Carbon Nanomaterials from Polyolefin Waste: Effective Catalysts for Quinoline Degradation through Catalytic Wet Peroxide Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Synthesis and Characterization
2.1.1. Catalyst for Chemical Vapor Deposition
2.1.2. Carbon Nanotubes
2.2. Catalytic Wet Peroxide Oxidation of Quinoline
3. Materials and Methods
3.1. Reagents and Materials
3.2. Synthesis of Carbon Nanotubes
3.3. Materials Characterization
3.4. Reaction Runs
3.4.1. Catalytic Wet Peroxide Oxidation (CWPO) of QN
3.4.2. Analytical Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Liu, Z.; Dong, H.; Yu, T.; Jiang, H.; Peng, Y.; Shi, L. Coupling quinoline degradation with Fe redox in clay minerals: A strategy integrating biological and physicochemical processes. Appl. Clay Sci. 2020, 188, 105504. [Google Scholar] [CrossRef]
- Saber, A.N.; Zhang, H.; Cervantes-Avilés, P.; Islam, A.; Gao, Y.; An, W.; Yang, M. Emerging concerns of VOCs and SVOCs in coking wastewater treatment processes: Distribution profile, emission characteristics, and health risk assessment. Environ. Pollut. 2020, 265, 114960. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Cui, Y.; Yang, Y.; Zhao, Z.; Wang, X.; Liu, X. An acid induction strategy to construct an ultralight and durable amino-functionalized graphene oxide aerogel for enhanced quinoline pollutants extraction from coking wastewater. Chem. Eng. J. 2021, 412, 128686. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhang, A.; Liu, Z. Treatment effects and genotoxicity relevance of the toxic organic pollutants in semi-coking wastewater by combined treatment process. Environ. Pollut. 2017, 220, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-B.; An, T.-C.; Xiao, X.-M.; Fu, J.-M.; Sheng, G.-Y.; Cui, M.-C. Photochemical degradation performance of quinoline aqueous solution in the presence of hydrogen peroxide. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2003, 38, 2599–2611. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, B.; Wang, B.; Cui, C.; Wang, S.; Wang, J.; Zhang, W. Enhanced degradation of quinoline in three-dimensional electro-Fenton system using catalytic Fe-Co-Ni-P/g-C3N4 particles. Int. J. Electrochem. Sci. 2022, 17, 221296. [Google Scholar] [CrossRef]
- Zhang, B.; You, H.; Wang, F. Microwave-enhanced catalytic wet peroxide oxidation of quinoline: The influence of pH and H2O2 dosage and identification of reactive oxygen species. RSC Adv. 2017, 7, 14769–14775. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, L.; Zheng, M.; Han, J.; Liu, R.; Yun, J. Catalytic Ozonation over Ca2Fe2O5 for the Degradation of Quinoline in an Aqueous Solution. Ind. Eng. Chem. Res. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, L.; Fan, J.; Li, Y.; Sun, P.; Han, J.; Fan, Z. Synergistic effects of Vis/H2O2/LaFe1-xCuxO3 and Vis/PDS/LaFe1−xCuxO3 for quinoline degradation: Performance and mechanism. J. Environ. Chem. Eng. 2022, 10, 108322. [Google Scholar] [CrossRef]
- Diaz de Tuesta, J.L.; Quintanilla, A.; Casas, J.A.; Morales-Torres, S.; Faria, J.L.; Silva, A.M.T.; Gomes, H.T. The pH effect on the kinetics of 4-nitrophenol removal by CWPO with doped carbon black catalysts. Catal. Today 2020, 356, 216–225. [Google Scholar] [CrossRef]
