Investigating the Impact of Stress on the Optical Properties of GaN-MX2 (M=Mo, W; X=S, Se) Heterojunctions Using the First Principles
Abstract
1. Introduction
2. Results
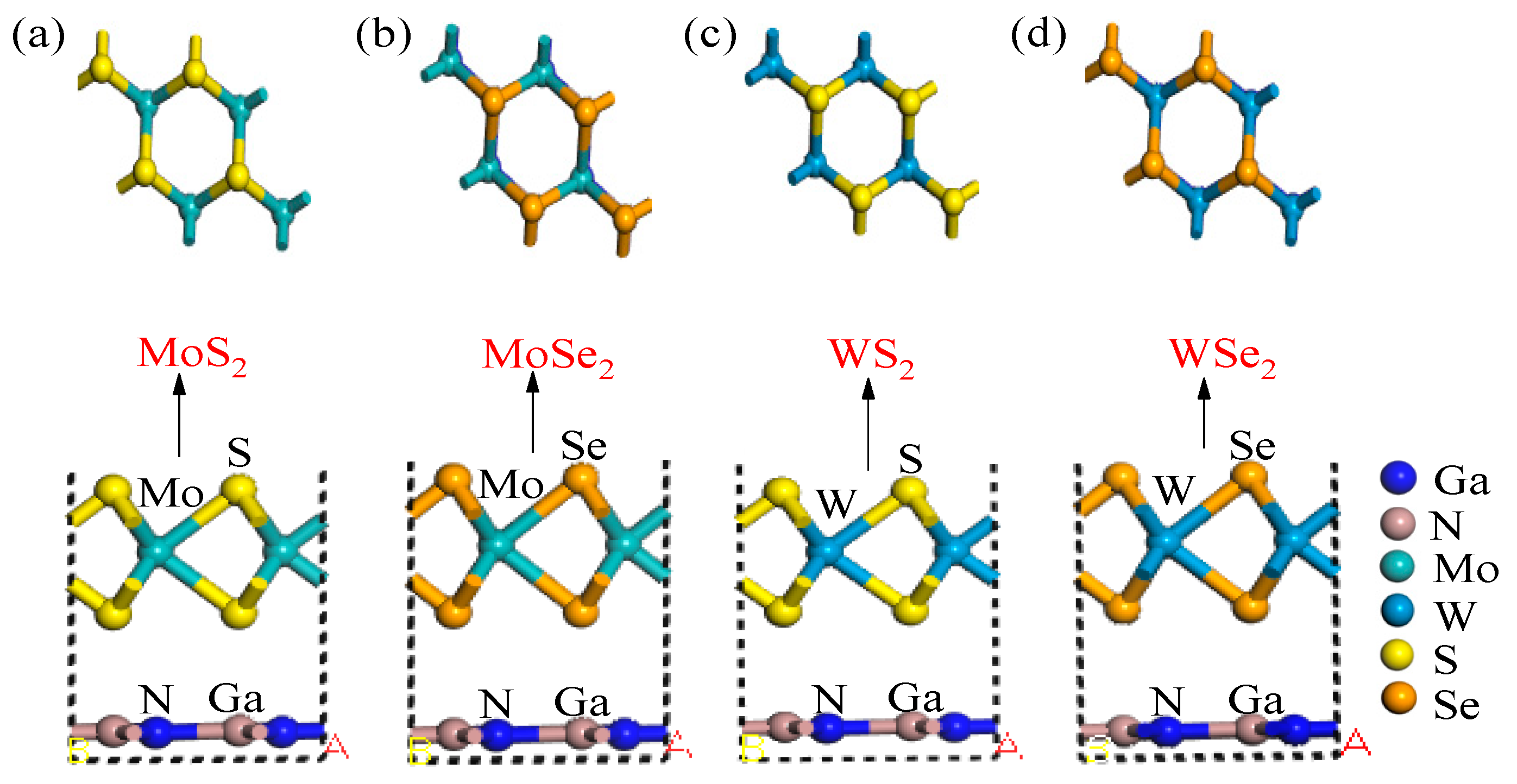
2.1. Geometric Parameters and System Stability
Geometric Parameters and Stability
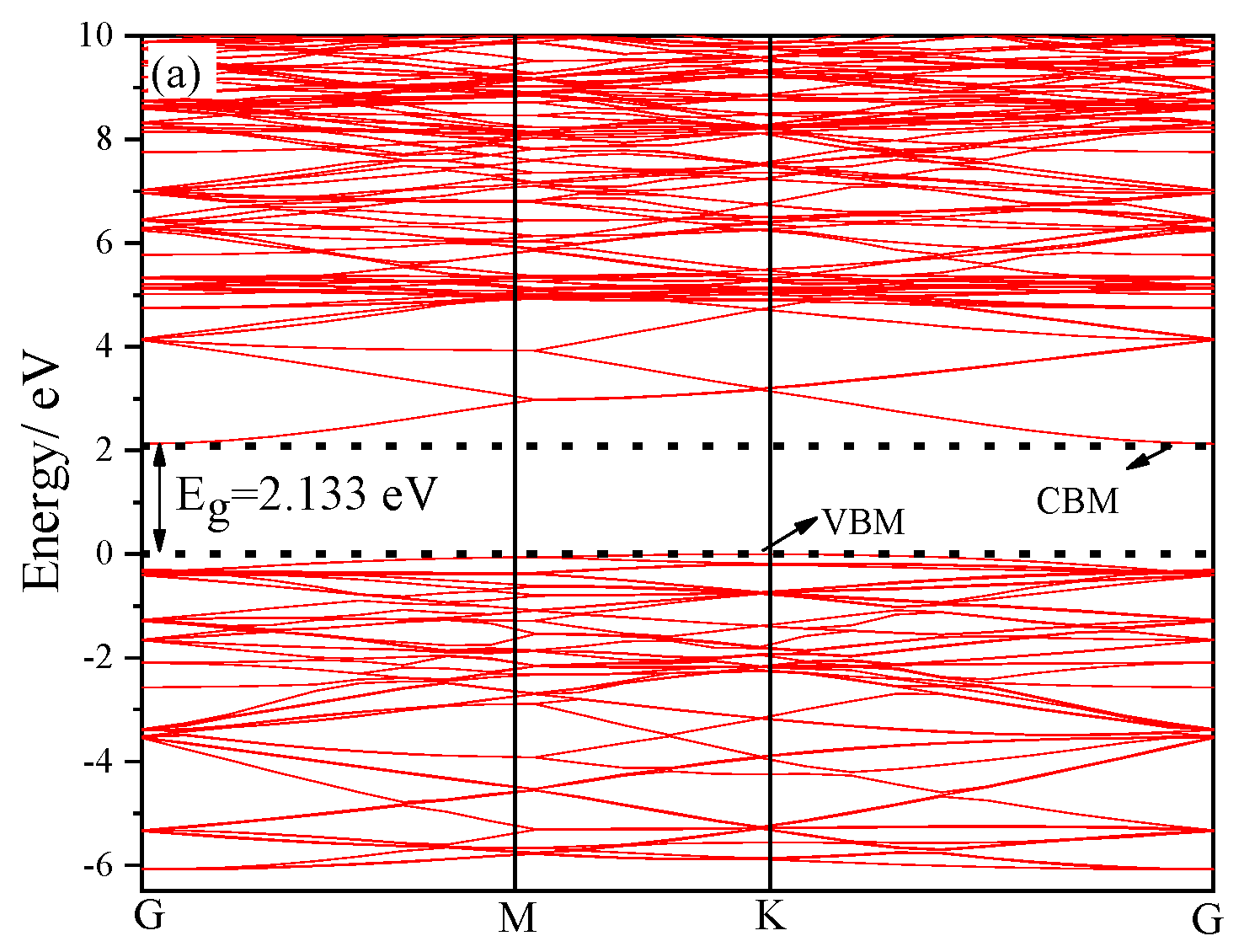
2.2. Electronic Structure Analysis
2.3. Work Function
2.4. Optical Properties
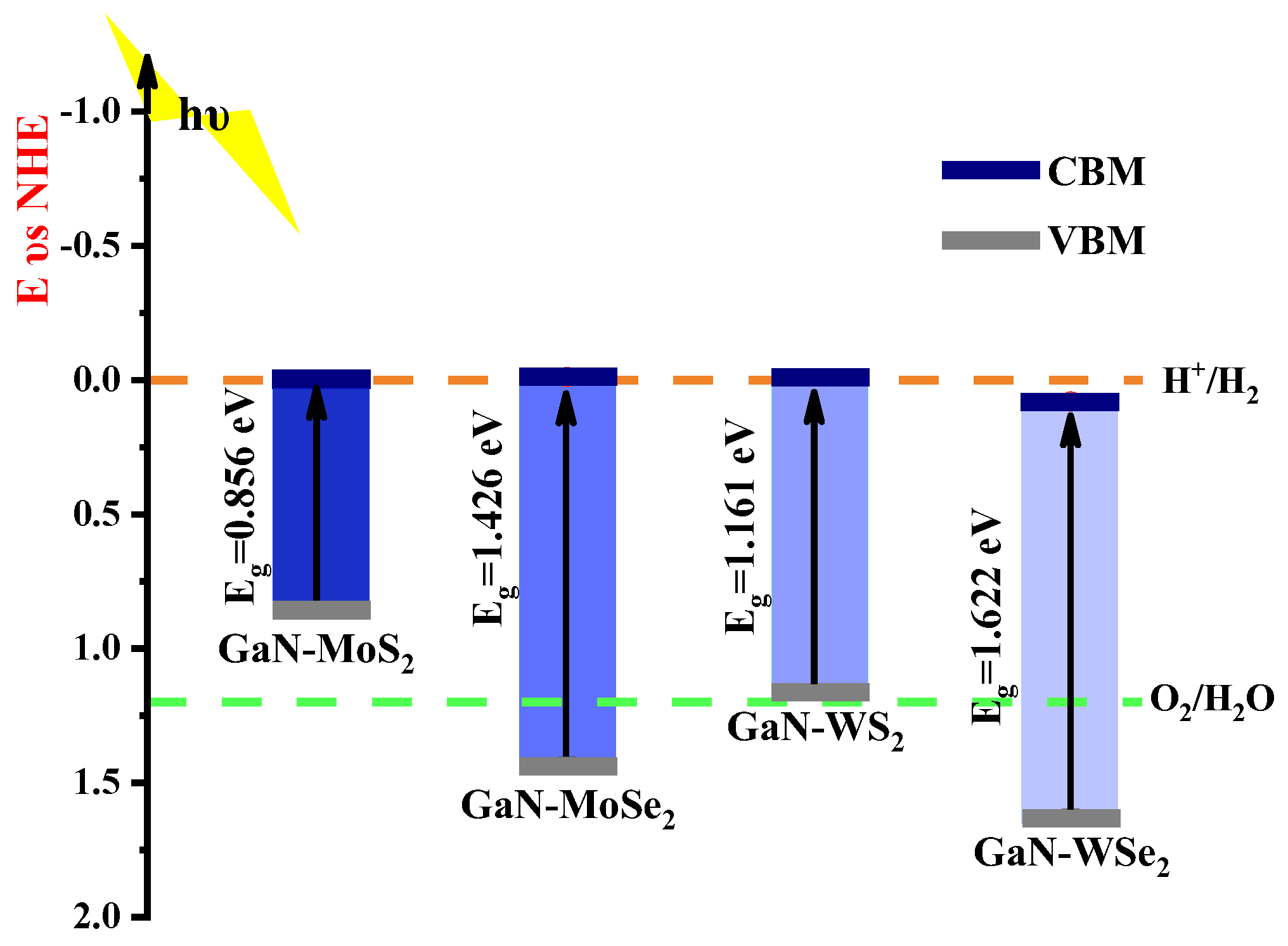
2.4.1. Alignment with Band Structure Edges
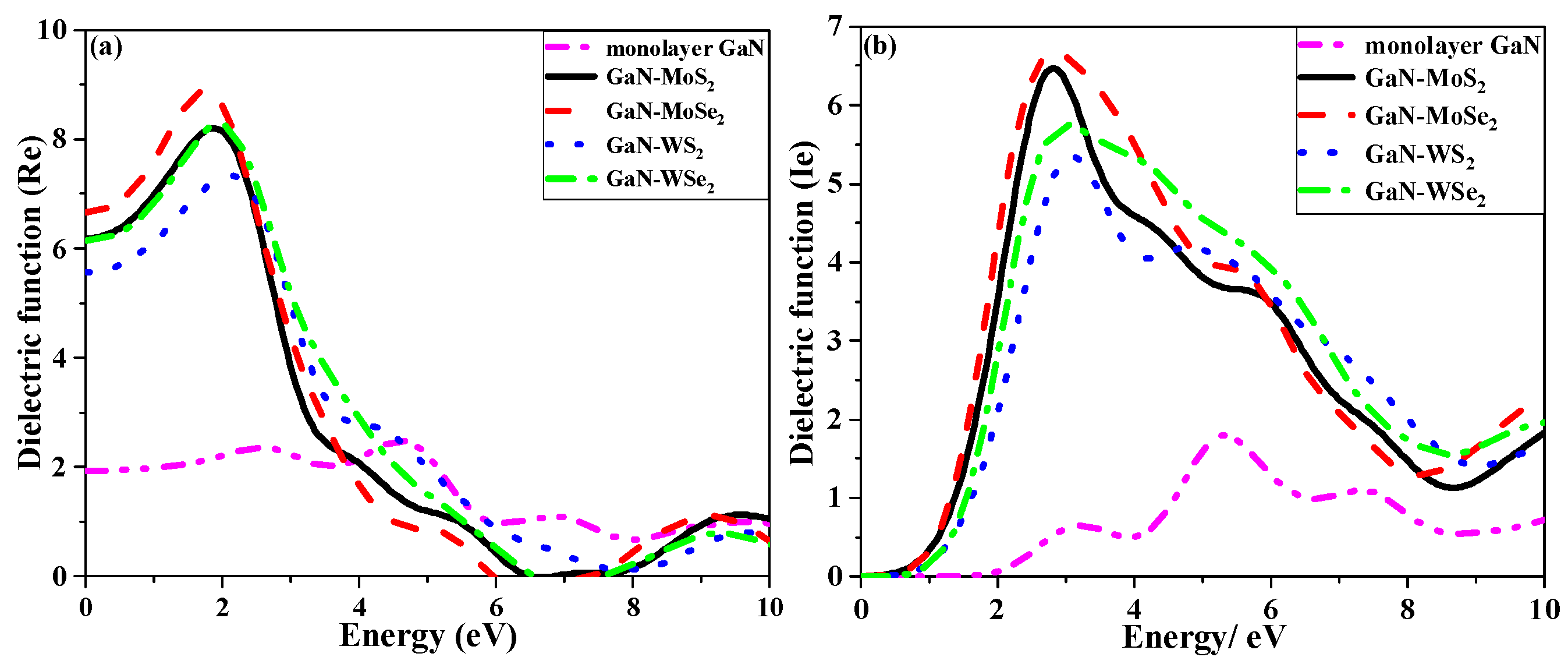
2.4.2. Dielectric Function
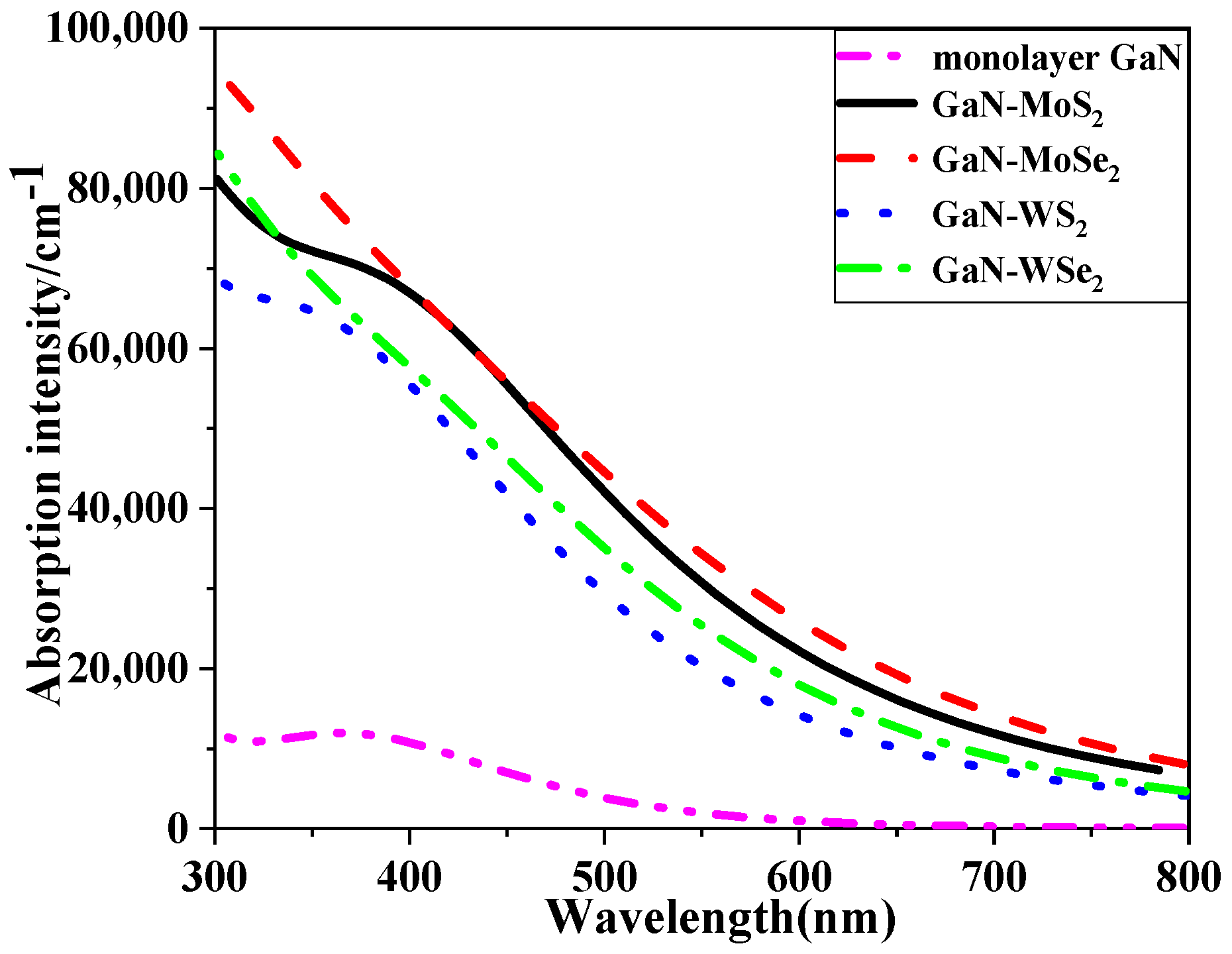
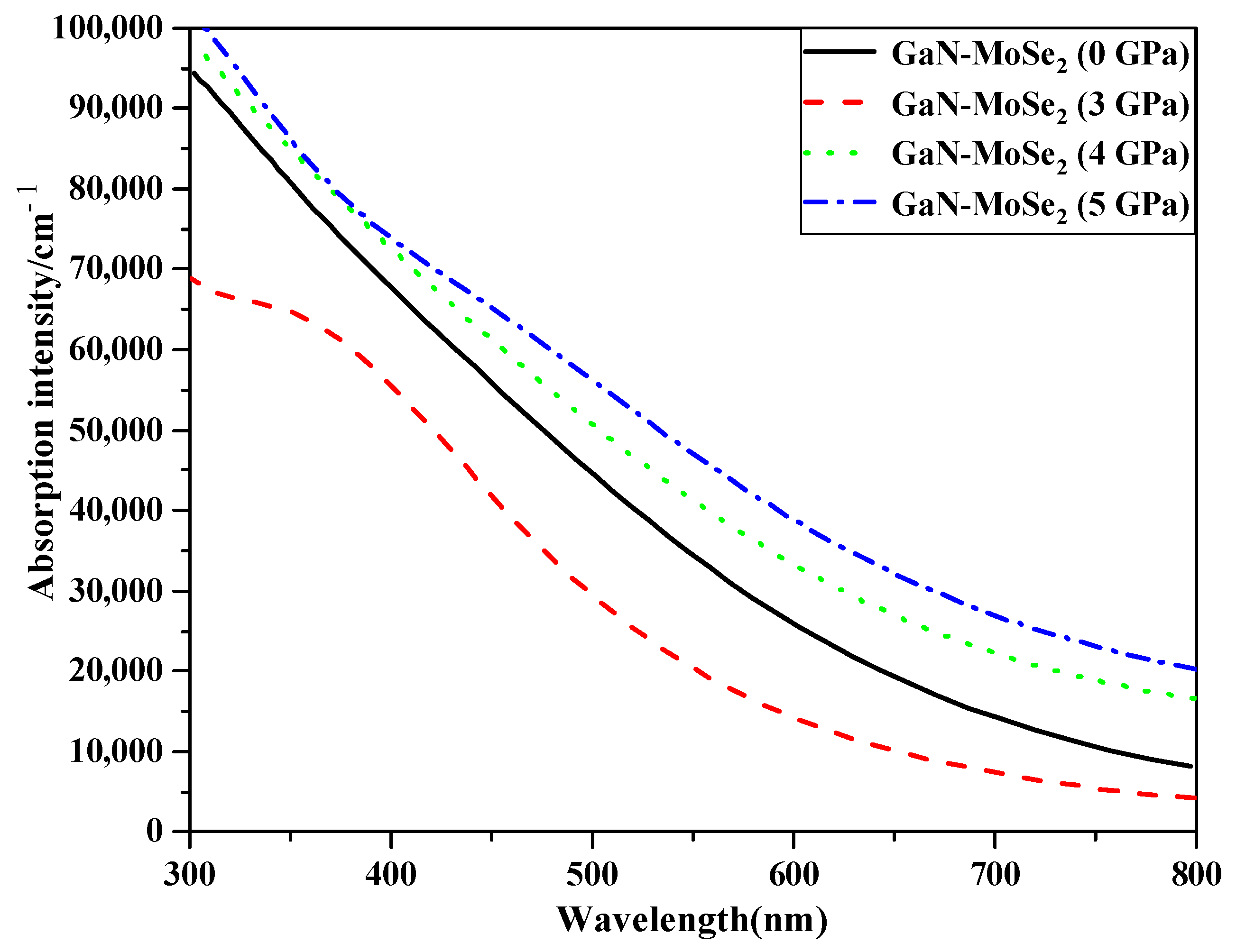
2.4.3. Absorption Spectra
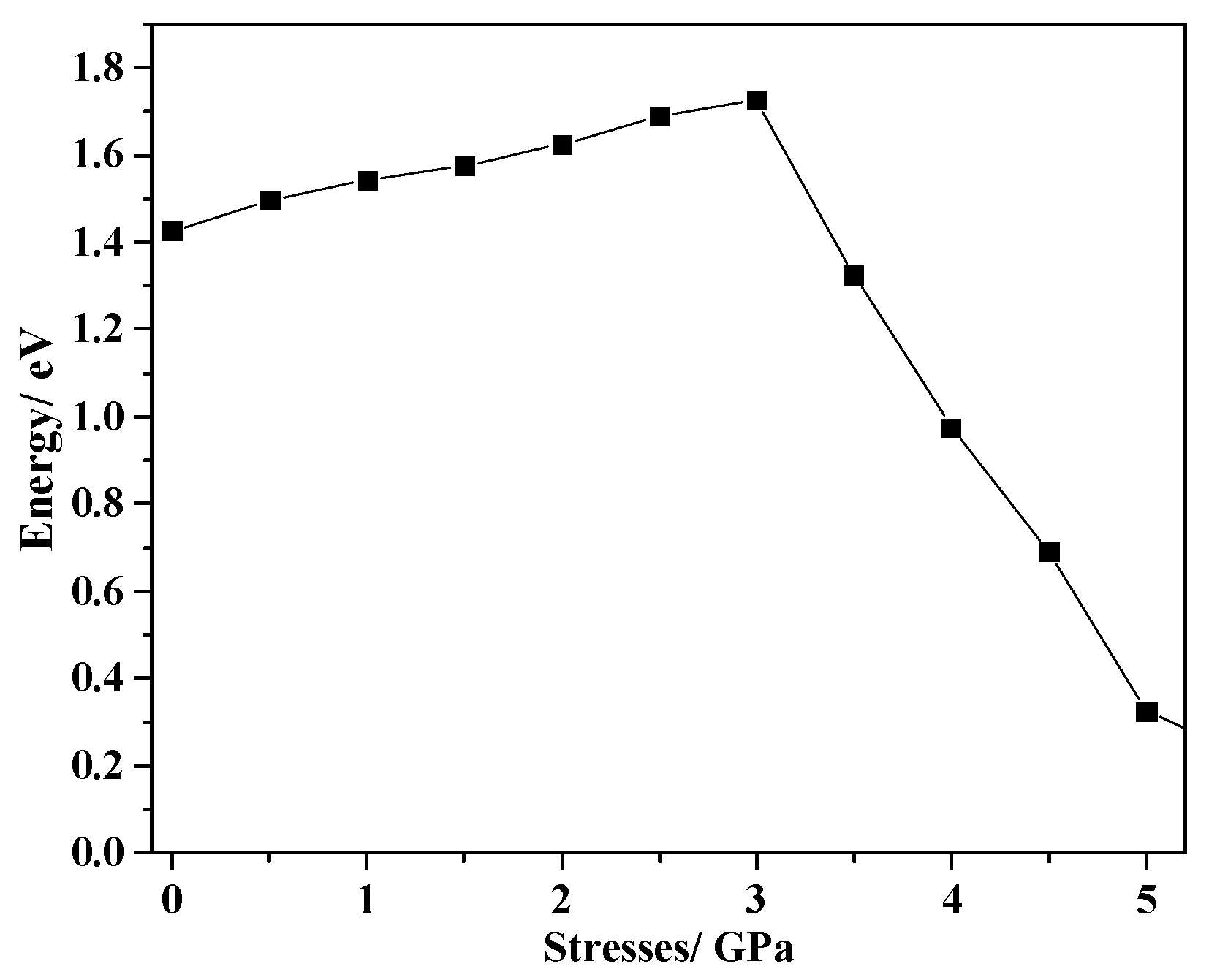
2.5. Applying Pressure
3. Computational Methods and Theoretical Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.R.; Li, S.T.; Huang, H.W. Metal-enhanced strategies for photocatalytic and photoelectrochemical CO2 reduction. Chem. Eng. J. 2023, 457, 141179. [Google Scholar] [CrossRef]
- Hu, Y.F.; Li, B.L.; Yu, C.L.; Fang, H.C.; Li, Z.S. Mechanochemical preparation of single atom catalysts for versatile catalytic applications: A perspective review. Mater. Today 2023, 63, 288–312. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, M.Y.; Tang, Z.R.; Xu, Y.J. Photoredox-catalyzed plastic waste conversion: Nonselective degradation versus selective synthesis. ACS Catal. 2023, 13, 3575–3590. [Google Scholar] [CrossRef]
