Cerium Doping Effect in 3DOM Perovskite-Type La2−xCexCoNiO6 Catalysts for Boosting Soot Oxidation
Abstract
:1. Introduction
2. Results and Discussions
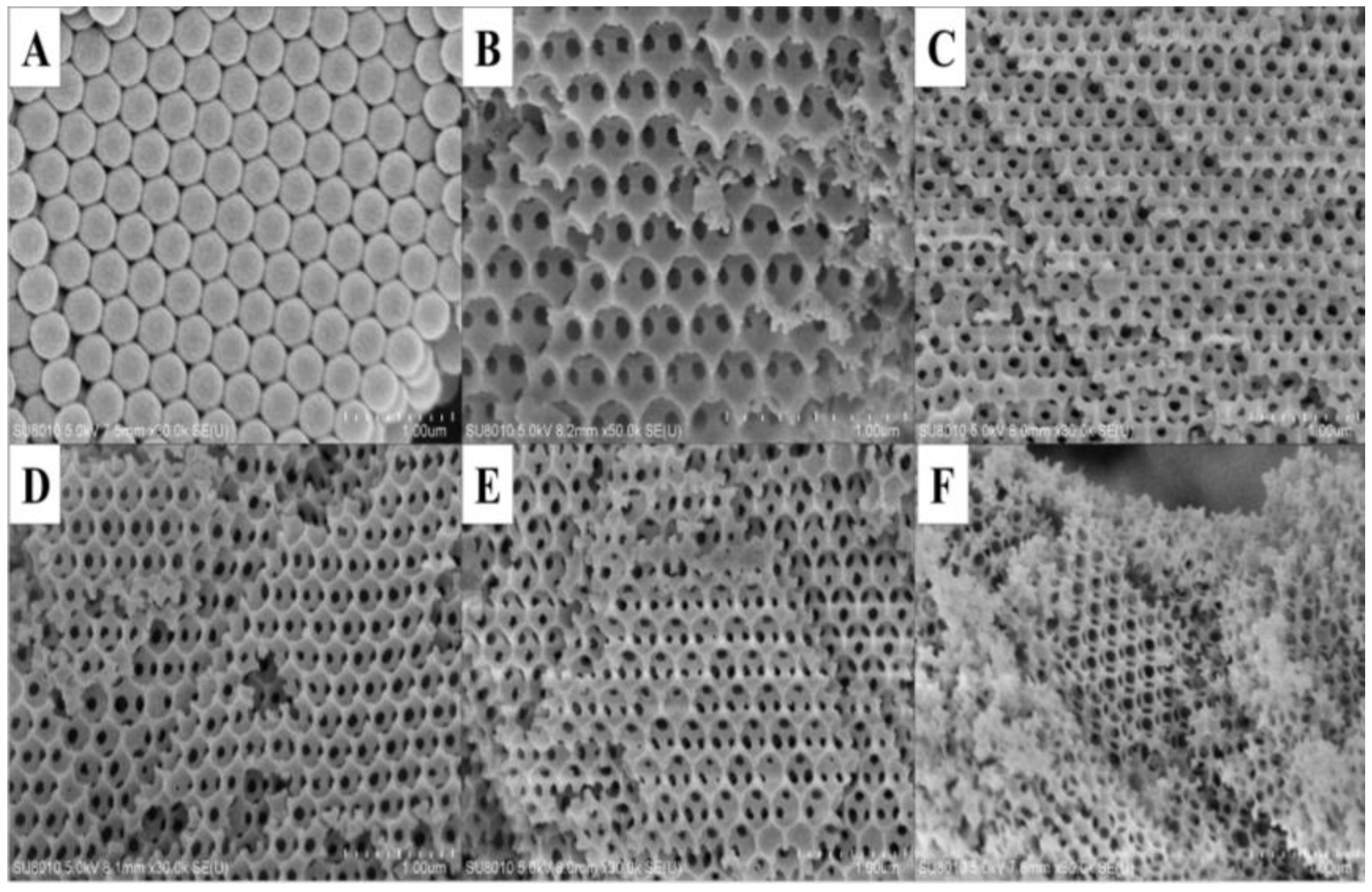
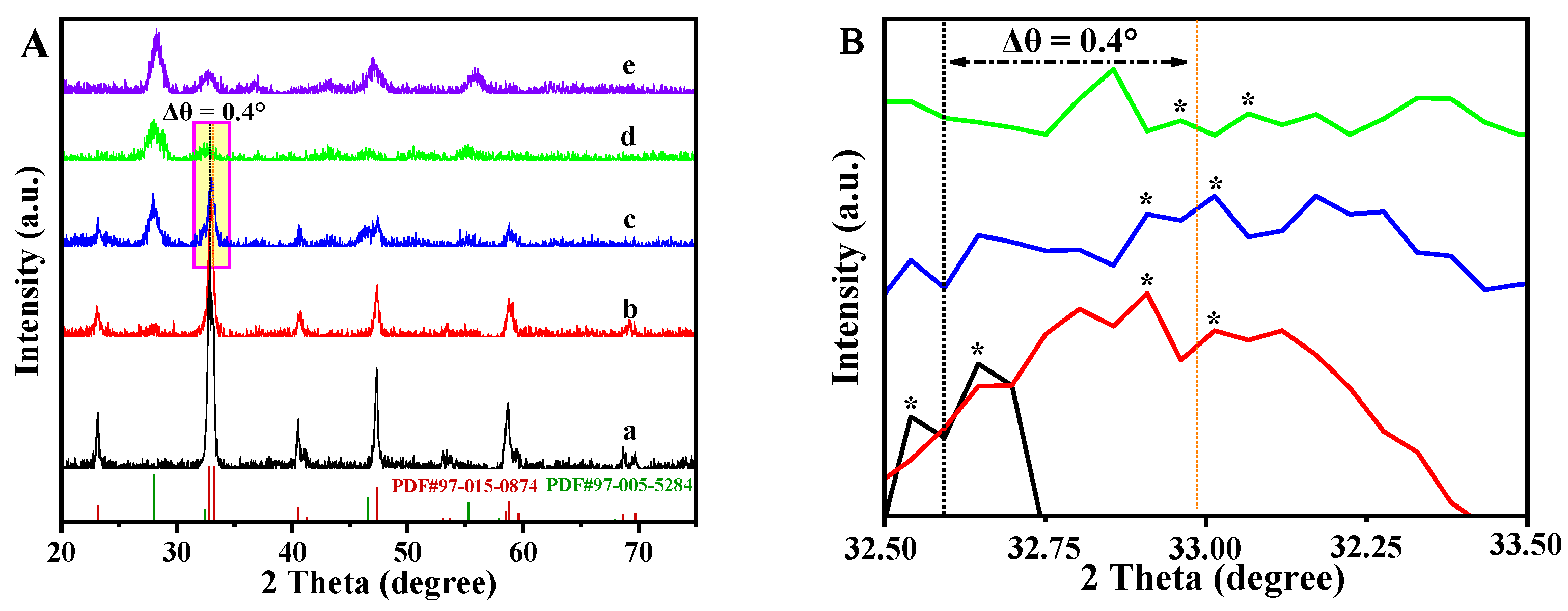
2.1. Structural Properties
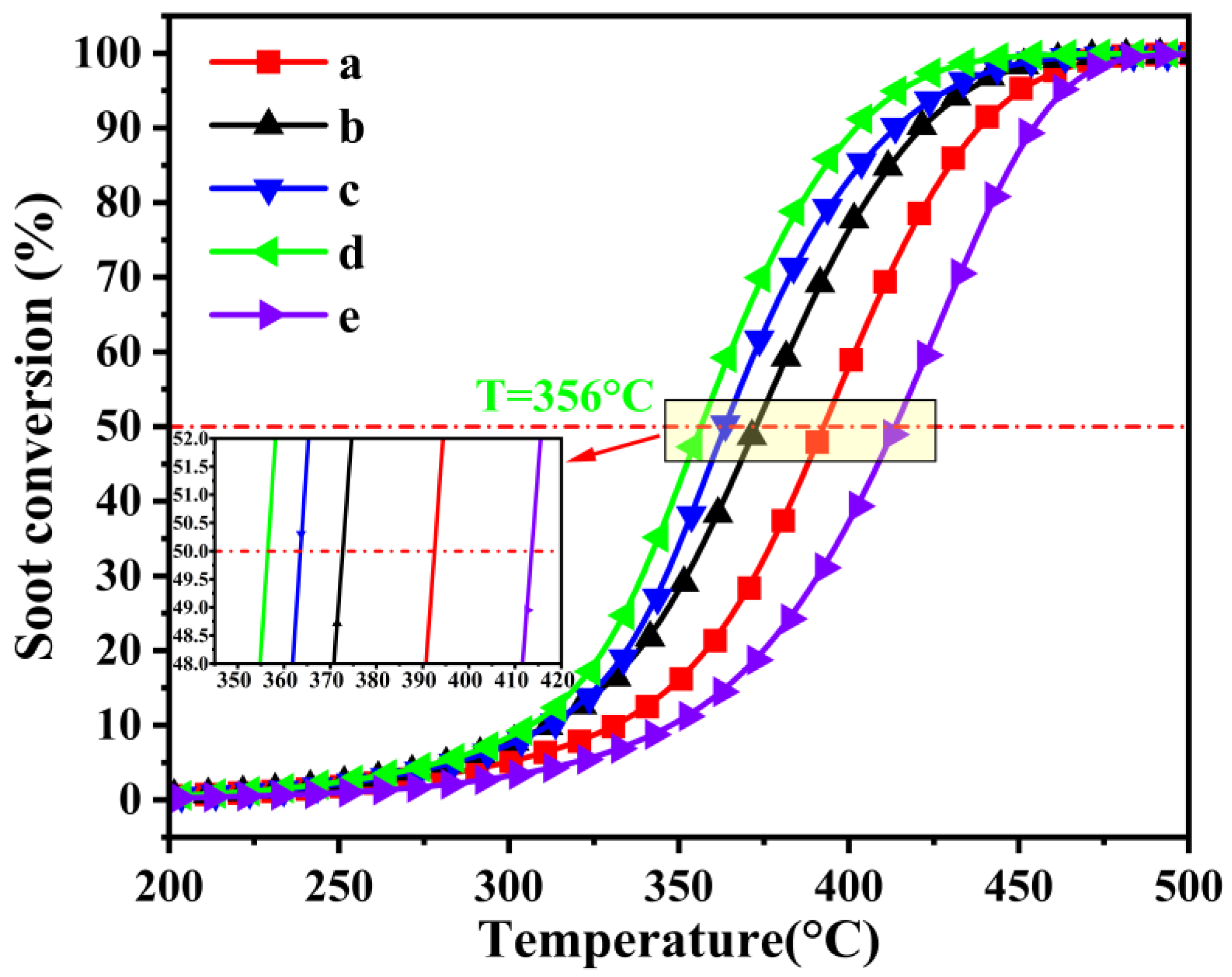
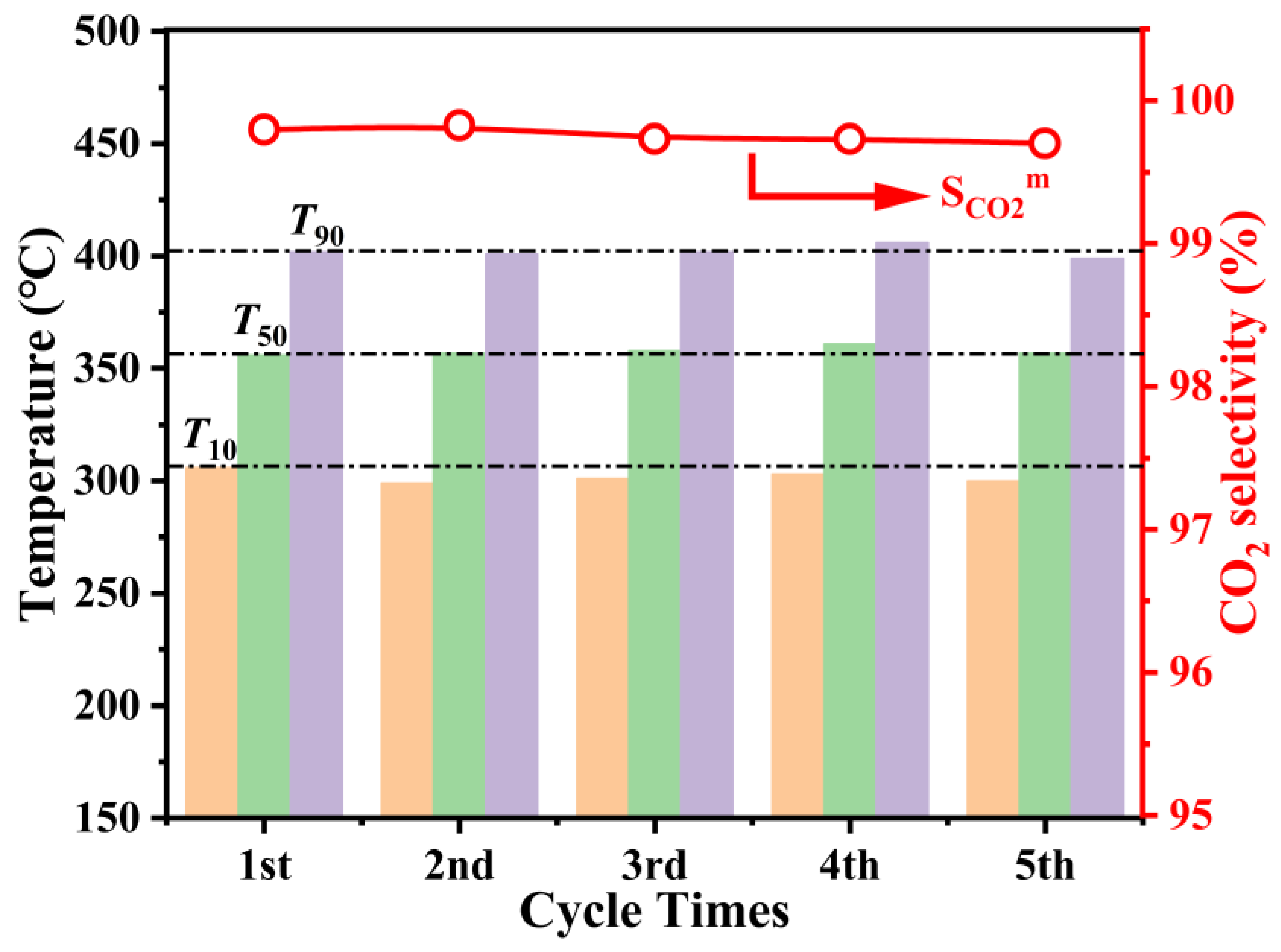
2.2. Catalytic Activity Performance
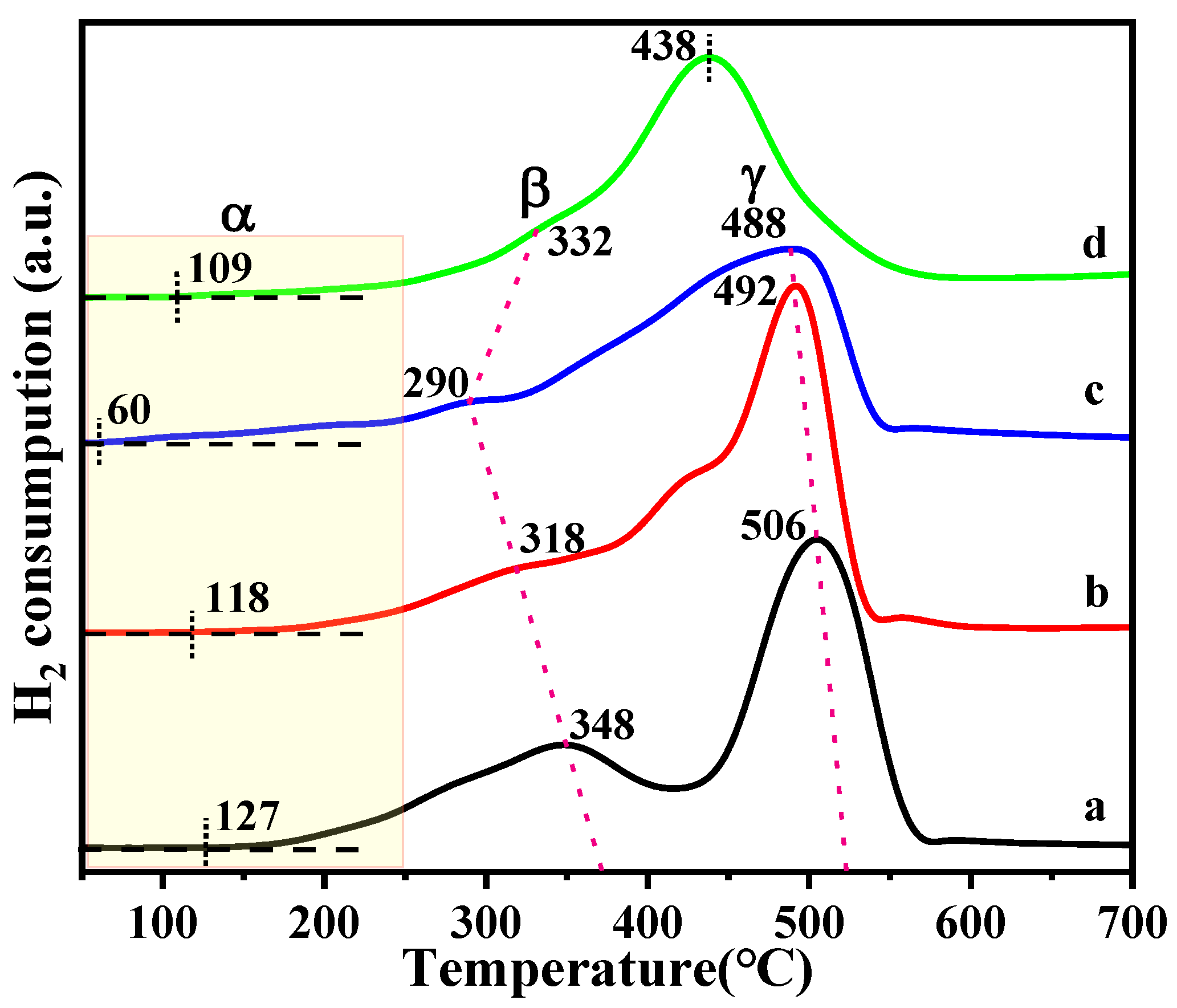
2.3. H2-TPR Profiles
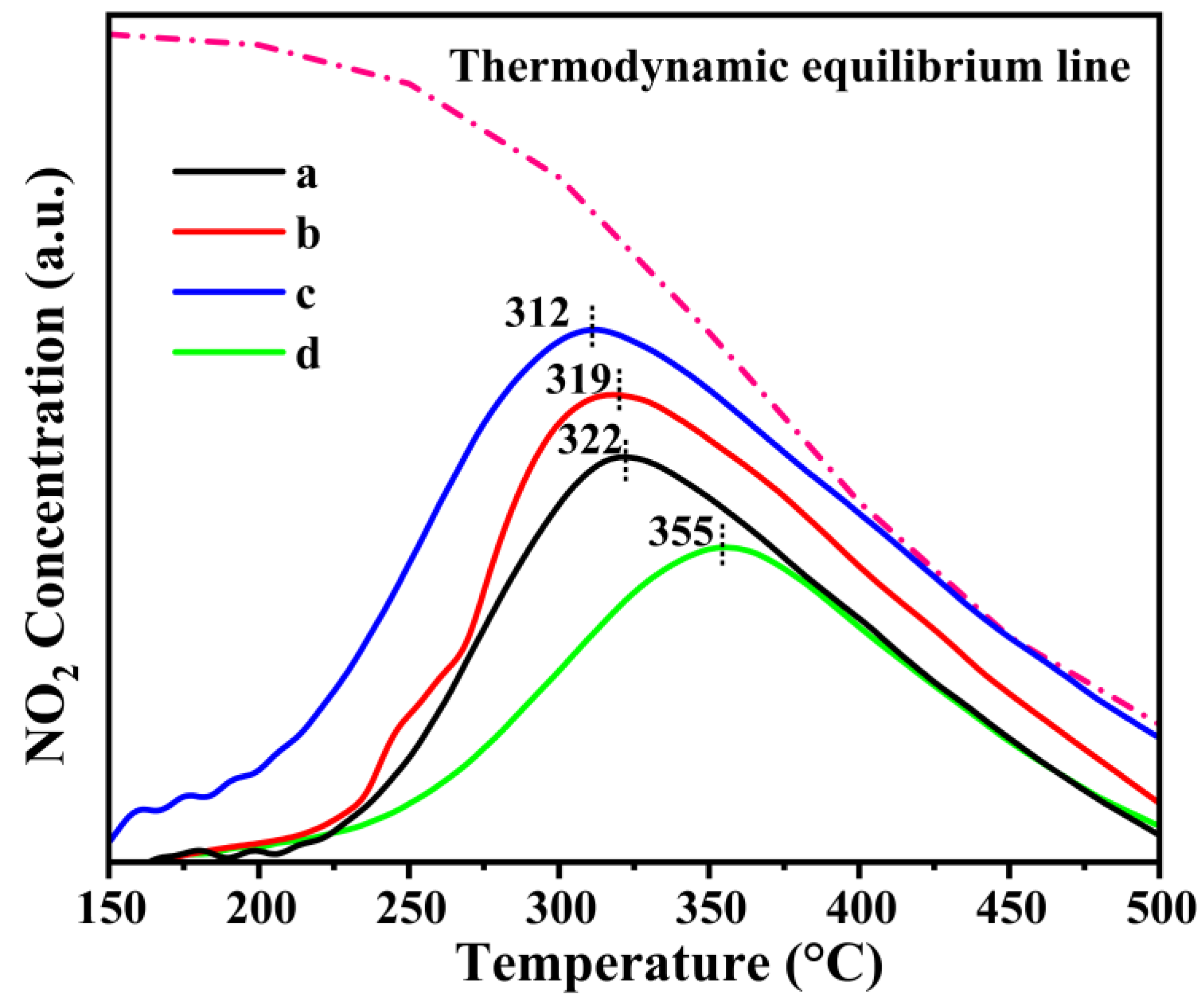
2.4. NO-TPO Measurement
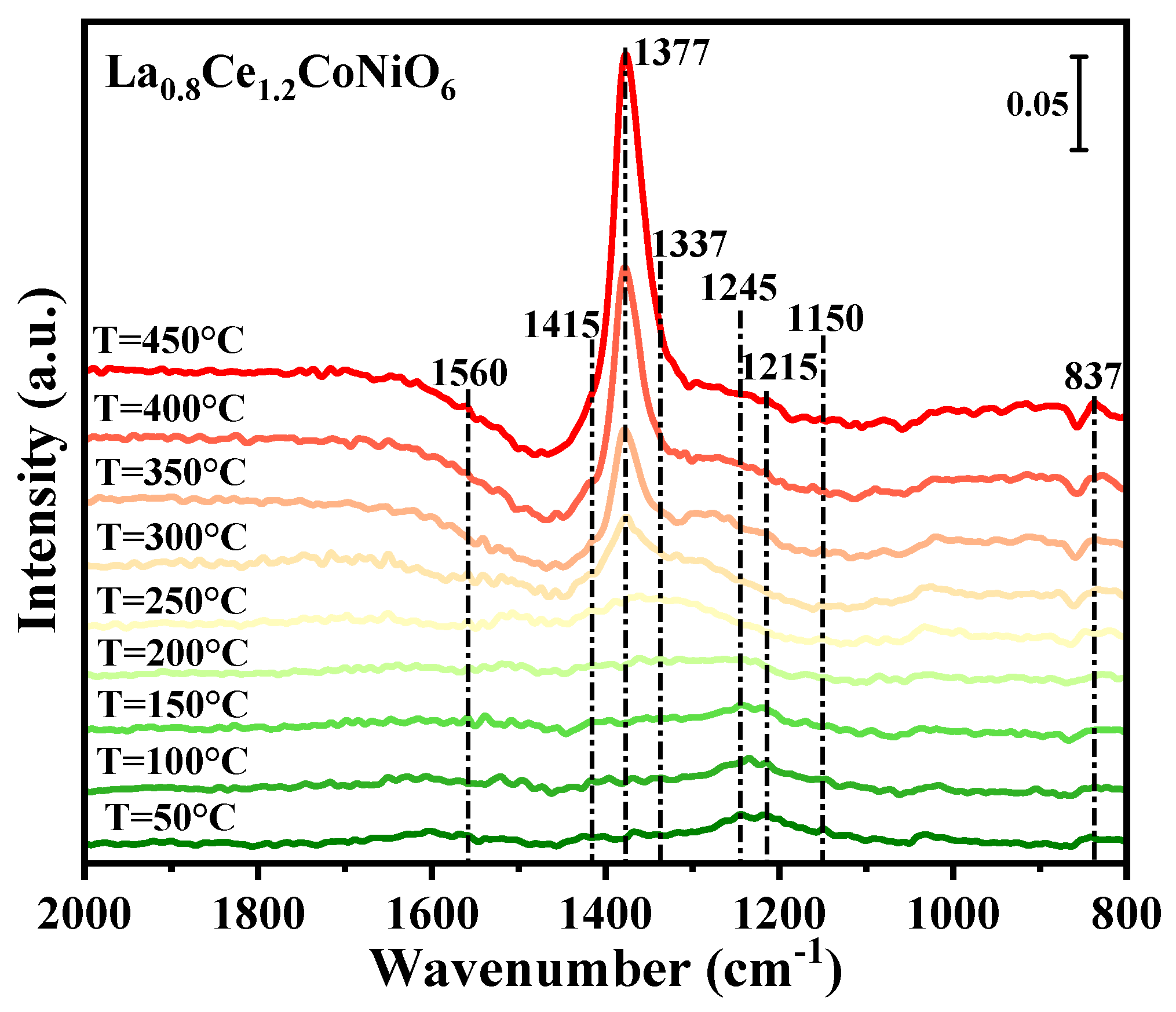
2.5. NO Oxidation In-Situ DRIFTS
3. Experimental Sections
3.1. Materials
3.2. Preparation Methods
3.2.1. Preparation of Polymethyl Methacrylate (PMMA) Microsphere Template
3.2.2. Syntheses of 3DOM La2−xCexCoNiO6 Catalysts
3.3. Catalysts Characterization
3.4. Evaluation of Catalytic Activity
Temperature-Programmed Co-Oxidation of Soot (Soot-TPO)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of the state-of-the-art of exhaust particulate filter technology in internal combustion engines. J. Environ. Manag. 2015, 154, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Fayyazbakhsh, A.; Bell, M.L.; Zhu, X.; Mei, X.; Koutný, M.; Hajinajaf, N.; Zhang, Y. Engine emissions with air pollutants and greenhouse gases and their control technologies. J. Clean. Prod. 2022, 376, 134260. [Google Scholar] [CrossRef]
- Lou, D.; Xiang, B.; Zhang, Y.; Fang, L.; Tan, P.; Hu, Z. Study on the Catalytic Characteristics of Noble Metal Catalysts with Different Pt/Pd Ratios for Soot Combustion. ACS Omega 2023, 8, 20834–20844. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Merkisz, J. Oxygenated Diesel Fuels and Their Effect on PM Emissions. Appl. Sci. 2022, 12, 7709. [Google Scholar] [CrossRef]
- Weng, R.; Mei, X.; Zhang, Z.; Xin, Y.; Xu, J.; Zhang, Y.; Zhang, J. Affecting factors of electrified soot combustion on potassium-supported antimony tin oxides. Chem. Eng. J. 2023, 465, 143046. [Google Scholar] [CrossRef]
- Feng, Q.; Lou, D.M.; Tan, P.Q.; Hu, Z.Y.; Cui, J.G. Effect of catalyzed diesel particulate filter on gaseous emissions from automobile diesel engine. J. Fuel Chem. Technol. 2014, 42, 1513–1521. [Google Scholar]