- Diaz de Tuesta, J.L.; Quintanilla, A.; Moreno, D.; Ferro, V.R.; Casas, J.A. Simulation and optimization of the CWPO process by combination of aspen plus and 6-factor doehlert matrix: Towards autothermal operation. Catalysts 2020, 10, 548. [Google Scholar] [CrossRef]
- Diaz de Tuesta, J.L.; Silva, A.S.; Roman, F.F.; Sanches, L.F.; da Silva, F.A.; Pereira, A.I.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Polyolefin-derived carbon nanotubes as magnetic catalysts for wet peroxide oxidation of paracetamol in aqueous solutions. Catal. Today 2023, 419, 114162. [Google Scholar] [CrossRef]
- Roman, F.F.; Diaz de Tuesta, J.L.; Sanches, F.K.K.; Silva, A.S.; Marin, P.; Machado, B.F.; Serp, P.; Pedrosa, M.; Silva, A.M.T.; Faria, J.L.; et al. Selective denitrification of simulated oily wastewater by oxidation using Janus-structured carbon nanotubes. Catal. Today 2023, 420, 114001. [Google Scholar] [CrossRef]
- Silva, A.S.; Kalmakhanova, M.S.; Massalimova, B.K.; de Tuesta, J.L.D.; Gomes, H.T. Wet peroxide oxidation of paracetamol using acid activated and Fe/Co-pillared clay catalysts prepared from natural clays. Catalysts 2019, 9, 705. [Google Scholar] [CrossRef]
- Guari, N.M.C.; Silva, A.S.; Diaz de Tuesta, J.L.; Pottker, W.E.; Cordeiro, P.Y.; Gomes, H.T. Magnetic CoFe2O4@carbon yolk-shell nanoparticles as catalysts for the catalytic wet peroxide oxidation of paracetamol: Kinetic insights. Glob. NEST J. 2022, 25, 57–66. [Google Scholar] [CrossRef]
- De Freitas Batista, G.; Roman, F.F.; de Tuesta, J.L.D.; Mambrini, R.V.; Praça, P.; Gomes, H.T. Assessment of Pretreatments for Highly Concentrated Leachate Waters to Enhance the Performance of Catalytic Wet Peroxide Oxidation with Sustainable Low-Cost Catalysts. Catalysts 2022, 12, 238. [Google Scholar] [CrossRef]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics–the Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 10 July 2023).
- Ribeiro, R.S.; Vieira, O.; Fernandes, R.; Roman, F.F.; Diaz de Tuesta, J.L.; Silva, A.M.T.T.; Gomes, H.T. Synthesis of low-density polyethylene derived carbon nanotubes for activation of persulfate and degradation of water organic micropollutants in continuous mode. J. Environ. Manag. 2022, 308, 114622. [Google Scholar] [CrossRef]
- Vieira, O.; Ribeiro, R.S.; Diaz de Tuesta, J.L.; Gomes, H.T.; Silva, A.M.T. A systematic literature review on the conversion of plastic wastes into valuable 2D graphene-based materials. Chem. Eng. J. 2022, 428, 131399. [Google Scholar] [CrossRef]
- Cai, N.; Li, X.; Xia, S.; Sun, L.; Hu, J.; Bartocci, P.; Fantozzi, F.; Williams, P.T.; Yang, H.; Chen, H. Pyrolysis-catalysis of different waste plastics over Fe/Al2O3 catalyst: High-value hydrogen, liquid fuels, carbon nanotubes and possible reaction mechanisms. Energy Convers. Manag. 2021, 229, 113794. [Google Scholar] [CrossRef]
- Mukherjee, A.; Debnath, B.; Ghosh, S.K. Carbon Nanotubes as a Resourceful Product Derived from Waste Plastic—A Review BT-Waste Management and Resource Efficiency; Ghosh, S.K., Ed.; Springer: Singapore, 2019; pp. 915–934. [Google Scholar]
- Aboul-Enein, A.A.; Awadallah, A.E.; Abdel-Rahman, A.A.H.; Haggar, A.M. Synthesis of multi-walled carbon nanotubes via pyrolysis of plastic waste using a two-stage process. Fullerenes Nanotub. Carbon Nanostruct. 2018, 26, 443–450. [Google Scholar] [CrossRef]
