A Highly Efficient Catalytic Co-Combustion of Aromatic and Oxygenated Volatile Organic Compounds (VOCs) via H2-Driven Onsite Heating
Abstract
1. Introduction
2. Results
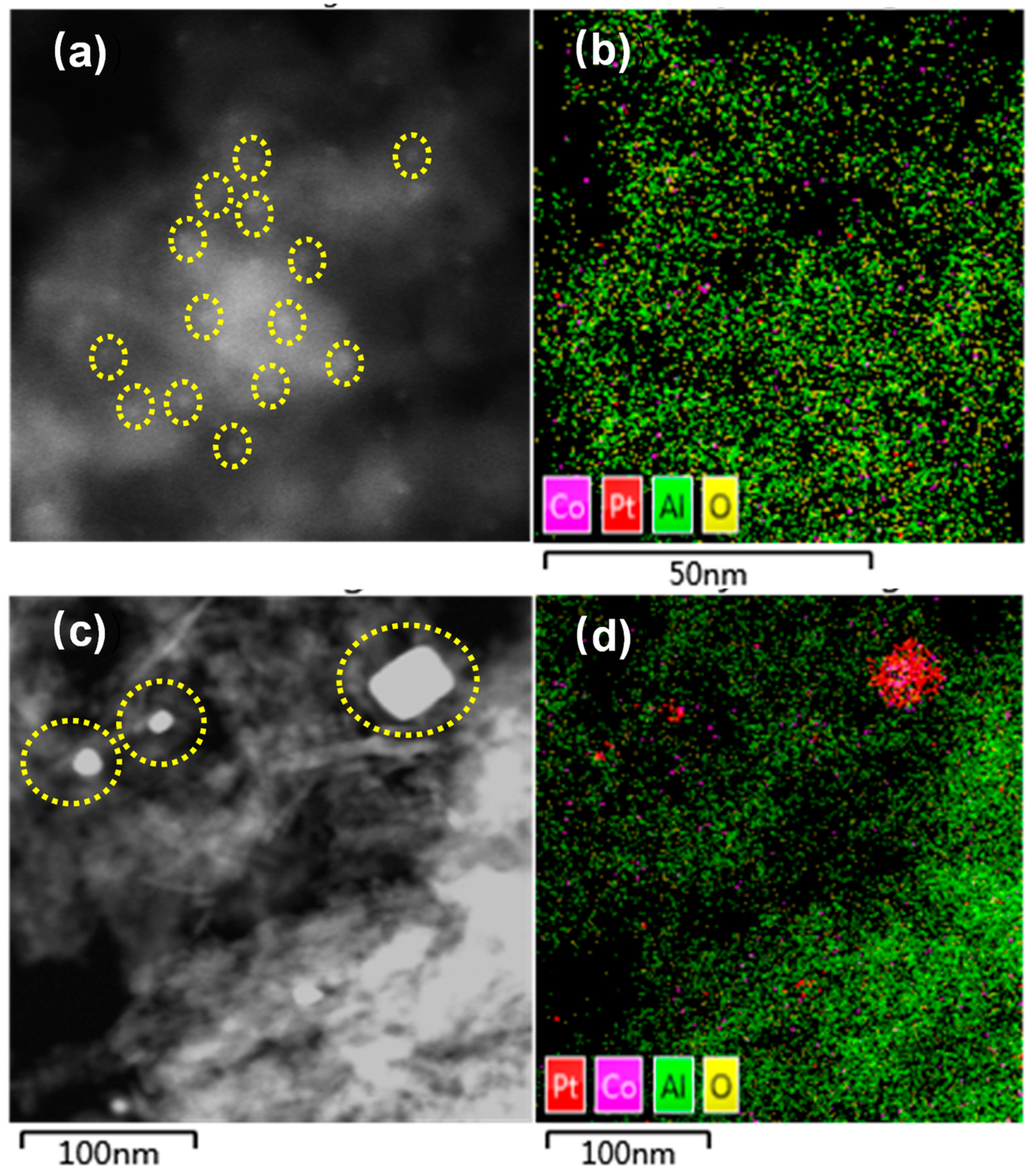
2.1. Structure Evolution of Catalyst
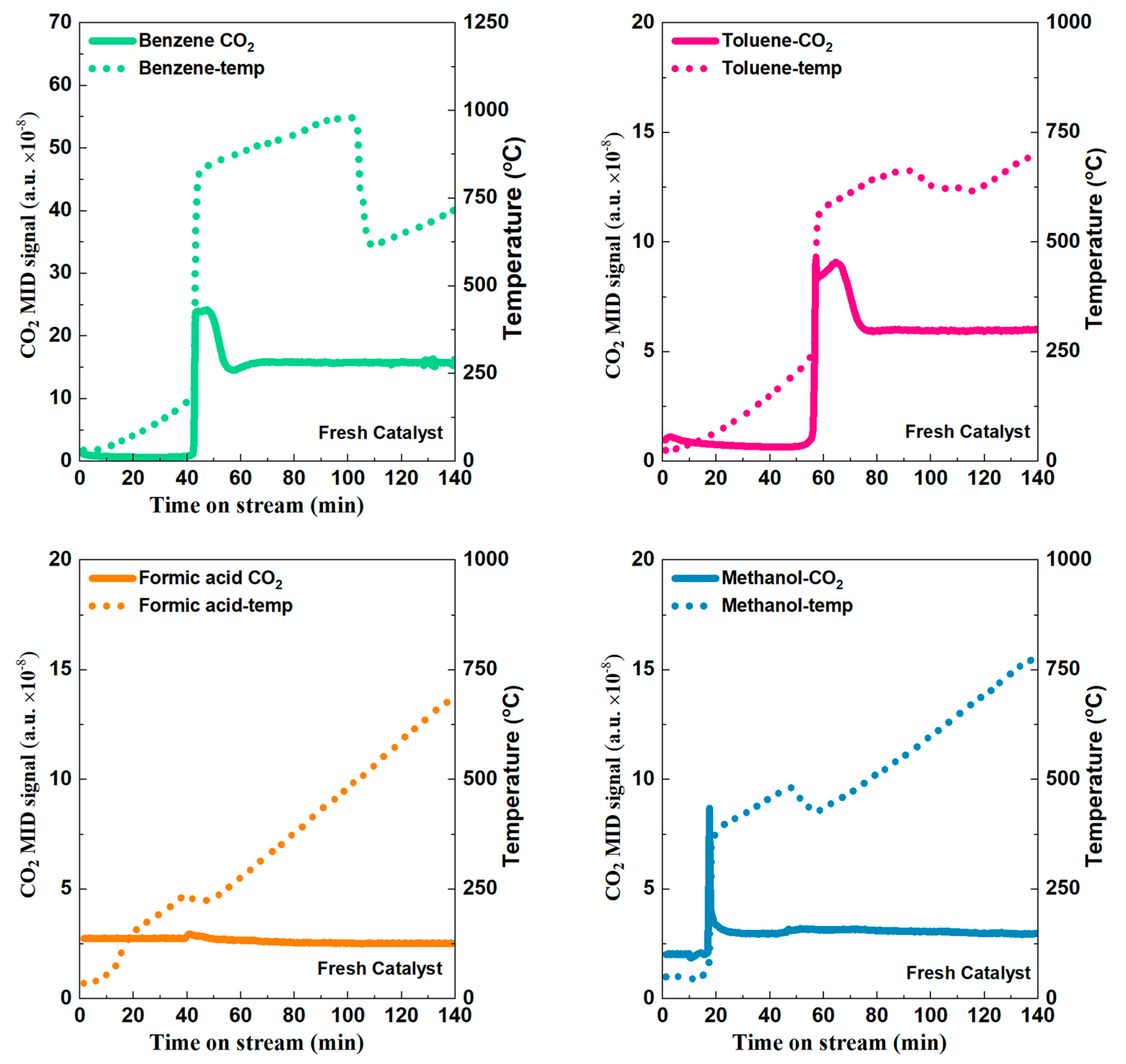
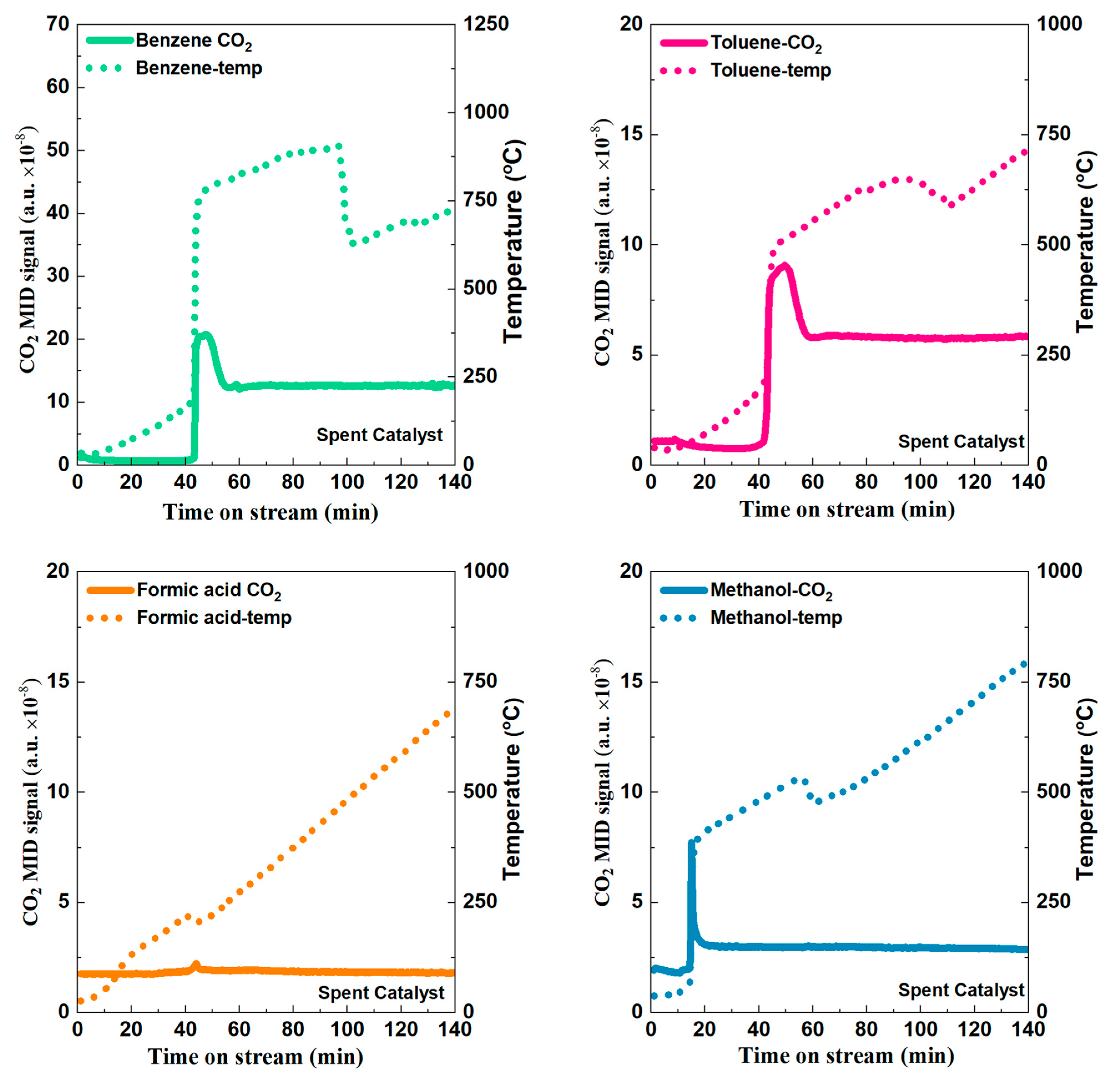
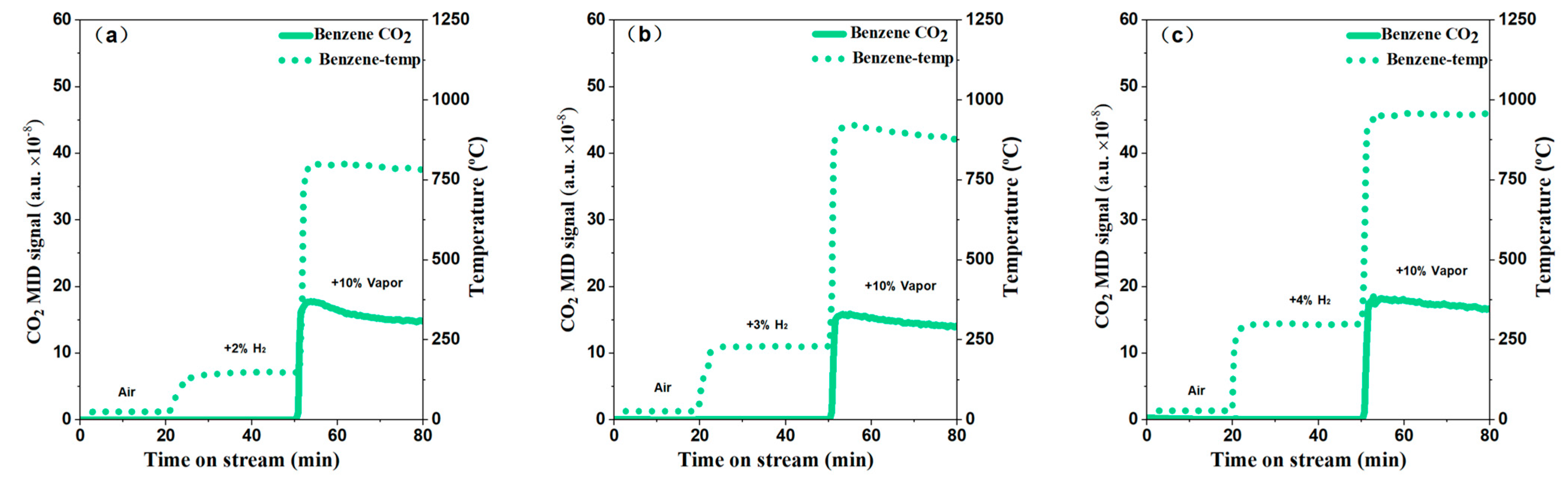
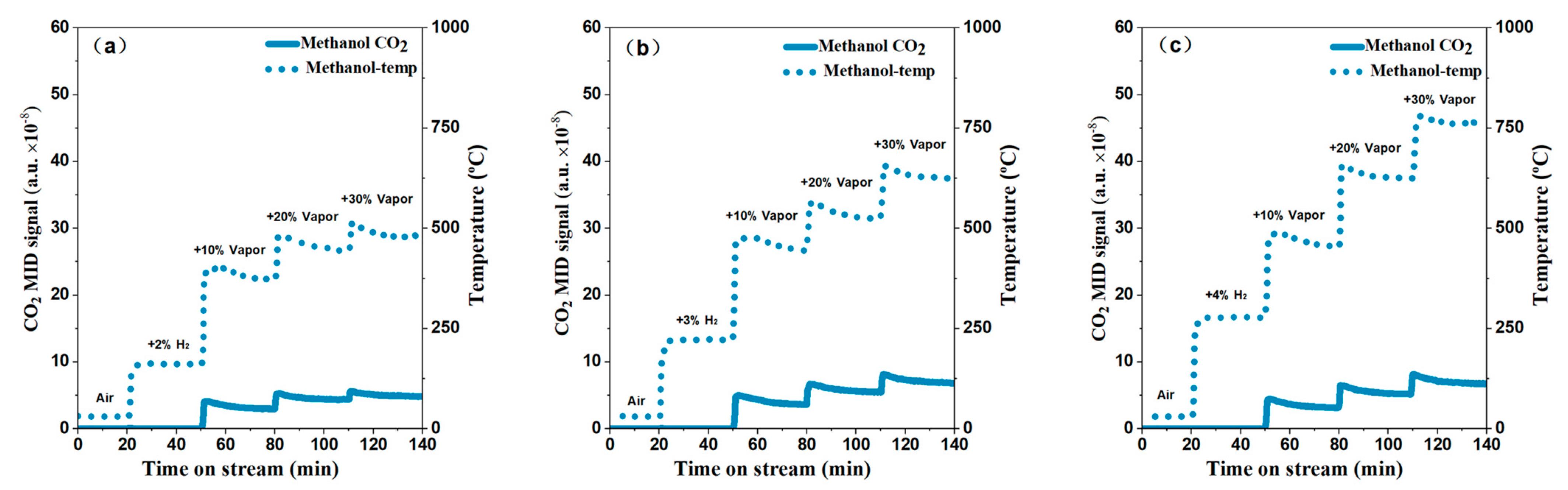
2.2. Catalytic Performance Test
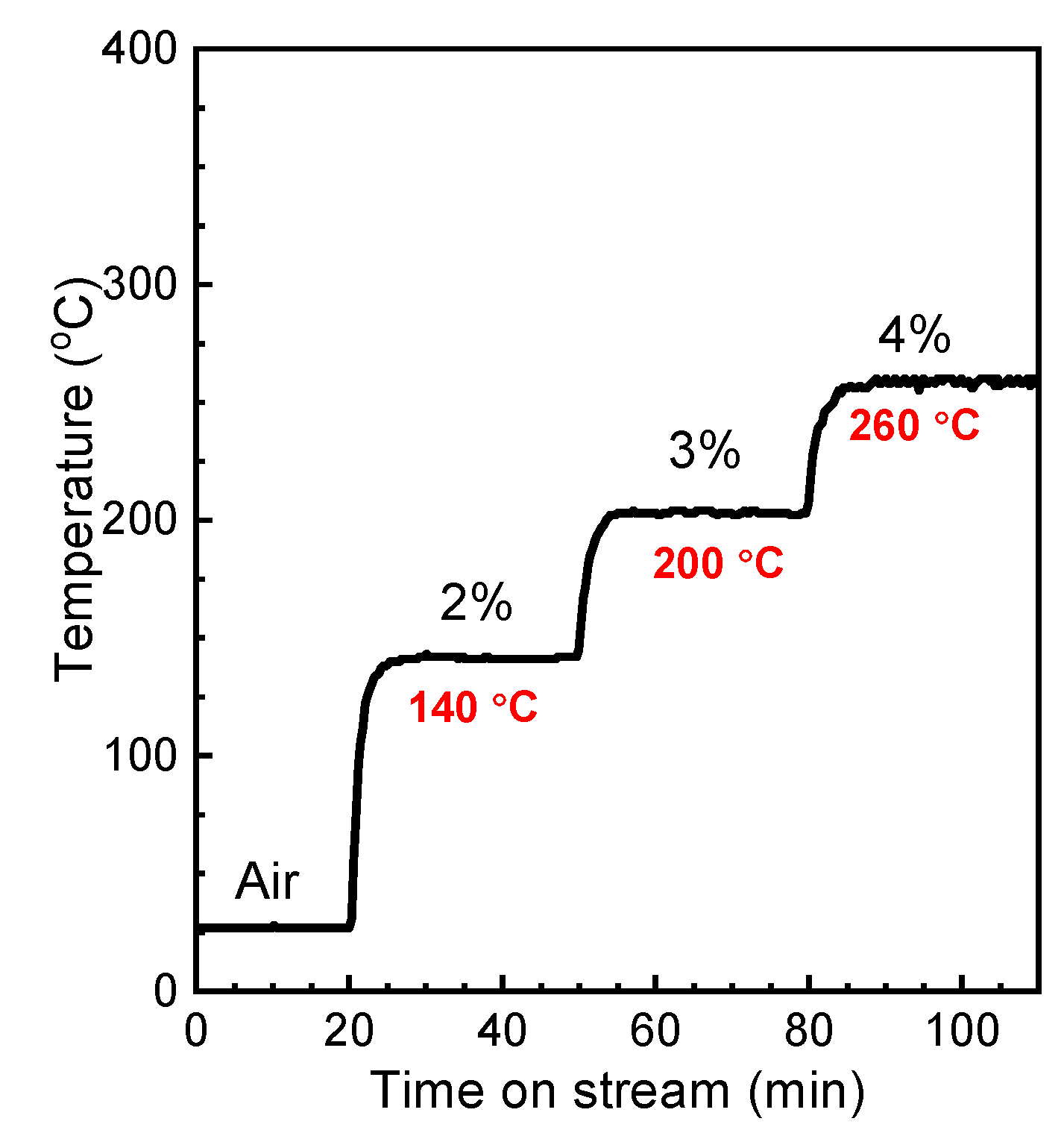
2.3. Electric Heating and Onsite Heating
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterizations
3.3. Reactor Setup and Performance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geng, W.; Zhang, J.; Yuan, D.; Sun, R.; Peng, L. Application Study on Three-Bed Regenerative Thermal Oxidizers to Treat Volatile Organic Compounds. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 042137. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, Z.; Li, N.; Yan, B.; He, C.; Hao, Z.; Chen, G. How to achieve complete elimination of Cl-VOCs: A critical review on byproducts formation and inhibition strategies during catalytic oxidation. Chem. Eng. J. 2021, 404, 126534. [Google Scholar] [CrossRef]
