Facile Synthesis of Metal/Carbide Hybrid toward Overall Water Splitting
Abstract
1. Introduction
2. Results and Discussion
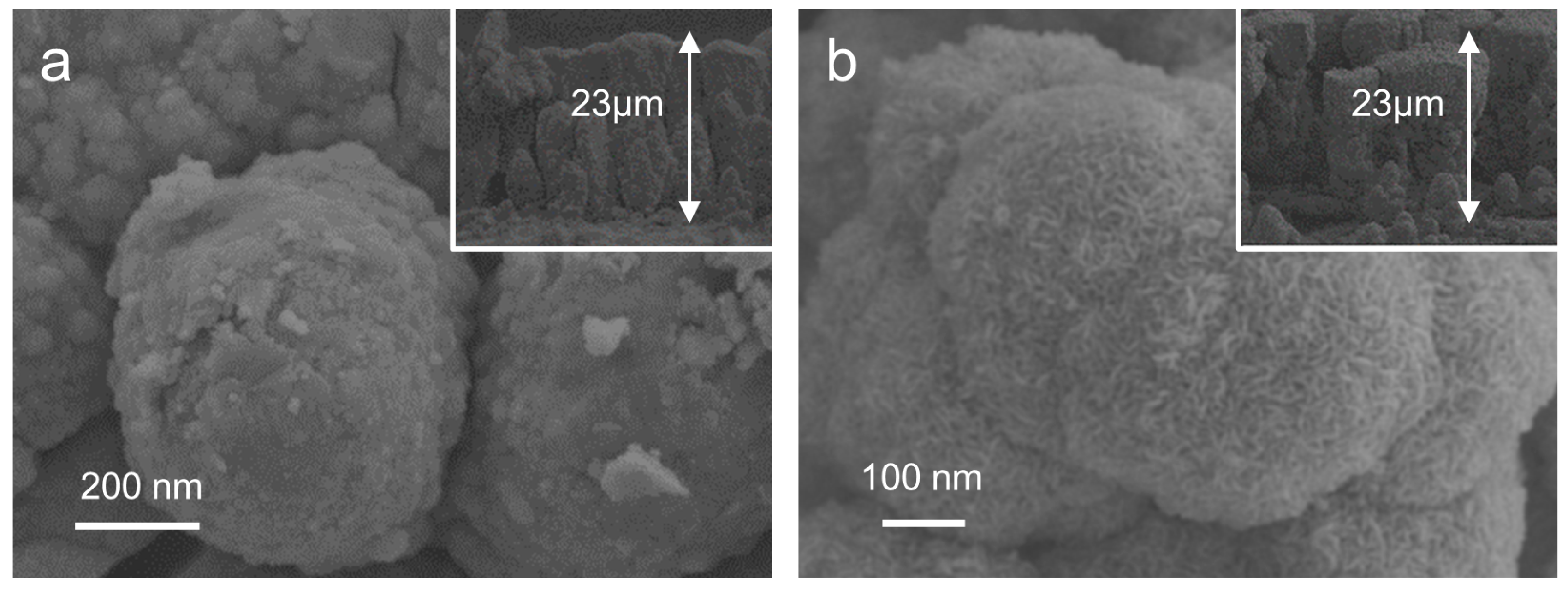
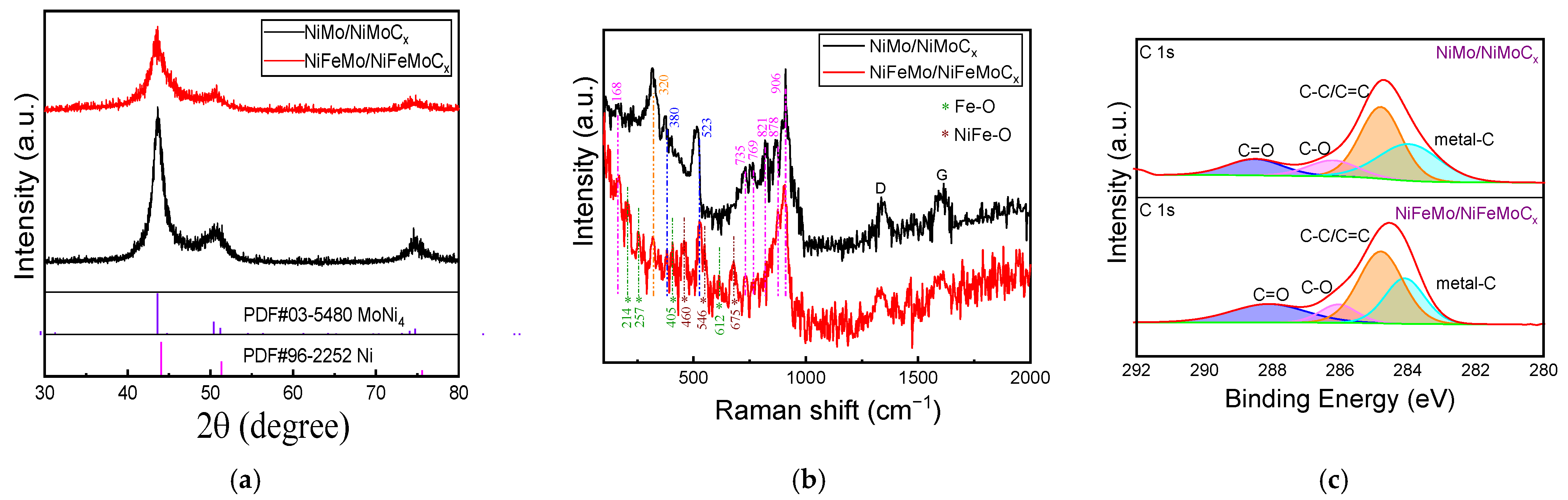
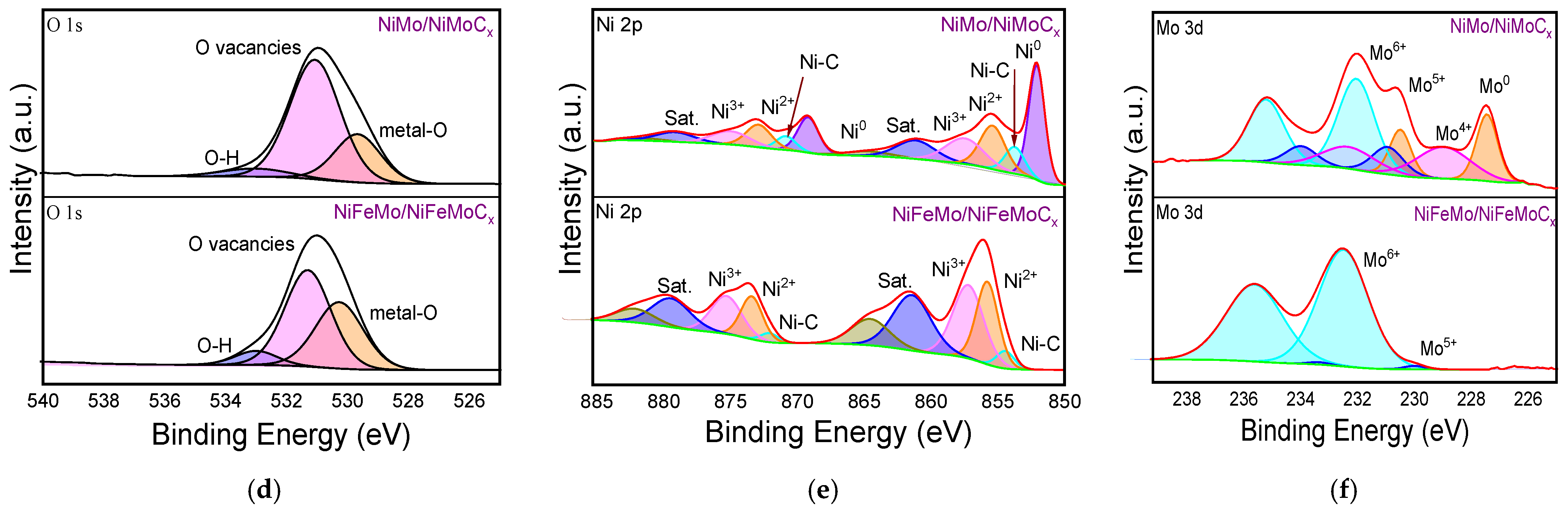
2.1. Morphological and Structural Characterization
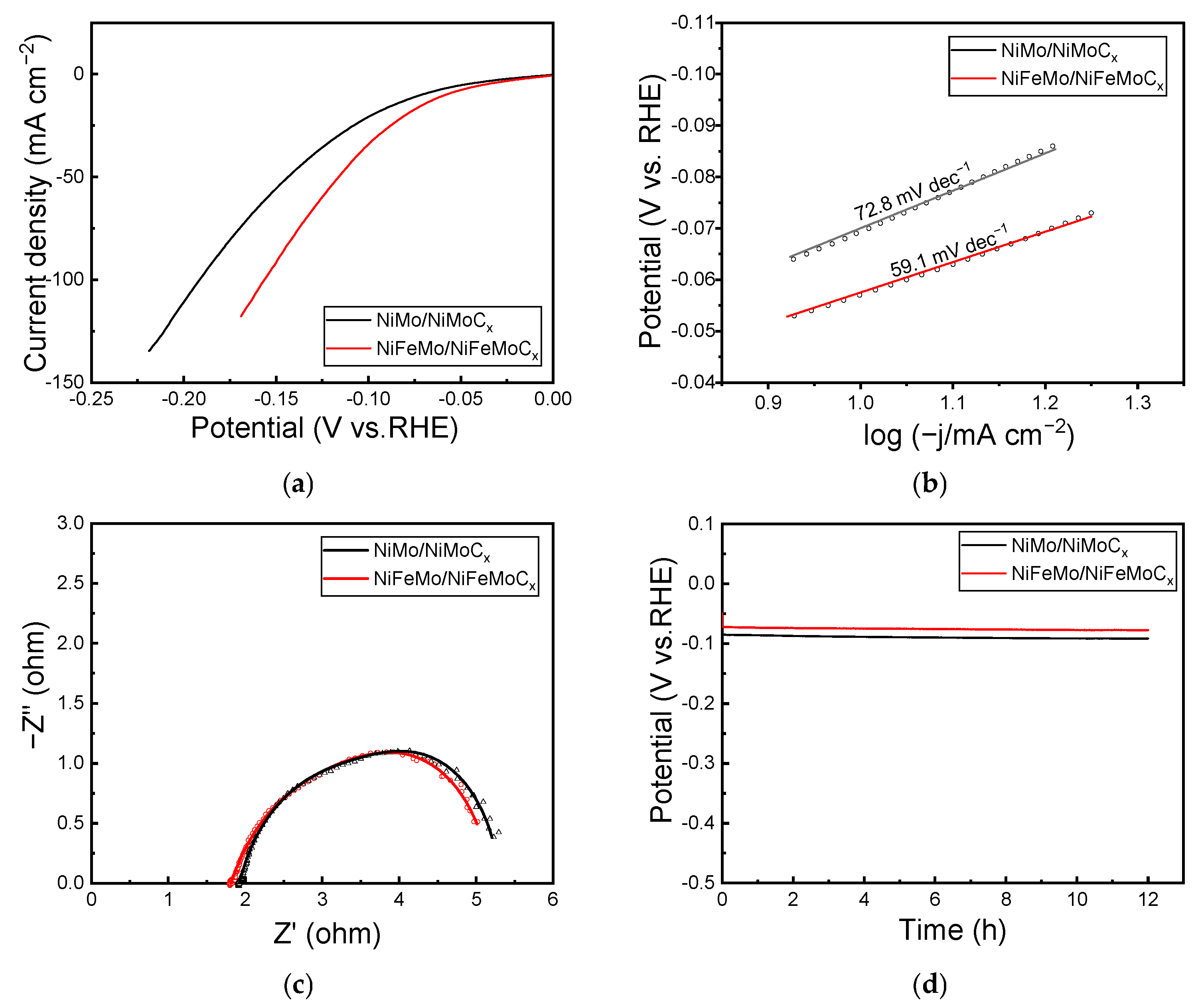
2.2. HER Performance
2.3. OER Performance
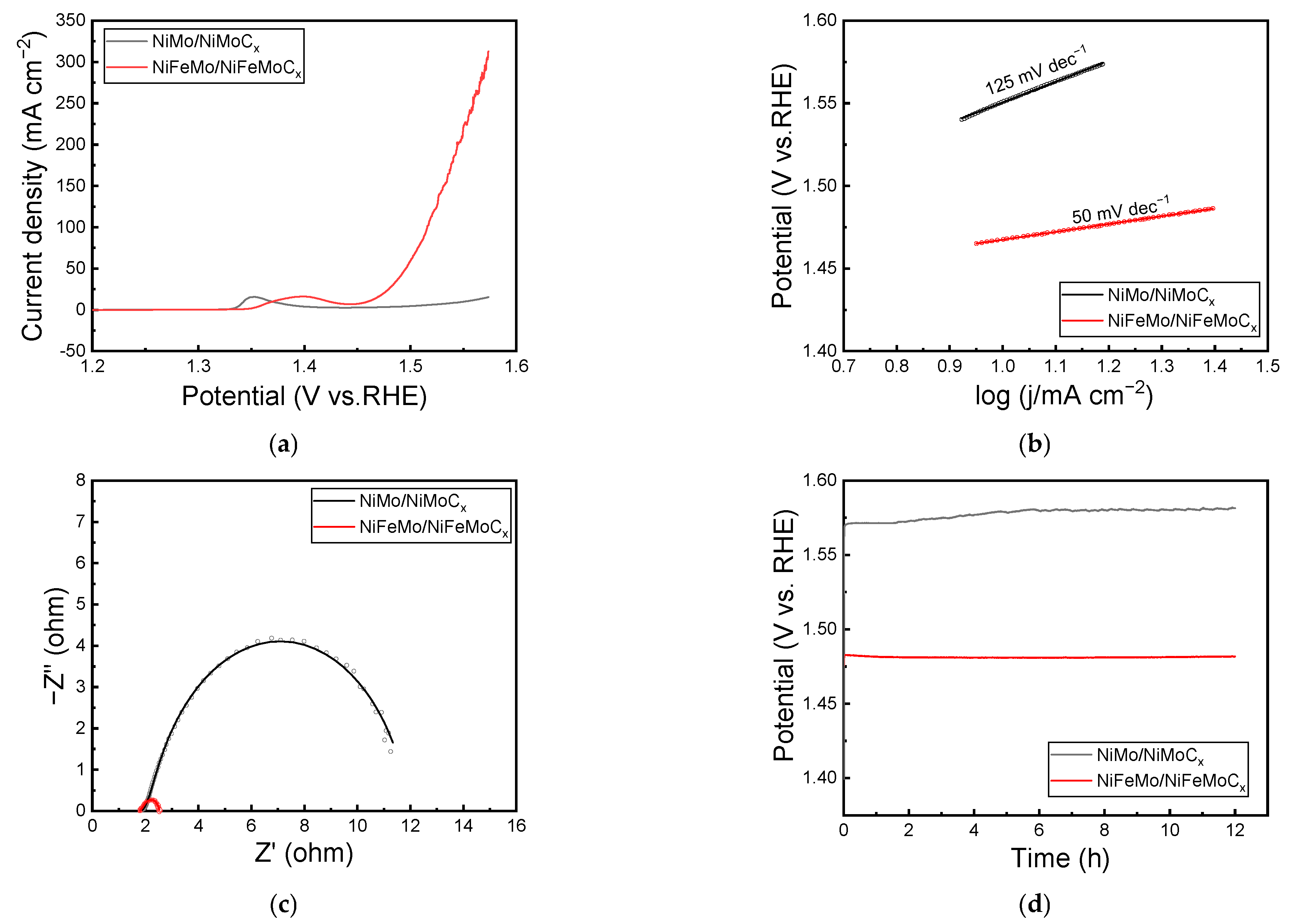
2.4. Overall Water Splitting Performance
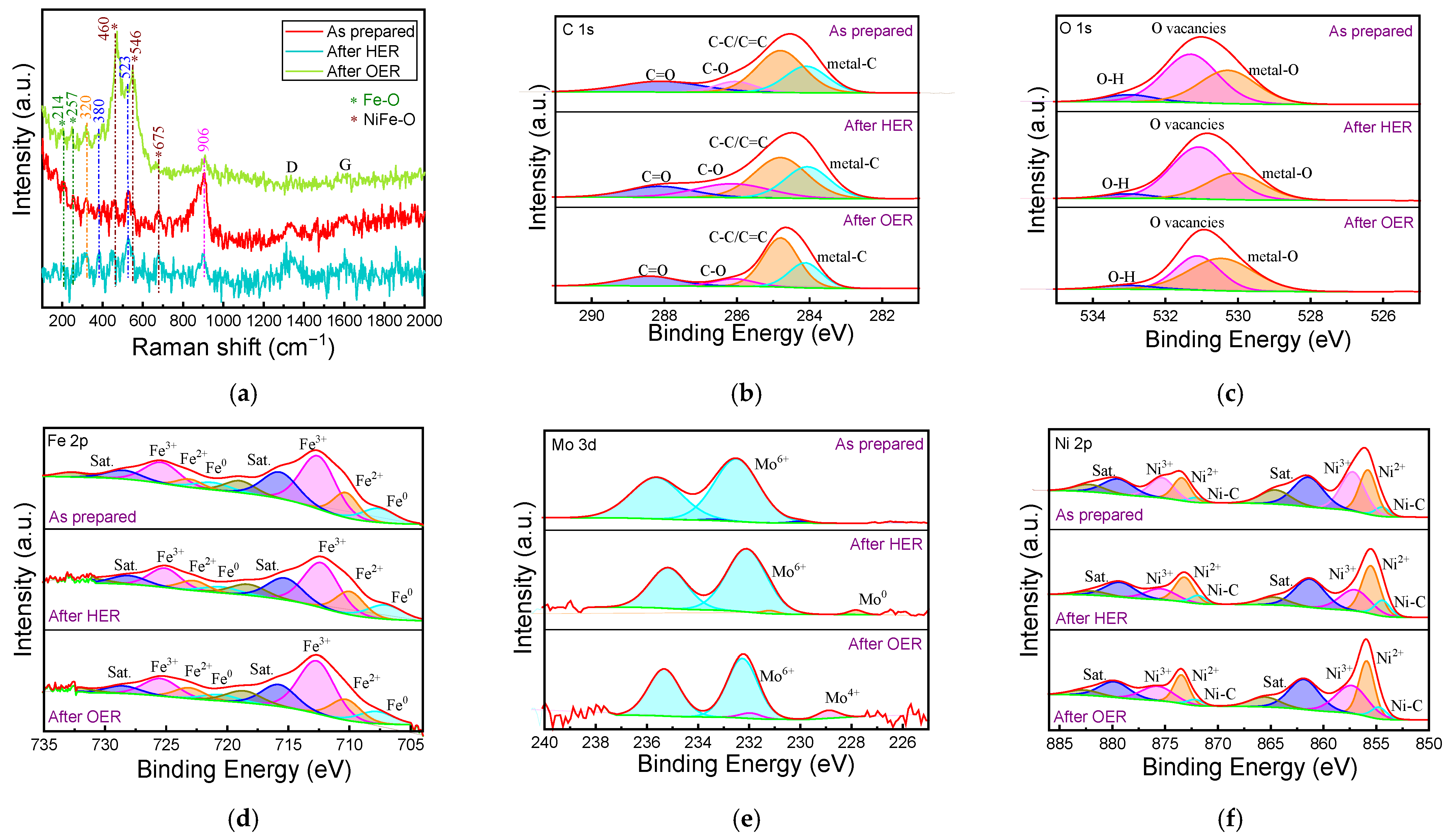
2.5. Mechanism of Stability Enhancement
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Material Characterization
3.4. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huang, D.; Fu, X.; Wen, M.; Jiang, X.; Lv, X.; Li, M.; Gao, L.; Liu, S.; Wang, M.; et al. Engineering NiS/Ni2P Heterostructures for Efficient Electrocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 4689–4696. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Thiehmed, Z.; Shakoor, A.; Altahtamouni, T. Recent Advances in WS2 and Its Based Heterostructures for Water-Splitting Applications. Catalysts 2021, 11, 1283. [Google Scholar] [CrossRef]
- Ibn Shamsah, S.M. Earth-Abundant Electrocatalysts for Water Splitting: Current and Future Directions. Catalysts 2021, 11, 429. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Du, X.; Wang, Q.; Zhang, X. Controllable synthesis of NiO/Ni3S2 hybrid arrays as efficient electrocatalysts for water splitting. New J. Chem. 2018, 42, 18201–18207. [Google Scholar] [CrossRef]
- Gu, J.; Duan, F.; Liu, S.; Cha, W.; Lu, J. Phase Engineering of Nanostructural Metallic Materials: Classification, Structures, and Applications. Chem. Rev. 2024, 124, 1247–1287. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Chen, G.; Shao, Z. Perovskite oxides as electrocatalysts for water electrolysis: From crystalline to amorphous. Carbon Energy 2024, e595. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, J.; Liu, Y.; Lu, W.; Fu, N.; Li, W.; Huang, H. Boosting the oxygen evolution reaction in non-precious catalysts by structural and electronic engineering. J. Mater. Chem. A 2018, 6, 10253–10263. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Shah, K.; Dai, R.; Mateen, M.; Hassan, Z.; Zhuang, Z.; Liu, C.; Israr, M.; Cheong, W.-C.; Hu, B.; Tu, R.; et al. Cobalt Single Atom Incorporated in Ruthenium Oxide Sphere: A Robust Bifunctional Electrocatalyst for HER and OER. Angew. Chem. Int. Ed. 2022, 61, e202114951. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New Nanocomposites Derived from Cation-Nonstoichiometric Bax(Co, Fe, Zr, Y)O3−δ as Efficient Electrocatalysts for Water Oxidation in Alkaline Solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Zhang, Z.; Chen, P.; Zhang, Y.; Dong, X. Dual-metal hydroxide@oxide heterojunction catalyst constructed via corrosion engineering for large-current oxygen evolution reaction. Appl. Catal. B 2023, 325, 122311. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Niu, S.; Sun, Y.; Sun, G.; Rakov, D.; Li, Y.; Ma, Y.; Chu, J.; Xu, P. Stepwise Electrochemical Construction of FeOOH/Ni(OH)2 on Ni Foam for Enhanced Electrocatalytic Oxygen Evolution. ACS Appl. Energy Mater. 2019, 2, 3927–3935. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Chen, N.; Che, S.; Liu, H.; Ta, N.; Li, G.; Chen, F.; Ma, G.; Yang, F.; Li, Y. In Situ Growth of Self-Supporting MOFs-Derived Ni2P on Hierarchical Doped Carbon for Efficient Overall Water Splitting. Catalysts 2022, 12, 1319. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Pei, L.; Huang, Y.; Chen, D.; Xie, Z. Hollow NiCoP Nanoprisms Derived from Prussian Blue Analogues as Bifunctional Electrocatalysts for Urea-Assisted Hydrogen Production in Alkaline Media. Small 2022, 18, 2205547. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Liu, K.R.; Chen, J.S.; Wei, X.J. A study on the electrodeposited Ni-S alloys as hydrogen evolution reaction cathodes. Int. J. Hydrogen Energy 2003, 28, 1207–1212. [Google Scholar] [CrossRef]
