The Research Progress of Ruthenium-Based Catalysts for the Alkaline Hydrogen Evolution Reaction in Water Electrolysis
Abstract
1. Introduction
2. Mechanism of Alkaline HER
3. Factors Affecting the Alkaline HER Performance of Ru-Based Catalysts and the Related Regulation Strategies
3.1. Regulation of H Adsorption Free Energy (ΔGH*) on Ru-Based Catalyst Surfaces
3.2. Regulation of H2O Adsorption and Activation on Ru-Based Catalyst Surfaces
3.3. Regulation of Adsorption and Coverage of -OH Species on Ru-Based Catalyst Surfaces
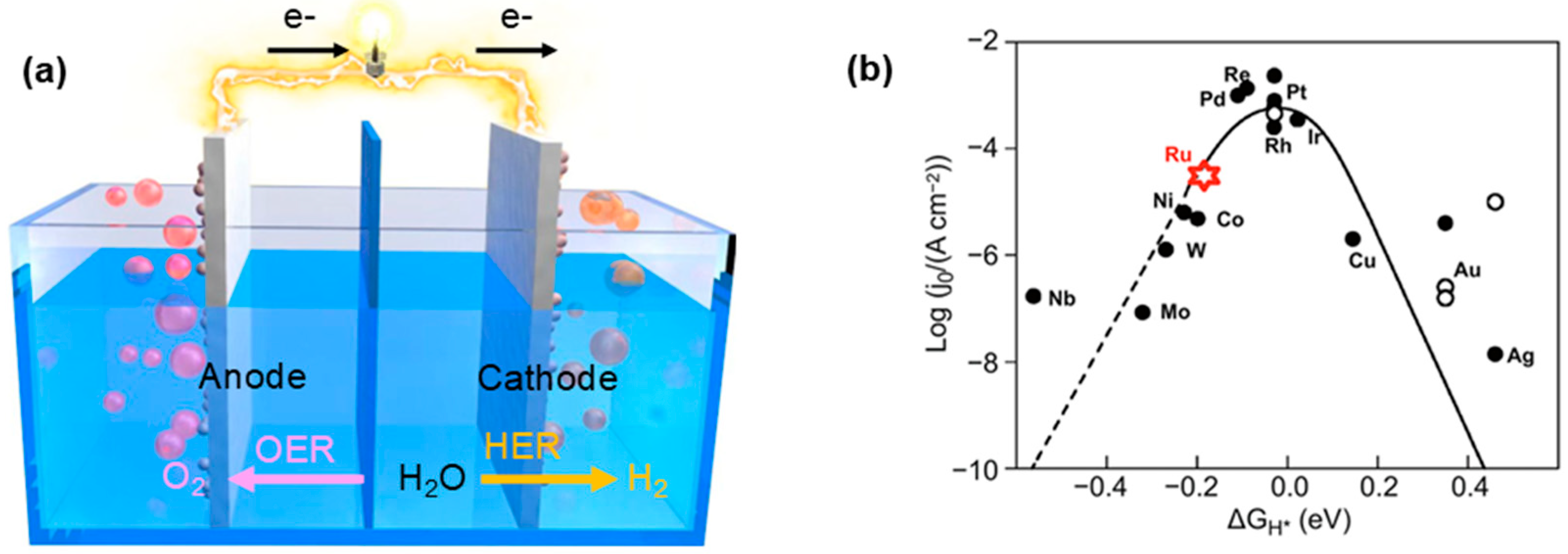
4. Research Progress of Ru-Based Catalysts in the Alkaline HER
4.1. Ruthenium Nanoparticles
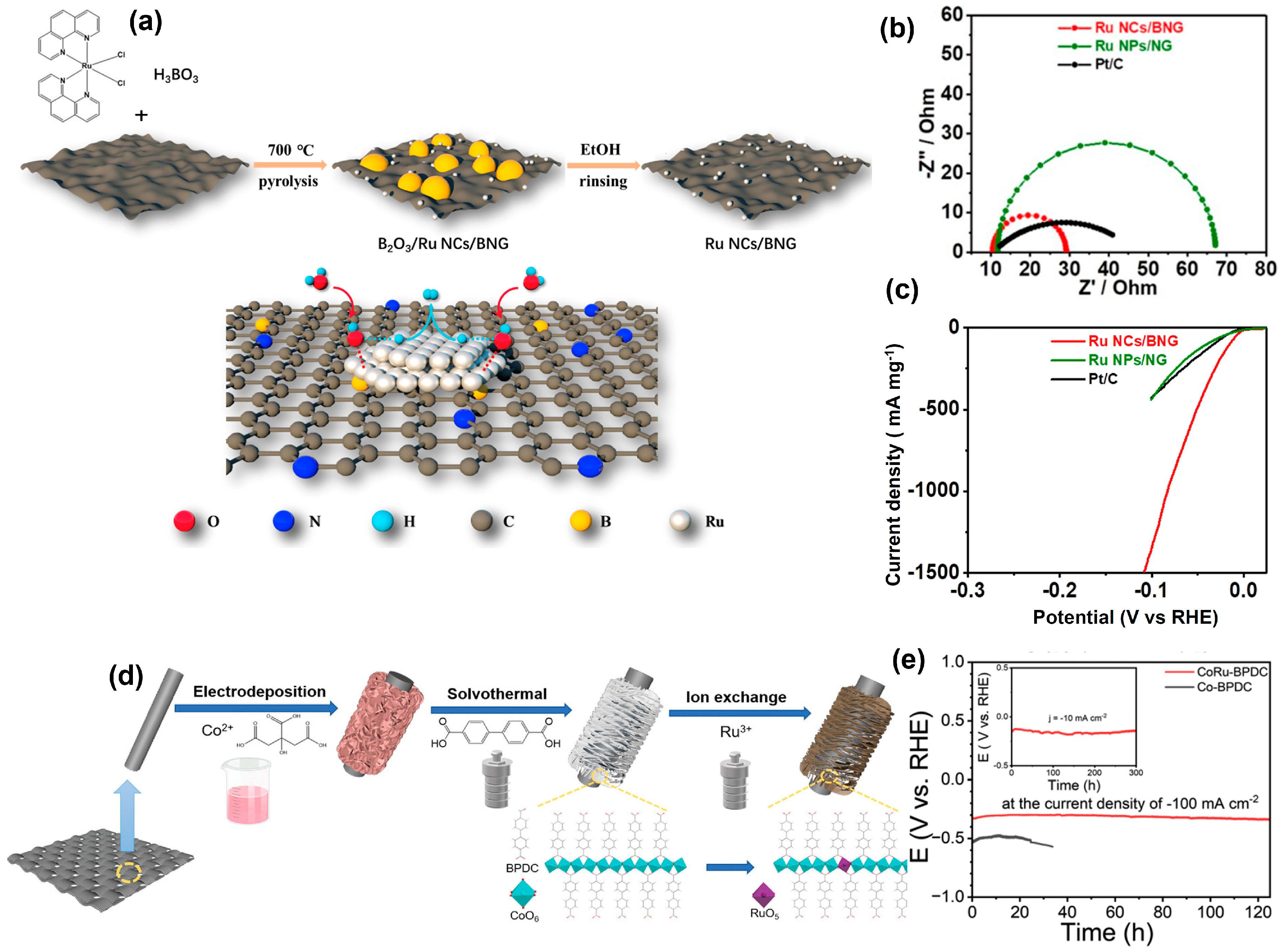
4.2. Ruthenium-M Heterogeneous Catalysts (M = Noble Metals and Transition Metals)
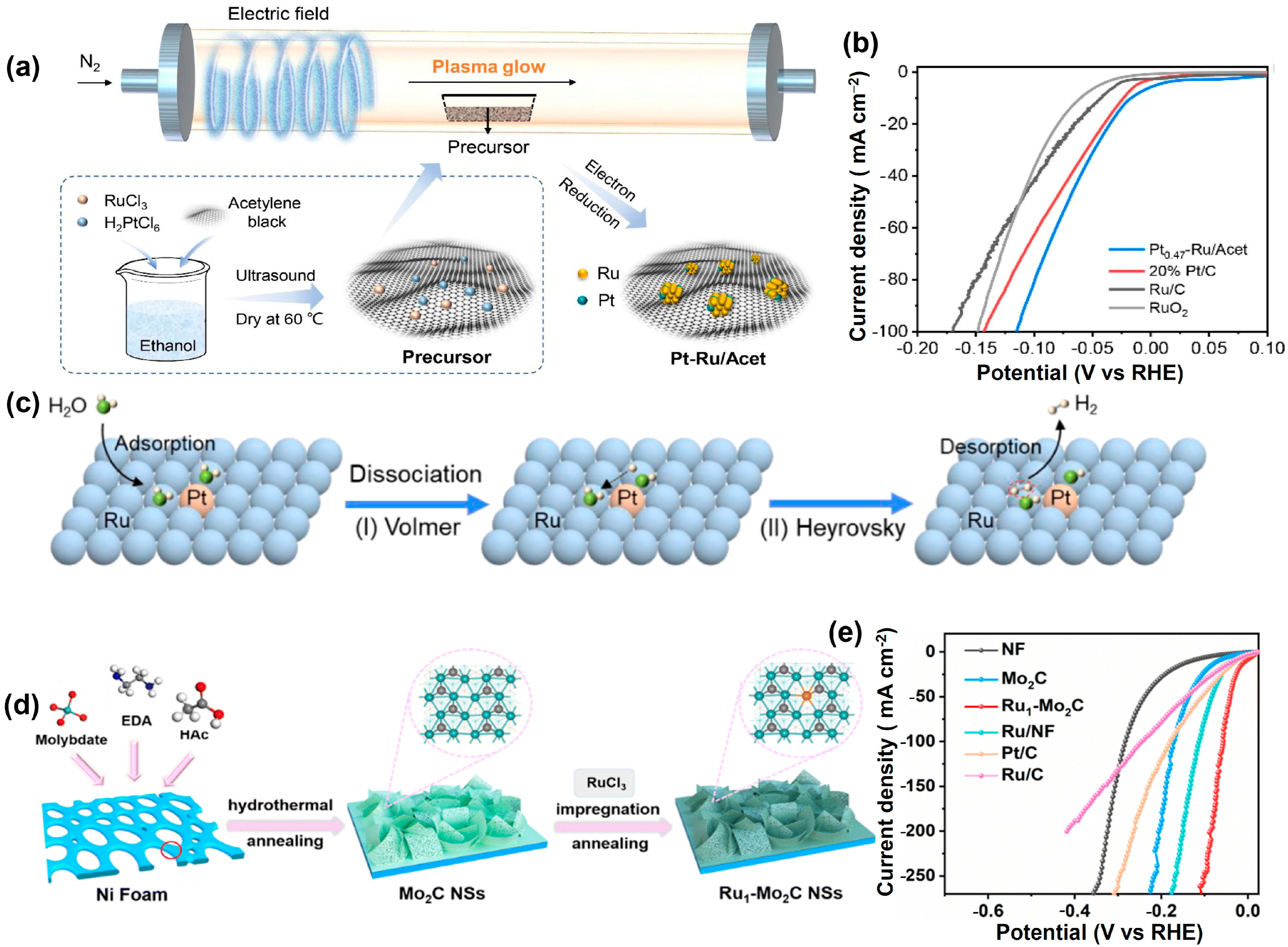
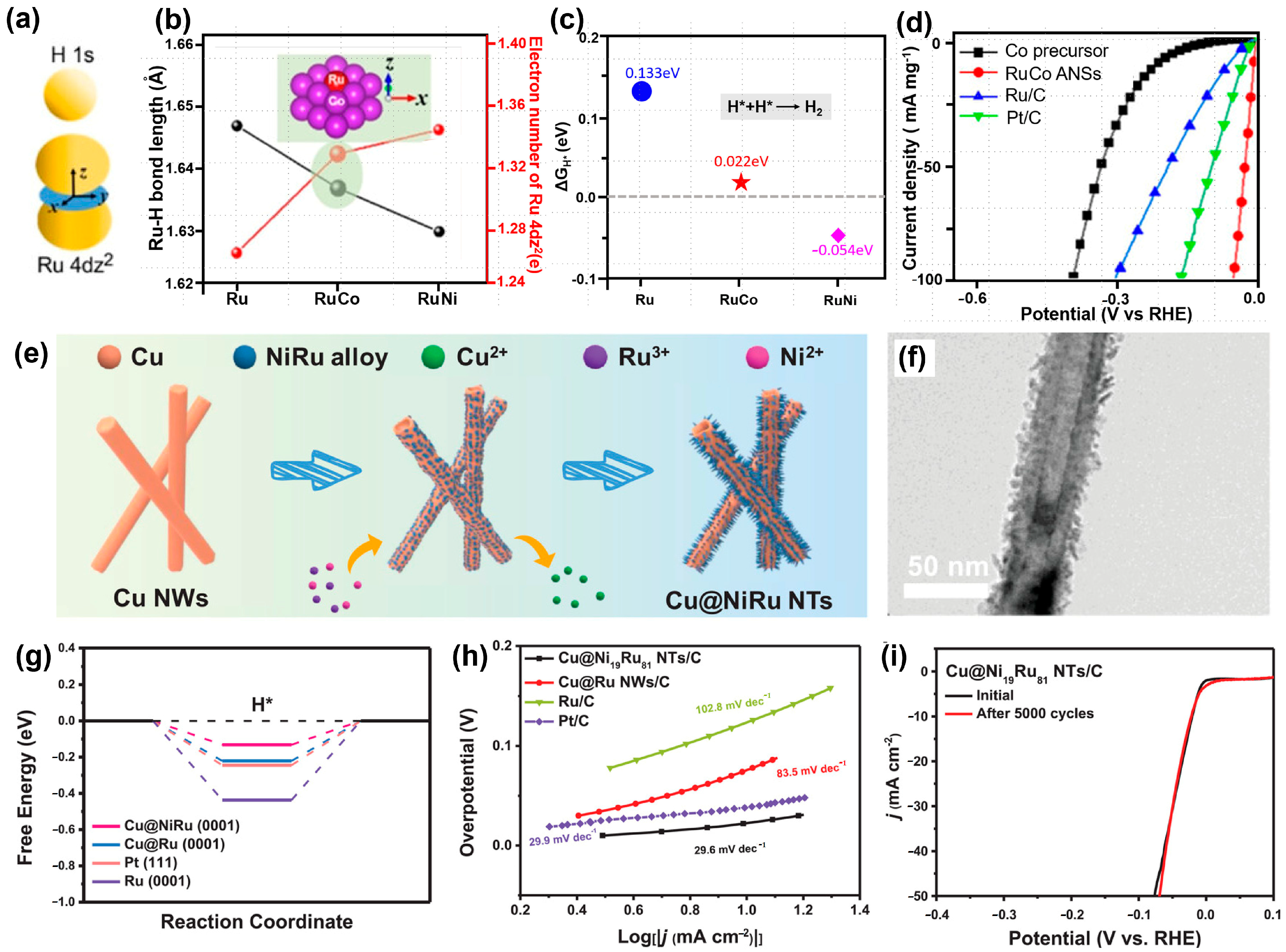
4.3. Ruthenium-Based Compounds
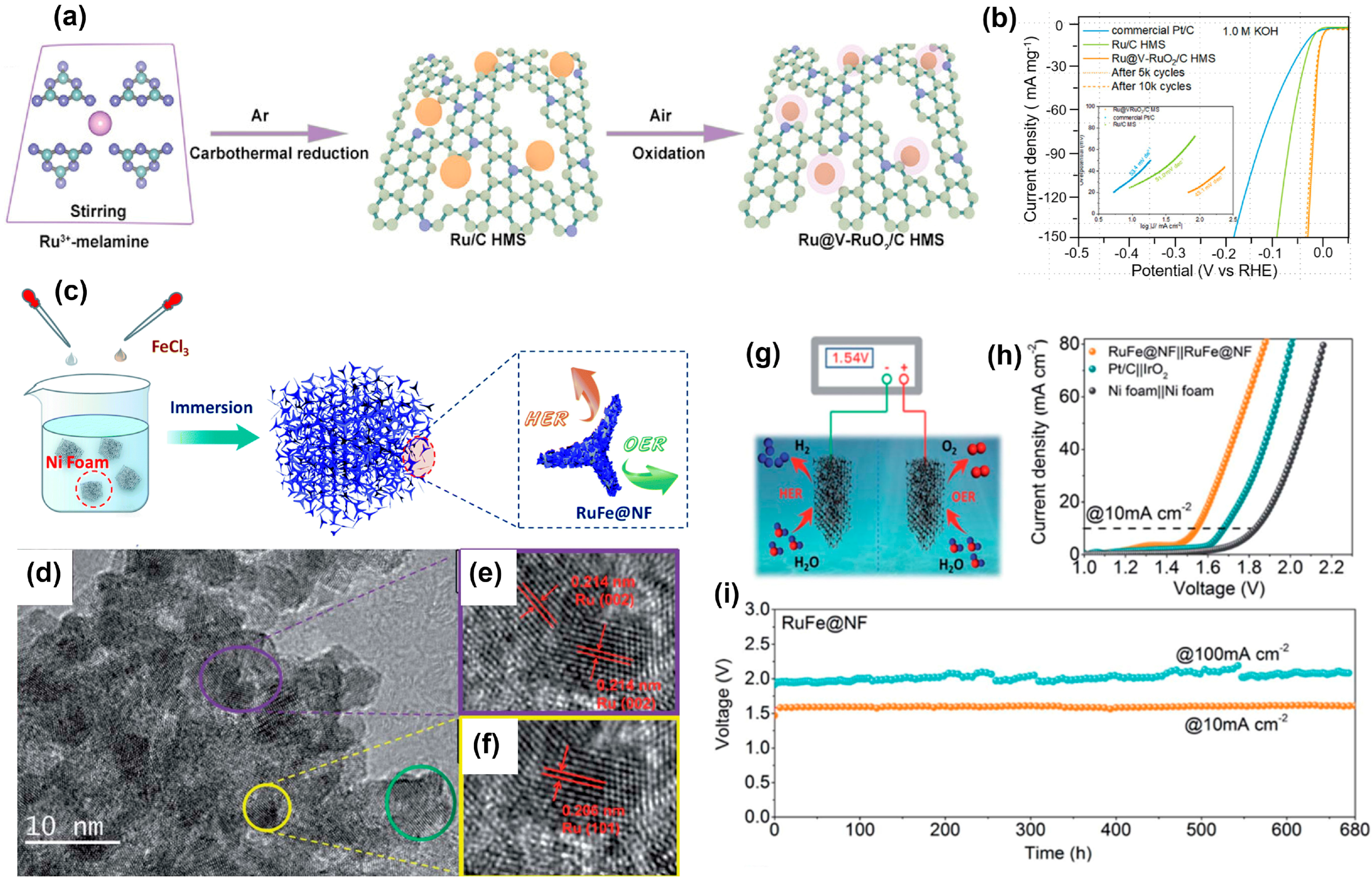

