Dimension Engineering in Noble-Metal-Based Nanocatalysts
Abstract
:1. Introduction
2. NMNs with Different Dimensions
2.1. 0D NMNs
2.2. 1D NMNs
2.3. 2D NMNs
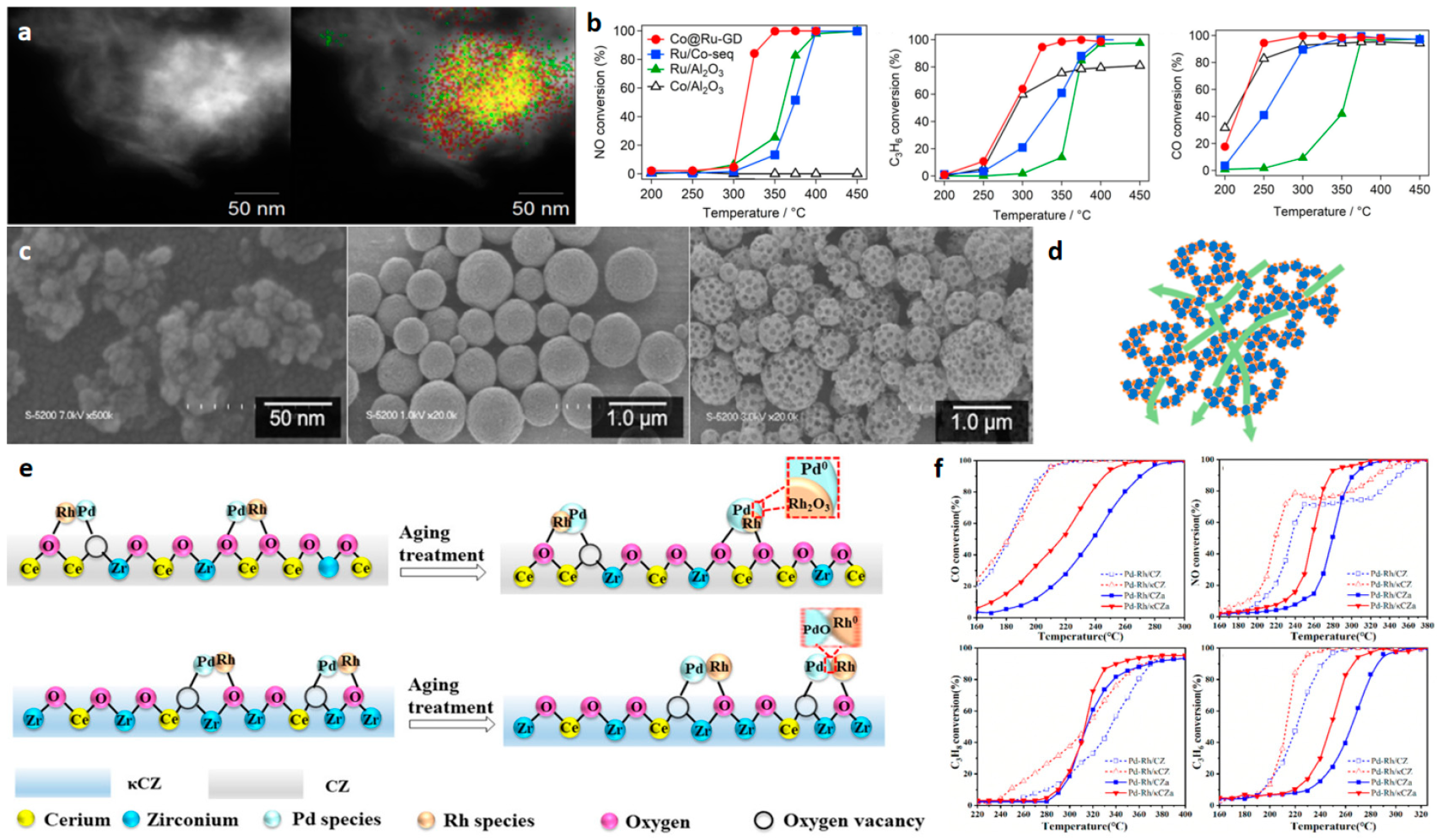
2.4. 3D NMNs
3. The Structure–Activity Relationship of NMNs
4. Applications
4.1. Direct Alcohol Fuel Cells (DAFCs)
4.2. Hydrogen Fuel Cells
4.3. Water Splitting
4.4. Selective Hydrogenation Reaction
4.5. Treatment of Automobile Exhaust
5. Challenges and Perspectives
- (1)
- Exploring general synthesis methods for multifunctional noble-metal-based catalysts. Developing general catalyst fabrication methods including adaptable surface properties and multiple active sites, as well as scalable manufacturing processes, is essential for diversified modern chemical industry. Universal synthetic strategies, such as the hydro/solvothermal approach, ligand-guided method, template-assisted route, epitaxial growth, and CO-confinement strategy, have been reported before. The innovative design of multifunctional catalysts should be versatile enough to satisfy various changing environmental conditions;
- (2)
- Enhancing catalytic efficiency and stability, as well as the service life. Improving the catalytic efficiency and stability of these catalysts is critical. Research should focus on optimizing the catalyst structure at the nanoscale, controlling active site densities, and enhancing the catalyst-support interaction to achieve higher efficiency and long-term stability under varying environmental conditions. Moreover, extending the service life of catalysts will contribute to their economic viability and environmental impact. Strategies such as developing robust protective coatings, designing self-regenerating catalyst systems, or exploring novel materials with inherent durability could be explored to enhance the longevity of these catalysts;
- (3)
- Revealing the intrinsic catalytic mechanism based on in situ analysis technology and simulation calculation. A comprehensive understanding of catalytic reaction mechanism is imperative for the rational design of catalysts with complex configurations and structures. In many cases of experimental research and chemical production, it is necessary to analyze the intermediate products of the reaction. The development of in situ analysis technology enables extensive testing and analysis under conditions closely resembling reality, thereby yielding more precise data and results. Moreover, revealing the surface reconstruction mechanism of the catalyst and correlating it with the distribution of active sites is also vital for improving catalyst activity. The revelation of intrinsic catalytic mechanisms through the use of both in situ analysis technology and simulation calculations is a crucial objective for catalyst manufacturing. In situ XRD and in situ XAF techniques can be used to characterize the dynamic phase changes of the reactants and observe the evolution of catalyst structure and, on this basis, analyze the restructuring phenomenon of the catalyst during the catalytic process. The in situ TEM technique can be used to observe the atomic arrangement on the catalyst surface in real time and further analyze the atomic rearrangement on the catalyst surface. In situ infrared technology and in situ Raman technology are used to observe the formation of active intermediates in the catalytic process in real time, which is very important and efficient in revealing the catalytic reaction mechanism.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
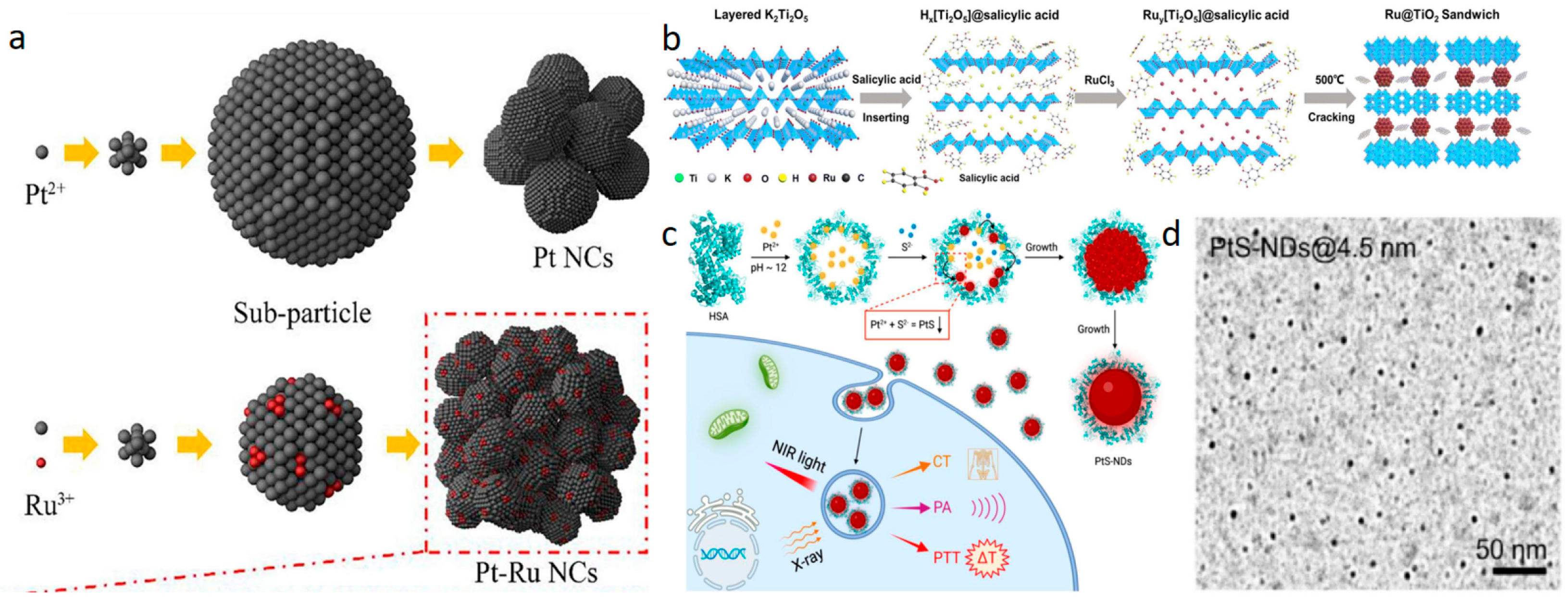
- Li, X.; Qian, C.; Tian, Y.; Yao, N.; Duan, Y.; Huang, Z. Pt-Ru Bimetallic Nanoclusters with Super Peroxidase-Like Activity for Ultra-Sensitive Lateral Flow Immunoassay. Chem. Eng. J. 2023, 457, 141324. [Google Scholar] [CrossRef]