- Tan, D.M.; Zhuang, R.; Chen, R.C.; Ban, M.H.; Feng, W.; Xu, F.; Chen, X.; Wang, Q.Y. Covalent organic frameworks enable sustainable solar to hydrogen peroxide. Adv. Funct. Mater. 2024, 34, 2311655. [Google Scholar] [CrossRef]
- Wu, Z.X.; Yang, P.F.; Li, Q.C.; Xiao, W.P.; Li, Z.J.; Xu, G.R.; Liu, F.S.; Jia, B.H.; Ma, T.Y.; Feng, S.H.; et al. Microwave synthesis of pt clusters on black TiO2 with abundant oxygen vacancies for efficient acidic electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300406. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-c3n4 fabricated by directly heating melamine. Langmuir ACS J. Surf. Colloids 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Bera, R.; Kundu, S.; Patra, A. 2d hybrid nanostructure of reduced graphene oxide-CdS nanosheet for enhanced photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 13251–13259. [Google Scholar] [CrossRef]
- Jung, H.S.; Hong, Y.J.; Li, Y.; Cho, J.; Kim, Y.; Yi, G. Photocatalysis using GaN nanowires. ACS Nano 2008, 2, 637–642. [Google Scholar] [CrossRef]
- Watanabe, K.; Taniguchi, T.; Kanda, H. Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal. Nat. Mater. 2004, 3, 404–409. [Google Scholar] [CrossRef]
