at 531.82 eV is associated with the -OH group in H2O molecules which can be attached to the catalyst surface [,].
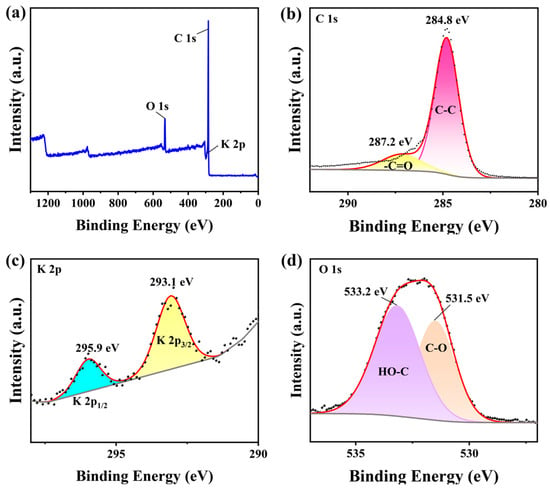
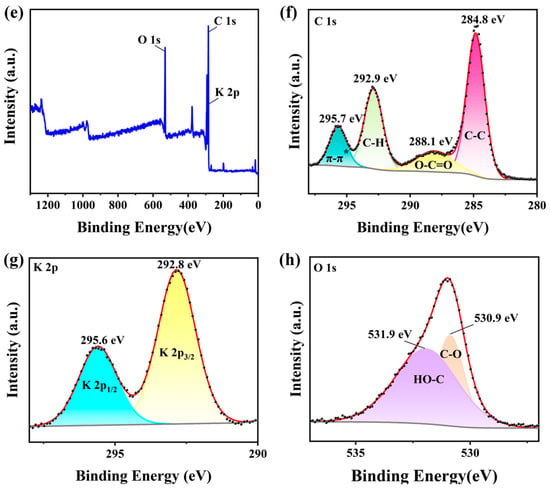
Figure 7.
X-ray photoelectron spectra (XPS) of the (a) Survey of the KXC (b) C 1s of the KXC (c) K 2p of the KXC (d) O 1s of the KXC (e) Survey of the 25KXC-600-4 catalyst (f) C 1s of the 25KXC-600-4 catalyst (g) K 2p of the 25KXC-600-4 catalyst and (h) O 1s of the 25KXC-600-4 catalyst.
2.8. Study of the Influence of Reaction Parameters for Biodiesel Synthesis
2.8.1. Effect of Methanol–Oil Molar Ratio (MOMR) on the Reaction
In order to evaluate the calcination effect of the 25KXC-600-4 catalyst, the conditions included a 25KXC-600-4 catalyst loading of 2 wt.%, a reaction time of 120 min, and a temperature of 65 °C; according to the literature, a methanol-to-oil molar ratio of 3:1 is required to acquire biodiesel from Xanthoceras sorbifolia bunge oil using a catalyst []. However, the reaction is reversible, so excessive amounts of alcohol are required. Therefore, the MOMRs of 6:1, 9:1, 12:1, 15:1, and 18:1 were selected for the reaction. The research shows that as the MOMR increased from 6:1 to 12:1, the reaction rate as well as the conversion of oil to biodiesel increased significantly from 51.75 ± 0.49 to 86.98 ± 1.6%. This shows that the positive effect of methanol in the transesterification reaction is positive. However, as the MOMR increased from 12:1 to 18:1, this does not indicate a significant rise in biodiesel production. This may be due to the reverse reaction of glycerol in the reaction mixture [,,]. Therefore, the MOMR of 12:1 was selected for further study.
2.8.2. Effect of Temperature on the Reaction
The impact of varying temperatures viz. 45, 55, 65, 75, and 85 °C on the reaction was investigated; the conditions included a 25KXC-600-4 catalyst loading of 2 wt.%, a reaction time of 120 min, and a MOMR of 12:1, whose results are presented in Figure 8b. This investigation reveals the significant increase in biodiesel yield from 44.42 ± 4.35% to 89.40 ± 1.42% when going from a 45 to 65 °C temperature. However, the reaction temperature reached 85 °C and did not show a remarkable impact on increasing the performance of the catalyst. This shows that the reaction temperature of 65 °C is more suitable for the performance of the catalyst, and also reduces the additional energy consumption caused by a high temperature [].
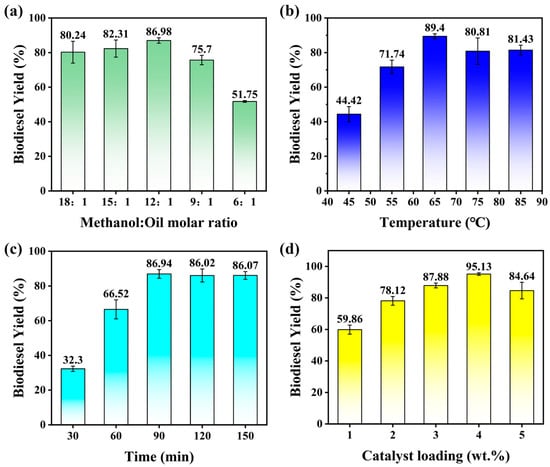
Figure 8.