- Zhang, Y.-H.; Lou, D.-M.; Tan, P.-Q.; Hu, Z.-Y. Effects of DOC+CDPF on Emission Characteristics of Heavy-duty Diesel Vehicle. Huan Jing Ke Xue 2017, 38, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lou, D.; Tan, P.; Hu, Z.; Fang, L. Effect of catalyzed diesel particulate filter and its catalyst loading on emission characteristics of a non-road diesel engine. J. Environ. Sci. 2023, 126, 794–805. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, T.; Li, Q.; Xin, Y.; Lu, X.; Tang, W.; Zhang, Z.; Gao, P.-X.; Anderson, J.A. Multiple strategies to decrease ignition temperature for soot combustion on ultrathin MnO2−x nanosheet array. Appl. Catal. B 2019, 246, 312–321. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, N.; Xu, H.; Li, Y.; Ren, L.; Liao, Y.; Chen, Y. Boosting diesel soot catalytic combustion via enhancement of solid(catalyst)-solid(soot) contact by tailoring micrometer scaled sheet-type agglomerations of CeO2-ZrO2 catalyst. Combust. Flame 2022, 235, 111700. [Google Scholar] [CrossRef]
- Mei, X.; Zhu, X.; Zhang, Y.; Zhang, Z.; Zhong, Z.; Xin, Y.; Zhang, J. Decreasing the catalytic ignition temperature of diesel soot using electrified conductive oxide catalysts. Nat. Catal. 2021, 4, 1002–1011. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Xiong, J.; Ma, Y.; Li, X.; Zhang, P.; Zhang, S.; Wei, Y. The Catalyst of Ruthenium Nanoparticles Decorated Silicalite-1 Zeolite for Boosting Catalytic Soot Oxidation. Catalysts 2023, 13, 1167. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Wei, Y.; Xiong, J.; Zhang, P.; Lai, K.; Chi, H.; Liu, X.; Chen, L.; Yu, X.; et al. A single site ruthenium catalyst for robust soot oxidation without platinum or palladium. Nat. Commun. 2023, 14, 7149. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jo, D.Y.; Choung, J.W.; Kim, C.H.; Ham, H.C.; Lee, K.-Y. Roles of noble metals (M = Ag, Au, Pd, Pt and Rh) on CeO2 in enhancing activity toward soot oxidation: Active oxygen species and DFT calculations. J. Hazard. Mater. 2021, 403, 124085. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, Y.; Zang, Y.; Liu, C.; Wang, W.; Han, R.; Ji, N.; Zhang, S.; Liu, Q. Promotion of A-Site Ag-Doped Perovskites for the Catalytic Oxidation of Soot: Synergistic Catalytic Effect of Dual Active Sites. ACS Catal. 2021, 11, 14224–14236. [Google Scholar] [CrossRef]