- Winnerl, J.; Kraut, M.; Artmeier, S.; Stutzmann, M. Selectively grown GaN nanowalls and nanogrids for photocatalysis: Growth and optical properties. Nanoscale 2019, 11, 4578–4584. [Google Scholar] [CrossRef]
- Tagliabue, G.; Duchene, J.S.; Abdellah, M.; Habib, A.; Gosztola, D.J.; Hattori, Y.; Cheng, W.; Zheng, K.; Canton, S.E.; Sundararaman, R.; et al. Ultrafast hot-hole injection modifies hot-electron dynamics in au/p-GaN heterostructures. Nat. Mater. 2020, 19, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Brown, E.R.; Growden, T.A.; Berger, P.R. Investigation of peak-to-valley current ratio of GaN/AlN resonant tunneling diodes. IEEE Trans. Electron Devices 2023, 70, 3483–3488. [Google Scholar] [CrossRef]
- Fu, S.; Xiao, Q.; Yao, Y.; Zou, M.; Tang, H.; Ye, J.; Xie, Q. First principles study of the effect of nitrogen defects on the electronic structure and optical property of GaN/g-c3n4 heterojunction. Chin. J. Inorg. Chem. 2023, 39, 1721–1728. [Google Scholar] [CrossRef]
- Han, W.; Liu, Z.; Yu, H. Synthesis and optical properties of GaN/ZnO solid solution nanocrystals. Appl. Phys. Lett. 2010, 96, 183112. [Google Scholar] [CrossRef]
- Jena, K.; Swain, R.; Lenka, T.R. Impact of a drain field plate on the breakdown characteristics of AlInN/GaN MOSHEMT. J. Korean Phys. Soc. 2015, 67, 1592–1596. [Google Scholar] [CrossRef]