- Cao, Y.G.; Liang, S.; Yan, Y.; Dong, W.J.; Dong, C.L.; Zheng, W.S.; Nong, S.Y.; Huang, F.Q. Unique Sandwich Structure of Ru@TiO2: Salicylic Acid Micro-Etching from K2Ti2O5 and High-Performance Electrocatalytic Hydrogen Evolution. Inorg. Chem. Front. 2023, 10, 3852–3859. [Google Scholar] [CrossRef]
- Fang, L.; Feng, J.J.; Shi, X.B.; Si, T.Z.; Song, Y.; Jia, H.; Li, Y.T.; Li, H.W.; Zhang, Q.G. Turning Bulk Materials into 0d, 1d and 2d Metallic Nanomaterials by Selective Aqueous Corrosion. Chem. Commun. 2019, 55, 10476–10479. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Li, T.; Zhang, J.; Wang, X.; Luo, J.; You, M.; Yang, T.; Deng, Y.B.; Yang, H.; et al. Albumin-templated platinum (II) sulfide nanodots for size-dependent cancer theranostics. Acta Biomater. 2023, 155, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.T.; Wang, A.Q.; Yang, X.F.; Allard, L.F.; Jiang, Z.; Cui, Y.T.; Liu, J.Y.; Li, J.; Zhang, T. Single-Atom Catalysis of Co Oxidation Using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Yuk, S.F.; Zheng, J.; Nguyen, M.T.; Lee, M.S.; Szanyi, J.; Kovarik, L.; Zhu, Z.H.; Balasubramanian, M.; Glezakou, V.A.; et al. Environment of Metal-O-Fe Bonds Enabling High Activity in CO2 Reduction on Single Metal Atoms and on Supported Nanoparticles. J. Am. Chem. Soc. 2021, 143, 5540–5549. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, C.; Inomata, Y.; Yasumura, S.; Lin, M.Y.; Taketoshi, A.; Honma, T.; Sakaguchi, N.; Haruta, M.; Shimizu, K.; Ishida, T.; et al. Defective Nio as a Stabilizer for Au Single-Atom Catalysts. ACS Catal. 2022, 12, 6149–6158. [Google Scholar] [CrossRef]
- Tian, S.B.; Wang, B.X.; Gong, W.B.; He, Z.Z.; Xu, Q.; Chen, W.X.; Zhang, Q.H.; Zhu, Y.Q.; Yang, J.R.; Fu, Q.; et al. Dual-Atom Pt Heterogeneous Catalyst with Excellent Catalytic Performances for the Selective Hydrogenation and Epoxidation. Nat. Commun. 2021, 12, 3181. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Guan, L.L.; Jiao, Y.J.; Yu, C.X.; Zhao, F.; Zhou, X.W.; Liu, Z. Pt, Ag and Au Nanoparticles on Hollow Carbon Spheres as Cathode ORR. Electron. Mater. Lett. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.F.; Yaseen, M.; Naeem, M.; Khan, A. Confinement of Au, Pd and Pt Nanoparticle with Reduced Sizes: Significant Improvement of Dispersion Degree and Catalytic Activity. Microporous Mesoporous Mater. 2022, 337, 111927. [Google Scholar] [CrossRef]
- Meng, G.H.; Zhang, X.Y.; Liu, C.; Wu, J.N.; Guo, X.H.; Liu, Z.Y. Ag Quantum Dot/Montmorillonite Composites with Fluorescent Properties: An Efficient Catalyst. Res. Chem. Intermed. 2017, 43, 7137–7145. [Google Scholar] [CrossRef]
- Nazarian-Samani, M.; Lim, H.D.; Haghighat-Shishavan, S.; Kim, H.K.; Ko, Y.M.; Kim, M.S.; Lee, S.W.; Kashani-Bozorg, S.F.; Abbasi, M.; Guim, H.U.; et al. A Robust Design of Ru Quantum Dot/N-Doped Holey Graphene for Efficient Li-O2 Batteries. J. Mater. Chem. A 2017, 5, 619–631. [Google Scholar] [CrossRef]
- Kou, J.H.; Varma, R.S. Beet Juice Utilization: Expeditious Green Synthesis of Noble Metal Nanoparticles (Ag, Au, Pt, and Pd) Using Microwaves. RSC Adv. 2012, 2, 10283–10290. [Google Scholar] [CrossRef]
- Xiong, Y.J.; Chen, J.Y.; Wiley, B.; Xia, Y.N.; Aloni, S.; Yin, Y.D. Understanding the Role of Oxidative Etching in the Polyol Synthesis of Pd Nanoparticles with Uniform Shape and Size. J. Am. Chem. Soc. 2005, 127, 7332–7333. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.S.; Zhang, H.; Xie, Z.X.; Xia, Y.N. Palladium Concave Nanocubes with High-Index Facets and Their Enhanced Catalytic Properties. Angew. Chem. Int. Ed. 2011, 50, 7850–7854. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.N.; Yang, P.D.; Sun, Y.G.; Wu, Y.Y.; Mayers, B.; Gates, B.; Yin, Y.D.; Kim, F.; Yan, Y.Q. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Huo, D.; Kim, M.J.; Lyu, Z.H.; Shi, Y.F.; Wiley, B.J.; Xia, Y.N. One-Dimensional Metal Nanostructures: From Colloidal Syntheses to Applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef]