- Li, C.; Li, R.; Wang, Y.; Niu, R.; Guo, Q.; Zhang, C. Transition metal modified manganese-based catalysts for soot oxidation promoted by noncompetitive adsorption of oxygen: Experiments and DFT calculations. J. Ind. Eng. Chem. 2023, 126, 454–464. [Google Scholar] [CrossRef]
- Portillo-Vélez, N.S.; Zanella, R. Comparative study of transition metal (Mn, Fe or Co) catalysts supported on titania: Effect of Au nanoparticles addition towards CO oxidation and soot combustion reactions. Chem. Eng. J. 2020, 385, 123848. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Z.; Li, H.; Wang, Q.; Liu, X.; Hu, Y.; Su, C.; Duan, R.; Chen, S.; Lan, L. Effect of oxygen vacancy and highly dispersed MnOx on soot combustion in cerium manganese catalyst. Sci. Rep. 2023, 13, 3386. [Google Scholar] [CrossRef]
- Cao, C.; Yang, H.; Xiao, J.; Yang, X.; Ren, B.; Xu, L.; Liu, G.; Li, X. Catalytic diesel soot elimination over potassium promoted transition metal oxide (Co/Mn/Fe) nanosheets monolithic catalysts. Fuel 2021, 305, 121446. [Google Scholar] [CrossRef]
- Wei, K.; Wang, X.; Shi, L.; Yang, S.; Xue, Z.; Qin, W.; Wang, J.; Xu, K.; Zhang, X. Potassium promoted Fe–Ce composite oxides monolithic catalysts for catalytic soot combustion. Chem. Pap. 2023, 77, 7045–7052. [Google Scholar] [CrossRef]
- Wang, M.; Han, Z.; Liu, Y.; Gao, C.; Pan, X.; Zhou, S. The influence of partial substitution of Ce with K in CeMO3 (M = Mn, Fe, Co, Ni, Cu) perovskite catalysts on soot combustion performance. J. Environ. Chem. Eng 2023, 11, 110850. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Sun, P.; Wang, P.; Zhang, C.; Ma, H. Experimental and theoretical study on La0.5K0.5Mn1−xFexO3 perovskite catalysts for mild temperature soot combustion and simultaneous removal of soot and NO. Fuel Process. Technol. 2023, 246, 107760. [Google Scholar] [CrossRef]
- Montilla-Verdú, S.; Torregrosa-Rivero, V.; Díaz-Verde, A.; Illán-Gómez, M.J. BaFe1−xNixO3 Catalysts for NOx-Assisted Diesel Soot Oxidation. Top. Catal. 2023, 66, 839–849. [Google Scholar] [CrossRef]
