Tailored Ni-MgO Catalysts: Unveiling Temperature-Driven Synergy in CH4-CO2 Reforming
Abstract
:1. Introduction
2. Characterization and Activity Results
2.1. Overview of Textural Analysis
2.2. X-ray Diffraction (XRD) Analysis
2.3. Temperature-Programmed Reduction and Desorption Study
2.4. Infrared (IR) Spectroscopy of Fresh and CO2-Treated Catalyst
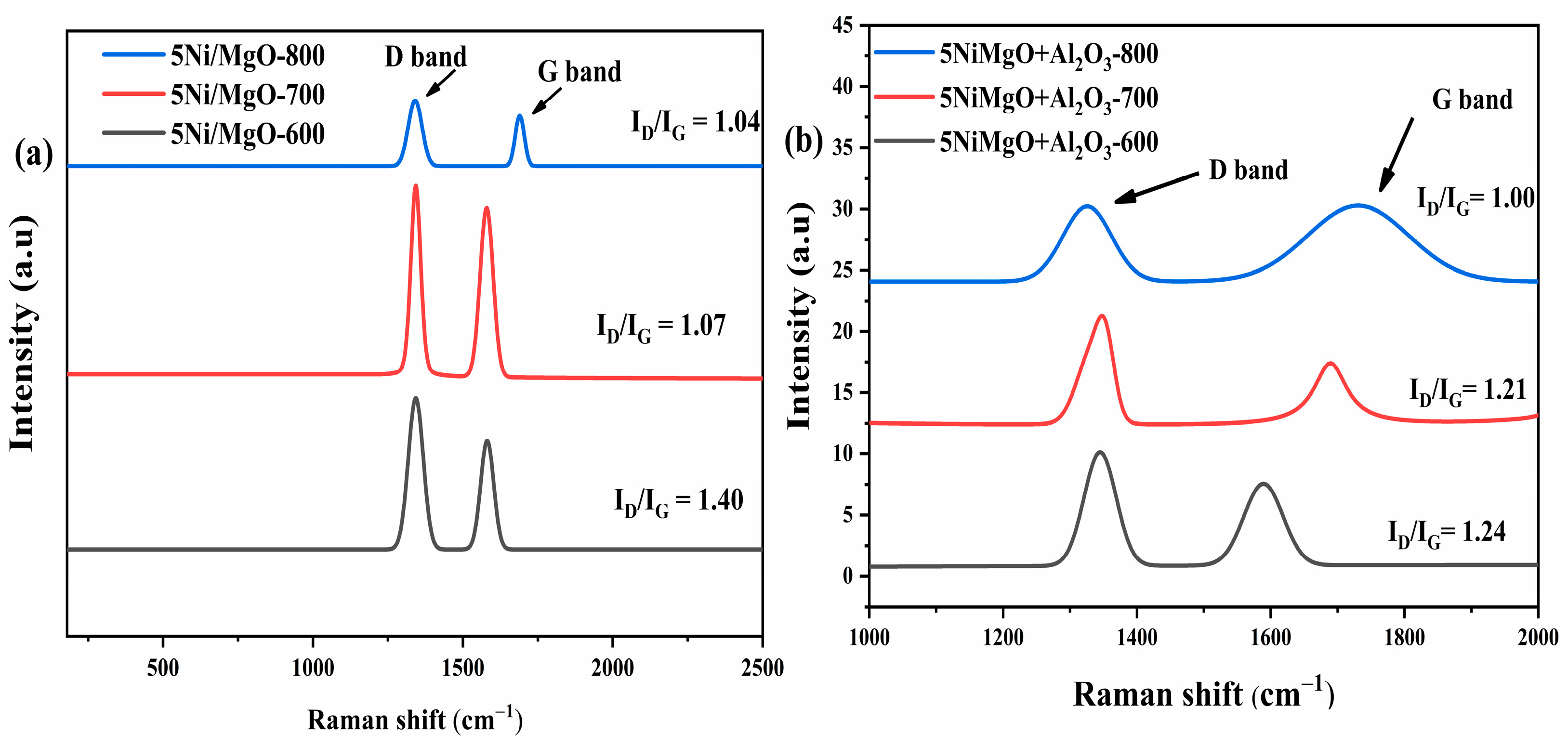
2.5. Raman Analysis of Spent Catalysts
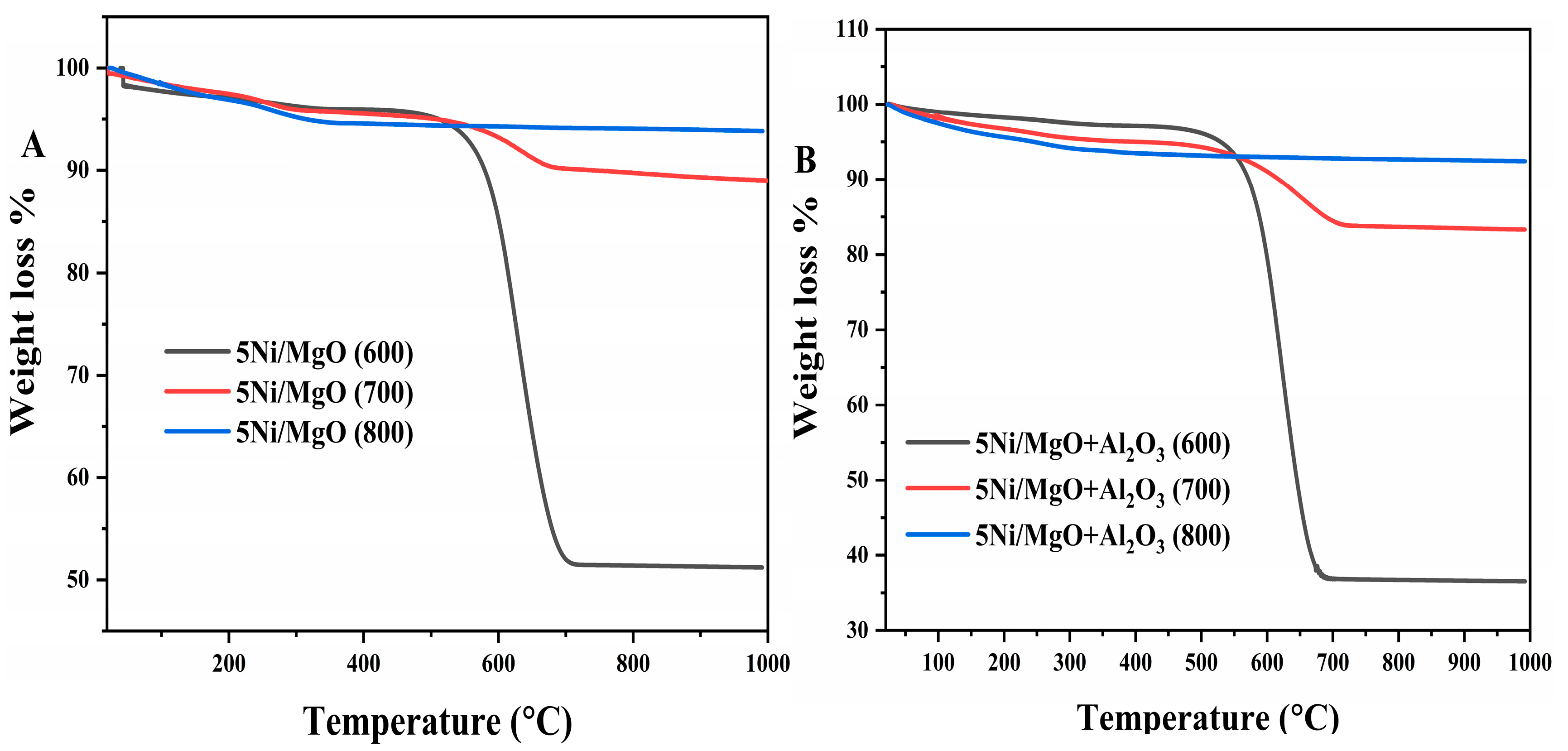
2.6. TGA Analysis of Spent Catalysts
2.7. Transmission Electron Microscopy
2.8. Catalytic Activity Results
2.9. Catalytic Activity Results at Different Reaction Temperatures
2.10. Temperature-Programmed Oxidation (TPO) Study over the Spent Catalyst
2.11. Impact of Reaction Temperature on the TGA Analysis
2.12. Raman Analysis of Spent Catalysts
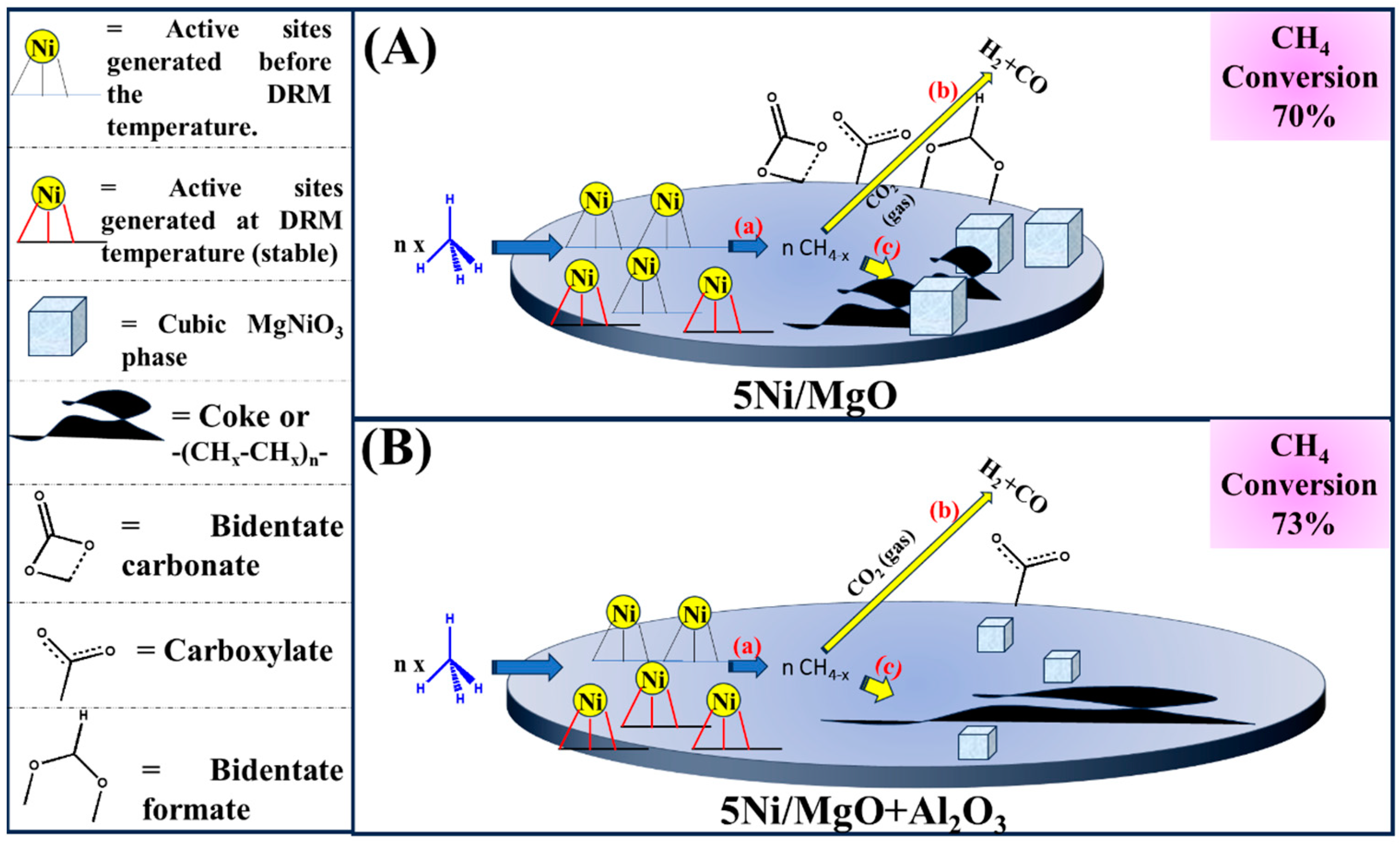
3. Discussion
4. Experiments
4.1. Materials
4.2. Catalyst Preparation
4.3. Catalytic Activity Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chatla, A.; Almanassra, I.W.; Kallem, P.; Atieh, M.A.; Alawadhi, H.; Akula, V.; Banat, F. Dry (CO2) Reforming of Methane over Zirconium Promoted Ni-MgO Mixed Oxide Catalyst: Effect of Zr Addition. J. CO2 Util. 2022, 62, 102082. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically Dispersed Nickel as Coke-Resistant Active Sites for Methane Dry Reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef] [PubMed]
- Bachiller-Baeza, B.; Mateos-Pedrero, C.; Soria, M.A.; Guerrero-Ruiz, A.; Rodemerck, U.; Rodríguez-Ramos, I. Transient Studies of Low-Temperature Dry Reforming of Methane over Ni-CaO/ZrO2-La2O3. Appl. Catal. B Environ. 2013, 129, 450–459. [Google Scholar] [CrossRef]
