Engineering Electrode Polarity for Enhancing In Situ Generation of Hydroxyl Radicals Using Granular Activated Carbon
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrogeneration of H2O2 and •OH by PR
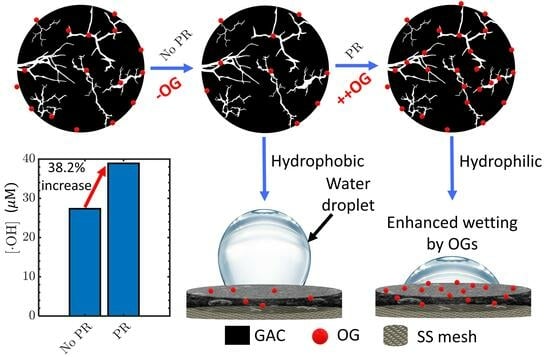
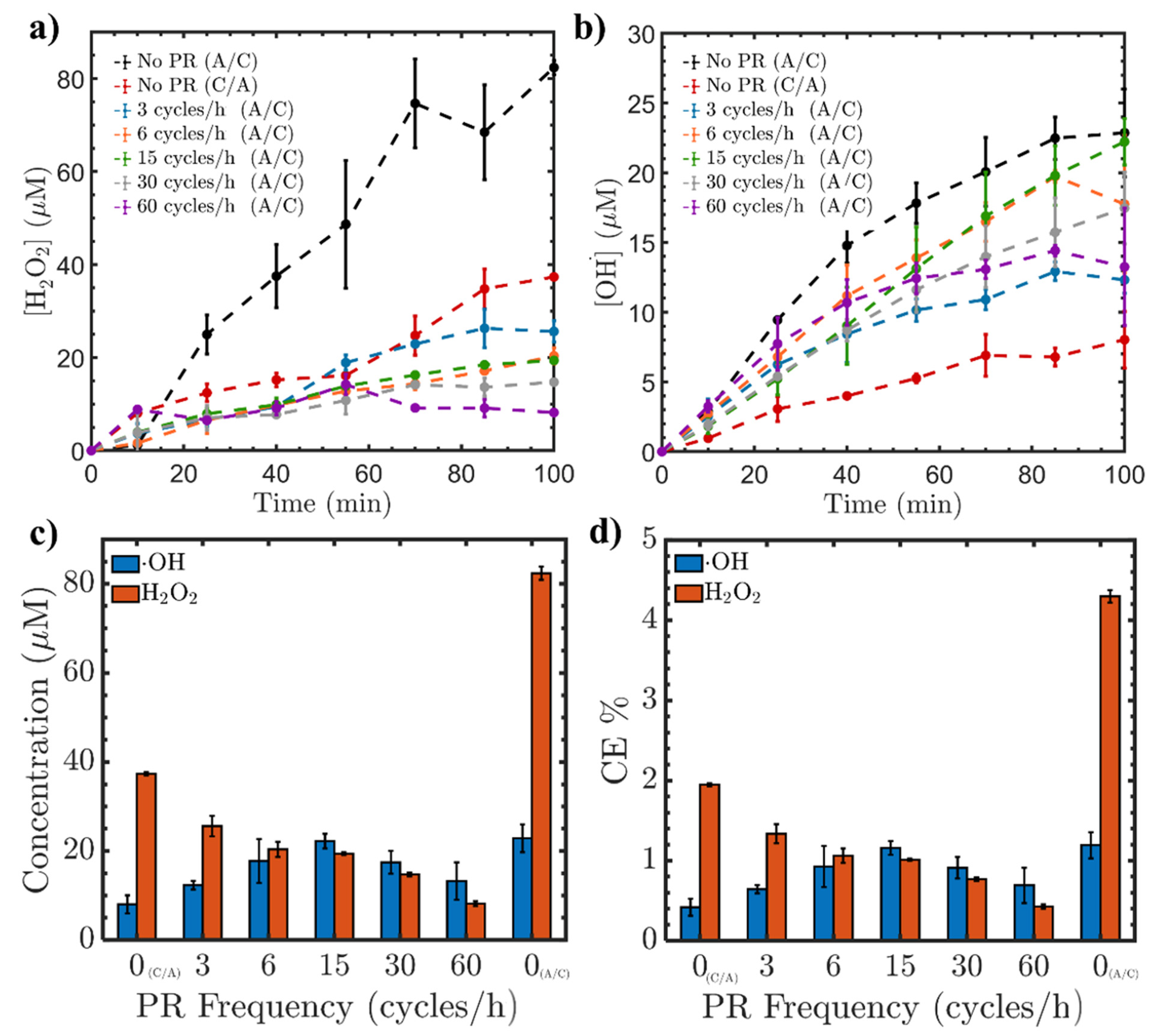
2.1.1. Influence of PR Modification
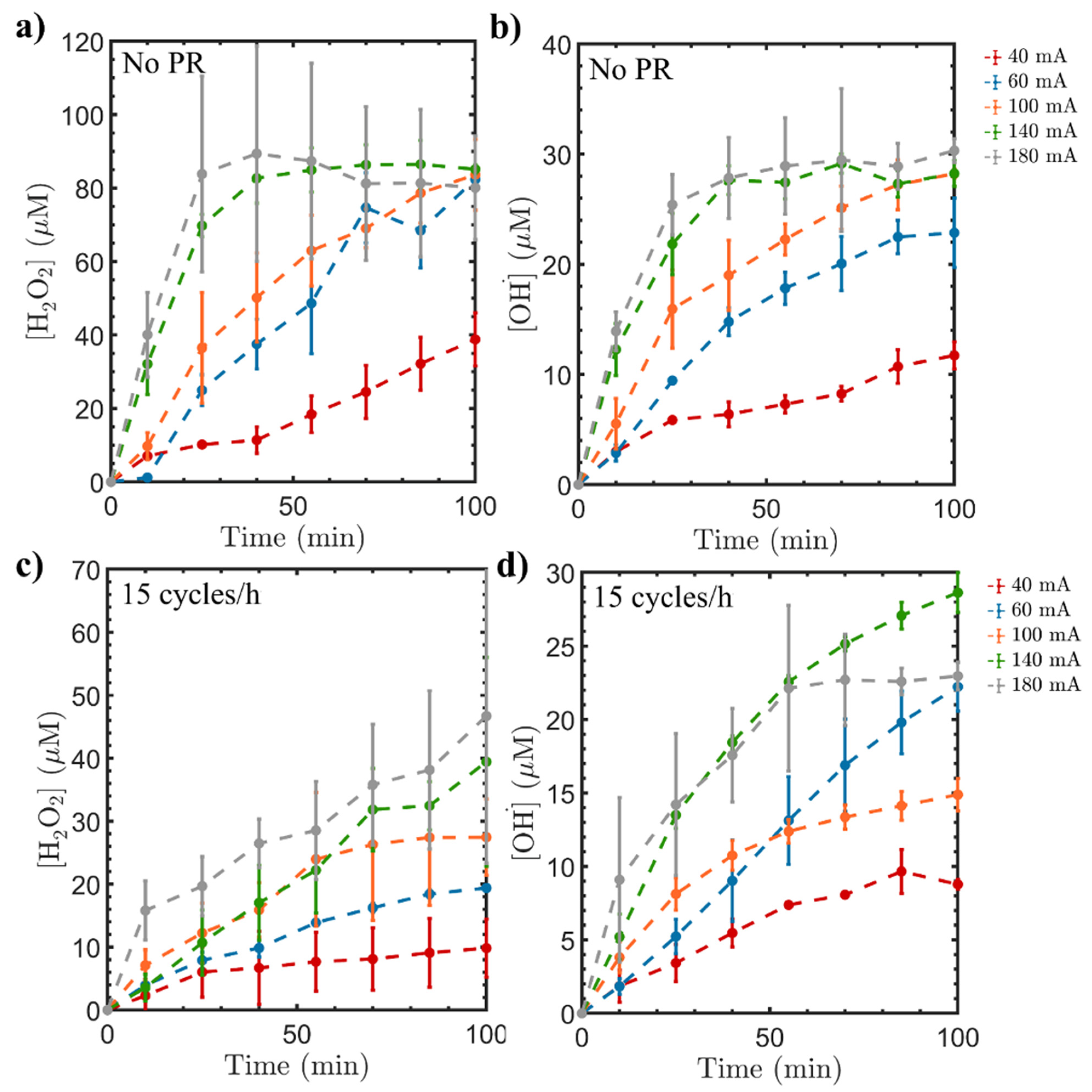
2.1.2. Influence of Current Intensity
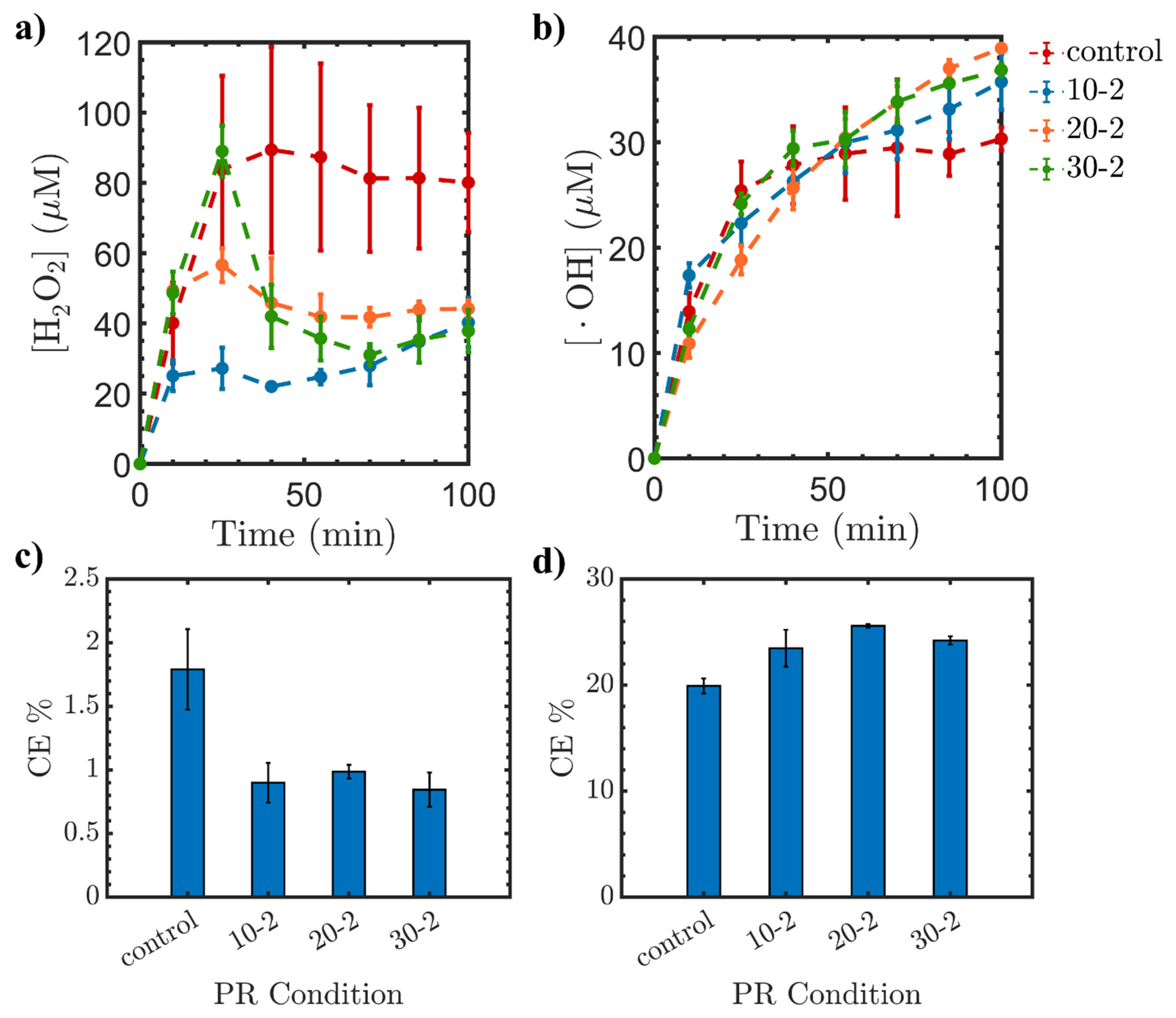
2.1.3. Influence of Reaction Time
2.2. Characterization: Contact Angle Measurement and Cyclic Voltammetry
3. Methodology
3.1. Preparation of GAC Cathode
3.2. Electrochemical Reactor
3.3. Characterization of the GAC-PTFE
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anglada, A.; Urtiaga, A.; Ortiz, I. Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J. Chem. Technol. Biotechnol. 2009, 84, 1747–1755. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Rajic, L.; Meng, X.; Nazari, R.; Zhao, Y.; Wang, Y.; Gao, J.; Qin, Y.; Alshawabkeh, A.N. Efficient H2O2 electrogeneration at graphite felt modified via electrode polarity reversal: Utilization for organic pollutants degradation. Chem. Eng. J. 2019, 364, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.S.; Silva, A.M.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. The influence of structure and surface chemistry of carbon materials on the decomposition of hydrogen peroxide. Carbon 2013, 62, 97–108. [Google Scholar] [CrossRef]
- Li, N.; Huang, C.; Wang, X.; Feng, Y.; An, J. Electrosynthesis of hydrogen peroxide via two-electron oxygen reduction reaction: A critical review focus on hydrophilicity/hydrophobicity of carbonaceous electrode. Chem. Eng. J. 2022, 450, 138246. [Google Scholar] [CrossRef]
- Tabti, Z.; Ruiz-Rosas, R.; Quijada, C.; Cazorla-Amoros, D.; Morallon, E. Tailoring the surface chemistry of activated carbon cloth by electrochemical methods. ACS Appl. Mater. Interfaces 2014, 6, 11682–11691. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, R.; Marco-Lozar, J.; Quijada, C.; Cazorla-Amorós, D.; Morallón, E. Effect of electrochemical treatments on the surface chemistry of activated carbon. Carbon 2009, 47, 1018–1027. [Google Scholar] [CrossRef]