- Sun, H.Y.; Guo, X.; Ye, W.; Kou, S.F.; Yang, J. Charge Transfer Accelerates Galvanic Replacement for PtAgAu Nanotubes with Enhanced Catalytic Activity. Nano Res. 2016, 9, 1173–1181. [Google Scholar] [CrossRef]
- Zhang, J.W.; Ye, J.Y.; Fan, Q.Y.; Jiang, Y.T.; Zhu, Y.F.; Li, H.Q.; Cao, Z.M.; Kuang, Q.; Cheng, J.; Zheng, J.; et al. Cyclic Penta-Twinned Rhodium Nanobranches as Superior Catalysts for Ethanol Electro-Oxidation. J. Am. Chem. Soc. 2018, 140, 11232–11240. [Google Scholar] [CrossRef]
- Xu, D.D.; Liu, X.L.; Han, M.; Bao, J.C. Facile Synthesis of Ultrathin Single-Crystalline Palladium Nanowires with Enhanced Electrocatalytic Activities. Chem. Commun. 2016, 52, 12996–12999. [Google Scholar] [CrossRef]
- Liu, J.W.; Niu, W.X.; Liu, G.G.; Chen, B.; Huang, J.T.; Cheng, H.F.; Hu, D.Y.; Wang, J.; Liu, Q.; Ge, J.J.; et al. Selective Epitaxial Growth of Rh Nanorods on 2h/Fcc Heterophase Au Nanosheets to Form 1D/2D Rh-Au Heterostructures for Highly Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2021, 143, 4387–4396. [Google Scholar] [CrossRef] [PubMed]
- Kichijo, R.; Miyajima, N.; Ogawa, D.; Sugimori, H.; Wang, K.H.; Imura, Y.; Kawai, T. Water-Phase Synthesis of Au and Au-Ag Nanowires and Their Sers Activity. RSC Adv. 2022, 12, 28937–28943. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.R.; Hu, Z.; Huang, M.; Zhao, Y.X.; Qu, J.Q.; Hu, S. Au Nanowires with High Aspect Ratio and Atomic Shell of Pt-Ru Alloy for Enhanced Methanol Oxidation Reaction. Chin. Chem. Lett. 2021, 32, 2033–2037. [Google Scholar] [CrossRef]
- Yin, S.L.; Wang, Z.Q.; Qian, X.Q.; Yang, D.D.; Xu, Y.; Li, X.N.; Wang, L.; Wang, H.J. PtM (M = Co, Ni) Mesoporous Nanotubes as Bifunctional Electrocatalysts for Oxygen Reduction and Methanol Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 7960–7968. [Google Scholar] [CrossRef]
- Hong, W.; Wang, J.; Wang, E.K. Rute/M (M=Pt, Pd) Nanoparticle Nanotubes with Enhanced Electrocatalytic Activity. J. Mater. Chem. A 2015, 3, 13642–13647. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.W.; Zhang, X.; Ye, J.Y.; Xue, F.; Zhen, C.; Liao, X.Y.; Li, H.Q.; Li, P.T.; Liu, M.C.; et al. Quatermetallic Pt-Based Ultrathin Nanowires Intensified by Rh Enable Highly Active and Robust Electrocatalysts for Methanol Oxidation. Nano Energy 2020, 71, 165705. [Google Scholar] [CrossRef]
- Li, M.F.; Zhao, Z.P.; Cheng, T.; Fortunelli, A.; Chen, C.Y.; Yu, R.; Zhang, Q.H.; Gu, L.; Merinov, B.V.; Lin, Z.Y.; et al. Ultrafine Jagged Platinum Nanowires Enable Ultrahigh Mass Activity for the Oxygen Reduction Reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Cui, X.Q.; Wei, S.T.; Zhang, Q.H.; Gu, L.; Meng, F.Q.; Fan, J.C.; Zheng, W.T. Highly Active Zigzag-Like Pt-Zn Alloy Nanowires with High-Index Facets for Alcohol Electrooxidation. Nano Res. 2019, 12, 1173–1179. [Google Scholar] [CrossRef]
- Wang, X.D.; Chen, S.T.; Reggiano, G.; Thota, S.; Wang, Y.C.; Kerns, P.; Suib, S.L.; Zhao, J. Au-Cu-M (M=Pt, Pd, Ag) Nanorods with Enhanced Catalytic Efficiency by Galvanic Replacement Reaction. Chem. Commun. 2019, 55, 1249–1252. [Google Scholar] [CrossRef]
- Yang, H.; He, L.Q.; Wang, Z.H.; Zheng, Y.Y.; Lu, X.H.; Li, G.R.; Fang, P.P.; Chen, J.; Tong, Y. Surface Plasmon Resonance Promoted Photoelectrocatalyst by Visible Light from Au Core Pd Shell Pt Cluster Nanoparticles. Electrochim. Acta 2016, 209, 591–598. [Google Scholar] [CrossRef]
- Guo, S.J.; Wang, L.; Wang, W.; Fang, Y.X.; Wang, E.K. Bifunctional Au@Pt Hybrid Nanorods. J. Colloid Interface Sci. 2007, 315, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.X.; Shifa, T.A.; Wen, Y.; Wang, F.M.; Zhan, X.Y.; Wang, Q.S.; Xu, K.; Huang, Y.; Yin, L.; et al. Two-Dimensional Non-Layered Materials: Synthesis, Properties and Applications. Adv. Funct. Mater. 2017, 27, 254. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Grzelczak, M. Growing Anisotropic Crystals at the Nanoscale. Science 2017, 356, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Q.; Zhou, J.Y.; Kong, Q.S.; Li, C.X. Two-Dimensional Au Nanocrystals: Shape/Size Controlling Synthesis, Morphologies, and Applications. Part. Part. Syst. Charact. 2015, 32, 796–808. [Google Scholar] [CrossRef]