- Jamsaz, A.; Pham-Ngoc, N.; Wang, M.; Jeong, D.H.; Oh, E.S.; Shin, E.W. Synergistic Effect of Macroporosity and Crystallinity on Catalyst Deactivation Behavior over Macroporous Ni/CexZr1-XO2–Al2O3 for Dry Reforming of Methane. Chem. Eng. J. 2023, 476, 146821. [Google Scholar] [CrossRef]
- de la Cruz-Flores, V.G.; Martinez-Hernandez, A.; Gracia-Pinilla, M.A. Deactivation of Ni-SiO2 Catalysts That Are Synthetized via a Modified Direct Synthesis Method during the Dry Reforming of Methane. Appl. Catal. A Gen. 2020, 594, 117455. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent Advances in Dry Reforming of Methane over Ni-Based Catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the Effects of the Oxygen Species over Ni/ZrO2 Catalyst Surface on Methane Reforming with Carbon Dioxide. Appl. Catal. B Environ. 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 Supported Nickel-Based Bimetallic Catalysts with Superior Stability during Carbon Dioxide Reforming of Methane: Effect of Strong Metal-Support Interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Yuan, J.; Lu, C.; Gu, Z.; Cai, J.; Zhao, H.; Li, D.; Jiang, L.; Xu, H.; Li, Z.q.; Li, K. Ni-Co Catalyst-Assisted Carbon Cycling for CH4-CO2 Reforming. Appl. Catal. B Environ. 2024, 341, 123318. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Chen, M.; Xie, X.; Li, C.; Wang, J.; Yuan, L. Dry Reforming of Methane for Syngas Production over Attapulgite-Derived MFI Zeolite Encapsulated Bimetallic Ni-Co Catalysts. Appl. Catal. B Environ. 2023, 322, 122088. [Google Scholar] [CrossRef]