- Zhao, Y.; Hojabri, S.; Sarrouf, S.; Alshawabkeh, A.N. Electrogeneration of H2O2 by graphite felt double coated with polytetrafluoroethylene and polydimethylsiloxane. J. Environ. Chem. Eng. 2022, 10, 108024. [Google Scholar] [CrossRef]
- Min, S.-J.; Kim, J.-G.; Baek, K. Role of carbon fiber electrodes and carbonate electrolytes in electrochemical phenol oxidation. J. Hazard. Mater. 2020, 400, 123083. [Google Scholar] [CrossRef]
- Khataee, A.; Safarpour, M.; Zarei, M.; Aber, S. Electrochemical generation of H2O2 using immobilized carbon nanotubes on graphite electrode fed with air: Investigation of operational parameters. J. Electroanal. Chem. 2011, 659, 63–68. [Google Scholar] [CrossRef]
- Qi, H.; Sun, X.; Sun, Z. Cu-doped Fe2O3 nanoparticles/etched graphite felt as bifunctional cathode for efficient degradation of sulfamethoxazole in the heterogeneous electro-Fenton process. Chem. Eng. J. 2022, 427, 131695. [Google Scholar] [CrossRef]
- Afanga, H.; Zazou, H.; Titchou, F.E.; Gaayda, J.E.; Sopaj, F.; Akbour, R.A.; Hamdani, M. Electrochemical oxidation of Naphthol Blue Black with different supporting electrolytes using a BDD/carbon felt cell. J. Environ. Chem. Eng. 2021, 9, 104498. [Google Scholar] [CrossRef]
- Li, D.; Zheng, T.; Liu, Y.; Hou, D.; He, H.; Song, H.; Zhang, J.; Tian, S.; Zhang, W.; Wang, L.; et al. A cost-effective Electro-Fenton process with graphite felt electrode aeration for degradation of dimethyl phthalate: Enhanced generation of H2O2 and iron recycling that simultaneously regenerates the electrode. Chem. Eng. J. 2020, 394, 125033. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, M.; Yu, X. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration. Electrochim. Acta 2015, 163, 182–189. [Google Scholar] [CrossRef]
- Özcan, A.; Şahin, Y.; Koparal, A.S.; Oturan, M.A. A comparative study on the efficiency of electro-Fenton process in the removal of propham from water. Appl. Catal. B Environ. 2009, 89, 620–626. [Google Scholar] [CrossRef]
- Wang, C.-T.; Hu, J.-L.; Chou, W.-L.; Kuo, Y.-M. Removal of color from real dyeing wastewater by Electro-Fenton technology using a three-dimensional graphite cathode. J. Hazard. Mater. 2008, 152, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Oturan, N.; Dezotti, M.; Oturan, M.A. Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode. Appl. Catal. B Environ. 2008, 83, 140–149. [Google Scholar] [CrossRef]