- Yu, X.; Yu, D.; Wang, L.; Ren, Y.; Chen, M.; Fan, X.; Zhao, Z.; Sojka, Z.; Kotarba, A.; Wei, Y.; et al. Ultralight and spongy La–Mn-based perovskite catalysts modified by alkali metals and Ce: Facile synthesis and excellent catalytic performance for soot combustion. Catal. Sci. Technol. 2023, 13, 1208–1220. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, X.; Wu, H.; Wu, X.; Zhou, Z.; Chen, G.; Yang, G. Activity and Stability of Cu-Based Spinel-Type Complex Oxides for Diesel Soot Combustion. ChemistrySelect 2021, 6, 14019–14026. [Google Scholar] [CrossRef]
- Chen, H.; Li, T.; Xu, Z.; Wang, W.; Wang, H. Oxidation of soot promoted by Fe-based spinel catalysts. Mater. Res. Express 2022, 9, 015502. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Zheng, C.; Hu, D.; Zhang, J.; Gao, X. Study on Catalytic Soot Oxidation over Spinel Type ACo2O4 (A = Co, Ni, Cu, Zn) Catalysts. Aerosol Air Qual. Res. 2017, 17, 2317–2327. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Kwon, E.; Park, Y.-K.; Nhat Huy, N.; Lisak, G.; Hsu, P.-S.; Hu, C.; Lin, K.-Y.A. Broccoli-like CeO2 with Hierarchical/Porous Structures, and promoted oxygen vacancy as an enhanced catalyst for catalytic diesel soot elimination. Sep. Purif. Technol. 2022, 281, 119867. [Google Scholar] [CrossRef]
- Alcalde-Santiago, V.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. On the soot combustion mechanism using 3DOM ceria catalysts. Appl. Catal. B 2018, 234, 187–197. [Google Scholar] [CrossRef]
- Woźniak, P.; Małecka, M.A.; Chinchilla, L.; Trasobares, S. 3D hierarchically structured Ce1−xGdxO2−x/2 mixed oxide particles: The role of microstructure, porosity and multi-level architecture stability in soot and propane oxidation. Mater. Res. Bull. 2022, 151, 111816. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, D.; Lee, J.; Baek, D.S.; Shin, T.J.; Kim, Y.-T.; Jeong, H.Y.; Kwak, J.H.; Kim, H.; Joo, S.H. A General Strategy to Atomically Dispersed Noble Metal Catalysts for Unravelling Their Catalytic Trends for Oxygen Reduction Reaction. ACS Nano 2020, 14, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Mañogil, J.; Quiles-Díaz, S.; Guillén-Hurtado, N.; García-García, A. Catalyzed Particulate Filter Regeneration by Platinum Versus Noble Metal-Free Catalysts: From Principles to Real Application. Top. Catal. 2017, 60, 2–12. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Deng, J.; Zhao, X.; Zhang, K.; Yang, J.; Han, Z.; Jiang, X.; Dai, H. Three-dimensionally ordered macroporous Cr2O3−CeO2: High-performance catalysts for the oxidative removal of trichloroethylene. Catal. Today 2020, 339, 200–209. [Google Scholar] [CrossRef]