- Mohanta, M.K.; Sarkar, A.D. Tweaking the physics of interfaces between monolayers of buckled cadmium sulfide for a superhigh piezoelectricity, excitonic solar cell efficiency, and thermoelectricity. ACS Appl. Mater. Interfaces 2020, 12, 18123–18137. [Google Scholar] [CrossRef]
- Rasool, A.; Anis, I.; Bhat, S.A.; Dar, M.A. Optimizing the NRR activity of single and double boron atom catalysts using a suitable support: A first principles investigation. Phys. Chem. Chem. Phys. 2023, 25, 22275–22285. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Hua, Z.; Liu, B. Enhanced photoelectrochemical performance of cobalt phosphate modified GaN-ZnO nanowires/GaN microrods heterostructure photoanode by vacancy defect modulation. Appl. Surf. Sci. 2024, 642, 158607. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhang, C.; Pu, H.; Zhang, Y.; Zhang, J.; Hao, Y. Transport properties of InGaN channel-based heterostructures with GaN interlayers. J. Electron. Mater. 2024, 53, 1105–1115. [Google Scholar] [CrossRef]
- Guan-Shi, W.; Yan-Ming, L.; Ya-Li, Z.; Zhen-Yi, J.; Xiao-Doug, Z. Electronic and optical performances of (Cu, N) codoped TiO2/MoS2 heterostructure photocatalyst: Hybrid DFT (HSE06) study. Acta Phys. Sin. 2018, 67, 233101. [Google Scholar] [CrossRef]