- Rangel-Mendez, J.R.; Streat, M. Adsorption of cadmium by activated carbon cloth: Influence of surface oxidation and solution pH. Water Res. 2002, 36, 1244–1252. [Google Scholar] [CrossRef]
- Isarain-Chávez, E.; Arias, C.; Cabot, P.L.; Centellas, F.; Rodríguez, R.M.; Garrido, J.A.; Brillas, E. Mineralization of the drug β-blocker atenolol by electro-Fenton and photoelectro-Fenton using an air-diffusion cathode for H2O2 electrogeneration combined with a carbon-felt cathode for Fe2+ regeneration. Appl. Catal. B Environ. 2010, 96, 361–369. [Google Scholar] [CrossRef]
- Zanella, O.; Tessaro, I.C.; Féris, L.A. Desorption-and decomposition-based techniques for the regeneration of activated carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Singh, A.R.; Dhumal, P.S.; Bhakare, M.A.; Lokhande, K.D.; Bondarde, M.P.; Some, S. In-situ synthesis of metal oxide and polymer decorated activated carbon-based photocatalyst for organic pollutants degradation. Sep. Purif. Technol. 2022, 286, 120380. [Google Scholar] [CrossRef]
- Fang, G.-D.; Liu, C.; Gao, J.; Zhou, D.-M. New insights into the mechanism of the catalytic decomposition of hydrogen peroxide by activated carbon: Implications for degradation of diethyl phthalate. Ind. Eng. Chem. Res. 2014, 53, 19925–19933. [Google Scholar] [CrossRef]
- Chang, C.; Lian, F.; Zhu, L. Simultaneous adsorption and degradation of γ-HCH by nZVI/Cu bimetallic nanoparticles with activated carbon support. Environ. Pollut. 2011, 159, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-H.; Hsu, M.-C. Regeneration of granular activated carbon by an electrochemical process. Sep. Purif. Technol. 2008, 64, 227–236. [Google Scholar] [CrossRef]
- Streat, M.; Horner, D.J. Adsorption of Highly Soluble Herbicides from Water Using Activated Carbon and Hypercrosslinked Polymers. Process Saf. Environ. Prot. 2000, 78, 363–382. [Google Scholar] [CrossRef]
- Wang, A.; Qu, J.; Ru, J.; Liu, H.; Ge, J. Mineralization of an azo dye Acid Red 14 by electro-Fenton’s reagent using an activated carbon fiber cathode. Dye. Pigment. 2005, 65, 227–233. [Google Scholar] [CrossRef]
- Quintanilla, A.; Menéndez, N.; Tornero, J.; Casas, J.A.; Rodríguez, J.J. Surface modification of carbon-supported iron catalyst during the wet air oxidation of phenol: Influence on activity, selectivity and stability. Appl. Catal. B Environ. 2008, 81, 105–114. [Google Scholar] [CrossRef]