5. Conclusions and Future Outlooks
- (1)
- The development of simple and economical synthesis methods for Ru-based catalysts. At this stage, the assessment of Ru-based catalysts remains in the laboratory stage. Most catalysts are prepared by complicated and cumbersome methods with high costs. In particular, the high-temperature pyrolysis of precursors has been commonly employed to produce Ru-based catalysts for hydrogen generation. At high temperatures, metallic Ru is highly susceptible to aggregation despite the use of a carrier with high conductivity and a large specific surface area. Accordingly, investigating low-temperature and scalable synthesis techniques, like electrochemical replacement reactions and wet chemical reduction, could help avoid the aggregation of Ru, thus exposing more catalytic sites and improving catalytic efficiency as well as lowering the cost, making such techniques more likely to enter into practical or industrial production and applications [94,95].
- (2)
- The standardization of electrochemical measurements and analysis. Standardized electrochemical measurements and analysis must be performed to accurately and fairly evaluate the performance of catalysts in different laboratories, including rational and accurate three-electrode configurations, the geometric area of electrodes, mass loading, normalized activity, and TOF calculation methods. The utilization of a rotating disk electrode (RDE) as the working electrode facilitates more precise outcomes for Ru-based powder catalysts. Furthermore, to obtain experimental results with good application prospects, the experimental scale should be expanded to narrow the gap between experimental research and industrial applications. It is recommended to conduct electrochemical measurements under actual equipment operation, such as a high current density at the ampere level [12,18,96].