- Ren, W.; Ding, T.; Yang, Y.; Xing, L.; Cheng, Q.; Zhao, D.; Zhang, Z.; Li, Q.; Zhang, J.; Zheng, L.; et al. Identifying Oxygen Activation/Oxidation Sites for Efficient Soot Combustion over Silver Catalysts Interacted with Nanoflower-Like Hydrotalcite-Derived CoAlO Metal Oxides. ACS Catal. 2019, 9, 8772–8784. [Google Scholar] [CrossRef]
- Qi, B.; Li, Z.; Lou, D.; Zhang, Y. Experimental investigation on the effects of DPF Cs-V-based non-noble metal catalysts and their coating forms on non-road diesel engine emission characteristics. Environ. Sci. Pollut. Res. 2023, 30, 9401–9415. [Google Scholar] [CrossRef] [PubMed]
- Libby, W.F. Promising Catalyst for Auto Exhaust. Science 1971, 171, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Voorhoeve, R.J.H.; Remeika, J.P.; Freeland, P.E.; Matthias, B.T. Rare-Earth Oxides of Manganese and Cobalt Rival Platinum for the Treatment of Carbon Monoxide in Auto Exhaust. Science 1972, 177, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Legutko, P.; Stelmachowski, P.; Yu, X.; Zhao, Z.; Sojka, Z.; Kotarba, A. Catalytic Soot Combustion—General Concepts and Alkali Promotion. ACS Catal. 2023, 13, 3395–3418. [Google Scholar] [CrossRef]
- Mei, X.; Xiong, J.; Wei, Y.; Zhang, Y.; Zhang, P.; Yu, Q.; Zhao, Z.; Liu, J. High-efficient non-noble metal catalysts of 3D ordered macroporous perovskite-type La2NiB’O6 for soot combustion: Insight into the synergistic effect of binary Ni and B’ sites. Appl. Catal. B 2020, 275, 119108. [Google Scholar] [CrossRef]
- Xing, L.; Yang, Y.; Ren, W.; Zhao, D.; Tian, Y.; Ding, T.; Zhang, J.; Zheng, L.; Li, X. Highly efficient catalytic soot combustion performance of hierarchically meso-macroporous Co3O4/CeO2 nanosheet monolithic catalysts. Catal. Today 2020, 351, 83–93. [Google Scholar] [CrossRef]
- Stegmayer, M.Á.; Irusta, S.; Miró, E.E.; Milt, V.G. Electrospinning synthesis and characterization of nanofibers of Co, Ce and mixed Co-Ce oxides. Their application to oxidation reactions of diesel soot and CO. Catal. Today 2022, 383, 266–276. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Z.; Feng, Y.; Xie, S.; Liu, Y.; Dai, H.; Deng, J. In situ molten salt derived iron oxide supported platinum catalyst with high catalytic performance for o-xylene elimination. Catal. Today 2020, 351, 30–36. [Google Scholar] [CrossRef]