- Ferrández-Gómez, B.; Ruiz-Rosas, R.; Beaumont, S.; Cazorla-Amorós, D.; Morallón, E. Electrochemical regeneration of spent activated carbon from drinking water treatment plant at different scale reactors. Chemosphere 2021, 264, 128399. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Xing, Z.-J.; Duan, Z.-K.; Meng, L.; Wang, Y. Effects of steam activation on the pore structure and surface chemistry of activated carbon derived from bamboo waste. Appl. Surf. Sci. 2014, 315, 279–286. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhao, Y.; Wang, X.; Wang, R.; Tang, T. Electrogeneration of H2O2 on a composite acetylene black–PTFE cathode consisting of a sheet active core and a dampproof coating. Electrochim. Acta 2014, 133, 414–421. [Google Scholar] [CrossRef]
- Aveiro, L.R.; da Silva, A.G.M.; Antonin, V.S.; Candido, E.G.; Parreira, L.S.; Geonmonond, R.S.; de Freitas, I.C.; Lanza, M.R.V.; Camargo, P.H.C.; Santos, M.C. Carbon-supported MnO2 nanoflowers: Introducing oxygen vacancies for optimized volcano-type electrocatalytic activities towards H2O2 generation. Electrochim. Acta 2018, 268, 101–110. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Teli, A.M.; Harale, N.S.; Patil, D.S.; Pawar, S.A.; Shin, J.C.; Patil, P.S. Fabrication of high energy density supercapacitor device based on hollow iridium oxide nanofibers by single nozzle electrospinning. Appl. Surf. Sci. 2021, 546, 149102. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Wang, Y.; Han, G.F.; Zhao, Y.; Ge, X.; Li, F.; Zhang, Z.; Zhong, Q.; Baek, J.B. Carbon-Based Electrocatalysts for Efficient Hydrogen Peroxide Production. Adv. Mater. 2021, 33, 2103266. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Fellinger, T.-P.; Hasché, F.; Strasser, P.; Antonietti, M. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 4072–4075. [Google Scholar] [CrossRef]
- Zhao, K.; Su, Y.; Quan, X.; Liu, Y.; Chen, S.; Yu, H. Enhanced H2O2 production by selective electrochemical reduction of O2 on fluorine-doped hierarchically porous carbon. J. Catal. 2018, 357, 118–126. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, M.; Zhang, C.; Jiang, Y.; Bi, Z.; Yang, J. Electro-Fenton degradation of p-nitrophenol using the anodized graphite felts. Chem. Eng. J. 2013, 233, 185–192. [Google Scholar] [CrossRef]