- (3)
- The combination of in situ/operando characterizations with theoretical calculations to elucidate the structure–performance relationships. In the alkaline HER, the intrinsic catalytic performance of catalysts is influenced by ΔGH*, the adsorption and dissociation of H2O, and the strength of -OH adsorption, and the related reaction mechanisms of different systems remain unclear. Therefore, it is necessary to characterize the reaction process in situ/operando to capture the information of interfacial water and intermediate species such as -H and -OH and, at the same time, combine this information with detailed and advanced theoretical calculations to deduce the reaction pathways and mechanisms, to reveal the structure–performance relationship and to provide guidance for the design of advanced catalysts. The advancement of material science, in situ/operando characterization methods, and theoretical simulations will facilitate a significant expansion in the production of hydrogen from alkaline water electrolysis. It is anticipated that the metal Ru, which has the potential to serve as an effective alternative to Pt, will be widely adopted in the large-scale design of alkaline HER catalysts [97,98,99].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chesshire, J. Energy crisis. Nature 1977, 269, 269. [Google Scholar] [CrossRef]
- Guerra, O.J.; Eichman, J.; Denholm, P. Optimal energy storage portfolio for high and ultrahigh carbon-free and renewable power systems. Energy Environ. Sci. 2021, 14, 5132–5146. [Google Scholar] [CrossRef]
- Mackey, B.; Lindenmayer, D. Fossil fuels’ future. Science 2014, 345, 739–740. [Google Scholar] [CrossRef]
- Gibson, L.; Wilman, E.N.; Laurance, W.F. How green is “green” energy? Trends Ecol. Evol. 2017, 32, 922–935. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhong, Y.J.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z.P. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Sun, H.N.; Kim, H.; Song, S.Z.; Jung, W. Copper foam-derived electrodes as efficient electrocatalysts for conventional and hybrid water electrolysis. Mater. Rep. Energy 2022, 2, 100092. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef]
- Hota, P.; Das, A.; Maiti, D.K. A short review on generation of green fuel hydrogen through water splitting. Int. J. Hydrogen Energy 2023, 48, 523–541. [Google Scholar] [CrossRef]
- Yan, X.Y.; Biemolt, J.; Zhao, K.; Zhao, Y.; Cao, X.J.; Yang, Y.; Wu, X.Y.; Rothenberg, G.; Yan, N. A membrane-free flow electrolyzer operating at high current density using earth-abundant catalysts for water splitting. Nat. Commun. 2021, 12, 4143. [Google Scholar] [CrossRef]
- He, Q.; Zhou, Y.Z.; Shou, H.W.; Wang, X.Y.; Zhang, P.J.; Xu, W.J.; Qiao, S.C.; Wu, C.Q.; Liu, H.J.; Liu, D.B.; et al. Synergic reaction kinetics over adjacent ruthenium sites for superb hydrogen generation in alkaline media. Adv. Mater. 2022, 34, 2110604. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.M.; Jiang, S.P.; Shao, Z.P. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Luo, W.J.; Wang, Y.J.; Cheng, C.W. Ru-based electrocatalysts for hydrogen evolution reaction: Recent research advances and perspectives. Mater. Today Phys. 2020, 15, 100274. [Google Scholar] [CrossRef]
- Hu, S.L.; Li, W.X. Sabatier principle of metal-support interaction for design of ultrastable metal nano-catalysts. Science 2021, 374, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tong, X.L.; Yang, N.J. Design and performance of Rh nanocatalysts for boosted H2 generation in alkaline media. Acc. Mater. Res. 2024, 5, 89–102. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.H.; Li, L.H.; Han, Y.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. High electrocatalytic hydrogen evolution activity of an anomalous ruthenium catalyst. J. Am. Chem. Soc. 2016, 138, 16174–16181. [Google Scholar] [CrossRef]
- Pan, S.Y.; Li, C.; Xiong, T.T.; Xie, Y.H.; Luo, F.; Yang, Z.H. Hydrogen spillover in MoOxRh hierarchical nanosheets boosts alkaline HER catalytic activity. Appl. Catal. B Environ. 2024, 341, 123275. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Q.X.; Shi, Y.Y.; Chang, J.W.; Lu, S.Y. Electrocatalytic water splitting: Mechanism and electrocatalyst design. Nano Res. 2023, 16, 9142–9157. [Google Scholar] [CrossRef]
- Guo, F.; Macdonald, T.J.; Sobrido, A.J.; Liu, L.X.; Feng, J.R.; He, G.J. Recent advances in ultralow-Pt-loading electrocatalysts for the efficient hydrogen evolution. Adv. Sci. 2023, 10, 2301098. [Google Scholar] [CrossRef]
- López, M.; Exner, K.S.; Viñes, F.; Illas, F. Theoretical study of the mechanism of the hydrogen evolution reaction on the V2C MXene: Thermodynamic and kinetic aspects. J. Catal. 2023, 421, 252–263. [Google Scholar] [CrossRef]
- Chen, D.C.; Chen, Z.W.; Zhang, X.X.; Lu, Z.L.; Xiao, S.; Xiao, B.B.; Singh, V.C. Exploring single atom catalysts of transition-metal doped phosphorus carbide monolayer for HER: A first-principles study. J. Energy Chem. 2021, 52, 155–162. [Google Scholar] [CrossRef]
- Wei, J.M.; Zhou, M.; Long, A.C.; Xue, Y.M.; Liao, H.B.; Wei, C.; Xu, J.Z. Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions. Nano Micro Lett. 2018, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.S.; Zhao, P.X.; Lee, L.Y.S.; Wong, K.Y. Recent advances in electrocatalytic hydrogen evolution using nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Yu, Y.H.; Li, J.; Chen, Q.R.; Du, Y.L.; Rao, P.; Li, R.S.; Jia, C.M.; Kang, Z.Y.; Deng, P.L.; et al. Engineering ruthenium-based electrocatalysts for effective hydrogen evolution reaction. Nano Micro Lett. 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Zhan, S.H.; Xia, Y.G.; Ma, S.L.; Zhou, Q.X.; Li, Y. The fundamental role and mechanism of reduced graphene oxide in rGO/Pt-TiO2 nanocomposite for high-performance photocatalytic water splitting. Appl. Catal. B Environ. 2017, 207, 335–346. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, K.R.; Wang, X.D.; Zheng, H.J.; Cao, J.Y.; Mi, L.R.; Huo, Q.H.; Yang, H.P.; Liu, J.H.; He, C.X. Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction. Nat. Commun. 2022, 13, 3958. [Google Scholar] [CrossRef]