- Chi, H.; Zhang, P.; Xiong, J.; Wei, Y.; Li, Y.; Zhao, Z.; Liu, J.; Jiao, J. Single-crystalline α-MnO2 catalysts with tailored exposed crystal facets for boosting catalytic soot oxidation: The crystal facet-dependent activity. Appl. Surf. Sci. 2023, 608, 155116. [Google Scholar] [CrossRef]
- Zhai, G.; Wang, J.; Chen, Z.; Yang, S.; Men, Y. Highly enhanced soot oxidation activity over 3DOM Co3O4-CeO2 catalysts by synergistic promoting effect. J. Hazard. Mater. 2019, 363, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Horyń, R.; Bukowska, E.; Sikora, A. Nature of structure defects in rhombohedral series of La1−xAxMnO3+δ (A = Na, K). J. Alloys Compd. 2002, 346, 107–115. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Tang, D.; Miao, Y.; Cao, Z.; Zhao, Z. Synergy of NTP-La1-xAgxMn1-yCoyO3-δ Hybrid for Soot Catalytic Combustion at Low Temperature. Plasma Chem. Plasma Process. 2021, 41, 1009–1019. [Google Scholar] [CrossRef]
- Uppara, H.P.; Singh, S.K.; Labhsetwar, N.K.; Murari, M.S.; Dasari, H. The decisive factor of hollow spherical network morphology of Nd1−xCexCo1−yCuyO3±δ perovskites towards soot oxidation. Chem. Pap. 2022, 76, 3771–3787. [Google Scholar] [CrossRef]
- Cao, C.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Zhang, J.; Hu, T.; Zheng, L.; Li, X. The monolithic transition metal oxide crossed nanosheets used for diesel soot combustion under gravitational contact mode. Appl. Surf. Sci. 2017, 406, 245–253. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Zhao, Z.; Au, C.T. Controlled preparation and high catalytic performance of three-dimensionally ordered macroporous LaMnO3 with nanovoid skeletons for the combustion of toluene. J. Catal. 2012, 287, 149–160. [Google Scholar] [CrossRef]
- Xie, S.; Deng, J.; Zang, S.; Yang, H.; Guo, G.; Arandiyan, H.; Dai, H. Au–Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J. Catal. 2015, 322, 38–48. [Google Scholar] [CrossRef]