- Guo, M.; Li, K.; Zhang, H.; Min, X.; Liang, J.; Hu, X.; Guo, W.; Jia, J.; Sun, T. Promotional removal of oxygenated VOC over manganese-based multi oxides from spent lithium-ions manganate batteries: Modification with Fe, Bi and Ce dopants. Sci. Total Environ. 2020, 740, 139951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chu, W.; Chen, F.; Li, L.; Jiang, R.; Yan, J. Effects of cerium precursors on surface properties of mesoporous CeMnOx catalysts for toluene combustion. J. Rare Earths 2020, 38, 70–75. [Google Scholar] [CrossRef]
- Schnelle Jr, K.B.; Brown, C.A. Air Pollution Control Technology Handbook; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Us, E. Control Technologies for Hazardous Air Pollutants; EPA/625/6-91/014; US Environmental Protection Agency: Washington, DC, USA, 1991. [Google Scholar]
- Liu, B.; Ji, J.; Zhang, B.; Huang, W.; Gan, Y.; Leung, D.Y.; Huang, H. Catalytic ozonation of VOCs at low temperature: A comprehensive review. J. Hazard. Mater. 2022, 422, 126847. [Google Scholar] [CrossRef]
- Brown, R.W.; Bull, I.D.; Journeaux, T.; Chadwick, D.R.; Jones, D.L. Volatile organic compounds (VOCs) allow sensitive differentiation of biological soil quality. Soil Biol. Biochem. 2021, 156, 108187. [Google Scholar] [CrossRef]
- Muir, B.; Sobczyk, M.; Bajda, T. Fundamental features of mesoporous functional materials influencing the efficiency of removal of VOCs from aqueous systems: A review. Sci. Total Environ. 2021, 784, 147121. [Google Scholar] [CrossRef]