- Youn, J.-S.; Jeong, S.; Oh, I.; Park, S.; Mai, H.D.; Jeon, K.-J. Enhanced Electrocatalytic Activity of Stainless Steel Substrate by Nickel Sulfides for Efficient Hydrogen Evolution. Catalysts 2020, 10, 1274. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, W.; Zhang, Y.; Dou, Y.; Jiang, Y.; Dou, S.X. Engineering Hierarchical Hollow Nickel Sulfide Spheres for High-Performance Sodium Storage. Adv. Funct. Mater. 2016, 26, 7479–7485. [Google Scholar] [CrossRef]
- Xing, Z.; Li, Q.; Wang, D.; Yang, X.; Sun, X. Self-supported nickel nitride as an efficient high-performance three-dimensional cathode for the alkaline hydrogen evolution reaction. Electrochim. Acta 2016, 191, 841–845. [Google Scholar] [CrossRef]
- Ma, Y.; He, Z.; Wu, Z.; Zhang, B.; Zhang, Y.; Ding, S.; Xiao, C. Galvanic-replacement mediated synthesis of copper-nickel nitrides as electrocatalyst for hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 24850–24858. [Google Scholar] [CrossRef]
- Hu, S.; Feng, C.; Wang, S.; Liu, J.; Wu, H.; Zhang, L.; Zhang, J. Ni3N/NF as Bifunctional Catalysts for Both Hydrogen Generation and Urea Decomposition. ACS Appl. Mater. Interfaces 2019, 11, 13168–13175. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, S.Y.; Kang, Y.C. First Introduction of NiSe2 to Anode Material for Sodium-Ion Batteries: A Hybrid of Graphene-Wrapped NiSe2/C Porous Nanofiber. Sci. Rep. 2016, 6, 23338. [Google Scholar] [CrossRef]
- Xu, K.; Ding, H.; Lv, H.; Tao, S.; Chen, P.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Understanding Structure-Dependent Catalytic Performance of Nickel Selenides for Electrochemical Water Oxidation. ACS Catal. 2017, 7, 310–315. [Google Scholar] [CrossRef]
- McKone, J.R.; Sadtler, B.F.; Werlang, C.A.; Lewis, N.S.; Gray, H.B. Ni-Mo Nanopowders for Efficient Electrochemical Hydrogen Evolution. ACS Catal. 2013, 3, 166–169. [Google Scholar] [CrossRef]