- Guo, W.; Luo, H.; Jiang, Z.; Shangguan, W. In-situ pressure-induced BiVO4/bi0. 6y0. 4VO4 s-scheme heterojunction for enhanced photocatalytic overall water splitting activity. Chin. J. Catal. 2022, 43, 316–328. [Google Scholar] [CrossRef]
- Ma, Q.F.; Ma, J.Y.; Sun, X.F.; Chen, X.J.; Liu, G.R.; Yang, H. Theoretical and experimental elucidation of synergistic enhanced piezo-photocatalysis and mechanism of BiVO4 for organic pollutant degradation. Mater. Res. Bull. 2024, 170, 112559. [Google Scholar] [CrossRef]
- Qiu, G.; Xiao, Q.; Hu, Y.; Qin, W.; Wang, D. Theoretical study of the surface energy and electronic structure of pyrite FeS2 (100) using a total-energy pseudopotential method, CASTEP. J. Colloid. Interface. Sci. 2004, 270, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhao, Q.; Tang, T.; Yin, J.; Quilty, C.D.; Renderos, G.D.; Liu, X.; Deng, Y.; Wang, L.; Bock, D.C.; et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019, 366, 645–648. [Google Scholar] [CrossRef]
- Kresse; Furthmuller Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Review. B Condens. Matter 1996, 54, 11169–11186. [CrossRef]
- Tian, F.; Kong, D.L.; Qiu, P.; Liu, H.; Zhu, X.L.; Wei, H.Y.; Song, Y.M.; Chen, H.; Zheng, X.H.; Peng, M.Z. Polarization modulation on charge transfer and band structures of GaN/MoS2 polar heterojunctions. Crystals 2023, 13, 563. [Google Scholar] [CrossRef]