- Huang, H.Z.; Liu, D.; Chen, L.W.; Zhu, Z.J.J.; Li, J.N.; Yu, Z.L.; Su, X.; Jing, X.T.; Wu, S.Q.; Tian, W.J.; et al. Ultrathin Dendritic Pd-Ag Nanoplates for Efficient and Durable Electrocatalytic Reduction of CO2 to Formate. Chem. Asian J. 2023, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.P.; Zhong, P.; Liu, K.; Han, L.; Zheng, H.Q.; Yin, Y.D.; Gao, C.B. Synthesis of Ultrathin Platinum Nanoplates for Enhanced Oxygen Reduction Activity. Chem. Sci. 2018, 9, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Tang, S.H.; Mu, X.L.; Dai, Y.; Chen, G.X.; Zhou, Z.Y.; Ruan, F.X.; Yang, Z.L.; Zheng, N.F. Freestanding Palladium Nanosheets with Plasmonic and Catalytic Properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.L.; Zhang, Z.C.; Chen, B.; Huang, Y.; Chen, J.Z.; Lai, Z.C.; Chen, Y.; Sindoro, M.; Wang, A.L.; Cheng, H.F.; et al. Synthesis of Ultrathin PdCu Alloy Nanosheets Used as a Highly Efficient Electrocatalyst for Formic Acid Oxidation. Adv. Mater. 2017, 29, 769. [Google Scholar] [CrossRef]
- Hu, P.F.; Yang, H.S.; Chen, S.L.; Xue, Y.F.; Zhu, Q.A.; Tang, M.Y.; Wang, H.; Liu, L.M.; Gao, P.; Duan, X.F.; et al. Hybrid Lamellar Superlattices with Monoatomic Platinum Layers and Programmable Organic Ligands. J. Am. Chem. Soc. 2023, 145, 717–724. [Google Scholar] [CrossRef]
- Sadikin, S.N.; Umar, M.I.A.; Hamzah, A.A.; Nurdin, M.; Umar, A.A. Formation of Ultimate Thin 2D Crystal of Pt in the Presence of Hexamethylenetetramine. Int. J. Mol. Sci. 2022, 23, 10239. [Google Scholar] [CrossRef]
- Yan, S.L.; Chen, C.Z.; Zhang, F.H.; Mahyoub, S.A.; Cheng, Z.M. High-Density Ag Nanosheets for Selective Electrochemical CO2 Reduction to CO. Nanotechnology 2021, 32, 165705. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Mahyoub, S.A.; Zhong, J.H.; Chen, C.Z.; Zhang, F.H.; Cheng, Z.M. Ultrathin and Dense Ag Nanosheets Synthesis under Suppressed Face (111) Growth and Surface Diffusion. J. Power Sources 2021, 488, 229484. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Zhang, H.Y.; Wu, P.S.; Zhang, F.Q.; Wei, S.H.; Sun, D.M.; Xu, L.; Tang, Y.W. One-Pot Synthesis of Freestanding Porous Palladium Nanosheets as Highly Efficient Electrocatalysts for Formic Acid Oxidation. Adv. Funct. Mater. 2017, 27, 1603852. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, J.; Wu, X.R.; Chen, P.; Jin, P.J.; Yao, H.C.; Chen, Y. Atomically Ultrathin Rhco Alloy Nanosheet Aggregates for Efficient Water Electrolysis in Broad Ph Range. J. Mater. Chem. A 2019, 7, 16437–16446. [Google Scholar] [CrossRef]
- Jin, C.H.; Fu, R.J.; Ran, L.Q.; Wang, W.H.; Wang, F.X.; Zheng, D.Z.; Feng, Q.; Wang, G.X. Facile Fabrication of Hierarchical Ultrathin Rh-Based Nanosheets for Efficient Hydrogen Evolution. RSC Adv. 2023, 13, 13985–13990. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Ham, S.; Acapulco, J.A.I.; Song, Y.; Hong, S.; Shuford, K.L.; Park, S. Fabrication of 2D Au Nanorings with Pt Framework. J. Am. Chem. Soc. 2014, 136, 17674–17680. [Google Scholar] [CrossRef]
- Sang, J.L.; Yu, L.; Song, X.W.; Geng, W.C.; Zhang, Y.; Li, Y.J. Nanoarchitectonics of 2D-Thin and Porous Ag-Au Nanostructures with Controllable Alloying Degrees toward Electrocatalytic CO2 Reduction. J. Alloys Compd. 2023, 944, 169155. [Google Scholar] [CrossRef]