- Liu, X.; Liu, P.; Wang, F.; Lv, X.; Yang, T.; Tian, W.; Wang, C.; Tan, S.; Ji, J. Plasma-Induced Defect Engineering and Cation Refilling of NiMoO4 Parallel Arrays for Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 41545–41554. [Google Scholar] [CrossRef] [PubMed]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.-J.; Sokaras, D.; Weng, T.-C.; Alonso-Mori, R.; et al. Identification of Highly Active Fe Sites in (Ni,Fe)OOH for Electrocatalytic Water Splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-T.; Yuan, G.-G.; Weng, C.-C.; Chen, L.; Yuan, Z.-Y. Uniquely integrated Fe-doped Ni(OH)2 nanosheets for highly efficient oxygen and hydrogen evolution reactions. Nanoscale 2018, 10, 10620–10628. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Oh, E.; Lim, C.; Shim, S.E.; Baeck, S.-H. Bimetallic NiFe alloys as highly efficient electrocatalysts for the oxygen evolution reaction. Catal. Today 2020, 352, 27–33. [Google Scholar] [CrossRef]
- Yang, N.; Tang, C.; Wang, K.; Du, G.; Asiri, A.M.; Sun, X. Iron-doped nickel disulfide nanoarray: A highly efficient and stable electrocatalyst for water splitting. Nano Res. 2016, 9, 3346–3354. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, Z.; Huang, J.; Gao, F. Fe-doped NiO mesoporous nanosheets array for highly efficient overall water splitting. J. Catal. 2018, 358, 243–252. [Google Scholar] [CrossRef]
- Gupta, P.; Tenhundfeld, G.; Daigle, E.O.; Ryabkov, D. Electrolytic plasma technology: Science and engineering—An overview. Surf. Coat. Technol. 2007, 201, 8746–8760. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Li, Y. A review of plasma-liquid interactions for nanomaterial synthesis. J. Phys. D Appl. Phys. 2015, 48, 424005. [Google Scholar] [CrossRef]
- Quan, C.; He, Y.; Zhang, J. High temperature oxidation behavior of a novel Ni-Cr binary alloy coating prepared by cathode plasma electrolytic deposition. Surf. Coat. Technol. 2016, 292, 11–19. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, X.; Ma, X.; Liu, Y.; Ying, Y.; Guo, X.; Fu, N.; Yu, F.; Wu, H.; Zhu, Y.; et al. A fast and general approach to produce a carbon coated Janus metal/oxide hybrid for catalytic water splitting. J. Mater. Chem. A 2021, 9, 7606–7616. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; Fu, N.; Mu, S.; Peng, J.; Liu, Y.; Zhang, G. Phase Engineering and Dispersion Stabilization of Cobalt toward Enhanced Hydrogen Evolution. Small 2024, 20, 2310499. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, H.-W.; Deng, Y.; Lu, Z.; Hsu, P.-C.; Liu, Y.; Lin, D.; Cui, Y. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 2015, 6, 7261. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; Joseph, B.; Caramazza, S.; Capitani, F.; Bendele, M.; Kantor, I.; Lotti, P.; Mathon, O.; Pascarelli, S.; Postorino, P. Local structure investigation of β-Ni(OH)2 under pressure using combined Raman and Ni K-edge extended x-ray absorption fine structure studies. High Pressure Res. 2017, 37, 1–10. [Google Scholar] [CrossRef]
- Dong, D.; Wang, W.; Barnabe, A.; Presmanes, L.; Rougier, A.; Dong, G.; Zhang, F.; Yu, H.; He, Y.; Diao, X. Enhanced electrochromism in short wavelengths for NiO:(Li, Mg) films in full inorganic device ITO/NiO:(Li, Mg)/Ta2O5/WO3/ITO. Electrochim. Acta 2018, 263, 277–285. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Komatsu, T.; Iordanova, R.; Dimitriev, Y. Study of molybdenum coordination state and crystallization behavior in MoO3–La2O3–B2O3 glasses by Raman spectroscopy. J. Phys. Chem. Solids 2011, 72, 263–268. [Google Scholar] [CrossRef]
- Rahman, G.; Joo, O.-S. Electrodeposited nanostructured α-Fe2O3 thin films for solar water splitting: Influence of Pt doping on photoelectrochemical performance. Mater. Chem. Phys. 2013, 140, 316–322. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Huang, J.; Gao, F. A Co-doped Ni-Fe mixed oxide mesoporous nanosheet array with low overpotential and high stability towards overall water splitting. J. Mater. Chem. A 2018, 6, 167–178. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Y.; Hu, K.; Tao, L.; Huang, X.; Huo, J.; Wang, S. In situ confined synthesis of molybdenum oxide decorated nickel–iron alloy nanosheets from MoO42− intercalated layered double hydroxides for the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 87–91. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, Y.; Edvinsson, T. In operando Raman investigation of Fe doping influence on catalytic NiO intermediates for enhanced overall water splitting. Nano Energy 2019, 66, 104118. [Google Scholar] [CrossRef]