- Khanam, R.; Hassan, A.; Nazir, Z.; Dar, M.A. Nickel single atom catalyst supported on the gallium nitride monolayer: First principles investigations on the decisive role of support in the electrocatalytic reduction of CO2. Sustain. Energ. Fuels 2023, 7, 5046–5056. [Google Scholar] [CrossRef]
- Zhang, X.; Matras-Postolek, K.; Yang, P.; Jiang, S.P. Z-scheme WOx/cu-g-c3n4 heterojunction nanoarchitectonics with promoted charge separation and transfer towards efficient full solar- spectrum photocatalysis. J. Colloid. Interface. Sci. 2023, 636, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, M.N.; Zhang, L.L. Electronic and optical properties of GaN/MoSe2 and its vacancy heterojunctions studied by first-principles. J. Appl. Phys. 2023, 133, 045307. [Google Scholar] [CrossRef]
- Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Recent progress on semiconductor heterogeneous photocatalysts in clean energy production and environmental remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Hermann, J.; Distasio, R.A.J.; Tkatchenko, A. First-principles models for van der waals interactions in molecules and materials: Concepts, theory, and applications. Chem. Rev. 2017, 117, 4714–4758. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Yan, Y.F. Optical activity of solids from first principles. Phys. Rev. B 2023, 107, 045201. [Google Scholar] [CrossRef]