- Chen, M.; Tang, S.H.; Guo, Z.D.; Wang, X.Y.; Mo, S.G.; Huang, X.Q.; Liu, G.; Zheng, N.F. Core-Shell Pd@Au Nanoplates as Theranostic Agents for in-Vivo Photoacoustic Imaging, CT Imaging, and Photothermal Therapy. Adv. Mater. 2014, 26, 8210–8216. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Wang, P.F.; Ye, F.; Tan, Q.Q.; Yang, J. Enhancing the Electrocatalytic Property of Hollow Structured Platinum Nanoparticles for Methanol Oxidation through a Hybrid Construction. Sci. Rep. 2014, 4, 6204. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, X.; Li, Y.; Li, P.; Liu, Q.; Fu, G.; Xu, L.; Sun, D.; Tang, Y. General Strategy for Synthesis of Pd3M (M = Co and Ni) Nanoassemblies as High-Performance Catalysts for Electrochemical Oxygen Reduction. Adv. Mater. Interfaces 2018, 5, 1701015. [Google Scholar] [CrossRef]
- Qiu, P.T.; Bi, J.L.; Zhang, X.J.; Yang, S.C. Organics- and Surfactant-Free Molten Salt Medium Controlled Synthesis of Pt-M (M = Cu and Pd) Bi- and Trimetallic Nanocubes and Nanosheets. ACS Sustain. Chem. Eng. 2017, 5, 4205–4213. [Google Scholar] [CrossRef]
- Yoo, S.; Cho, S.; Kim, D.; Ih, S.; Lee, S.; Zhang, L.Q.; Li, H.; Lee, J.Y.; Liu, L.C.; Park, S. 3D PtAu Nanoframe Superstructure as a High-Performance Carbon-Free Electrocatalyst. Nanoscale 2019, 11, 2840–2847. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Z.; Scudiero, L.; Ha, S. Effect of Au and Ru in Au@Pd and Ru@Pd Core@Shell Nanoparticles for Direct Formic Acid Fuel Cells. ECS Trans. 2014, 64, 1121–1127. [Google Scholar] [CrossRef]
- Song, C.Y.; Sun, Y.Z.; Li, J.X.; Dong, C.; Zhang, J.J.; Jiang, X.Y.; Wang, L.H. Silver-Mediated Temperature-Controlled Selective Deposition of Pt on Hexoctahedral Au Nanoparticles and the High Performance of Au@AgPt NPs in Catalysis and Sers. Nanoscale 2019, 11, 18881–18893. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, Y.P.; Ren, F.F.; Shiraishi, Y.; Du, Y.K. Universal Surfactant-Free Strategy for Self-Standing 3D Tremella-Like Pd-M (M = Ag, Pb, and Au) Nanosheets for Superior Alcohols Electrocatalysis. Adv. Funct. Mater. 2020, 30, 2000255. [Google Scholar] [CrossRef]
- Nahar, L.; Farghaly, A.A.; Esteves, R.J.A.; Arachchige, I.U. Shape Controlled Synthesis of Au/Ag/Pd Nanoalloys and Their Oxidation-Induced Self-Assembly into Electrocatalytically Active Aerogel Monoliths. Chem. Mater. 2017, 29, 7704–7715. [Google Scholar] [CrossRef]
- Pan, C.X.; Zheng, Y.Y.; Yang, J.; Lou, D.Y.; Li, J.; Sun, Y.J.; Liu, W. Pt-Pd Bimetallic Aerogel as High-Performance Electrocatalyst for Nonenzymatic Detection of Hydrogen Peroxide. Catalysts 2022, 12, 528. [Google Scholar] [CrossRef]
- Farsadrooh, M.; Saravani, H.; Mehdizadeh, K.; Elyasi, Z.; Javadian, H.; Douk, A.S. Growing Nanoparticles to Assemble a Modern Three-Dimensional Nanoarchitecture of Pt-Ru Aerogel for Advanced Electrocatalysis. J. Mol. Liq. 2023, 384, 122047. [Google Scholar] [CrossRef]
- Oh, A.; Sa, Y.J.; Hwang, H.; Baik, H.; Kim, J.; Kim, B.; Joo, S.H.; Lee, K. Rational Design of Pt-Ni-Co Ternary Alloy Nanoframe Crystals as Highly Efficient Catalysts toward the Alkaline Hydrogen Evolution Reaction. Nanoscale 2016, 8, 16379–16386. [Google Scholar] [CrossRef]
- Chen, G.Z.; Wang, R.Y.; Zhao, W.; Kang, B.T.; Gao, D.W.; Li, C.C.; Lee, J.Y. Effect of Ru Crystal Phase on the Catalytic Activity of Hydrolytic Dehydrogenation of Ammonia Borane. J. Power. Sources 2018, 396, 148–154. [Google Scholar] [CrossRef]
- Tanabe, I.; Ryoki, T.; Ozaki, Y. The Effects of Au Nanoparticle Size (5–60 Nm) and Shape (Sphere, Rod, Cube) over Electronic States and Photocatalytic Activities of TiO2 Studied by Far- and Deep-Ultraviolet Spectroscopy. RSC Adv. 2015, 5, 13648–13652. [Google Scholar] [CrossRef]
- Si, F.Z.; Chen, X.M.; Liang, L.; Li, C.Y.; Liao, J.H.; Liu, C.P.; Zhang, X.B.; Xing, W. Pt/C Anodic Catalysts with Controlled Morphology for Direct Dimethyl Ether Fuel Cell: The Role of Consecutive Surface. Electrochim. Acta 2011, 56, 5966–5971. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, T.T.; Zhang, Z.Q.; Zhang, G.D.; Wang, F.; Wang, X.F.; Liu, S.Z.; Zhang, H.Z.; Wang, S.S.; Ma, J. Investigation on Catalytic Properties of Au Nanorods with Different Aspect Ratios by Kinetic and Thermodynamic Analysis. J. Solid State Chem. 2018, 263, 11–17. [Google Scholar] [CrossRef]