Effect of process variables on biodiesel yield. (a) Methanol–oil molar ratio; (b) temperature; (c) time; (d) catalyst loading.
2.8.3. Effect of Time on the Reaction
The impact of varying times viz. 30, 60, 90, 120, and 150 min on the reaction was investigated; the conditions included a 25KXC-600-4 catalyst loading of 2 wt.%, a reaction temperature of 65 °C, and a MOMR of 12:1, whose results are presented in Figure 8c. This confirmed that a 86.94 ± 2.51% yield had been achieved after 90 min of reaction time and that the yield began to decrease gradually after 90 min, as esters may undergo hydrolysis over time, leading to the formation of soap [,]. Therefore, when the reaction reached equilibrium, the reaction was stopped immediately to ensure that the transesterification reaction was maximized in the positive direction.
2.8.4. Effect of Catalyst Loading
Figure 8d shows the results of the catalyst amounts (1, 2, 3, 4, and 5 wt.%) in a biodiesel production process using a calcined 25KXC-600-4 catalyst with a MOMR of 12:1, a reaction time of 120 min, and a temperature of 65 °C. When the catalyst amount was raised from 1 wt.% to 4 wt.%, a marginal rise in biodiesel yield from 59.86 ± 2.87% to 95.13 ± 0.82% was observed. Moreover, the yield of biodiesel does not increase but decreases with the increase in catalyst loading while maintaining the same reaction conditions. It shows that too many catalysts may cause a blockage of active sites, or it may be that the loading of the catalyst leads to a more viscous reaction system and reduces the mass transfer rate. Accordingly, in this work, the 25KXC-600-4 catalyst amount of 4 wt.% was found to be produce the best experimental conditions for Xanthoceras sorbifolia bunge oil transesterification to biodiesel [,].
2.8.5. Investigation of Catalyst Reusability
The reusability test for the 25KXC-600-4 catalyst was also studied with optimized reaction conditions. The catalyst was retrieved through centrifugation, washed with three batches of n-hexane, and dried in an oven at 105 °C for 12 h. The recovered catalyst was applied to a continuous reaction cycle, and the outcomes are presented in Figure 9. Eventually, biodiesel yield decreased to 58.11 ± 3.8% in the fifth cycle. The decrease in catalytic activity might be related to the leaching of certain elements that can diminish the active sites of the catalyst [,]. The slight decrease in biodiesel yield observed after each cycle may result from a reduction in the catalyst’s active sites, which can be blocked by organic substances such as K+ and CH3O−. This blockage might lead to the leaching of active metal components into the reaction mixture [,], which implies the excellent catalytic stability of the 25KXC-600-4 catalyst.
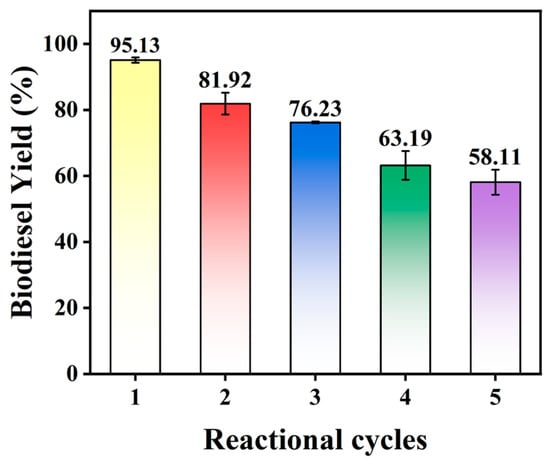
Figure 9.
Reusability of calcined 25KXC-600-4 catalyst (catalyst load = 4 wt.%, MOMR = 12:1, time, and temperature = 65 °C).
2.9. Characterization of Xanthoceras sorbifolia Biodiesel
2.9.1. FTIR Analysis Xanthoceras sorbifolia Biodiesel
The FT-IR spectra of the Xanthoceras sorbifolia bunge oil and its biodiesel are depicted in Figure 10. The wavelength signals of Xanthoceras sorbifolia bunge oil at 714 cm−1 and biodiesel at 707 cm−1 were caused by the swing of fatty acid chains []. The wavelength signals of Xanthoceras sorbifolia bunge oil at 1161 cm−1 and biodiesel at 1175 cm−1 were caused by the C-O stretching band of ester molecules and triglyceride, respectively []. The wavelength signals of Xanthoceras sorbifolia bunge oil at 1462 cm−1 and biodiesel at 1448 cm−1 were caused by the bending vibration of -CH3 []. The C-O stretching vibration of Xanthoceras sorbifolia bunge oil at 1741 cm−1 was replaced by methyl ester at 1746 cm−1 of biodiesel and this signal indicated the formation of biodiesel. The wavelength signals of Xanthoceras sorbifolia bunge oil at 2846 cm−1 and 2916 cm−1 and biodiesel at 2854 cm−1 and 2923 cm−1 are caused by C-H stretching vibration in methyl and methylene. The wavelength signal of Xanthoceras sorbifolia bunge oil at 3011 cm−1 and biodiesel at 3077 cm−1 is caused by the =C-H stretching band. Therefore, the product synthesized from Xanthoceras sorbifolia bunge oil is thus identified as a blend of various fatty acid methyl esters [,,].