- Gangadharan, P.; Kanchi, K.C.; Lou, H.H. Evaluation of the Economic and Environmental Impact of Combining Dry Reforming with Steam Reforming of Methane. Chem. Eng. Res. Des. 2012, 90, 1956–1968. [Google Scholar] [CrossRef]
- Zhao, B.; Yan, B.; Yao, S.; Xie, Z.; Wu, Q.; Ran, R.; Weng, D.; Zhang, C.; Chen, J.G. LaFe0.9Ni0.1O3 Perovskite Catalyst with Enhanced Activity and Coke-Resistance for Dry Reforming of Ethane. J. Catal. 2018, 358, 168–178. [Google Scholar] [CrossRef]
- Lenggoro, I.W.; Itoh, Y.; Iida, N.; Okuyama, K. Control of Size and Morphology in NiO Particles Prepared by a Low-Pressure Spray Pyrolysis. Mater. Res. Bull. 2003, 38, 1819–1827. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Mesbah, A.W.; Muzart, J.; Sahebdelfar, S. NiO-MgO Solid Solution Prepared by Sol-Gel Method as Precursor for Ni/MgO Methane Dry Reforming Catalyst: Effect of Calcination Temperature on Catalytic Performance. Catal. Lett. 2016, 146, 238–248. [Google Scholar] [CrossRef]
- Gao, X.; Ge, Z.; Zhu, G.; Wang, Z.; Ashok, J.; Kawi, S. Reforming of Methane: Recent Progress and Prospects. Catalysts 2021, 11, 1003. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, M.; Yu, J.; Zhan, W.; Wang, L.; Guo, Y.; Guo, Y. NixAl1O2-δ Mesoporous Catalysts for Dry Reforming of Methane: The Special Role of NiAl2O4 Spinel Phase and Its Reaction Mechanism. Appl. Catal. B Environ. 2021, 291, 120074. [Google Scholar] [CrossRef]
- Rogers, J.L.; Mangarella, M.C.; D’Amico, A.D.; Gallagher, J.R.; Dutzer, M.R.; Stavitski, E.; Miller, J.T.; Sievers, C. Differences in the Nature of Active Sites for Methane Dry Reforming and Methane Steam Reforming over Nickel Aluminate Catalysts. ACS Catal. 2016, 6, 5873–5886. [Google Scholar] [CrossRef]