- Duan, D.; Guo, D.; Gao, J.; Liu, S.; Wang, Y. Electrodeposition of cobalt-iron bimetal phosphide on Ni foam as a bifunctional electrocatalyst for efficient overall water splitting. J. Colloid Interface Sci. 2022, 622, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Han, R.; Wang, J.; Yang, L.; Fan, G.; Li, F. In situ synthesis of nickel carbide-promoted nickel/carbon nanofibers nanocomposite catalysts for catalytic applications. Chem. Eng. J. 2015, 275, 36–44. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, W.; Shi, Z.; Yang, L.; Tang, Y. Structural Design and Electronic Modulation of Transition-Metal-Carbide Electrocatalysts toward Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1802880. [Google Scholar] [CrossRef]
- Das, D.; Santra, S.; Nanda, K.K. In Situ Fabrication of a Nickel/Molybdenum Carbide-Anchored N-Doped Graphene/CNT Hybrid: An Efficient (Pre)catalyst for OER and HER. ACS Appl. Mater. Interfaces 2018, 10, 35025–35038. [Google Scholar] [CrossRef]
- Yuan, Z.; Yao, X.; Zhang, G.; Fu, N.; Liu, Y.; Ye, F. Regulating the Heterostructure of Metal/Oxide toward the Enhanced Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 5644–5651. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Wang, J.; Wang, Y.; Li, L. Fe3+ doped amorphous Co2BOy(OH)z with enhanced activity for oxygen evolution reaction. Electrochim. Acta 2018, 280, 1–8. [Google Scholar] [CrossRef]
- Gibson, J.S.; Uddin, J.; Cundari, T.R.; Bodiford, N.K.; Wilson, A.K. First-principle study of structure and stability of nickel carbides. J. Phys. Condens. Matter 2010, 22, 445503. [Google Scholar] [CrossRef]