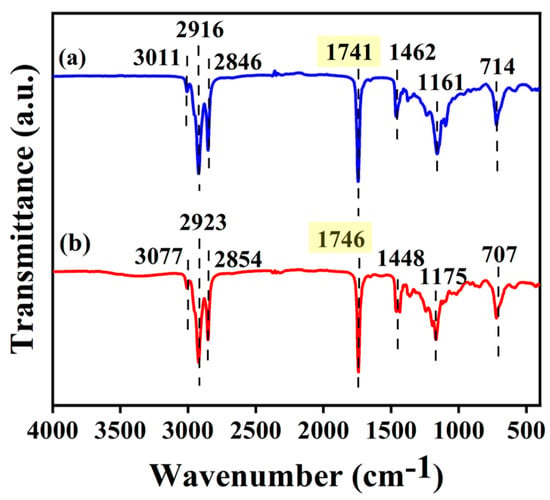
Figure 10.
FT-IR spectra of the (a) Xanthoceras sorbifolia bunge oil and (b) biodiesel.
2.9.2. GC-MS Analysis
To better identify the types and amounts of methyl ester in biodiesel, the product underwent GC-MS analysis, and the gas chromatogram of biodiesel was obtained, as shown in Table 1 and Figure 11. As a result, it was discovered that Xanthoceras sorbifolia bunge biodiesel consists primarily of C19 fatty acid methyl esters, which are, respectively, methyl palmitate (4.33%), methyl linoleate (37.86%), methyl oleate (46.28%), methyl stearate (2.60%), methyl cis-11-eicosenoate (4.95%), and methyl cis-13-docosenoat (3.99%).

Table 1.
Composition of the resultant biodiesel from Xanthoceras sorbifolia bunge oil.
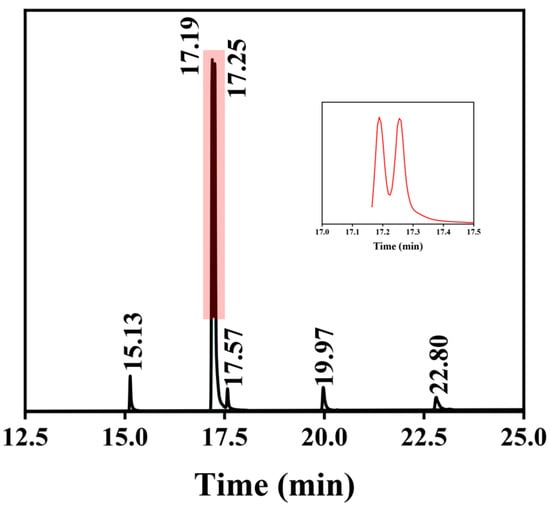
Figure 11.
GC-MS of Xanthoceras sorbifolia bunge oil biodiesel.
2.9.3. Xanthoceras sorbifolia Bunge Oil Biodiesel Properties
The biodiesel produced using the 25KXC-600-4 catalyst under optimal reaction conditions was physically and chemically characterized following the ASTM D6751 standard [,]. The properties of biodiesel produced using Xanthoceras sorbifolia bunge oil is tabulated in Table 2. Xanthoceras sorbifolia bunge oil biodiesel’s characteristic produced in this study was within the limits of the standard method [].

Table 2.
Properties of Xanthoceras sorbifolia bunge oil biodiesel.
2.10. Proposed Reaction Mechanisms
The possible reaction mechanism for the production of biodiesel by the 25KXC-600-4 catalyst is presented in Figure 12. The 25KXC-600-4 catalyst mainly uses K2CO3 and K2O as active sites. In addition, there are M-O bonds in these active sites. The electronegativity difference between alkali metals and oxygen atoms leads to the Lewis acid and Brønsted base properties of metal cations (Mσ+) and oxygen anions (Oσ−), respectively []. When the methanol interacts with the K-O bond, the nucleophilic alcohol generated from methanol attacks the electrophilic part of the triglyceride carbonyl group to form an intermediate tetrahedron, which is rearranged. The intermediate tetrahedron is converted into diacylglycerol anion and a fatty acid methyl ester. Under the action of the 25KXC-600-4 catalyst, the hydrogen ion obtains a diacylglycerol, and this process is repeated continuously to generate monoglyceride and glycerol. In this process, a total of three fatty acid methyl esters and one glycerol are produced.