- Yang, R.X.; Chuang, K.H.; Wey, M.Y. Effects of Temperature and Equivalence Ratio on Carbon Nanotubes and Hydrogen Production from Waste Plastic Gasification in Fluidized Bed. Energy Fuels 2018, 32, 5462–5470. [Google Scholar] [CrossRef]
- Liu, X.; Shen, B.; Wu, Z.; Parlett, C.M.A.; Han, Z.; George, A.; Yuan, P.; Patel, D.; Wu, C. Producing carbon nanotubes from thermochemical conversion of waste plastics using Ni/ceramic based catalyst. Chem. Eng. Sci. 2018, 192, 882–891. [Google Scholar] [CrossRef]
- Wyss, K.M.; De Kleine, R.D.; Couvreur, R.L.; Kiziltas, A.; Mielewski, D.F.; Tour, J.M. Upcycling end-of-life vehicle waste plastic into flash graphene. Commun. Eng. 2022, 1, 3. [Google Scholar] [CrossRef]
- Dai, L.; Karakas, O.; Cheng, Y.; Cobb, K.; Chen, P.; Ruan, R. A review on carbon materials production from plastic wastes. Chem. Eng. J. 2023, 453, 139725. [Google Scholar] [CrossRef]
- Zhuo, C.; Levendis, Y.A. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2014, 131, 1–14. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P. Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: Graphene and CNTs. Renew. Sustain. Energy Rev. 2016, 58, 976–1006. [Google Scholar] [CrossRef]
- Hazan, M.A.; Chan, K.F.; Jofri, K.A.; Mamat, M.S.; Endot, N.A.; Liza, S.; Ismail, I.; Hussein, M.Z.; Tanemura, M.; Yaakob, Y. Waste nr latex based-precursors as carbon source for cnts eco-fabrications. Polymers 2021, 13, 3409. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Lv, Y.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. Pyrolysis technology for plastic waste recycling: A state-of-the-art review. Prog. Energy Combust. Sci. 2022, 93, 101021. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Qu, J.; Wang, Z.; Qiu, J. Fabrication and characterization of magnetic Fe3O4-CNT composites. J. Phys. Chem. Solids 2010, 71, 673–676. [Google Scholar] [CrossRef]
- Kazemifard, S.; Nayebzadeh, H.; Saghatoleslami, N.; Safakish, E. Assessment the activity of magnetic KOH/Fe3O4@Al2O3 core–shell nanocatalyst in transesterification reaction: Effect of Fe/Al ratio on structural and performance. Environ. Sci. Pollut. Res. 2018, 25, 32811–32821. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, A.R.; Pradhan, B.K.; Kim, U.J.; Chen, G.; Eklund, P.C. CVD Synthesis of Single Wall Carbon Nanotubes under “Soft” Conditions. Nano Lett. 2002, 2, 525–530. [Google Scholar] [CrossRef]
- Gakis, G.P.; Termine, S.; Trompeta, A.F.A.; Aviziotis, I.G.; Charitidis, C.A. Unraveling the mechanisms of carbon nanotube growth by chemical vapor deposition. Chem. Eng. J. 2022, 445, 136807. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J.; Yu, J.A. Catalyst effect on carbon nanotubes synthesized by thermal chemical vapor deposition. Chem. Phys. Lett. 2002, 360, 250–255. [Google Scholar] [CrossRef]
- Veksha, A.; Chen, W.; Liang, L.; Lisak, G. Converting polyolefin plastics into few-walled carbon nanotubes via a tandem catalytic process: Importance of gas composition and system configuration. J. Hazard. Mater. 2022, 435, 128949. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Tan, S.; Song, R. Universal Ni-Mo-Mg catalysts combined with carbon blacks for the preparation of carbon nanotubes from polyolefins. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Pinho, M.T.; Gomes, H.T.; Ribeiro, R.S.; Faria, J.L.; Silva, A.M.T. Carbon nanotubes as catalysts for catalytic wet peroxide oxidation of highly concentrated phenol solutions: Towards process intensification. Appl. Catal. B Environ. 2015, 165, 706–714. [Google Scholar] [CrossRef]