- Oni, B.A.; Tomomewo, O.S.; Sanni, S.E.; Ojo, V.O. Dry Reforming of Methane with CO2 over Co-La1−xCaxNiO3 Perovskite-Type Oxides Supported on ZrO2. Mater. Today Commun. 2023, 36, 106802. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, X.; Yang, J.; Yang, T.; Liu, Z.; Han, Y. Deciphering the ZrO2 Phase Engineering Effects on Dry Reforming of Methane over the Ni/ZrO2 Catalysts. Fuel 2023, 349, 128705. [Google Scholar] [CrossRef]
- Androulakis, A.; Yentekakis, I.V.; Panagiotopoulou, P. Dry Reforming of Methane over Supported Rh and Ru Catalysts: Effect of the Support (Al2O3, TiO2, ZrO2, YSZ) on the Activity and Reaction Pathway. Int. J. Hydrogen Energy 2023, 48, 33886–33902. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; González-Carrazán, S.R.; Soria, M.A.; Ruíz, P. Effect of the Nature of TiO2 Support over the Performances of Rh/TiO2 Catalysts in the Partial Oxidation of Methane. Catal. Today 2013, 203, 158–162. [Google Scholar] [CrossRef]
- Yokota, S.; Okumura, K.; Niwa, M. Support Effect of Metal Oxide on Rh Catalysts in the CH4-CO2 Reforming Reaction. Catal. Lett. 2002, 84, 131–134. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, L.; Zheng, X.; Liu, W.; Cao, Z.; Peng, H. Coke-Resistance over Rh–Ni Bimetallic Catalyst for Low Temperature Dry Reforming of Methane. Int. J. Hydrogen Energy 2023, 48, 13890–13901. [Google Scholar] [CrossRef]