- Ma, X.F.; Xiao, H.; Gao, Y.; Zhao, M.; Zhang, L.; Zhang, J.; Jia, J.F.; Wu, H.S. Enhancement of pore confinement caused by the mosaic structure on Ru nanoparticles for pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2023, 11, 3524–3534. [Google Scholar] [CrossRef]
- Li, L.L.; Tian, F.Y.; Qiu, L.Y.; Wu, F.Y.; Yang, W.W.; Yu, Y.S. Recent progress on ruthenium-based electrocatalysts towards the hydrogen evolution reaction. Catalysts 2023, 13, 1497. [Google Scholar] [CrossRef]
- Zhou, B.H.; Gao, R.J.; Zou, J.J.; Yang, H.M. Surface design strategy of catalysts for water electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef]
- Luo, M.; Cai, J.Y.; Zou, J.S.; Jiang, Z.; Wang, G.M.; Kang, X.W. Promoted alkaline hydrogen evolution by an N-doped Pt–Ru single atom alloy. J. Mater. Chem. A 2021, 9, 14941–14947. [Google Scholar] [CrossRef]
- Alsabban, M.M.; Eswaran, M.K.; Peramaiah, K.; Wahyudi, W.; Yang, X.L.; Ramalingam, V.; Hedhili, M.N.; Miao, X.H.; Schwingenschlögl, U.; Li, L.J.; et al. Unusual activity of rationally designed cobalt phosphide/oxide heterostructure composite for hydrogen production in alkaline medium. ACS Nano 2022, 16, 3906–3916. [Google Scholar] [CrossRef]
- Huang, X.X.; Lu, R.H.; Cen, Y.P.; Wang, D.C.; Jin, S.; Chen, W.X.; Geoffrey, I.; Waterhouse, N.; Wang, Z.Y.; Tian, S.B.; et al. Micropore-confined Ru nanoclusters catalyst for efficient pH-universal hydrogen evolution reaction. Nano Res. 2023, 16, 9073–9080. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, J.H.; Sun, M.Z.; Lin, F.X.; Huang, B.L.; Lv, F.; Zeng, L.Y.; Zhang, Q.H.; Gu, L.; Luo, M.C.; et al. Cu-doped heterointerfaced Ru/RuSe2 nanosheets with optimized H and H2O adsorption boost hydrogen evolution catalysis. Adv. Mater. 2023, 35, 2300980. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, P.; Li, M.X.; Li, Y.R.; Zhang, X.; Chen, J.; Fang, X.; Liu, Y.J.; Yuan, X.L.; Dai, X.P.; et al. Interfacial synergy between dispersed Ru sub-nanoclusters and porous NiFe layered double hydroxide on accelerated overall water splitting by intermediate modulation. Nanoscale 2020, 12, 9669–9679. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, W.; Wang, H.N.; Lu, S.F.; Li, Y.Q.; Lu, L.; Yu, L.F.; Xiang, Y. Insight into in situ grown Ru-based integrated electrodes for hydrogen evolution reaction: Boosted mass transfer efficiency and promoted intrinsic activity. Chem. Eng. J. 2024, 485, 149807. [Google Scholar] [CrossRef]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef]
- Sarawutanukul, S.; Phattharasupakun, N.; Sawangphruk, M. 3D CVD graphene oxide-coated Ni foam as carbo-and electro-catalyst towards hydrogen evolution reaction in acidic solution: In situ electrochemical gas chromatograph. Carbon 2019, 151, 109–119. [Google Scholar] [CrossRef]
- Ye, S.H.; Luo, F.Y.; Xu, T.T.; Zhang, P.Y.; Shi, H.D.; Qin, S.Q.; Wu, J.P.; He, C.X.; Ouyang, X.P.; Zhang, Q.L.; et al. Boosting the alkaline hydrogen evolution of Ru nanoclusters anchored on B/N–doped graphene by accelerating water dissociation. Nano Energy 2020, 68, 104301. [Google Scholar] [CrossRef]
- Li, C.F.; Zhao, J.W.; Xie, L.J.; Wang, Y.; Tang, H.B.; Zheng, L.R.; Li, G.R. N coupling with S-coordinated Ru nanoclusters for highly efficient hydrogen evolution in alkaline media. J. Mater. Chem. A 2021, 9, 12659–12669. [Google Scholar] [CrossRef]
- Liao, P.S.; Kang, J.W.; Zhong, Y.C.; Xiang, R.N.; Wang, S.H.; Li, S.S.; Liu, X.L.; Li, G.Q. Recent advances of two-dimensional metal-organic frameworks in alkaline electrolysis water for hydrogen production. Sci. China Chem. 2023, 66, 1924–1939. [Google Scholar] [CrossRef]
- Sun, Y.M.; Xue, Z.Q.; Liu, Q.L.; Jia, Y.L.; Li, Y.L.; Liu, K.; Lin, Y.Y.; Liu, M.; Li, G.Q.; Su, C.Y. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12, 1369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef] [PubMed]
- Thalluri, S.M.; Rodriguez-Pereira, J.; Zazpe, R.; Bawa, B.; Kolíbalová, E.; Jelinek, L.; Macak, J.M. Enhanced C-O functionality on carbon papers ensures lowering nucleation delay of ALD for Ru towards unprecedented alkaline HER activity. Small 2023, 19, 2300974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.R.; An, L.L.; Song, M.; Zhao, T.H.; Zhang, J.J.; Zhang, C.; Li, Z.Z.; Wang, D.L. Tuning the hydrogen and hydroxyl adsorption on Ru nanoparticles for hydrogen electrode reactions via size controlling. Chin. Chem. Lett. 2023, 34, 107622. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Wang, X.W.; Cheng, G.Z.; Lou, W. Phosphorus-induced activation of ruthenium for boosting hydrogen oxidation and evolution electrocatalysis. ACS Catal. 2020, 10, 11751–11757. [Google Scholar] [CrossRef]
- Ajmal, Z.; Arif, M.; Kumar, A.; Haq, M.U.; Du, Y.F.; Zhang, Y.C.; Abboud, M.; Qian, J.; Chen, Z.J.; Ni, B.J.; et al. Uniformaly distributed Ru nanoparticles over N, P co-doped porous carbon as a highly active trifunctional electrocatalyst. Int. J. Hydrogen Energy 2024, 73, 768–774. [Google Scholar] [CrossRef]