- Xu, Z.; Ying, Y.; Zhang, G.; Li, K.; Liu, Y.; Fu, N.; Guo, X.; Yu, F.; Huang, H. Engineering NiFe layered double hydroxide by valence control and intermediate stabilization toward the oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 26130–26138. [Google Scholar] [CrossRef]
- Han, N.; Luo, S.; Deng, C.; Zhu, S.; Xu, Q.; Min, Y. Defect-Rich FeN0.023Mo2C Heterostructure as a Highly Efficient Bifunctional Catalyst for Overall Water-Splitting. ACS Appl. Mater. Interfaces 2021, 13, 8306–8314. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Zhou, Y.; Zhao, S.; Chen, J.; Dai, R.; Zhou, W.; Yang, P.; Zhang, H.; Chen, A. Bimetallic co-doped CoP nanoflower arrays for efficient and stable overall water splitting. Int. J. Hydrogen Energy 2024, 51, 276–284. [Google Scholar] [CrossRef]
- Li, D.; Wu, S.; Jiang, T.; Huang, S.; Wang, Z.; Wu, H.; Cai, G.; Ren, F. A highly efficient heterostructure nanorod bifunctional electrocatalyst for realizing enhanced overall water splitting at a large current density. J. Mater. Chem. A 2023, 11, 18158–18167. [Google Scholar] [CrossRef]
- Ren, F.; Xu, J.; Feng, L. An effective bimetallic oxide catalyst of RuO2-Co3O4 for alkaline overall water splitting. Nano Res. 2023, 17, 3785–3793. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Tang, K.; Li, Q.; Yan, C. Blending Fe3O4 into a Ni/NiO composite for efficient and stable bifunctional electrocatalyst. Electrochim. Acta 2018, 264, 225–232. [Google Scholar] [CrossRef]
- Suryanto, B.H.R.; Wang, Y.; Hocking, R.K.; Adamson, W.; Zhao, C. Overall electrochemical splitting of water at the heterogeneous interface of nickel and iron oxide. Nat. Commun. 2019, 10, 5599. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharyya, S. Porous NiFe-Oxide Nanocubes as Bifunctional Electrocatalysts for Efficient Water-Splitting. ACS Appl. Mater. Interfaces 2017, 9, 41906–41915. [Google Scholar] [CrossRef]
- Gurusamy, P.; Perumalsamy, S.V.; Gnanasekar, P.; Kulandaivel, J.; Paramasivam, T.; Pandian, T. Synthesis of Ni-doped 1T/2H MoS2 nanoparticles as an efficient and stable bifunctional electrocatalyst for overall water-splitting. Int. J. Hydrogen Energy 2024, 77, 244–252. [Google Scholar] [CrossRef]