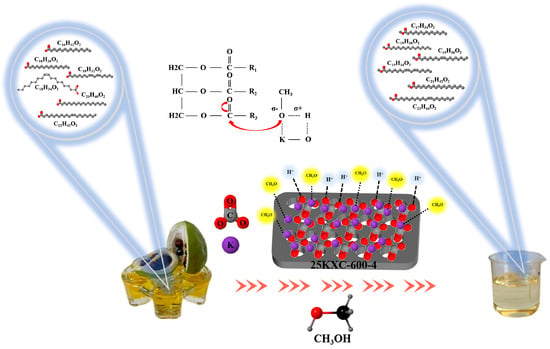
Figure 12.
Reaction mechanism proposed for the 25KXC-600-4 catalyst assisted transesterification.
3. Materials and Methods
3.1. Materials
Xanthoceras sorbifolia bunge shell was collected from the planting area of Hohhot, Inner Mongolia Autonomous Region. Xanthoceras sorbifolia bunge oil was acquired from Hohhot, Inner Mongolia Autonomous Region. Methanol (99.5% purity), KOH (85% purity), n-hexane (98% purity), and K2CO3 (99% purity) were acquired from Tianjin XinPlatt Chemical and Fuchen Chemical Reagent Co., Ltd., in Tianjin, China. All chemicals employed in this study were of analytical quality and used as received, without additional purification. These reagents were used without further purification prior to the transesterification.
3.2. Preparation of the Catalysts
The Xanthoceras sorbifolia bunge shells were initially cleaned by washing with distilled water several times to remove all impurities of sand and dust. After this, they were dried for 24 h in an oven at 60 °C. The clean and completely dry Xanthoceras sorbifolia bunge shells were then crushed and pulverized in a grinder, then sieved in a 40-mesh standard sieve. The dried Xanthoceras sorbifolia bunge shells were subsequently calcined at 600 °C for 2 h within a nitrogen atmosphere to generate the Xanthoceras sorbifolia bunge shells carbon (XC). Then, the XC was chemically activated. The suspension was filtered to recover the XC. The filtered XC was then washed multiple times with distilled water until the pH reached neutrality. Finally, the KXC that was obtained was dried overnight in an oven at 105 °C. The KXC was chemically modified by K2CO3, and the KXC was mixed with various concentrations of K2CO3 at room temperature at 800 rpm for a certain time to obtain sufficient impregnation. Next, it was placed in an oven for aging for 24 h, and then activated in a tubular furnace (Figure 13). The as-synthesized catalysts were designated as YKXC-T-t, where Y, T, and t represented the K2CO3 concentrations (wt.%), calcination temperature, and calcination time.
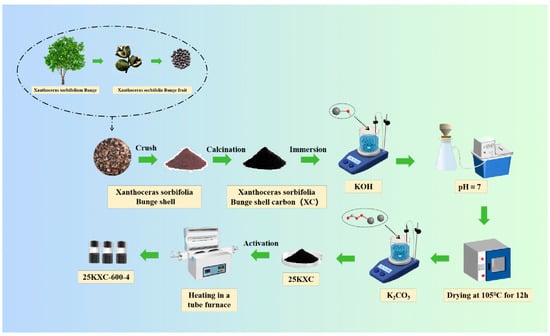
Figure 13.
Flow chart of preparation of 25KXC-600-4 catalyst.
3.3. Catalyst Characterization
For X-ray powder diffraction, Cu Kα radiation was used at 2θ = 5–80°, with the operating voltage set at 40 kV and the current at 40 mA (XRD, Tongda TD-3700, Dandong, China). The chemical composition of the catalyst was analyzed in the range of 4000~400 cm−1 by Fourier transform infrared spectroscopy (FTIR, Bruker SOR-27, Karlsruhe, Germany). SEM combined with an energy dispersive spectrometer (EDS). Before the SEM test, the catalyst was sprayed with gold in a vacuum (SEM-EDS, Phenom-World S-3400N, Breda, Netherlands). Thermogravimetric analysis was used to thermally evaluate the catalyst. The determination was achieved by heating the specimen gradually to 850 °C at 30 °C/min under a N2 stream of 50 mL/min (TGA, Hengjiu HCT-4, Beijing, China). To evaluate the catalyst’s surface properties, the nitrogen adsorption–desorption isotherm was examined utilizing the Brunauer–Emmett–Teller method within a pressure range (P/P0) of 0.1–1.0, beginning at −196 °C. The volatile species that were adsorbed onto the surface of the catalyst were removed through a vacuum outgassing process overnight at a 200 °C for 3 h (BET, BSD-PSI, Beijing, China). X-ray photoelectron spectroscopy was used to analyze the surface elemental composition and valence states of the catalyst; the C1s peak was calibrated at 284.80 eV (XPS, Thermofisher, Waltham, MA, USA).
3.4. Transesterification and Characterization of Biodiesel
The transesterification of Xanthoceras sorbifolia bunge oil (10 g) was performed in a 50 mL reactor at a temperature of 600 rpm/min for a certain time to produce biodiesel with methanol and a KXC catalyst (Figure 14). The reactor was reduced to room temperature to obtain the mixed product. The product was centrifuged at 8000 rpm for 10 min for solid–liquid separation, and the liquid product was placed in a separation funnel for 12 h to separate the biodiesel layer and the glycerol layer. The obtained solid catalyst was washed with n-hexane and dried overnight in an oven. The biodiesel layer was finally sealed in a glass bottle containing anhydrous sodium sulfate to eliminate any remaining water (Figure 15). The biodiesel production was determined using Equation (1), where m1 stands for the weight of biodiesel produced and m2 stands for the weight of the Xanthoceras sorbifolia oil used [].