- Shen, H.; Li, H.; Yang, Z.; Li, C. Magic of Hydrogen Spillover: Understanding and Application. Green Energy Environ. 2022, 7, 1161–1198. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Kasim, S.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Alrasheed, R.; Bagabas, A.; Chaudhary, M.L.; Frusteri, F.; et al. The Effect of Modifier Identity on the Performance of Ni-Based Catalyst Supported on γ-Al2O3 in Dry Reforming of Methane. Catal. Today 2020, 348, 236–242. [Google Scholar] [CrossRef]
- de Bokx, P.K.; Bonne, R.L.C.; Geus, J.W. Strong Metal-Support Interaction in Ni/TiO2 Catalysts: The Origin of TiOx Moieties on the Surface of Nickel Particles. Appl. Catal. 1987, 30, 33–46. [Google Scholar] [CrossRef]
- Khatri, J.; Fakeeha, A.H.; Kasim, S.O.; Lanre, M.S.; Abasaeed, A.E.; Ibrahim, A.A.; Kumar, R.; Al-Fatesh, A.S. Ceria Promoted Phosphate-Zirconia Supported Ni Catalyst for Hydrogen Rich Syngas Production through Dry Reforming of Methane. Int. J. Energy Res. 2021, 45, 19289–19302. [Google Scholar] [CrossRef]
- Al-Fatish, A.S.A.; Ibrahim, A.A.; Fakeeha, A.H.; Soliman, M.A.; Siddiqui, M.R.H.; Abasaeed, A.E. Coke Formation during CO2 Reforming of CH4 over Alumina-Supported Nickel Catalysts. Appl. Catal. A Gen. 2009, 364, 150–155. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H. In Situ Auto-Gasification of Coke Deposits over a Novel Ni-Ce/W-Zr Catalyst by Sequential Generation of Oxygen Vacancies for Remarkably Stable Syngas Production via CO2-Reforming of Methane. Appl. Catal. B Environ. 2021, 280, 119445. [Google Scholar] [CrossRef]