- Sun, X.Z.; Gao, X.Y.; Chen, J.; Wang, X.; Chang, H.X.; Li, B.; Song, D.M.; Li, J.; Li, H.S.; Wang, N. Ultrasmall Ru nanoparticles highly dispersed on sulfur-doped graphene for HER with high electrocatalytic performance. ACS Appl. Mater. Interfaces 2020, 12, 48591–48597. [Google Scholar] [CrossRef]
- Jin, S. How to effectively utilize MOFs for electrocatalysis. ACS Energy Lett. 2019, 4, 1443–1445. [Google Scholar] [CrossRef]
- He, Y.Q.; Yan, F.; Zhang, X.; Zhu, C.L.; Zhao, Y.Y.; Geng, B.; Chou, S.L.; Xie, Y.; Chen, Y.J. Creating dual active sites in conductive metal-organic frameworks for efficient water splitting. Adv. Energy Mater. 2023, 13, 2204177. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Klingenhof, M.; Gao, C.L.; Koketsu, T.; Weiser, G.; Pi, Y.C.; Liu, S.H.; Sui, L.J.; Hou, J.R.; Li, J.L.; et al. Facilitating alkaline hydrogen evolution reaction on the hetero-interfaced Ru/RuO2 through Pt single atoms doping. Nat. Commun. 2024, 15, 1447. [Google Scholar] [CrossRef]
- Li, X.Y.; Luo, D.; Jiang, F.; Zhang, K.J.; Wang, S.X.; Li, S.F.; Zha, Q.Q.; Huang, Y.C.; Ni, Y.H. Electronic modulation of metal-organic frameworks caused by atomically dispersed Ru for efficient hydrogen evolution. Small 2023, 19, 2301850. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Hu, Y.P.; Fu, Y.Y.; Yang, J.; Song, D.M.; Li, B.; Xu, W.H.; Wang, N. Single Ru sites on covalent organic framework-coated carbon nanotubes for highly efficient electrocatalytic hydrogen evolution. Small 2024, 20, 2305978. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Li, J.; Wang, N.; Zhou, Y.N.; Zheng, J.; Chu, W. Plasma-assisted highly dispersed Pt single atoms on Ru nanoclusters electrocatalyst for pH-universal hydrogen evolution. Chem. Eng. J. 2022, 448, 137611. [Google Scholar] [CrossRef]
- Yang, H.B.; Ma, D.W.; Li, Y.; Zhao, Q.H.; Pan, F.; Zheng, S.S.; Lou, Z.R. Mo doped Ru-based cluster to promote alkaline hydrogen evolution with ultra-low Ru loading. Chin. J. Struct. Chem. 2023, 42, 100031. [Google Scholar] [CrossRef]
- Pang, B.B.; Liu, X.K.; Liu, T.Y.; Chen, T.; Shen, X.Y.; Zhang, W.; Wang, S.C.; Liu, T.; Liu, D.; Ding, T.; et al. Laser-assisted high-performance PtRu alloy for pH-universal hydrogen evolution. Energ. Environ. Sci. 2022, 15, 102–108. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.W.; Wang, B.; Yang, T.; Yang, S.C. Electrochemical formation of PtRu bimetallic nanoparticles for highly efficient and pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 2090–2098. [Google Scholar] [CrossRef]
- Li, C.; Jang, H.; Liu, S.G.; Kim, M.G.; Hou, L.Q.; Liu, X.E.; Cho, J. P and Mo dual doped Ru ultrasmall nanoclusters embedded in P-doped porous carbon toward efficient hydrogen evolution reaction. Adv. Energy Mater. 2022, 12, 2200029. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, L.S.; Xu, S.L.; Cui, K.; Wang, T.S.; Jiang, Z.; Li, J.Y. Hydrogen spillover mechanism at the metal–metal interface in electrocatalytic hydrogenation. Angew. Chem. 2024, 136, e202407810. [Google Scholar] [CrossRef]
- Gao, X.R.; Xing, Z.; Wang, M.Y.; Nie, C.H.; Shang, Z.C.; Bai, Z.C.; Dou, S.X.; Wang, N.N. Comprehensive insights into solid-state electrolytes and electrode-electrolyte interfaces in all-solid-state sodium-ion batteries. Energy Storage Mater. 2023, 60, 102821. [Google Scholar] [CrossRef]
- Chao, T.T.; Xie, W.B.; Hu, Y.M.; Zhao, T.H.; Chen, C.; Zhang, Z.D.; Yu, G.; Hong, X.; Jin, H.L.; Wang, D.S.; et al. Reversible hydrogen spillover at the atomic interface for efficient alkaline hydrogen evolution. Energy Environ. Sci. 2024, 17, 1397–1406. [Google Scholar] [CrossRef]
- Okazoe, S.; Kusada, K.; Wu, D.S.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kawaguchi, S.; Kubota, Y.; Kitagawa, H. Synthesis of Mo and Ru solid-solution alloy NPs and their hydrogen evolution reaction activity. Chem. Commun. 2020, 56, 14475–14478. [Google Scholar] [CrossRef] [PubMed]
- Li, L.G.; Liu, S.H.; Zhan, C.H.; Wen, Y.; Sun, Z.F.; Han, J.J.; Chan, T.S.; Zhang, Q.B.; Hud, Z.W.; Huang, X.Q. Surface and lattice engineered ruthenium superstructures towards high-performance bifunctional hydrogen catalysis. Energ. Environ. Sci. 2023, 16, 157–166. [Google Scholar] [CrossRef]
- Kutyła, D.; Pajić, M.N.K.; Lačnjevac, U.Č.; Marzec, M.M.; NElezović, N.R.; Żabiński, P. Ru–Co alloy coatings electrodeposited on a MAX phase substrate as efficient catalysts for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 56, 28–40. [Google Scholar] [CrossRef]
- Cai, C.; Liu, K.; Zhu, Y.M.; Li, P.C.; Wang, Q.Y.; Liu, B.; Chen, S.Y.; Li, H.J.W.; Zhu, L.; Li, H.M.; et al. Optimizing hydrogen binding on Ru sites with RuCo alloy nanosheets for efficient alkaline hydrogen evolution. Angew. Chem. 2022, 61, e202113664. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.X.; Zhang, W.Y.; Liu, R.; Lv, F.; Chao, Y.G.; Wang, Z.C.; Guo, S.J. Amorphous Ru nanoclusters onto Co-doped 1D carbon nanocages enables efficient hydrogen evolution catalysis. Chin. J. Catal. 2022, 43, 110–115. [Google Scholar] [CrossRef]
- Zhang, H.; Su, H.; Soldatov, M.A.; Li, Y.L.; Zhao, X.; Liu, M.H.; Zhou, W.L.; Zhang, X.X.; Sun, X.; Xu, Y.Z.; et al. Dynamic Co-Ru bond shrinkage at atomically dispersed Ru sites for alkaline hydrogen evolution reaction. Small 2021, 17, 2105231. [Google Scholar] [CrossRef]
- Gao, X.R.; Zang, W.J.; Li, X.; Wang, Z.K.; Zheng, L.R.; Kou, Z.K. Achieving efficient alkaline hydrogen evolution reaction on long-range Ni sites in Ru clusters-immobilized Ni3N array catalyst. Chem. Eng. J. 2023, 451, 138698. [Google Scholar] [CrossRef]
- Ojha, K.; Saha, S.; Dagar, P.; Ganguli, A.K. Nanocatalysts for hydrogen evolution reactions. Phys. Chem. Chem. Phys. 2018, 20, 6777–6799. [Google Scholar] [CrossRef]