YBiodiesel yield(%) = m1/m2

Figure 14.
Reactor apparatus diagram. ① Pressure gauge. ② Explosion-proof film. ③ Temperature sensor. ④ Intake valve. ⑤ Relief valve. ⑥ Handle. ⑦ Heating and insulation module. ⑧ Intelligent regulation chassis.
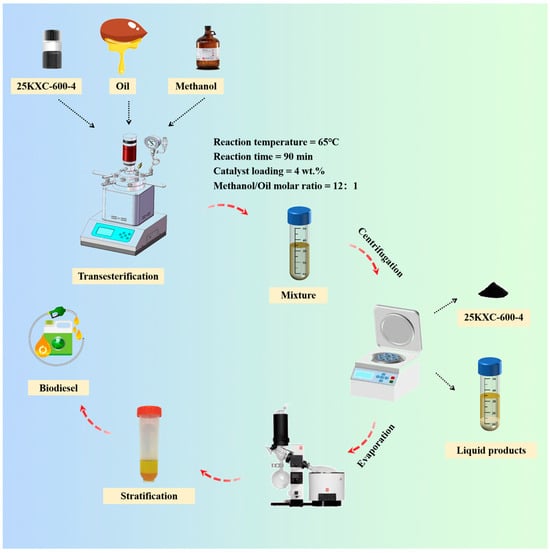
Figure 15.
Schematic diagram for the overall biodiesel manufacture process in this study.
The FAME composition of the produced biodiesel was analyzed using a GC setup (GC, Agilent 8890-5977B, Santa Clara, CA, USA). The temperature programming conditions of the column box were as follows: the initial temperature was maintained at 50 °C for 1 min, increased to 150 °C at 10 °C/min for 2 min, and increased to 250 °C at 5 °C/min for 10 min. The injection volume was 1.0 μL, the inlet temperature was 240 °C, the carrier gas was helium, the flow rate was 1.0 mL/min, and the ionization energy was 70 eV. The FAME composition was examined by comparing it with the mass spectra of the standard FAME mixture and its retention time.
4. Conclusions
The research demonstrated the promise of the XC-supported K2CO3 catalyst in producing biodiesel from Xanthoceras sorbifolia bunge oil. The conditions for synthesizing a new basic heterogeneous catalyst from KXC were analyzed by varying the K2CO3 loading, calcination temperature, and calcination time. The catalyst synthesized by a K2CO3 loading of 25% and a calcination at 600 °C for 4 h demonstrated a high transesterification reaction activity. The catalyst characterization revealed that the primary compounds are metal oxides and carbonates, along with basic surface sites, which may account for its strong catalytic activity. The optimized reaction conditions of the 25KXC-600-4 catalyst loading of 4 wt.%, the MOMR of 12:1, and the reaction time of 120 min at 65 °C resulted in a biodiesel yield of 95.13%. The error of three repeated experiments was 0.82%. The biodiesel yield of the catalyst was still 58.11 ± 3.80% after five times of recycling; the main reason for its deactivation was the leaching of potassium ions into the solution. The 25KXC-600-4 catalyst is a cost-effective and efficient option, offering an innovative green approach to biodiesel production.
Author Contributions
J.W.: writing, conceptualization, methodology, and formal analysis. J.S. and K.Z.: investigation. M.L., R.D. and Z.L.: editing. Y.H.: conceptualization, supervision, review, and investigation. X.W.: conceptualization, supervision, methodology, data curation, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jungar Banner technology research and development project (No. 2023YY–12), the Natural Science Foundation of Inner Mongolia (No. 2024MS03068), and the College Student Innovation and Entrepreneurship Program of Inner Mongolia (No. 202410129010 and No. 202410129031), the Science and technology projects of the Inner Mongolia Autonomous Region (No. 2022YFDZ0045).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Karkal, S.S.; Rathod, D.R.; Jamadar, A.S.; Mamatha, S.S.; Kudre, T.G. Production optimization, scale-up, and characterization of biodiesel from marine fishmeal plant oil using Portunus sanguinolentus crab shell derived heterogeneous catalyst. Biocatal. Agric. Biotechnol. 2023, 47, 102571. [Google Scholar] [CrossRef]
- Masera, K.; Hossain, A.K. Advancement of biodiesel fuel quality and NOx emission control techniques. Renew. Sustain. Energy Rev. 2023, 178, 113235. [Google Scholar] [CrossRef]
- Bahadorizadeh, O.; Sobati, M.A.; Shahnazari, S. Emission characteristics of a semi-industrial boiler fueled by waste cooking oil biodiesel containing different metal oxide nanoparticles. Process Saf. Environ. Prot. 2022, 158, 199–209. [Google Scholar] [CrossRef]
- Shokrani, R.; Haghighi, M. Textural evolution of hierarchical nanostructured ZSM-5 via sono-hydrothermal design by various carbon shapes for efficient biodiesel production. Appl. Catal. B Environ. 2020, 271, 118940. [Google Scholar] [CrossRef]