- Yang, Y.; Si, W.; Peng, Y.; Chen, J.; Wang, Y.; Chen, D.; Tian, Z.; Wang, J.; Li, J. Oxygen vacancy engineering on copper-manganese spinel surface for enhancing toluene catalytic combustion: A comparative study of acid treatment and alkali treatment. Appl. Catal. B Environ. 2024, 340, 123142. [Google Scholar] [CrossRef]
- Chen, L.; Liao, Y.; Chen, Y.; Wu, J.; Ma, X. Performance of Ce-modified VW-Ti type catalyst on simultaneous control of NO and typical VOCS. Fuel Process. Technol. 2020, 207, 106483. [Google Scholar] [CrossRef]
- Shayegan, Z.; Haghighat, F.; Lee, C.-S. Surface fluorinated Ce-doped TiO2 nanostructure photocatalyst: A trap and remove strategy to enhance the VOC removal from indoor air environment. Chem. Eng. J. 2020, 401, 125932. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Wu, X.; Chen, Y. Recent advance on VOCs oxidation over layered double hydroxides derived mixed metal oxides. Chin. J. Catal. 2020, 41, 550–560. [Google Scholar] [CrossRef]
- Ren, S.; Liang, W.; Fang, H.; Zhu, Y. Performance and poisoning analysis of organic sulfur resistance of Pd-Ce catalyst in catalytic oxidation of VOCs. J. Environ. Chem. Eng. 2021, 9, 106640. [Google Scholar] [CrossRef]
- Zhang, S.; Pu, W.; Chen, A.; Xu, Y.; Wang, Y.; Yang, C.; Gong, J. Oxygen vacancies enhanced photocatalytic activity towards VOCs oxidation over Pt deposited Bi2WO6 under visible light. J. Hazard. Mater. 2020, 384, 121478. [Google Scholar] [CrossRef]