- Miao, J.; Zhu, H.; Tang, Y.; Chen, Y.; Wan, P. Graphite felt electrochemically modified in H2SO4 solution used as a cathode to produce H2O2 for pre-oxidation of drinking water. Chem. Eng. J. 2014, 250, 312–318. [Google Scholar] [CrossRef]
- Barton, S.; Evans, M.; Halliop, E.; MacDonald, J. Anodic oxidation of porous carbon. Langmuir 1997, 13, 1332–1336. [Google Scholar] [CrossRef]
- Pletcher, D.; Walsh, F.C. Industrial Electrochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yoon, C.-M.; Long, D.; Jang, S.-M.; Qiao, W.; Ling, L.; Miyawaki, J.; Rhee, C.-K.; Mochida, I.; Yoon, S.-H. Electrochemical surface oxidation of carbon nanofibers. Carbon 2011, 49, 96–105. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Nunell, G.V.; Fernandez, M.E.; Bonelli, P.R.; Cukierman, A.L. Nitrate uptake improvement by modified activated carbons developed from two species of pine cones. J. Colloid Interface Sci. 2015, 440, 102–108. [Google Scholar] [CrossRef]
- Sun, H.; Li, A.; Zhu, Z.; Liang, W.; Zhao, X.; La, P.; Deng, W. Superhydrophobic activated carbon-coated sponges for separation and absorption. ChemSusChem 2013, 6, 1057–1062. [Google Scholar] [CrossRef]
- Mazarji, M.; Aminzadeh, B.; Baghdadi, M.; Bhatnagar, A. Removal of nitrate from aqueous solution using modified granular activated carbon. J. Mol. Liq. 2017, 233, 139–148. [Google Scholar] [CrossRef]
- Rajic, L.; Fallahpour, N.; Yuan, S.; Alshawabkeh, A.N. Electrochemical transformation of trichloroethylene in aqueous solution by electrode polarity reversal. Water Res. 2014, 67, 267–275. [Google Scholar] [CrossRef]
- Pazos, M.; Sanroman, M.; Cameselle, C. Improvement in electrokinetic remediation of heavy metal spiked kaolin with the polarity exchange technique. Chemosphere 2006, 62, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.N.; Sarrouf, S.; Ehsan, M.F.; Manzoor, S.; Ashiq, M.N.; Alshawabkeh, A.N. Polarity reversal for enhanced in-situ electrochemical synthesis of H2O2 over banana-peel derived biochar cathode for water remediation. Electrochim. Acta 2023, 453, 142351. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Fan, Y.; Zhang, Y.; Tong, M.; Liao, P. Pd-Catalytic In Situ Generation of H2O2 from H2 and O2 Produced by Water Electrolysis for the Efficient Electro-Fenton Degradation of Rhodamine B. Environ. Sci. Technol. 