- Barnett, R.L.; Polkovnikov, A.; Demler, E.; Yin, W.; Ku, W. Coexistence of gapless excitations and commensurate charge-density wave in the 2h transition metal dichalcogenides. Phys. Rev. Lett. 2006, 96, 26406. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, K.; Tavazza, F. Convergence and machine learning predictions of monkhorst-pack k-points and plane-wave cut-off in high-throughput DFT calculations. Comput. Mater. Sci. 2019, 161, 300–308. [Google Scholar] [CrossRef]
- Faccio, R.; Denis, P.A.; Pardo, H.; Goyenola, C.; Mombru, A.W. Mechanical properties of graphene nanoribbons. J. Phys. Condens. Matter Inst. Phys. J. 2009, 21, 285304. [Google Scholar] [CrossRef]
- Armakovic, S.J.; Savanovic, M.M.; Armakovic, S. Titanium dioxide as the most used photocatalyst for water purification: An overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic degradation of organic and inorganic pollutants to harmless end products: Assessment of practical application potential for water and air cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]



| Model | GaN-MoS2 | GaN-MoSe2 | GaN-WS2 | GaN-WSe2 |
|---|---|---|---|---|
| σ /% | 0.5 | 3.3 | 0.1 | 3.2 |
| Eb/eV | −0.1385 | −0.1512 | −0.1347 | −0.1498 |
| Cell volume/Å3 | 786.404 | 826.206 | 825.014 | 846.888 |