- Liu, G.; Tian, Y.; Zhang, B.; Wang, L.; Zhang, X. Catalytic combustion of VOC on sandwich-structured Pt@ ZSM-5 nanosheets prepared by controllable intercalation. J. Hazard. Mater. 2019, 367, 568–576. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Zhou, R. Pt-support interaction and nanoparticle size effect in Pt/CeO2–TiO2 catalysts for low temperature VOCs removal. Chemosphere 2021, 265, 129127. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; He, Z.; Wang, D.; Bo, Q.; Fan, T.; Jiang, Y. Mo-modified Pd/Al2O3 catalysts for benzene catalytic combustion. J. Environ. Sci. 2014, 26, 1481–1487. [Google Scholar] [CrossRef]
- Tahsini, N.; Yang, A.-C.; Streibel, V.; Werghi, B.; Goodman, E.D.; Aitbekova, A.; Bare, S.R.; Li, Y.; Abild-Pedersen, F.; Cargnello, M. Colloidal Platinum–Copper Nanocrystal Alloy Catalysts Surpass Platinum in Low-Temperature Propene Combustion. J. Am. Chem. Soc. 2022, 144, 1612–1621. [Google Scholar] [CrossRef]
- Dietrich, P.J.; Akatay, M.C.; Sollberger, F.G.; Stach, E.A.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Effect of Co Loading on the Activity and Selectivity of PtCo Aqueous Phase Reforming Catalysts. ACS Catal. 2014, 4, 480–491. [Google Scholar] [CrossRef]
- Wu, D.; Jia, R.; Wen, M.; Zhong, S.; Wu, Q.; Fu, Y.; Yu, S. Ultrastable PtCo/Co3O4-SiO2 Nanocomposite with Active Lattice Oxygen for Superior Catalytic Activity toward CO Oxidation. Inorg. Chem. 2020, 59, 1218–1226. [Google Scholar] [CrossRef]