- Mierczynski, P.; Szkudlarek, L.; Chalupka, K.; Maniukiewicz, W.; Wahono, S.K.; Vasilev, K.; Szynkowska-Jozwik, M.I. The Effect of the Activation Process and Metal Oxide Addition (CaO, MgO, SrO) on the Catalytic and Physicochemical Properties of Natural Zeolite in Transesterification Reaction. Materials 2021, 14, 2415. [Google Scholar] [CrossRef]
- Basumatary, B.; Basumatary, S.; Das, B.; Nath, B.; Kalita, P. Waste Musa paradisiaca plant: An efficient heterogeneous base catalyst for fast production of biodiesel. J. Clean. Prod. 2021, 305, 127089. [Google Scholar] [CrossRef]
- Das, A.; Shi, D.; Halder, G.; Lalthazuala Rokhum, S. Microwave-assisted synthesis of glycerol carbonate by transesterification of glycerol using Mangifera indica peel calcined ash as catalyst. Fuel 2022, 330, 125511. [Google Scholar] [CrossRef]
- Rhithuparna, D.; Ghosh, N.; Khatoon, R.; Rokhum, S.L.; Halder, G. Evaluating the commercial potential of Cocos nucifera derived biochar catalyst in biodiesel synthesis from Kanuga oil: Optimization, kinetics, thermodynamics, and process cost analysis. Process Saf. Environ. Prot. 2024, 183, 859–874. [Google Scholar] [CrossRef]
- Adepoju, T.F.; Olatunbosun, B.E.; Olatunji, O.M.; Ibeh, M.A. Brette Pearl Spar Mable (BPSM): A potential recoverable catalyst as a renewable source of biodiesel from Thevetia peruviana seed oil for the benefit of sustainable development in West Africa. Energ. Sustain. Soc. 2018, 8, 23. [Google Scholar] [CrossRef]
- Eldiehy, K.S.H.; Gohain, M.; Daimary, N.; Borah, D.; Mandal, M.; Deka, D. Radish (Raphanus sativus L.) leaves: A novel source for a highly efficient heterogeneous base catalyst for biodiesel production using waste soybean cooking oil and Scenedesmus obliquus oil. Renew. Energy 2022, 191, 888–901. [Google Scholar] [CrossRef]
- Asuquo, A.J.; Zhang, X.; Lin, L.; Li, J. Green heterogeneous catalysts derived from fermented kola nut pod husk for sustainable biodiesel production. Int. J. Green Energy 2024, 21, 2218–2227. [Google Scholar] [CrossRef]
- Mulkan, A.; Zulkifli, N.W.M.; Husin, H.; Ahmadi; Dahlan, I.; Syafiie, S. Development of jackfruit (Artocarpus heterophyllus) peel waste as a new solid catalyst: Biodiesel synthesis, optimization and characterization. Process Saf. Environ. Prot. 2023, 177, 152–168. [Google Scholar] [CrossRef]
- Elendu, C.C.; Liu, C.; Aleem, R.D.; Shan, Y.; Cao, C.; Ramzan, N.; Duan, P.-G. Optimization and Deep Learning Modeling of the Yield and Properties of Baobab-Derived Biodiesel Catalyzed by Waste Banana Bunch Stalk Biochar. ACS Omega 2024, 9, 12941–12955. [Google Scholar] [CrossRef] [PubMed]
- Saetiao, P.; Kongrit, N.; Jitjamnong, J.; Direksilp, C.; Cheng, C.K.; Khantikulanon, N. Enhancing Sustainable Production of Fatty Acid Methyl Ester from Palm Oil Using Bio-Based Heterogeneous Catalyst: Process Simulation and Techno-Economic AnaK2CO3lysis. ACS Omega 2023, 8, 30598–30611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Razeghi, J.; Ramavandi, B. Biodiesel production from edible oils using algal biochar/CaO/ as a heterogeneous and recyclable catalyst. Renew. Energy 2021, 168, 1207–1216. [Google Scholar] [CrossRef]
- Haghighi, M.; Zare, L.B.; Ghiasi, M. Biodiesel production from Spirulina algae oil over [Cu(H2PDC)(H2O)2] complex using transesterification reaction: Experimental study and DFT approach. Chem. Eng. J. 2022, 430, 132777. [Google Scholar] [CrossRef]
- Amadine, O.; Essamlali, Y.; Fihri, A.; Larzek, M.; Zahouily, M. Effect of calcination temperature on the structure and catalytic performance of copper–ceria mixed oxide catalysts in phenol hydroxylation. RSC Adv. 2017, 7, 12586–12597. [Google Scholar] [CrossRef]
- Mawlid, O.A.; Abdelhady, H.H.; Abd El-Moghny, M.G.; Hamada, A.; Abdelnaby, F.; Kased, M.; Al-Bajouri, S.; Elbohy, R.A.; El-Deab, M.S. Clean approach for catalytic biodiesel production from waste frying oil utilizing K2CO3/Orange peel derived hydrochar via RSM Optimization. J. Clean. Prod. 2024, 442, 140947. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, A.A.; Puri, S.K.; Tuli, D.K. Wood ash as a potential heterogeneous catalyst for biodiesel synthesis. Biomass Bioenergy 2012, 41, 94–106. [Google Scholar] [CrossRef]