- Jin, B.; Wu, X.; Weng, D.; Liu, S.; Yu, T.; Zhao, Z.; Wei, Y. Roles of cobalt and cerium species in three-dimensionally ordered macroporous CoxCe1−xOδ catalysts for the catalytic oxidation of diesel soot. J. Colloid Interface Sci. 2018, 532, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Weng, J.; Lu, X.; Wen, L.; Suburamanian, A.; Nam, C.-Y.; Gao, P.-X. Alkali-metal poisoning effect of total CO and propane oxidation over Co3O4 nanocatalysts. Appl. Catal. B 2019, 256, 117859. [Google Scholar] [CrossRef]
- Kostin, G.; Borodin, A.; Emely’anov, V.; Naumov, D.; Virovets, A.; Rohmer, M.M.; Varnek, A. Synthesis and structure of heterometallic compounds of [RuNO(NO2)4OH]2− with triphenyl phosphine oxide complexes of Co(II), Ni (II), and Zn(II). J. Mol. Struct. 2007, 837, 63–71. [Google Scholar] [CrossRef]
- Chaisriratanakul, W.; Bunjongpru, W.; Pankiew, A.; Srisuwan, A.; Jeamsaksiri, W.; Chaowicharat, E.; Thornyanadacha, N.; Pengpad, P.; Horprathum, M.; Phromyothin, D. Modification of polyvinyl chloride ion-selective membrane for nitrate ISFET sensors. Appl. Surf. Sci. 2020, 512, 145664. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Species formed during NO adsorption and NO + O2 co-adsorption on ceria: A combined DRIFTS and DFT study. Mol. Catal. 2018, 451, 114–124. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T. Removal of NO by Reversible Adsorption on Fe−Mn Based Transition Metal Oxides. Langmuir 2001, 17, 4997–5003. [Google Scholar] [CrossRef]
- Dai, X.; Jiang, W.; Wang, W.; Weng, X.; Shang, Y.; Xue, Y.; Wu, Z. Supercritical water syntheses of transition metal-doped CeO2 nano-catalysts for selective catalytic reduction of NO by CO: An in situ diffuse reflectance Fourier transform infrared spectroscopy study. Chin. J. Catal. 2018, 39, 728–735. [Google Scholar] [CrossRef]
- Mihaylov, M.; Hadjiivanov, K.; Panayotov, D. DRIFTS mechanistic studies on the selective catalytic reduction of NOx with methane over Ni-containing zeolites: Comparison between NiY and Ni-ZSM-5. Appl. Catal. B 2004, 51, 33–42. [Google Scholar] [CrossRef]
- Penkova, A.; Hadjiivanov, K.; Mihaylov, M.; Daturi, M.; Saussey, J.; Lavalley, J.C. DRIFTS Spectroscopic Study of Low Temperature NO Adsorption and NO + O2 Coadsorption on H−ZSM-5. Langmuir 2004, 20, 5425–5431. [Google Scholar] [CrossRef]