- Mark, M.F.; Mark, F.; Maier, W.F. Reaction Kinetics of the CO2 Reforming of Methane. Chem. Eng. Technol. 1997, 20, 361–370. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.; Kawi, S. Kinetic and Mechanistic Aspects for CO2 Reforming of Methane over Ni Based Catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Bayahia, H.; Fakeeha, A.H.; Al-Zahrani, S.A.; Alreshaidan, S.B.; Al-Awadi, A.S.; Alotibi, M.F.; Kumar, R.; Al-Fatesh, A.S. COx -Free H2 Production via Catalytic Decomposition of CH4 over Fe Supported on Tungsten Oxide-Activated Carbon Catalyst: Effect of Tungsten Loading. Arab. J. Chem. 2023, 16, 104781. [Google Scholar] [CrossRef]
- Shah, M.; Bordoloi, A.; Nayak, A.K.; Mondal, P. Effect of Ti/Al ratio on the performance of Ni/TiO2-Al2O3 catalyst for methane reforming with CO2. Fuel Process. Technol. 2019, 192, 21–35. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y.; Wang, Z.; Jiang, B.; Kawi, S. Dry reforming of methane on Ni/mesoporous-Al2O3 catalysts: Effect of calcination temperature. Int. J. Hydrogen Energy 2021, 46, 31041–31053. [Google Scholar] [CrossRef]
- Yao, L.; Galvez, M.E.; Hu, C.; Da Costa, P. Mo-promoted Ni/Al2O3 catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 23500–23507. [Google Scholar] [CrossRef]
- Jeon, K.-W.; Kim, H.-M.; Kim, B.-J.; Lee, Y.-L.; Na, H.-S.; Shim, J.-O.; Jang, W.-J.; Roh, H.-S. Synthesis gas production from carbon dioxide reforming of methane over Ni-MgO catalyst: Combined effects of titration rate during co-precipitation and CeO2. Fuel Process. Technol. 2021, 219, 106877. [Google Scholar] [CrossRef]
- Khan, M.M.; Jin, L.; Khan, M.M.; Li, Y.; Saulat, H.; Zhang, Y.; Sarfraz, M.; Zhu, J.; Hu, H. CO2 reforming of methane over activated carbon-Ni/MgO-Al2O3 composite catalysts for syngas production. Fuel Process. Technol. 2021, 211, 106595. [Google Scholar] [CrossRef]