| Length (Å) | 2.9505 | 2.5270 | 2.4218 | 2.4838 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.-C.; Dai, M.-Y.; Lang, F.-M.; Zhao, C.; Chen, Q.-Y.; Zhang, L.-L.; Huang, Y.-N.; Lu, H.-M.; Qin, X.-C. Investigating the Impact of Stress on the Optical Properties of GaN-MX2 (M=Mo, W; X=S, Se) Heterojunctions Using the First Principles. Catalysts 2024, 14, 732. https://doi.org/10.3390/catal14100732
Zhao X-C, Dai M-Y, Lang F-M, Zhao C, Chen Q-Y, Zhang L-L, Huang Y-N, Lu H-M, Qin X-C. Investigating the Impact of Stress on the Optical Properties of GaN-MX2 (M=Mo, W; X=S, Se) Heterojunctions Using the First Principles. Catalysts. 2024; 14(10):732. https://doi.org/10.3390/catal14100732
Chicago/Turabian StyleZhao, Xu-Cai, Meng-Yao Dai, Fu-Mei Lang, Can Zhao, Qiao-Yue Chen, Li-Li Zhang, Yi-Neng Huang, Hai-Ming Lu, and Xiao-Chuan Qin. 2024. "Investigating the Impact of Stress on the Optical Properties of GaN-MX2 (M=Mo, W; X=S, Se) Heterojunctions Using the First Principles" Catalysts 14, no. 10: 732. https://doi.org/10.3390/catal14100732
APA StyleZhao, X.-C., Dai, M.-Y., Lang, F.-M., Zhao, C., Chen, Q.-Y., Zhang, L.-L., Huang, Y.-N., Lu, H.-M., & Qin, X.-C. (2024). Investigating the Impact of Stress on the Optical Properties of GaN-MX2 (M=Mo, W; X=S, Se) Heterojunctions Using the First Principles. Catalysts, 14(10), 732. https://doi.org/10.3390/catal14100732