- Xue, H.; Guo, X.; Meng, T.; Mao, D.; Ma, Z. NH3-SCR of NO over M/ZSM-5 (M = Mn, Co, Cu) catalysts: An in-situ DRIFTS study. Surf. Interfaces 2022, 29, 101722. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Ivanova, E.Z.; Vayssilov, G.N.; Hadjiivanov, K.I. Revisiting ceria-NOx interaction: DRIFTS studies. Catal. Today 2020, 357, 613–620. [Google Scholar] [CrossRef]
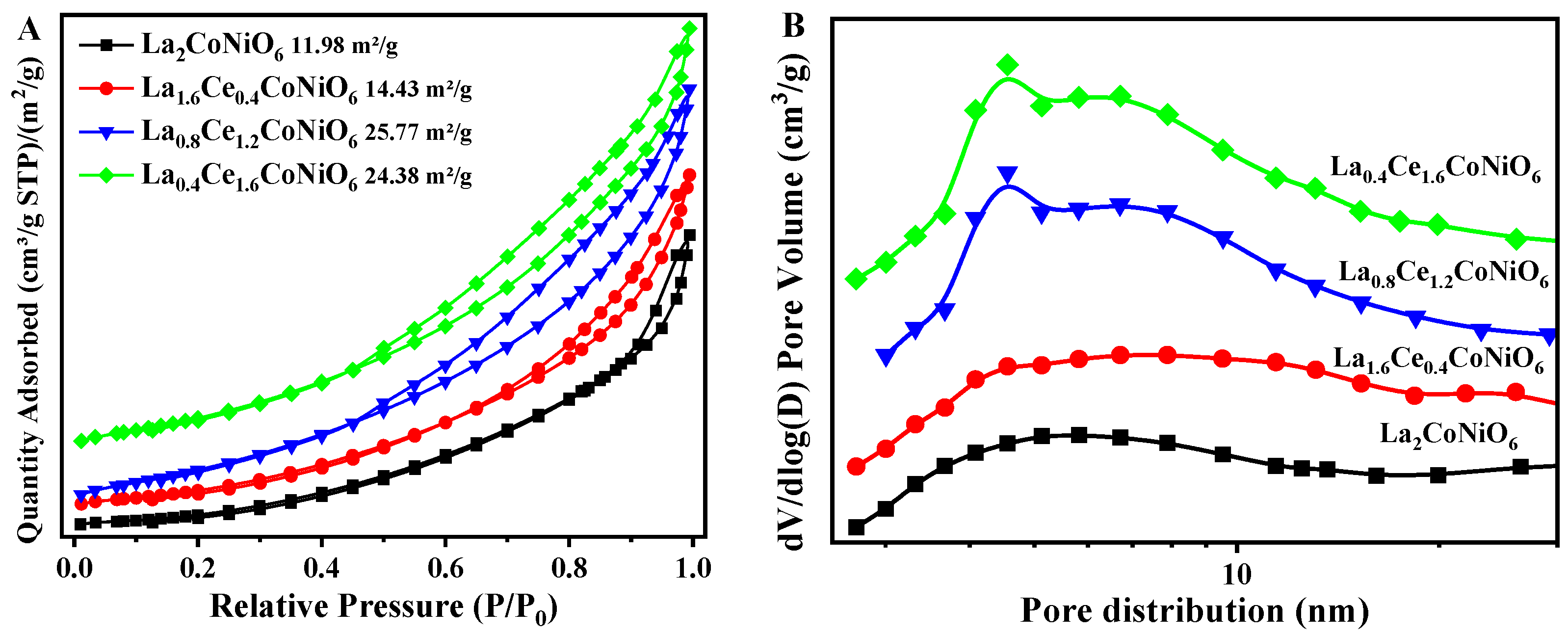
| 3DOM Catalysts | SBET (m2/g) | Average Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| La2CoNiO6 | 12 | 0.07 | 9.2 |
| La1.6Ce0.4CoNiO6 | 16 | 0.08 | 8.8 |
| La0.8Ce1.2CoNiO6 | 26 | 0.10 | 7.7 |
| La0.4Ce1.6CoNiO6 | 24 | 0.11 | 7.6 |
| 3DOM Catalysts | T10 (°C) | T50 (°C) | T90 (°C) | SCO2m (%) |
|---|---|---|---|---|
| La2CoNiO6 | 331 | 400 | 439 | 99.64 |
| La1.9Ce0.1CoNiO6 | 318 | 388 | 437 | 99.99 |
| La1.6Ce0.4CoNiO6 | 311 | 372 | 420 | 99.75 |
| La1.2Ce0.8CoNiO6 | 312 | 363 | 413 | 99.67 |
| La1Ce1Co1Ni1O6 | 303 | 361 | 404 | 99.86 |
| La0.8Ce1.2CoNiO6 | 306 | 356 | 402 | 99.80 |
| La0.4Ce1.6CoNiO6 | 348 | 414 | 452 | 98.73 |
| LaCoO3 | 345 | 427 | 468 | 97.21 |
| LaNiO3 | 364 | 437 | 486 | 97.90 |
| soot (no catalyst) | 487 | 599 | 651 | 30.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Xu, L.; Li, Y.; Xiong, J.; Han, D.; Ma, Y.; Zhang, P.; Guo, H.; Wei, Y. Cerium Doping Effect in 3DOM Perovskite-Type La2−xCexCoNiO6 Catalysts for Boosting Soot Oxidation. Catalysts 2024, 14, 18. https://doi.org/10.3390/catal14010018
Chen K, Xu L, Li Y, Xiong J, Han D, Ma Y, Zhang P, Guo H, Wei Y. Cerium Doping Effect in 3DOM Perovskite-Type La2−xCexCoNiO6 Catalysts for Boosting Soot Oxidation. Catalysts. 2024; 14(1):18. https://doi.org/10.3390/catal14010018
Chicago/Turabian StyleChen, Kaixuan, Linsheng Xu, Yuanfeng Li, Jing Xiong, Dawei Han, Yaxiao Ma, Peng Zhang, Haoqi Guo, and Yuechang Wei. 2024. "Cerium Doping Effect in 3DOM Perovskite-Type La2−xCexCoNiO6 Catalysts for Boosting Soot Oxidation" Catalysts 14, no. 1: 18. https://doi.org/10.3390/catal14010018
APA StyleChen, K., Xu, L., Li, Y., Xiong, J., Han, D., Ma, Y., Zhang, P., Guo, H., & Wei, Y. (2024). Cerium Doping Effect in 3DOM Perovskite-Type La2−xCexCoNiO6 Catalysts for Boosting Soot Oxidation. Catalysts, 14(1), 18. https://doi.org/10.3390/catal14010018