- Janani, G.; Yuvaraj, S.; Surendran, S.; Chae, Y.; Sim, Y.; Song, S.-J.; Park, W.; Kim, M.-J.; Sim, U. Enhanced bifunctional electrocatalytic activity of Ni-Co bimetallic chalcogenides for efficient water-splitting application. J. Alloys Compd. 2020, 846, 156389. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Wei, Y.; Xia, L.; Pi, C.; Song, H.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K. General synthesis of NiCo alloy nanochain arrays with thin oxide coating: A highly efficient bifunctional electrocatalyst for overall water splitting. J. Alloys Compd. 2019, 797, 1216–1223. [Google Scholar] [CrossRef]
- Xu, T.; Jiao, D.; Liu, M.; Zhang, L.; Fan, X.; Zheng, L.; Zheng, W.; Cui, X. Ni Center Coordination Reconstructed Nanocorals for Efficient Water Splitting. Adv. Sci. 2023, 10, 2205605. [Google Scholar] [CrossRef]
- Srinivas, K.; Chen, Y.; Su, Z.; Yu, B.; Karpuraranjith, M.; Ma, F.; Wang, X.; Zhang, W.; Yang, D. Heterostructural CoFe2O4/CoO nanoparticles-embedded carbon nanotubes network for boosted overall water-splitting performance. Electrochim. Acta 2022, 404, 139745. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, J.; Fu, N.; Mu, S.; Peng, J.; Liu, Y.; Zhang, G. Facile Synthesis of Metal/Carbide Hybrid toward Overall Water Splitting. Catalysts 2024, 14, 730. https://doi.org/10.3390/catal14100730
Mo J, Fu N, Mu S, Peng J, Liu Y, Zhang G. Facile Synthesis of Metal/Carbide Hybrid toward Overall Water Splitting. Catalysts. 2024; 14(10):730. https://doi.org/10.3390/catal14100730
Chicago/Turabian StyleMo, Junxiang, Nianqing Fu, Songlin Mu, Jihua Peng, Yan Liu, and Guoge Zhang. 2024. "Facile Synthesis of Metal/Carbide Hybrid toward Overall Water Splitting" Catalysts 14, no. 10: 730. https://doi.org/10.3390/catal14100730
APA StyleMo, J., Fu, N., Mu, S., Peng, J., Liu, Y., & Zhang, G. (2024). Facile Synthesis of Metal/Carbide Hybrid toward Overall Water Splitting. Catalysts, 14(10), 730. https://doi.org/10.3390/catal14100730