- Raji, K.; Thomas, S.; Sobhan, C.B. A chemical kinetic model for chemical vapor deposition of carbon nanotubes. Appl. Surf. Sci. 2011, 257, 10562–10570. [Google Scholar] [CrossRef]
- Toussi, S.M.; Fakhru’L-Razi, A.; Chuah, A.L.; Suraya, A.R. Effect of synthesis condition on the growth of SWCNTs via catalytic chemical vapour deposition. Sains Malays. 2011, 40, 197–201. [Google Scholar]
- Niu, Z.; Fang, Y. Effect of temperature for synthesizing single-walled carbon nanotubes by catalytic chemical vapor deposition over Mo-Co-MgO catalyst. Mater. Res. Bull. 2008, 43, 1393–1400. [Google Scholar] [CrossRef]
- Williams, P.T. Hydrogen and Carbon Nanotubes from Pyrolysis-Catalysis of Waste Plastics: A Review. Waste Biomass Valorization 2021, 12, 1–28. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M.; Garagorri, E.; Fernández, J.A. Catalytic conversion of polyolefins into fuels over zeolite beta. Polym. Degrad. Stab. 2000, 69, 11–16. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, L.; Wang, Y.Z.; Zhao, G.M.; Wang, B. Catalytic degradation of low-density polyethylene and polypropylene using modified ZSM-5 zeolites. Polym. Degrad. Stab. 2004, 84, 493–497. [Google Scholar] [CrossRef]
- Yan, G.; Jing, X.; Wen, H.; Xiang, S. Thermal cracking of virgin and waste plastics of PP and LDPE in a semibatch reactor under atmospheric pressure. Energy Fuels 2015, 29, 2289–2298. [Google Scholar] [CrossRef]
- Sengupta, J.; Jacob, C. Growth temperature dependence of partially Fe filled MWCNT using chemical vapor deposition. J. Cryst. Growth 2009, 311, 4692–4697. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, B.; Borowiak-Palen, E.; Kalenczuk, R.J. Oxidation and reduction of multiwalled carbon nanotubes-preparation and characterization. Mater. Charact. 2010, 61, 185–191. [Google Scholar] [CrossRef]
- Zhong, B.; Huang, R.; Su, D.S.; Liu, H. Effect of graphitization of oxygen-modified carbon nanotubes in selective oxidation of acrolein. Catal. Today 2019, 330, 142–148. [Google Scholar] [CrossRef]
- Likodimos, V.; Steriotis, T.A.; Papageorgiou, S.K.; Romanos, G.E.; Marques, R.R.N.; Rocha, R.P.; Faria, J.L.; Pereira, M.F.R.; Figueiredo, J.L.; Silva, A.M.T.; et al. Controlled surface functionalization of multiwall carbon nanotubes by HNO3 hydrothermal oxidation. Carbon 2014, 69, 311–326. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Teixeira, I.F.; Christofani, T.; Tristão, J.C.; Guimarães, I.R.; Moura, F.C.C. Biphasic oxidation reactions promoted by amphiphilic catalysts based on red mud residue. Appl. Catal. B Environ. 2014, 144, 144–151. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Han, Y.; Li, Y.; Gao, P. Review on coal-based reduction and magnetic separation for refractory iron-bearing resources. Int. J. Miner. Metall. Mater. 2022, 29, 2087–2105. [Google Scholar] [CrossRef]