- Alabi, W.O.; Adesanmi, B.M.; Wang, H.; Patzig, C. Correlation of MgO loading to spinel inversion, octahedral site occupancy, site generation and performance of bimetal CoeNi catalyst for dry reforming of CH4. Int. J. Hydrogen Energy 2024, 51, 1087–1098. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Hernández, P.S.; Anzures, F.M.; Martínez, A.G.; Galicia, G.M.; García, M.E.F.; Escobar-Alarcón, L.; Morales, F.J.T.; Pérez-Hernández, R. MgO impregnation to Al2O3 supported Ni catalyst for SYNGAS production using greenhouse gases: Some aspects of chemical state of Ni species. Int. J. Hydrogen Energy 2024, 52, 1131–1140. [Google Scholar] [CrossRef]
- binti Rosdin, R.D.; Yusuf, M.; Abdullah, B. Dry reforming of methane over Ni-based catalysts: Effect of ZrO2 and MgO addition as support. Mater. Lett. 2021, 12, 100095. [Google Scholar] [CrossRef]
- Kim, B.-J.; Park, H.-R.; Lee, Y.-L.; Ahn, S.-Y.; Kim, K.-J.; Hong, G.-R.; Roh, H.-S. Customized Ni-MgO-ZrO2 catalysts for the dry reforming of methane using coke oven gas: Optimizing the MgO content. J. CO2 Util. 2023, 68, 102379. [Google Scholar] [CrossRef]

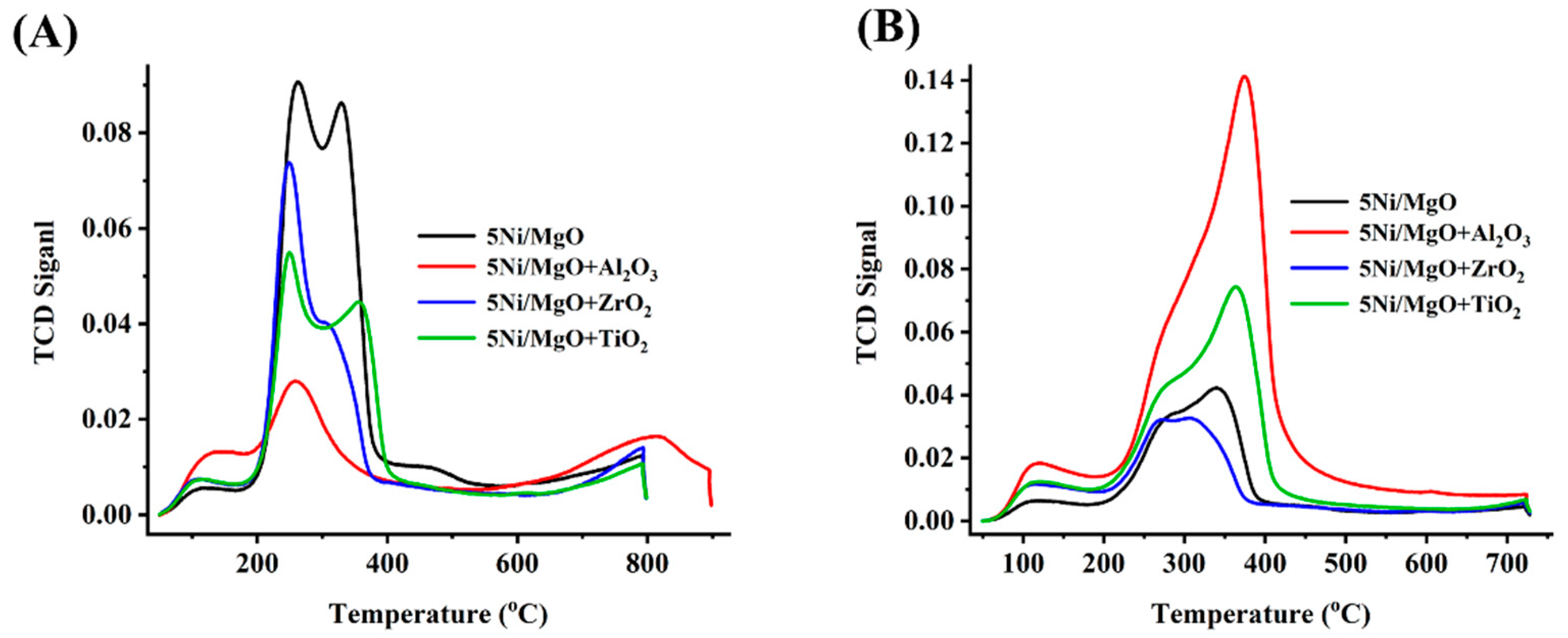

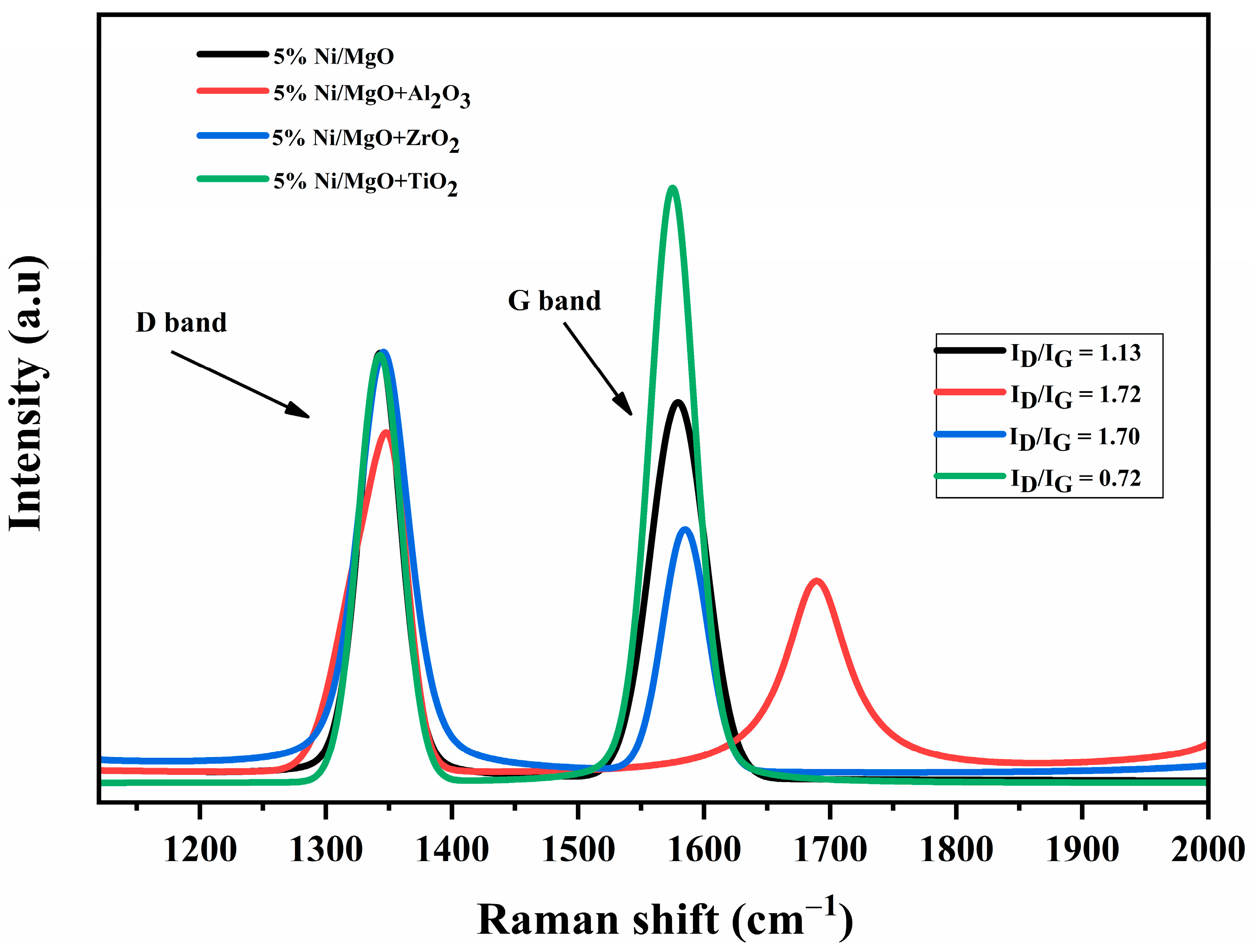
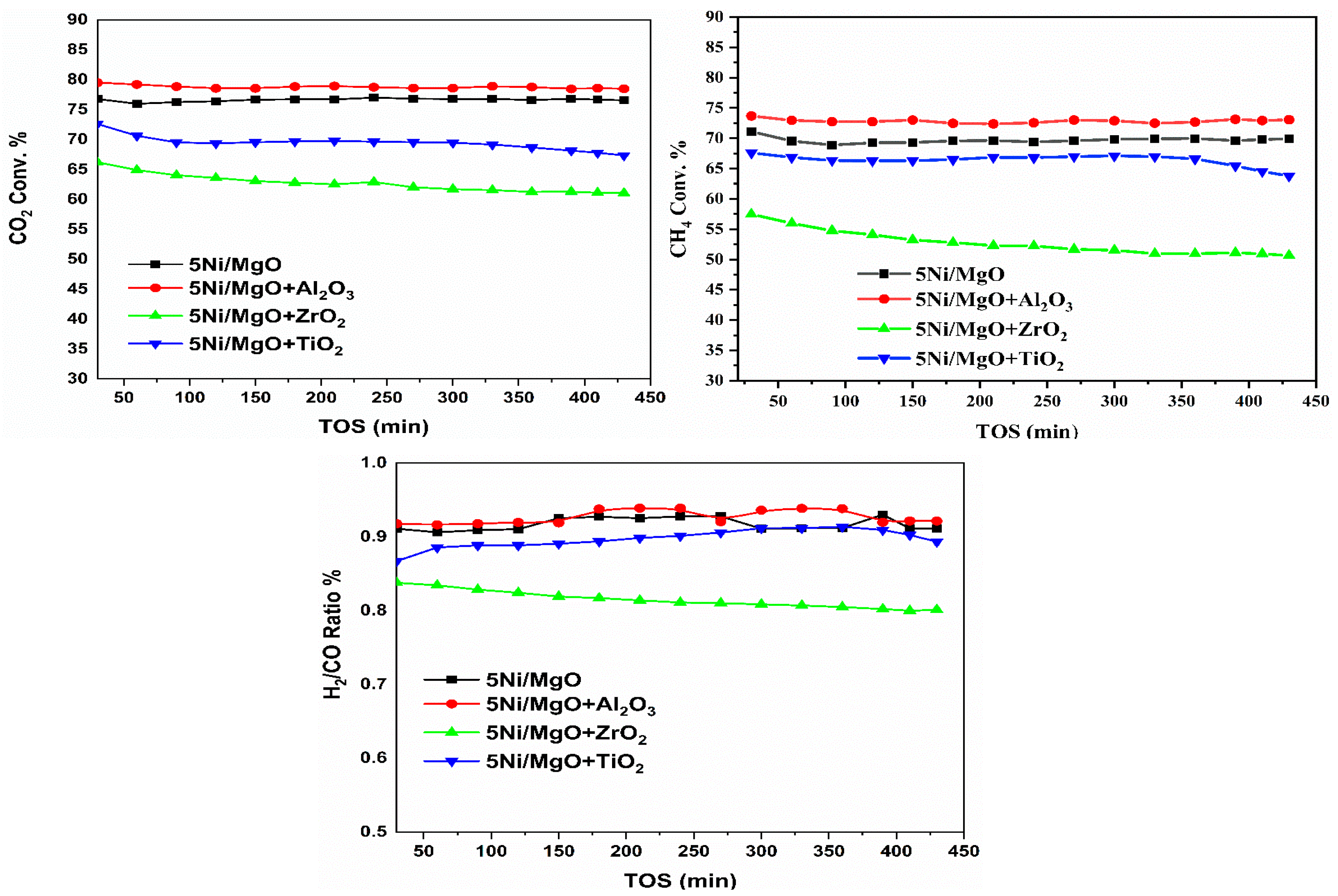

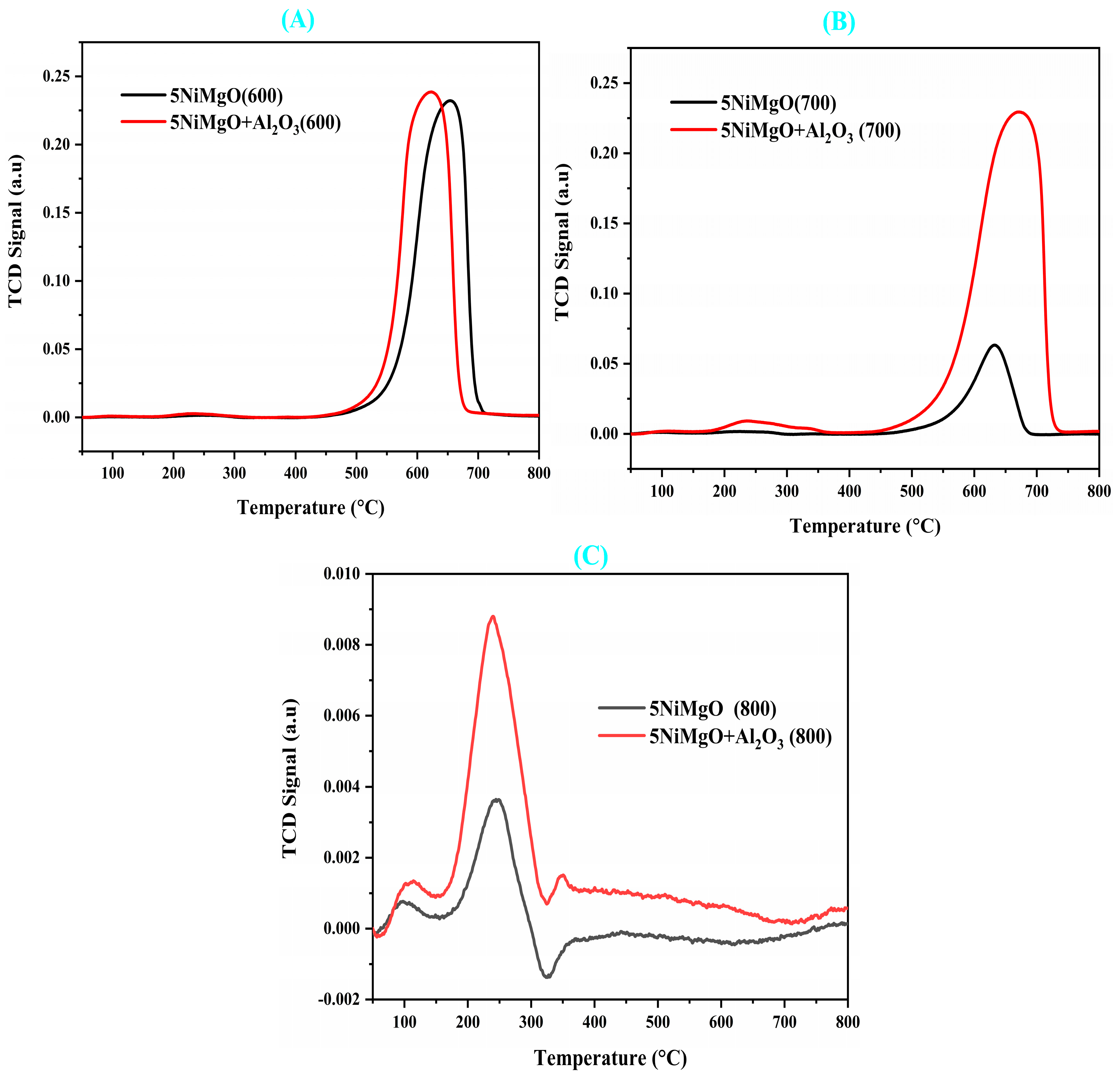
| Samples | SA m2/g | cm3/g | Nm | Total H2 Quantity cm3/g | Total CO2 Quantity cm3/g | Total NH3 Quantity cm3/g | % | D.F a | TOF b h−1 | WL % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | F | ||||||||||
| 5Ni/MgO | 55 | 0.52 | 39.8 | 11.11 | 4.49 | 8.40 | 71.3 | 70.0 | 1.82 | 46.30 | 11.0 |