2011, 45, 8514–8520. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jiang, B.; Yuan, J.; Liu, Y.; Bi, X.; Xin, S. Cost-effective electrogeneration of H2O2 utilizing HNO3 modified graphite/polytetrafluoroethylene cathode with exterior hydrophobic film. J. Colloid Interface Sci. 2019, 533, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, A.; Cano, M.; Calsolaro, F.; Santiago, A.R.P.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Berlier, G.; Cravotto, G.; Martina, K.; Luque, R. Improving the electrocatalytic performance of sustainable Co/carbon materials for the oxygen evolution reaction by ultrasound and microwave assisted synthesis. Sustain. Energy Fuels 2021, 5, 720–731. [Google Scholar] [CrossRef]
- Zhou, W.; Rajic, L.; Chen, L.; Kou, K.; Ding, Y.; Meng, X.; Wang, Y.; Mulaw, B.; Gao, J.; Qin, Y.; et al. Activated carbon as effective cathode material in iron-free Electro-Fenton process: Integrated H2O2 electrogeneration, activation, and pollutants adsorption. Electrochim. Acta 2019, 296, 317–326. [Google Scholar] [CrossRef]
- Canizares, P.; Dominguez, J.; Rodrigo, M.; Villasenor, J.; Rodriguez, J. Effect of the current intensity in the electrochemical oxidation of aqueous phenol wastes at an activated carbon and steel anode. Ind. Eng. Chem. Res. 1999, 38, 3779–3785. [Google Scholar] [CrossRef]
- Yuan, S.; Gou, N.; Alshawabkeh, A.N.; Gu, A.Z. Efficient degradation of contaminants of emerging concerns by a new electro-Fenton process with Ti/MMO cathode. Chemosphere 2013, 93, 2796–2804. [Google Scholar] [CrossRef]
- Lakshmipathiraj, P.; Raju, G.B.; Sakai, Y.; Takuma, Y.; Yamasaki, A.; Kato, S.; Kojima, T. Studies on electrochemical detoxification of trichloroethene (TCE) on Ti/IrO2–Ta2O5 electrode from aqueous solution. Chem. Eng. J. 2012, 198, 211–218. [Google Scholar] [CrossRef]
- Wüthrich, R.; Comninellis, C.; Bleuler, H. Bubble evolution on vertical electrodes under extreme current densities. Electrochim. Acta 2005, 50, 5242–5246. [Google Scholar] [CrossRef]
- Taqieddin, A.; Nazari, R.; Rajic, L.; Alshawabkeh, A. Physicochemical hydrodynamics of gas bubbles in two phase electrochemical systems. J. Electrochem. Soc. 2017, 164, E448. [Google Scholar] [CrossRef] [PubMed]