- Chen, X.W.; Ye, L.F.; Wu, W.; Chen, S.H.; Wang, Z.C.; Zhu, Y.; Jiang, H.R.; Chen, R.Z.; Cheng, N.C. Compressed Ru skin on atomic-ordered hexagonal Ru-Ni enabling rapid Volmer-Tafel kinetics for efficient alkaline hydrogen evolution. Chem. Eng. J. 2024, 487, 150457. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.N.; Chen, Z.F.; Dai, Q.Z.; Dong, C.L.; Yang, B.; Li, Z.J.; Hu, X.B.; Lei, L.C.; Hou, Y. Theory-guided design of electron-deficient ruthenium cluster for ampere-level current density electrochemical hydrogen evolution. Nano Energy 2023, 115, 108694. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.Y.; Liang, J.S.; Li, S.Z.; Shi, H.; Liu, J.J.; Wang, T.Y.; Han, J.T.; Li, Q. Protrusion-rich Cu@NiRu core@shell nanotubes for efficient alkaline hydrogen evolution electrocatalysis. Small 2022, 18, 2202496. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.T.; Wang, L.; Chen, L.; Song, X.L.; Kong, Q.H.; Wang, H.Y.; Yuan, Z.Y. Interface metal oxides regulating electronic state around nickel species for efficient alkaline hydrogen electrocatalysis. Small 2023, 19, 2206196. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.S.; Zheng, X.Q.; Li, L.; Zhang, L.; Yang, N.; Xiong, K.; Chen, H.M.; Li, J.; Wei, Z.D. Chimney effect of the interface in metal oxide/metal composite catalysts on the hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 245, 122–129. [Google Scholar] [CrossRef]
- Dang, Y.L.; Wu, T.L.; Tan, H.Y.; Wang, J.L.; Cui, C.; Kerns, P.; Zhao, W.; Posada, L.; Wen, L.Y.; Suib, S.L. Partially reduced Ru/RuO2 composites as efficient and pH-universal electrocatalysts for hydrogen evolution. Energy Environ. Sci. 2021, 14, 5433–5443. [Google Scholar] [CrossRef]
- Li, Y.P.; Wang, W.T.; Cheng, M.Y.; Feng, Y.F.; Han, X.; Qian, Q.Z.; Zhu, Y.; Zhang, G.Q. Arming Ru with oxygen-vacancy-enriched RuO2 sub-nanometer skin activates superior bifunctionality for pH-universal overall water splitting. Adv. Mater. 2023, 35, 2206351. [Google Scholar] [CrossRef]
- Chen, J.L.; Feng, S.Y.; Lu, C.J.; Huang, F.J. Janus Ru/RuO2 nano-boomerangs on carbon as pH-universal electrocatalysts with bifunctional activity toward the hydrogen/oxygen evolution reaction. Chem. Eng. J. 2023, 468, 143761. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, X.P.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nanowires for highly efficient and stable overall water splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Lin, J.J.; Chen, J.; Tan, C.H.; Zhang, Y.Z.; Li, Y.C. Ruthenium-doped Ni(OH)2 to enhance the activity of methanol oxidation reaction and promote the efficiency of hydrogen production. RSC Adv. 2024, 14, 18695–18702. [Google Scholar] [CrossRef]
- Liu, H.B.; Jia, Q.H.; Huang, S.Q.; Yang, L.; Wang, S.T.; Zheng, L.R.; Cao, D.P. Ultra-small Ru nanoparticles embedded on Fe–Ni(OH)2 nanosheets for efficient water splitting at a large current density with long-term stability of 680 hours. J. Mater. Chem. A 2022, 10, 4817–4824. [Google Scholar] [CrossRef]
- Mo, Y.F.; Du, D.D.; Du, Y.Y.; Feng, Y.J.; Tang, P.G.; Li, D.Q. Fe (OH)x modified ultra-small Ru nanoparticles for highly efficient hydrogen evolution reaction and its application in water splitting. J. Colloid Interf. Sci. 2024, 659, 697–706. [Google Scholar] [CrossRef]
- Anantharaj, S. Hydrogen evolution reaction on Pt and Ru in alkali with volmer-step promotors and electronic structure modulators. Curr. Opin. Electrochem. 2022, 33, 100961. [Google Scholar] [CrossRef]
- Li, D.H.; Cai, R.S.; Zheng, D.Y.; Ren, J.; Dong, C.L.; Huang, Y.C.; Haigh, S.J.; Liu, X.E.; Gong, F.L.; Liu, Y.M.; et al. A sustainable route to ruthenium phosphide (RuP)/Ru heterostructures with electron-shuttling of interfacial Ru for efficient hydrogen evolution. Adv. Sci. 2024, 11, 2309869. [Google Scholar] [CrossRef]
- Li, P.; Li, W.Q.; Zhao, S.E.; Huang, T.Q.; Tian, S.H.; Huang, X.F. Advanced hydrogen evolution electrocatalysis enabled by ruthenium phosphide with tailored hydrogen binding strength via interfacial electronic interaction. Chem. Eng. J. 2022, 429, 132557. [Google Scholar] [CrossRef]
- Zhou, S.Z.; Jang, H.; Qin, Q.; Hou, L.Q.; Kim, M.G.; Liu, S.G.; Liu, X.E.; Cho, J. Boosting hydrogen evolution reaction by phase engineering and phosphorus doping on Ru/P-TiO2. Angew. Chem. Int. Ed. 2022, 61, e202212196. [Google Scholar] [CrossRef]
- El-Refaei, S.M.; Russo, P.A.; Schultz, T.; Chen, Z.N.; Amsalem, P.; Koch, N.; Pinna, N. Activating Ru in the pyramidal sites of Ru2P-type structures with earth-abundant transition metals for achieving extremely high HER activity while minimizing noble metal content. Carbon Energy 2024, e556. [Google Scholar] [CrossRef]
- Li, J.; Miró, R.; Wrzesińska-Lashkova, A.; Yu, J.; Arbiol, J.; Vaynzof, Y.; Shavel, A.; Lesnyak, V. Aqueous room-temperature synthesis of transition metal dichalcogenide nanoparticles: A sustainable route to efficient hydrogen evolution. Adv. Funct. Mater. 2024, 2404565. [Google Scholar] [CrossRef]
- Pinto, F.M.; de Conti, M.C.M.D.; Pereira, W.S.; Sczancoski, J.C.; Medina, M.; Corradini, P.G.; de Brito, J.F.; Nogueira, A.E.; Góes, M.S.; Ferreira, O.P.; et al. Recent advances in layered MX2-based materials (M = Mo, W and X = S, Se, Te) for emerging optoelectronic and photo (electro) catalytic applications. Catalysts 2024, 14, 388. [Google Scholar] [CrossRef]
- Xu, C.Y.; Yu, H.Z.; Huang, H.M.; Huang, W.C.; Li, S.; Cao, Y.H.; Lu, H.F.; Li, G.M.; Li, Y.J.; Li, X.K.; et al. Boosting electrocatalytic activity of Ru for hydrogen evolution through engineering Ru on multiple interfaces of 1T-MoS2 and carbon. Chem. Eng. J. 2024, 489, 151295. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, F.; Zhang, S.; Liang, Y.Y.; Wang, R.H. Engineering MoS2 basal planes for hydrogen evolution via synergistic ruthenium doping and nanocarbon hybridization. Adv. Sci. 2019, 6, 1900090. [Google Scholar] [CrossRef]