| 5Ni/MgO + Al2O3 | 85 | 0.53 | 23.6 | 9.72 | 2.90 | 12.40 | 73.8 | 73.1 | 0.95 | 48.43 | 13.0 |
| 5Ni/MgO + ZrO2 | 53 | 0.39 | 33.0 | 2.22 | 2.95 | 8.60 | 57.5 | 50.8 | 11.65 | 34.88 | 12.5 |
| 5Ni/MgO + TiO2 | 58 | 0.48 | 36.3 | 13.32 | 3.83 | 8.12 | 67.9 | 63.9 | 5.89 | 44.58 | 63.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.M.; Ibrahim, A.A.; Ali, F.A.A.; Bamatraf, N.A.; Fakeeha, A.H.; Osman, A.I.; Alreshaidan, S.B.; Fadhillah, F.; Al-Zahrani, S.A.; Al-Fatesh, A.S. Tailored Ni-MgO Catalysts: Unveiling Temperature-Driven Synergy in CH4-CO2 Reforming. Catalysts 2024, 14, 33. https://doi.org/10.3390/catal14010033
Alghamdi AM, Ibrahim AA, Ali FAA, Bamatraf NA, Fakeeha AH, Osman AI, Alreshaidan SB, Fadhillah F, Al-Zahrani SA, Al-Fatesh AS. Tailored Ni-MgO Catalysts: Unveiling Temperature-Driven Synergy in CH4-CO2 Reforming. Catalysts. 2024; 14(1):33. https://doi.org/10.3390/catal14010033
Chicago/Turabian StyleAlghamdi, Ahmad M., Ahmed A. Ibrahim, Fekri Abdulraqeb Ahmed Ali, Nouf A. Bamatraf, Anis H. Fakeeha, Ahmed I. Osman, Salwa B. Alreshaidan, Farid Fadhillah, Salma A. Al-Zahrani, and Ahmed S. Al-Fatesh. 2024. "Tailored Ni-MgO Catalysts: Unveiling Temperature-Driven Synergy in CH4-CO2 Reforming" Catalysts 14, no. 1: 33. https://doi.org/10.3390/catal14010033
APA StyleAlghamdi, A. M., Ibrahim, A. A., Ali, F. A. A., Bamatraf, N. A., Fakeeha, A. H., Osman, A. I., Alreshaidan, S. B., Fadhillah, F., Al-Zahrani, S. A., & Al-Fatesh, A. S. (2024). Tailored Ni-MgO Catalysts: Unveiling Temperature-Driven Synergy in CH4-CO2 Reforming. Catalysts, 14(1), 33. https://doi.org/10.3390/catal14010033