- Betiku, E.; Etim, A.O.; Pereao, O.; Ojumu, T.V. Two-Step Conversion of Neem (Azadirachta indica) Seed Oil into Fatty Methyl Esters Using a Heterogeneous Biomass-Based Catalyst: An Example of Cocoa Pod Husk. Energy Fuels 2017, 31, 6182–6193. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Devaraj, K.; Veerasamy, M.; Aathika, S.; Mani, Y.; Thanarasu, A.; Dhanasekaran, A.; Subramanian, S. Study on effectiveness of activated calcium oxide in pilot plant biodiesel production. J. Clean. Prod. 2019, 225, 18–26. [Google Scholar] [CrossRef]
- Joshi, G.; Rawat, D.S.; Lamba, B.Y.; Bisht, K.K.; Kumar, P.; Kumar, N.; Kumar, S. Transesterification of Jatropha and Karanja oils by using waste egg shell derived calcium based mixed metal oxides. Energy Convers. Manag. 2015, 96, 258–267. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.C. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Al-Hamamre, Z. Biodiesel production from waste cooking oil using heterogeneous KNO3/Oil shale ash catalyst. Renew. Energy 2023, 211, 470–483. [Google Scholar] [CrossRef]
- Betiku, E.; Okeleye, A.A.; Ishola, N.B.; Osunleke, A.S.; Ojumu, T.V. Development of a Novel Mesoporous Biocatalyst Derived from Kola Nut Pod Husk for Conversion of Kariya Seed Oil to Methyl Esters: A Case of Synthesis, Modeling and Optimization Studies. Catal. Lett. 2019, 149, 1772–1787. [Google Scholar] [CrossRef]
- Naghipour, A.; Ghorbani-Choghamarani, A.; Taherinia, Z. Novel hybrid materials based on mesoporous Gamma-Alumina@ Riboflavin@ vanadium for biodiesel production. Fuel 2023, 334, 126674. [Google Scholar] [CrossRef]
- Wang, S. A novel peat biochar supported catalyst for the transesterification reaction. Energy Convers. Manag. 2017, 139, 89–96. [Google Scholar] [CrossRef]
- Nath, B.; Basumatary, B.; Wary, N.; Basumatary, U.R.; Basumatary, J.; Rokhum, S.L.; Azam, M.; Min, K.; Basumatary, S. Agricultural Waste-Based Heterogeneous Catalyst for the Production of Biodiesel: A Ranking Study via the VIKOR Method. Int. J. Energy Res. 2023, 2023, 1–23. [Google Scholar] [CrossRef]
- Abu-Ghazala, A.H.; Abdelhady, H.H.; Mazhar, A.A.; El-Deab, M.S. Exceptional room temperature catalytic transesterification of waste cooking oil to biodiesel using environmentally-benign K2CO3/γ-Al2O3 nano-catalyst. Chem. Eng. J. 2023, 474, 145784. [Google Scholar] [CrossRef]
- Lu, S.; Cui, W.; Wang, R.; Zhang, C.; Yan, P. Biomimetic mineralization and characterization of hierarchically structured hydrated magnesium carbonates: The effects of sodium alginate. J. CO2 Util. 2022, 56, 101848. [Google Scholar] [CrossRef]
- Lu, S.; Cui, W.; Zhang, C.; Yan, P. Controllable biomimetic mineralization and characterization of hydrated magnesium carbonates using sodium carboxymethyl cellulose. J. Cryst. Growth 2022, 589, 126693. [Google Scholar] [CrossRef]
- Abdelrahim, A.M.; Abd El-Moghny, M.G.; El-Shakre, M.E.; El-Deab, M.S. Robust electrolytic oxygen evolution at nanostructured NiFe LDH@ in-situ functionalized graphite felt. J. Alloys Compd. 2023, 967, 171771. [Google Scholar] [CrossRef]
- Kaur, N.; Ali, A. Biodiesel production via ethanolysis of jatropha oil using molybdenum impregnated calcium oxide as solid catalyst. RSC Adv. 2015, 5, 13285–13295. [Google Scholar] [CrossRef]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J. Clean. Prod. 2019, 239, 118112. [Google Scholar] [CrossRef]
- Gohain, M.; Devi, A.; Deka, D. Musa balbisiana Colla peel as highly effective renewable heterogeneous base catalyst for biodiesel production. Ind. Crops Prod. 2017, 109, 8–18. [Google Scholar] [CrossRef]
- Miladinović, M.R.; Zdujić, M.V.; Veljović, D.N.; Krstić, J.B.; Banković-Ilić, I.B.; Veljković, V.B.; Stamenković, O.S. Valorization of walnut shell ash as a catalyst for biodiesel production. Renew. Energy 2020, 147, 1033–1043. [Google Scholar] [CrossRef]