- Islam, S.E.; Hang, D.R.; Liang, C.T.; Sharma, K.H.; Huang, H.C.; Chou, M.M.C. Tuning the electrocatalytic activity of MoS2 nanosheets via the in situ hybridization with ruthenium and graphene network. Chem. Eng. J. 2024, 488, 150950. [Google Scholar] [CrossRef]
- Li, X.H.; Han, S.H.; Qiao, Z.L.; Zeng, X.F.; Cao, D.P.; Chen, J.F. Ru monolayer island doped MoS2 catalysts for efficient hydrogen evolution reaction. Chem. Eng. J. 2023, 453, 139803. [Google Scholar] [CrossRef]
- Cai, J.L.; Ding, J.; Wei, D.H.; Xie, X.; Li, B.J.; Lu, S.Y.; Zhang, J.M.; Liu, Y.S.; Cai, Q.; Zang, S.Q. Coupling of Ru and O-vacancy on 2D Mo-based electrocatalyst via a solid-phase interface reaction strategy for hydrogen evolution reaction. Adv. Energy Mater. 2021, 11, 2100141. [Google Scholar] [CrossRef]
- Zhao, J.J.; Guo, Y.N.; Li, S.J.; Wang, J.Q.; Liu, K.H.; Dai, L.J.; Dai, Y.; Jiang, B.; Li, H.X. Amorphous-crystalline porous ruthenium selenide as highly efficient electrocatalysts for alkaline hydrogen evolution. Chem. Eng. J. 2024, 485, 150074. [Google Scholar] [CrossRef]
- Kuang, Y.B.; Yang, F.L.; Feng, L.G. Advancements in ruthenium (Ru)-based heterostructure catalysts: Overcoming bottlenecks in catalysis for hydrogen evolution reaction. Adv. Energy Mater. 2024, 14, 2402043. [Google Scholar] [CrossRef]
- Bae, S.Y.; Mahmood, J.; Jeon, I.Y.; Baek, J.B. Recent advances in ruthenium-based electrocatalysts for the hydrogen evolution reaction. Nanoscale Horiz. 2020, 5, 43–56. [Google Scholar] [CrossRef]
- Li, Y.Q.; Liu, X.; Xu, J.L.; Chen, S.R. Ruthenium-based electrocatalysts for hydrogen evolution reaction: From nanoparticles to single atoms. Small 2024, 2402846. [Google Scholar] [CrossRef]
- Song, J.T.; Qian, Z.X.; Yang, J.; Lin, X.M.; Xu, Q.C.; Li, J.F. In situ/operando investigation for heterogeneous electro-catalysts: From model catalysts to state-of-the-art catalysts. ACS Energy Lett. 2024, 9, 4414–4440. [Google Scholar] [CrossRef]
- Sun, Y.L.; Deng, Y.L.; Chen, H.N.; Yang, X.T.; Lin, X.M.; Li, J.F. Design strategies and in situ infrared, Raman, and X-ray absorption spectroscopy techniques insight into the electrocatalysts of hydrogen energy system. Small Struct. 2023, 4, 2200201. [Google Scholar] [CrossRef]
- Lin, X.M.; Li, J.F. Applications of in situ Raman spectroscopy on rechargeable batteries and hydrogen energy systems. ChemElectroChem 2023, 10, e202201003. [Google Scholar] [CrossRef]
| Catalyst | Electrolyte | Tafel Plot (mV·dec−1) | Overpotential (mV) @10 mA·cm−2 | Reference |
|---|---|---|---|---|
| CP100C Ru | 1 M KOH | 20.0 | 4.7 | [43] |
| Ru/C-600 | 1 M KOH | 33.7 | 30.0 | [44] |
| Ru NCs/NC | 1 M KOH | 64.7 | 14.0 | [31] |
| Ru NCs/BNG | 1 M KOH | 28.9 | 14.0 | [38] |
| P-Ru-3/C | 1 M KOH | 105.0 | 31.0 | [45] |
| Ru@N-P-C-800 | 1 M KOH | 115.0 | 45.0 | [46] |
| Ru-S/N-C | 1 M KOH | 36.0 | 10.0 | [39] |
| RuCo-CAT/CC | 1 M KOH | 32.1 | 38.0 | [49] |
| CoRu-BPDC | 1 M KOH | 73.2 | 37.0 | [51] |
| Pt0.47-Ru/Acet | 1 M KOH | 66.0 | 17.0 | [53] |
| PtRu/mCNTs | 1 M KOH | 33.5 | 15.0 | [55] |
| PtRu/CC 1500 | 1 M KOH | 25.0 | 19.0 | [56] |
| RuMo/NC | 1 M KOH | 52.8 | 24.0 | [54] |
| P,Mo-Ru@PC | 1 M KOH | 21.7 | 21.0 | [57] |
| Ru1-Mo2C NPs | 1 M KOH | 38.5 | 10.8 | [60] |
| Mo-Ru NSAs | 1 M KOH | 16.1 | 16.0 | [62] |
| RuCo ANSs | 1 M KOH | 20.6 | 10.0 | [64] |
| Ru-Co@Ti2AlC | 1 M KOH | 105.0 | 25.0 | [63] |
| a-Ru@Co-DHC | 1 M KOH | 62.0 | 40.0 | [65] |
| M-Co NPs@Ru SAs/NC | 1 M KOH | 55.0 | 34.0 | [66] |
| Ru-Ni | 1 M KOH | 25.9 | 23.0 | [69] |
| Ru/Ni@C | 1 M KOH | 27.0 | 15.0 | [70] |
| Cu@Ni19Ru81 NTs/C | 1 M KOH | 29.6 | 22.0 | [71] |
| RuO2-300Ar | 1 M KOH | 35.0 | 17.0 | [74] |
| Ru@V-RuO2/C HMS | 1 M KOH | 45.1 | 6.0 | [75] |
| Ru/RuO2 NB/C | 1 M KOH | 45.1 | 54.0 | [76] |
| RuFe@NF | 1 M KOH | 63.4 | 28.0 | [79] |
| RuP/Ru@CNS | 1 M KOH | 32.0 | 15.0 | [82] |
| RuP/NP-C | 1 M KOH | 49.0 | 40.0 | [83] |
| Ru/P-TiO2 | 1 M KOH | 28.3 | 27.0 | [84] |
| CoRuP | 1 M KOH | 30.0 | 12.9 | [85] |
| Ru-MoS2/CNT | 1 M KOH | 62.0 | 50.0 | [89] |
| Ru@MoS2/GO | 1 M KOH | 38.0 | 60.0 | [90] |
| Ru MIs-MoS2 | 1 M KOH | 63.0 | 17.0 | [91] |
| MiSC-1 | 1 M KOH | 50.0 | 12.0 | [92] |
| RuSe2 | 1 M KOH | 39.2 | 29.5 | [86] |
| RuSe2-400 C | 1 M KOH | 39.2 | 27.0 | [93] |
| Cu-doped Ru/RuSe2 | 1 M KOH | 58.5 | 23.0 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.-L.; Chen, X.; Niu, B.-T.; Lin, Y.-T.; Chen, Y.-X.; Lin, X.-M. The Research Progress of Ruthenium-Based Catalysts for the Alkaline Hydrogen Evolution Reaction in Water Electrolysis. Catalysts 2024, 14, 671. https://doi.org/10.3390/catal14100671
Lin B-L, Chen X, Niu B-T, Lin Y-T, Chen Y-X, Lin X-M. The Research Progress of Ruthenium-Based Catalysts for the Alkaline Hydrogen Evolution Reaction in Water Electrolysis. Catalysts. 2024; 14(10):671. https://doi.org/10.3390/catal14100671
Chicago/Turabian StyleLin, Bi-Li, Xing Chen, Bai-Tong Niu, Yuan-Ting Lin, Yan-Xin Chen, and Xiu-Mei Lin. 2024. "The Research Progress of Ruthenium-Based Catalysts for the Alkaline Hydrogen Evolution Reaction in Water Electrolysis" Catalysts 14, no. 10: 671. https://doi.org/10.3390/catal14100671
APA StyleLin, B.-L., Chen, X., Niu, B.-T., Lin, Y.-T., Chen, Y.-X., & Lin, X.-M. (2024). The Research Progress of Ruthenium-Based Catalysts for the Alkaline Hydrogen Evolution Reaction in Water Electrolysis. Catalysts, 14(10), 671. https://doi.org/10.3390/catal14100671