- Guelcher, S.A.; Solomentsev, Y.E.; Sides, P.J.; Anderson, J.L. Thermocapillary phenomena and bubble coalescence during electrolytic gas evolution. J. Electrochem. Soc. 1998, 145, 1848. [Google Scholar] [CrossRef]
- Gilbert, D.M.; Sale, T.C. Sequential electrolytic oxidation and reduction of aqueous phase energetic compounds. Environ. Sci. Technol. 2005, 39, 9270–9277. [Google Scholar] [CrossRef] [PubMed]
- Georgi, A.; Kopinke, F.-D. Interaction of adsorption and catalytic reactions in water decontamination processes: Part I. Oxidation of organic contaminants with hydrogen peroxide catalyzed by activated carbon. Appl. Catal. B Environ. 2005, 58, 9–18. [Google Scholar] [CrossRef]
- Aguinaco, A.; Pocostales, J.P.; García-Araya, J.F.; Beltran, F.J. Decomposition of hydrogen peroxide in the presence of activated carbons with different characteristics. J. Chem. Technol. Biotechnol. 2011, 86, 595–600. [Google Scholar] [CrossRef]
- Khalil, L.B.; Girgis, B.S.; Tawfik, T.A.M. Decomposition of H2O2 on activated carbon obtained from olive stones. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2001, 76, 1132–1140. [Google Scholar] [CrossRef]
- Vega, E.; Valdés, H. New evidence of the effect of the chemical structure of activated carbon on the activity to promote radical generation in an advanced oxidation process using hydrogen peroxide. Microporous Mesoporous Mater. 2018, 259, 1–8. [Google Scholar] [CrossRef]
- Abdulrasheed, A.A.; Jalil, A.A.; Triwahyono, S.; Zaini, M.A.A.; Gambo, Y.; Ibrahim, M. Surface modification of activated carbon for adsorption of SO2 and NOX: A review of existing and emerging technologies. Renew. Sustain. Energy Rev. 2018, 94, 1067–1085. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Gao, J.; Sun, F.; Zhao, G. Janus graphite felt cathode dramatically enhance the H2O2 yield from O2 electroreduction by the hydrophilicity-hydrophobicity regulation. Chemosphere 2021, 278, 130382. [Google Scholar] [CrossRef]
- Chen, P.-A.; Cheng, H.-C.; Wang, H.P. Activated carbon recycled from bitter-tea and palm shell wastes for capacitive desalination of salt water. J. Clean. Prod. 2018, 174, 927–932. [Google Scholar] [CrossRef]
- Leaf-nosed bat. In Encyclopædia Britannica; Encyclopædia Britannica Online: Chatswood West, NSW, Australia, 2009.