- Khalil, L.; Sabahat, S.; Ahmed, W. Effect of Aspect Ratio on the Catalytic Activities of Gold Nanorods. Catal. Lett. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Sun, S.G.; Xu, H.Y.; Tang, S.H.; Guo, J.S.; Li, H.Q.; Cao, L.; Zhou, B.; Xin, Q.; Sun, G.Q. Synthesis of PtRu Nanowires and Their Catalytic Activity in the Anode of Direct Methanol Fuel Cells. Chin. J. Catal. 2006, 27, 932–936. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.H.; Zheng, Y.Y.; He, L.Q.; Zhan, C.; Lu, X.H.; Tian, Z.Q.; Fang, P.P.; Tong, Y.X. Tunable Wavelength Enhanced Photoelectrochemical Cells from Surface Plasmon Resonance. J. Am. Chem. Soc. 2016, 138, 16204–16207. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Zhou, Y.D.; Villarreal, E.; Lin, Y.; Zou, S.L.; Wang, H. Faceted Gold Nanorods: Nanocuboids, Convex Nanocuboids, and Concave Nanocuboids. Nano Lett. 2015, 15, 4161–4169. [Google Scholar] [CrossRef]
- Yue, G.C.; Li, S.; Li, D.M.; Liu, J.; Wang, Y.Q.; Zhao, Y.C.; Wang, N.; Cui, Z.M.; Zhao, Y. Coral-Like Au/TiO2 Hollow Nanofibers with through-Holes as a High-Efficient Catalyst through Mass Transfer Enhancement. Langmuir 2019, 35, 4843–4848. [Google Scholar] [CrossRef]
- Sadeghi, B.; Meskinfam, M. A Direct Comparison of Nanosilver Particles and Nanosilver Plates for the Oxidation of Ascorbic Acid. Spectrochim. Acta A 2012, 97, 326–328. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Burueva, D.B.; Prosvirin, I.P.; Klyushin, A.Y.; Panafidin, M.A.; Kovtunov, K.V.; Bukhtiyarov, V.I.; Koptyug, I.V. Bimetallic Pd-Au/Highly Oriented Pyrolytic Graphite Catalysts: From Composition to Pairwise Parahydrogen Addition Selectivity. J. Phys. Chem. C 2018, 122, 18588–18595. [Google Scholar] [CrossRef]
- Elbert, K.; Hu, J.; Ma, Z.; Zhang, Y.; Chen, G.Y.; An, W.; Liu, P.; Isaacs, H.S.; Adzic, R.R.; Wang, J.X. Elucidating Hydrogen Oxidation/Evolution Kinetics in Base and Acid by Enhanced Activities at the Optimized Pt Shell Thickness on the Ru Core. ACS Catal. 2015, 5, 6764–6772. [Google Scholar] [CrossRef]
- Bellini, M.; Pagliaro, M.V.; Lenarda, A.; Fornasiero, P.; Marelli, M.; Evangelisti, C.; Innocenti, M.; Jia, Q.Y.; Mukerjee, S.; Jankovic, J.; et al. Palladium-Ceria Catalysts with Enhanced Alkaline Hydrogen Oxidation Activity for Anion Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2019, 2, 4999–5008. [Google Scholar] [CrossRef]
- Mao, J.J.; He, C.T.; Pei, J.J.; Liu, Y.; Li, J.; Chen, W.X.; He, D.S.; Wang, D.S.; Li, Y.D. Isolated Ni Atoms Dispersed on Ru Nanosheets: High-Performance Electrocatalysts toward Hydrogen Oxidation Reaction. Nano Lett. 2020, 20, 3442–3448. [Google Scholar] [CrossRef] [PubMed]
- Alia, S.M.; Pivovar, B.S.; Yan, Y.S. Platinum-Coated Copper Nanowires with High Activity for Hydrogen Oxidation Reaction in Base. J. Am. Chem. Soc. 2013, 135, 13473–13478. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, A.R.; Ganguly, D.; Sundara, R. High temperature annealed (002) oriented WO3 nanoplatelets with uniform Pt decoration as durable carbon free anode electrocatalyst for PEMFC application. Int. J. Hydrogen Energy 2022, 47, 24978–24990. [Google Scholar]
- Li, H.; Wang, X.; Gong, X.; Liu, C.; Ge, J.J.; Song, P.; Xu, W.L. “One Stone Three Birds” of a Synergetic Effect between Pt Single Atoms and Clusters Makes an Ideal Anode Catalyst for Fuel Cells. J. Mater. Chem. A 2023, 11, 14826–14832. [Google Scholar] [CrossRef]
- Norskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Norskov, J.K. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23–J26. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.D.; Zhang, J.W.; Pan, L.; Zhang, X.W.; Zou, J.J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 464. [Google Scholar] [CrossRef]