- Gao, L.; Liu, J.; Zhong, X.; Zhang, Y.; Chen, J.; Shakeri, M.; Zhang, X.; Zhang, B. Structural Regulation of PtCo/Y Zeolite Catalysts for the Selective Hydrogenation of 3-Nitrostyrene to 3-Vinylaniline. ACS Appl. Nano Mater. 2023, 6, 5685–5691. [Google Scholar] [CrossRef]
- Zhu, A.; Zhou, Y.; Wang, Y.; Zhu, Q.; Liu, H.; Zhang, Z.; Lu, H. Catalytic combustion of VOCs on Pt/CuMnCe and Pt/CeY honeycomb monolithic catalysts. J. Rare Earths 2018, 36, 1272–1277. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Catalytic hydrogen combustion for domestic and safety applications: A critical review of catalyst materials and technologies. Energies 2021, 14, 4897. [Google Scholar] [CrossRef]
- Zhong, B.-J.; Yang, Q.-T.; Yang, F. Hydrogen-assisted catalytic ignition characteristics of different fuels. Combust. Flame 2010, 157, 2005–2007. [Google Scholar] [CrossRef]
- Deutschmann, O.; Maier, L.; Riedel, U.; Stroemman, A.; Dibble, R. Hydrogen assisted catalytic combustion of methane on platinum. Catal. Today 2000, 59, 141–150. [Google Scholar] [CrossRef]
- Yuan, L.-J.; Zhao, Z.-C.; Wang, W.-Q.; Wang, Y.-F.; Liu, Y.-J. Review of Catalysts, Substrates, and Fabrication Methods in Catalytic Hydrogen Combustion with Further Challenges and Applications. Energy Fuels 2024, 38, 4881–4903. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Ullah, L.; Munsif, S.; Cao, L.; Zhang, J.-C.; Li, W.-Z. Facile Abatement of Oxygenated Volatile Organic Compounds via Hydrogen Co-Combustion over Pd/Al2O3 Catalyst as Onsite Heating Source. Catalysts 2024, 14, 372. [Google Scholar] [CrossRef]
- Ullah, L.; Munsif, S.; Cao, L.; Murthy, P.R.; Zhang, J.-C.; Li, W.-Z. Hydrogen Co-Combustion of Aromatic Volatile Organic Compounds over Pd/Al2O3 Catalyst. Catalysts 2024, 14, 563. [Google Scholar] [CrossRef]
- Ladacki, M.; Houser, T.J.; Roberts, R.W. The catalyzed low-temperature hydrogen-oxygen reaction. J. Catal. 1965, 4, 239–247. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Properties and performance of Pd based catalysts for catalytic oxidation of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 429–436. [Google Scholar] [CrossRef]
- Kim, J.; Yu, J.; Lee, S.; Tahmasebi, A.; Jeon, C.-H.; Lucas, J. Advances in catalytic hydrogen combustion research: Catalysts, mechanism, kinetics, and reactor designs. Int. J. Hydrogen Energy 2021, 46, 40073–40104. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Y.; Yang, D.P.; Dai, J.; Liu, Z.; Chen, Y.; Huang, J.; Li, Q. Biogenic Pt/CaCO3 Nanocomposite as a Robust Catalyst toward Benzene Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Lu, Y.; Liu, Y.; Liu, N.; Hu, J.; Wei, L.; Li, Z.; Tian, X.; Gao, R.; Yu, X.; et al. A General Dual-Metal Nanocrystal Dissociation Strategy to Generate Robust High-Temperature-Stable Alumina-Supported Single-Atom Catalysts. J. Am. Chem. Soc. 2023, 145, 15869–15878. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Jian, Y.; Liu, Q.; Liu, Y.; Wang, J.; Chai, S.; Jing, M.; Albilali, R.; He, C. Boosted Light Alkane Deep Oxidation via Metal Bond Length Modulation-Induced C–C Bond Preferential Activation. Environ. Sci. Technol. 2024, 58, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Deng, J.; Liu, Y.; Jing, L.; Wang, J.; Wang, Z.; Dai, H. Mesoporous NaxMnOy-Supported Platinum–Cobalt Bimetallic Single-Atom Catalysts with Good Sulfur Dioxide Tolerance in Propane Oxidation. ACS Sustain. Chem. Eng. 2022, 10, 8326–8341. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Hua, D.; Zhou, S. Enhanced Catalytic Hydrogenation Activity and Selectivity of Pt-MxOy/Al2O3 (M = Ni, Fe, Co) Heteroaggregate Catalysts by in Situ Transformation of PtM Alloy Nanoparticles. J. Phys. Chem. C 2013, 117, 7294–7302. [Google Scholar] [CrossRef]



| Chemical Hydrogen | Vapor Pressure (20 °C, kPa) 286 | Heat of Combustion (25 °C, kJ/mol) | Nominal Concentration (%) | Calorific Value (kJ/m3) | ||||
|---|---|---|---|---|---|---|---|---|
| 10% Vapor | 20% Vapor | 30% Vapor | 10% Vapor | 20% Vapor | 30% Vapor | |||
| Benzene | 10 | 3263 | 0.99 | 1.98 | 2.99 | 1435.2 | 2284 | 4335 |