- Saeed, A.M.M.; Sharma, S.; Hassan, S.Z.; Ghaleb, A.M.; Cao, G.-P. Intensification and Optimization of FAME Synthesis via Acid-Catalyzed Esterification Using Central Composite Design (CCD). ACS Omega 2023, 8, 26206–26217. [Google Scholar] [CrossRef]
- Laskar, I.B.; Gupta, R.; Chatterjee, S.; Vanlalveni, C.; Rokhum, S.L. Taming waste: Waste Mangifera indica peel as a sustainable catalyst for biodiesel production at room temperature. Renew. Energy 2020, 161, 207–220. [Google Scholar] [CrossRef]
- Abu-Jrai, A.M.; Jamil, F.; Al-Muhtaseb, A.H.; Baawain, M.; Al-Haj, L.; Al-Hinai, M.; Al-Abri, M.; Rafiq, S. Valorization of waste Date pits biomass for biodiesel production in presence of green carbon catalyst. Energy Convers. Manag. 2017, 135, 236–243. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Biodiesel synthesis from Hevea brasiliensis oil employing carbon supported heterogeneous catalyst: Optimization by Taguchi method. Renew. Energy 2016, 89, 506–514. [Google Scholar] [CrossRef]
- Buasri, A.; Ksapabutr, B.; Panapoy, M.; Chaiyut, N. Biodiesel production from waste cooking palm oil using calcium oxide supported on activated carbon as catalyst in a fixed bed reactor. Korean J. Chem. Eng. 2012, 29, 1708–1712. [Google Scholar] [CrossRef]
- Betiku, E.; Oraegbunam, J.C.; Falowo, O.A.; Ojumu, T.V.; Latinwo, L.M. Sustainable microwave-supported biodiesel production using sandbox oil and its waste shell as a nanoparticle green alkali heterogeneous catalyst. Process Biochem. 2024, 142, 1–12. [Google Scholar] [CrossRef]
- Fadara, O.A.; Falowo, O.A.; Ojumu, T.V.; Betiku, E. Process optimization of microwave irradiation-aided transesterification of kariya seed oil by Taguchi orthogonal array: Pawpaw trunk as a novel biocatalyst. Biofuels Bioprod. Biorefin. 2021, 15, 1006–1020. [Google Scholar] [CrossRef]
- Falowo, O.A.; Betiku, E. A novel heterogeneous catalyst synthesis from agrowastes mixture and application in transesterification of yellow oleander-rubber oil: Optimization by Taguchi approach. Fuel 2022, 312, 122999. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry, 1st ed.; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Basumatary, S.F.; Brahma, S.; Das, B.; Basumatary, S. A mixture of three agrowastes-K2CO3 as a solid catalyst for biodiesel synthesis from a binary blend of two non-edible oils. J. Indian Chem. Soc. 2024, 101, 101195. [Google Scholar] [CrossRef]
- Prajapati, P.; Shrivastava, S.; Sharma, V.; Srivastava, P.; Shankhwar, V.; Sharma, A.; Srivastava, S.; Agarwal, D. Karanja seed shell ash: A sustainable green heterogeneous catalyst for biodiesel production. Results Eng. 2023, 18, 101063. [Google Scholar] [CrossRef]
- Chutia, G.P.; Phukan, K. Jute leaves ash@Fe3O4 as efficient nanocatalyst for sustainable biodiesel production: Characterization, optimization and kinetic investigation. J. Ind. Eng. Chem. 2024, 131, 288–304. [Google Scholar] [CrossRef]
- ASTM D6751; Standard Specification for Biodiesel Fuel Blendstock(B100) for Middle Distillate Fuels. ASTM international: West Conshohocken, PA, USA, 2023.
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Jitjamnong, J.; Thunyaratchatanon, C.; Luengnaruemitchai, A.; Kongrit, N.; Kasetsomboon, N.; Sopajarn, A.; Chuaykarn, N.; Khantikulanon, N. Response surface optimization of biodiesel synthesis over a novel biochar-based heterogeneous catalyst from cultivated (Musa sapientum) banana peels. Biomass Conv. Bioref. 2021, 11, 2795–2811. [Google Scholar] [CrossRef]
- Bekele, D.T.; Shibeshi, N.T.; Reshad, A.S. Heterogeneous Catalysts from Metallic Oxides and Lignocellulosic Biomasses Ash for the Valorization of Feedstocks into Biodiesel: An Overview. Bioenerg. Res. 2023, 16, 1361–1379. [Google Scholar] [CrossRef]
- Alsaiari, R.A.; Musa, E.M.; Rizk, M.A. Biodiesel production from date seed oil using hydroxyapatite-derived catalyst from waste camel bone. Heliyon 2023, 9, e15606. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).