- Li, C.J.; Xu, Y.; Yang, D.D.; Qian, X.Q.; Chai, X.J.; Wang, Z.Q.; Li, X.N.; Wang, L.; Wang, H.J. Boosting Electrocatalytic Activities of Pt-Based Mesoporous Nanoparticles for Overall Water Splitting by a Facile Ni, P Co-Incorporation Strategy. ACS Sustain. Chem. Eng. 2019, 7, 9709–9716. [Google Scholar] [CrossRef]
- Zhang, N.; Shao, Q.; Pi, Y.C.; Guo, J.; Huang, X.Q. Solvent-Mediated Shape Tuning of Well-Defined Rhodium Nanocrystals for Efficient Electrochemical Water Splitting. Chem. Mater. 2017, 29, 5009–5015. [Google Scholar] [CrossRef]
- Wang, Q.; Ming, M.; Niu, S.; Zhang, Y.; Fan, G.Y.; Hu, J.S. Scalable Solid-State Synthesis of Highly Dispersed Uncapped Metal (Rh, Ru, Ir) Nanoparticles for Efficient Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 698. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Zhan, G.M.; Zhang, L.Z. Solar Water Splitting and Nitrogen Fixation with Layered Bismuth Oxyhalides. Acc. Chem. Res. 2017, 50, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, C.; Lu, G.X. Visible Photocatalytic Water Splitting and Photocatalytic Two-Electron Oxygen Formation over Cu- and Fe-Doped g-C3N4. J. Phys. Chem. C 2016, 120, 56–63. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, H.W.; Du, X.; Zhang, T.R.; Tian, N.; Guo, Y.X.; Zhang, Y.H. In Situ CO-Crystallization for Fabrication of g-C3N4/Bi5O7 Heterojunction for Enhanced Visible-Light Photocatalysis. J. Phys. Chem. C 2015, 119, 17156–17165. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhang, G.G.; Lan, Z.A.; Lin, L.H.; Lin, S.; Wang, X.C. Overall Water Splitting by Pt/G-C3N4 Photocatalysts without Using Sacrificial Agents. Chem. Sci. 2016, 7, 3062–3066. [Google Scholar] [CrossRef]
- Claus, P.; Kraak, P.; Schodel, R. Selective Hydrogenation of α,β-Unsaturated Aldehydes to Allylic Alcohols over Supported Monometallic and Bimetallic Ag Catalysts. Stud. Surf. Sci. Catal. 1997, 108, 281–288. [Google Scholar]
- Wei, S.J.; Li, A.; Liu, J.C.; Li, Z.; Chen, W.X.; Gong, Y.; Zhang, Q.H.; Cheong, W.C.; Wang, Y.; Zheng, L.R.; et al. Direct Observation of Noble Metal Nanoparticles Transforming to Thermally Stable Single Atoms. Nat. Nanotechnol. 2018, 13, 856–861. [Google Scholar] [CrossRef]
- Xu, E.Z.; Feng, H.S.; Wang, L.; Zhang, Y.J.; Liu, K.L.; Cui, S.; Meng, H.; Wang, G.R.; Yang, Y.S. Pt Single Atoms and Nanosized Clusters as Catalytic Reaction Platforms for Selective Hydrogenation Applications. ACS Appl. Nano Mater. 2023, 16, 14991–15001. [Google Scholar] [CrossRef]
- Mitsudome, T.; Urayama, T.; Yamazaki, K.; Maehara, Y.; Yamasaki, J.; Gohara, K.; Maeno, Z.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Design of Core-Pd/Shell-Ag Nanocomposite Catalyst for Selective Semihydrogenation of Alkynes. ACS Catal. 2016, 6, 666–670. [Google Scholar] [CrossRef]
- Chen, M.; Li, B.L.; Wang, F.; Fang, J.H.; Li, K.; Zhang, C.B. Enhanced CH4 Selectivity in CO2 Hydrogenation on Bimetallic Pt-Ni Catalysts with Pt Nanoparticles Modified by Isolated Ni Atoms. ACS Appl. Nano Mater. 2023, 6, 5826–5834. [Google Scholar] [CrossRef]
- Wang, S.H.; Xin, Z.L.; Huang, X.; Yu, W.Z.; Niu, S.; Shao, L.D. Nanosized Pd-Au Bimetallic Phases on Carbon Nanotubes for Selective Phenylacetylene Hydrogenation. Phys. Chem. Chem. Phys. 2017, 19, 6164–6168. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.I. Recent Advances in Automobile Exhaust Catalysts. Catal. Today 2004, 90, 183–190. [Google Scholar] [CrossRef]
- Mahara, Y.; Ohyama, J.; Sawabe, K.; Satsuma, A. Synthesis of Supported Bimetal Catalysts Using Galvanic Deposition Method. Chem. Rec. 2018, 18, 1306–1313. [Google Scholar] [CrossRef]
- Le, P.H.; Yamashita, S.; Cao, K.L.A.; Hirano, T.; Tsunoji, N.; Kautsar, D.B.; Ogi, T. Co Oxidation Enabled by Three-Way Catalysts Comprising Pd/Rh Nanoparticles Supported on Al2O3 and CeZrO4 Confined in Macroporous Polystyrene Latex Templates. ACS Appl. Nano Mater. 2023, 6, 17324–17335. [Google Scholar] [CrossRef]