| Toluene | 9 | 3910 | 0.29 | 0.58 | 0.57 | 504 | 1012 | 1517 |
| Methanol | 13 | 725 | 1.3 | 2.6 | 3.9 | 420.8 | 841.5 | 1262.3 |
| Formic Acid | 4.6 | 254.6 | 0.5 | 0.9 | 1.4 | 56.8 | 102.3 | 159.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munsif, S.; Ullah, L.; Cao, L.; Murthy, P.R.; Zhang, J.-C.; Li, W.-Z. A Highly Efficient Catalytic Co-Combustion of Aromatic and Oxygenated Volatile Organic Compounds (VOCs) via H2-Driven Onsite Heating. Catalysts 2024, 14, 729. https://doi.org/10.3390/catal14100729
Munsif S, Ullah L, Cao L, Murthy PR, Zhang J-C, Li W-Z. A Highly Efficient Catalytic Co-Combustion of Aromatic and Oxygenated Volatile Organic Compounds (VOCs) via H2-Driven Onsite Heating. Catalysts. 2024; 14(10):729. https://doi.org/10.3390/catal14100729
Chicago/Turabian StyleMunsif, Sehrish, Lutf Ullah, Long Cao, Palle Ramana Murthy, Jing-Cai Zhang, and Wei-Zhen Li. 2024. "A Highly Efficient Catalytic Co-Combustion of Aromatic and Oxygenated Volatile Organic Compounds (VOCs) via H2-Driven Onsite Heating" Catalysts 14, no. 10: 729. https://doi.org/10.3390/catal14100729
APA StyleMunsif, S., Ullah, L., Cao, L., Murthy, P. R., Zhang, J.-C., & Li, W.-Z. (2024). A Highly Efficient Catalytic Co-Combustion of Aromatic and Oxygenated Volatile Organic Compounds (VOCs) via H2-Driven Onsite Heating. Catalysts, 14(10), 729. https://doi.org/10.3390/catal14100729