- Bury, N.A.; Mumford, K.A.; Stevens, G.W. The electro-Fenton regeneration of Granular Activated Carbons: Degradation of organic contaminants and the relationship to the carbon surface. J. Hazard. Mater. 2021, 416, 125792. [Google Scholar] [CrossRef] [PubMed]
- Trellu, C.; Oturan, N.; Keita, F.K.; Fourdrin, C.; Pechaud, Y.; Oturan, M.A. Regeneration of Activated Carbon Fiber by the Electro-Fenton Process. Environ. Sci. Technol. 2018, 52, 7450–7457. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Lin, C.; Qi, C.; Zhang, H.; Ma, J. Enhanced activation of periodate by iodine-doped granular activated carbon for organic contaminant degradation. Chemosphere 2017, 181, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Mines, P.D.; Uthuppu, B.; Thirion, D.; Jakobsen, M.H.; Yavuz, C.T.; Andersen, H.R.; Hwang, Y. Granular activated carbon with grafted nanoporous polymer enhances nanoscale zero-valent iron impregnation and water contaminant removal. Chem. Eng. J. 2018, 339, 22–31. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Zhao, Y.; Wu, Y.; Liu, Y.; Wang, R.; Yang, Y.; Chen, J. Research status and progress in degradation of organic pollutants via hydrogen evolution reaction and oxygen evolution reaction in wastewater electrochemical treatment. Int. J. Hydrog. Energy 2023, 48, 33746–33762. [Google Scholar] [CrossRef]


| Variable | Unit | Values | Conditions |
|---|---|---|---|
| PR frequency | Cycles/h | 3; 6; 15; 30; 60 | 10 mM BA; 60 mA |
| Current intensity | mA | 40; 60; 100; 140; 180 | 10 mM BA; No PR; 15 cycles/h |
| PR | - | A/C; C/A | 10 mM BA; 60 mA |
| Time interval | mins | 10-2; 20-2; 30-2 | 10 mM BA; 140 mA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarrouf, S.; Taqieddin, A.; Ehsan, M.F.; Alshawabkeh, A.N. Engineering Electrode Polarity for Enhancing In Situ Generation of Hydroxyl Radicals Using Granular Activated Carbon. Catalysts 2024, 14, 52. https://doi.org/10.3390/catal14010052
Sarrouf S, Taqieddin A, Ehsan MF, Alshawabkeh AN. Engineering Electrode Polarity for Enhancing In Situ Generation of Hydroxyl Radicals Using Granular Activated Carbon. Catalysts. 2024; 14(1):52. https://doi.org/10.3390/catal14010052
Chicago/Turabian StyleSarrouf, Stephanie, Amir Taqieddin, Muhammad Fahad Ehsan, and Akram N. Alshawabkeh. 2024. "Engineering Electrode Polarity for Enhancing In Situ Generation of Hydroxyl Radicals Using Granular Activated Carbon" Catalysts 14, no. 1: 52. https://doi.org/10.3390/catal14010052
APA StyleSarrouf, S., Taqieddin, A., Ehsan, M. F., & Alshawabkeh, A. N. (2024). Engineering Electrode Polarity for Enhancing In Situ Generation of Hydroxyl Radicals Using Granular Activated Carbon. Catalysts, 14(1), 52. https://doi.org/10.3390/catal14010052