- Li, L.C.; Zhang, N.Q.; Wu, R.; Song, L.Y.; Zhang, G.Z.; He, H. Comparative Study of Moisture-Treated Pd@CeO2/Al2O3 and Pd/CeO2/Al2O3 Catalysts for Automobile Exhaust Emission Reactions: Effect of Core-Shell Interface. ACS Appl. Mater. Interfaces 2020, 12, 10350–10358. [Google Scholar] [CrossRef]
- Yin, X.Y.; Li, S.S.; Deng, J.; Wang, Y.; Li, M.C.; Zhao, Y.; Wang, W.; Wang, J.L.; Chen, Y.Q. Superior Pd-Rh Three-Way Catalyst: Modulating the Surface Composition by Introducing Ceria-Zirconia with Partial Κ-Ce2Zr2O8 Structure as Support. Ind. Eng. Chem. Res. 2022, 61, 13011–13020. [Google Scholar] [CrossRef]

| Element | Morphology | Diameter |
|---|---|---|
| Pt-Ru [1] | nanoclusters | 46.78 nm (overall) |
| 2.67 nm (single) | ||
| Pt [1] | nanoclusters | 32.85 nm (overall) |
| 3.74 nm (single) | ||
| Ru [2] | NPs | 1.6 nm |
| Ag [3] | spherical NPs | 18~26 nm |
| PtS [4] | NDs | 2.1 nm, 3.2 nm, 4.5 nm |
| Pt [9] | NPs | 11 ± 6 nm |
| Ag [9] | NPs | 25 ± 4 nm |
| Au [9] | NPs | 32 ± 18 nm |
| Pd [10] | NPs | 3.6 nm |
| Pt [10] | NPs | 4.5 nm |
| Ag [11] | quantum dots | 2~5 nm |
| Ru [12] | quantum dots | 1~5 nm |
| Ag [13] | spherical NPs | 20~40 nm |
| Pd [14] | cuboctahedrons | 8~10 nm |
| Pd [15] | concave nanocubes | 37 nm |
| Element | Morphology | Diameter |
|---|---|---|
| Pt-Au-Ag [18] | porous NTs | |
| Rh [19] | CPT NBs | 4.3 nm |
| Pd [20] | NWs | 3.5 nm |
| Au [22] | NWs | 2.7 nm |
| Au-Ag [22] | NWs | 3.3 nm |
| Au@Pt [23] | core–shell NWs | 6.8 nm |
| Rh [21] | NRs | |
| PtM (M=Co, Ni) [24] | mesoporous NTs | 100 nm |
| RuTe [25] | NTs | 14 nm |
| PtCoNiRh [26] | ultrathin nanowires | 1.5 nm |
| Pt [27] | ultrafine jagged nanowires | 2.2 nm |
| Pt-Zn [28] | zigzag-like NWs | 6.24 nm |
| Au–Cu–M (X=Pt, Pd, Ag) [29] | NRs | |
| Au@Pd@Pt [30] | core–shell NRs | 49 nm |
| Au@Pt [31] | hybrid nanorods | 17 nm |
| Element | Morphology | Thickness |
|---|---|---|
| PdAg [35] | nanoplates | 1.7 nm |
| Au [21] | NSs | 8 nm |
| Pt [36] | nanoplates | 2 nm |
| Pd [37] | NSs | 1.8 nm |
| PdCu [38] | NSs | 2.8 ± 0.3 nm |
| Pt [40] | ultimate thin NSs | 1.55 nm |
| Ag [41] | high-density NSs | 20 nm, 50 nm |
| Ag [42] | ultrathin and dense NSs | 11 nm |
| Pd [43] | porous nanoplates | 10 nm |
| RhCo [44] | ultrathin NSs | 1.3 nm |
| Rh [45] | hierarchical ultrathin NSs | |
| Pt@Au [46] | core–shell nanorings | 41 nm |
| AgAu [47] | porous nanomeshes | 3 nm |
| Pd@Au [48] | core–shell nanoplates | 4 nm |
| Element | Morphology | Size |
|---|---|---|
| Ag@Pt [49] | core–shell NPs | 12.6 nm |
| PtCu [51] | NCs | 26.54 nm |
| PtPdCu [51] | NCs | 40.86 nm |
| PtAu [52] | TOh NFs | 72 ± 2 nm |
| Pd3Co [50] | nanoassemblies | 44.9 nm |
| Au@Pd [53] | core–shell NPs | 10.14 nm |
| Ru@Pd [53] | core–shell NPs | 11.36 nm |
| Au@AgPt [54] | complex hexoctahedral NPs | |
| Pd-M (M=Ag, Pb, Au) [55] | NSs with tremella-like superstructures | |
| Au/Ag/Pd [56] | self-assembled aerogel | 4.8 nm |
| PtPd [57] | aerogel | 4.9 nm |
| Pt-Ru [58] | assembled aerogel | |
| Pt-Ni-Co [59] | nanoframe crystals | 20 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Yang, H.; Hu, P.; Wang, G.-S.; Guo, Y.; Zhao, H. Dimension Engineering in Noble-Metal-Based Nanocatalysts. Catalysts 2024, 14, 9. https://doi.org/10.3390/catal14010009
Liu B, Yang H, Hu P, Wang G-S, Guo Y, Zhao H. Dimension Engineering in Noble-Metal-Based Nanocatalysts. Catalysts. 2024; 14(1):9. https://doi.org/10.3390/catal14010009
Chicago/Turabian StyleLiu, Bei, Haosen Yang, Pengfei Hu, Guang-Sheng Wang, Yongqiang Guo, and Hewei Zhao. 2024. "Dimension Engineering in Noble-Metal-Based Nanocatalysts" Catalysts 14, no. 1: 9. https://doi.org/10.3390/catal14010009
APA StyleLiu, B., Yang, H., Hu, P., Wang, G.-S., Guo, Y., & Zhao, H. (2024). Dimension Engineering in Noble-Metal-Based Nanocatalysts. Catalysts, 14(1), 9. https://doi.org/10.3390/catal14010009