- Leventis, N.; Donthula, S.; Mandal, C.; Ding, M.S.; Sotiriou-Leventis, C. Explosive versus Thermite Behavior in Iron(0) Aerogels Infiltrated with Perchlorates. Chem. Mater. 2015, 27, 8126–8137. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Hooker, S.A. Development of stabilized zero valent iron nanoparticles. Desalin. Water Treat. 2012, 37, 114–121. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Venieri, D.; Antonopoulou, M.; Konstantinou, I.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Magnetic carbon xerogels for the catalytic wet peroxide oxidation of sulfamethoxazole in environmentally relevant water matrices. Appl. Catal. B Environ. 2016, 199, 170–186. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, Y.; Xian, H.; Yan, L.; Fu, H.; Zhu, G.; Xi, Y.; Zhu, J.; He, H. CNTs/ferrihydrite as a highly efficient heterogeneous Fenton catalyst for the degradation of bisphenol A: The important role of CNTs in accelerating Fe(III)/Fe(II) cycling. Appl. Catal. B Environ. 2020, 270, 118891. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Catalytic wet peroxide oxidation: A route towards the application of hybrid magnetic carbon nanocomposites for the degradation of organic pollutants. A review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Song, Y.; Wei, Y.; He, L.; Lan, B.; He, X.; Wang, J. Effective mineralization of quinoline and bio-treated coking wastewater by catalytic ozonation using CuFe2O4/Sepiolite catalyst: Efficiency and mechanism. Chemosphere 2019, 227, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Bian, L.; Ding, J.; Wu, Y.; Xia, H.; Li, J. Electrochemical oxidation of quinoline aqueous solution on ?-PbO2 anode and the evolution of phytotoxicity on duckweed. Water Sci. Technol. 2017, 75, 1820–1829. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Gallo, J.; Bañobre-López, M.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Enhanced performance of cobalt ferrite encapsulated in graphitic shell by means of AC magnetically activated catalytic wet peroxide oxidation of 4-nitrophenol. Chem. Eng. J. 2019, 376, 120012. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X. Toxicity change patterns and its mechanism during the degradation of nitrogen-heterocyclic compounds by O(3)/UV. Chemosphere 2007, 69, 747–754. [Google Scholar] [CrossRef]
- Wei, Q.; Qiao, S.; Sun, B.; Zou, H.; Chen, J.; Shao, L. Study on the treatment of simulated coking wastewater by O3 and O3/Fenton processes in a rotating packed bed. RSC Adv. 2015, 5, 93386–93393. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J.; Dong, J.; Liu, H.; Sun, X. Treatment of coking wastewater by an advanced Fenton oxidation process using iron powder and hydrogen peroxide. Chemosphere 2012, 86, 409–414. [Google Scholar] [CrossRef]
- Silva, A.S.; Diaz de Tuesta, J.L.; Sayuri Berberich, T.; Delezuk Inglez, S.; Bertão, A.R.; Çaha, I.; Deepak, F.L.; Bañobre-López, M.; Gomes, H.T. Doxorubicin delivery performance of superparamagnetic carbon multi-core shell nanoparticles: pH dependence, stability and kinetic insight. Nanoscale 2022, 14, 7220–7232. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Kompotiatis, L.; Kontogeorgakos, A.; Kordas, G. Microwave behavior of ferrites prepared via sol-gel method. J. Magn. Magn. Mater. 2002, 246, 360–365. [Google Scholar] [CrossRef]
- Silva, A.S.; Roman, F.F.; Dias, A.V.; Diaz de Tuesta, J.L.; Narcizo, A.; da Silva, A.P.F.; Çaha, I.; Deepak, F.L.; Bañobre-López, M.; Ferrari, A.M.C.; et al. Hybrid multi-core shell magnetic nanoparticles for wet peroxide oxidation of paracetamol: Application in synthetic and real matrices. J. Environ. Chem. Eng. 2023, 11, 110806. [Google Scholar] [CrossRef]
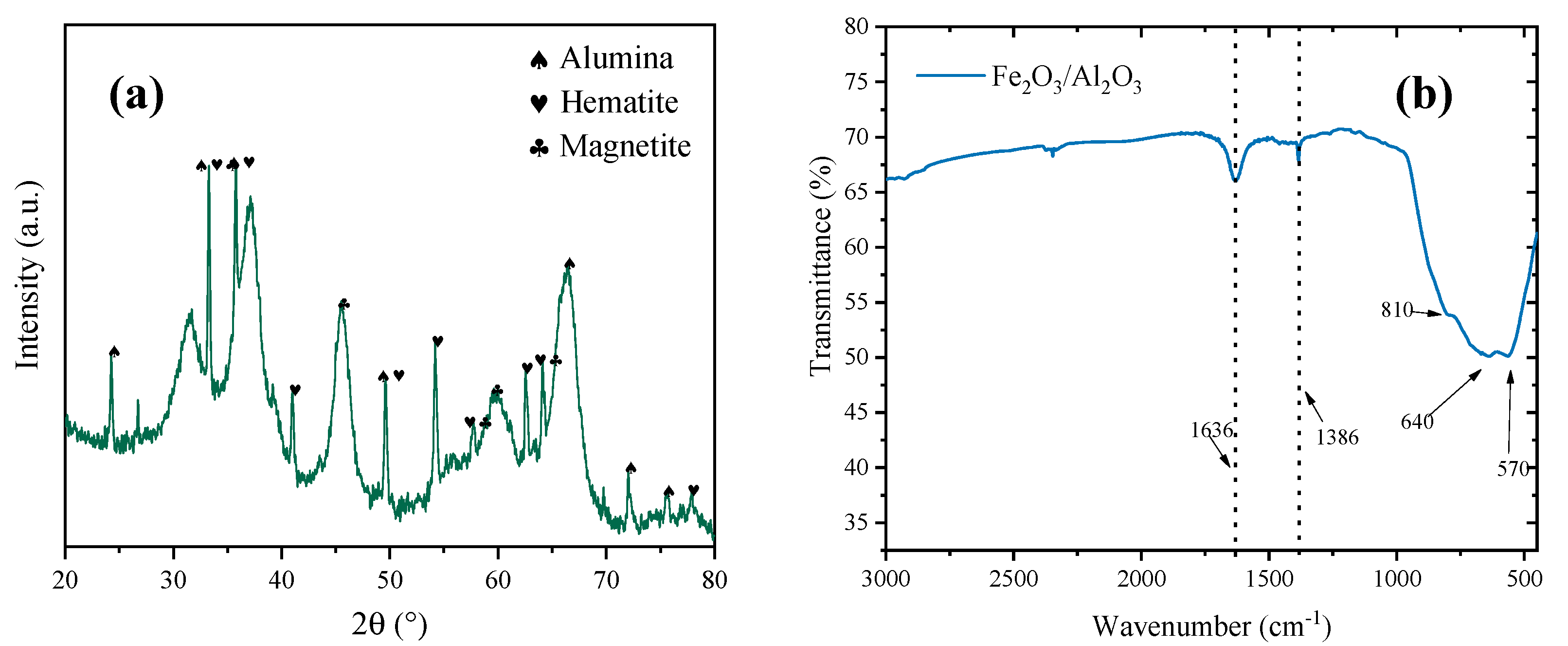


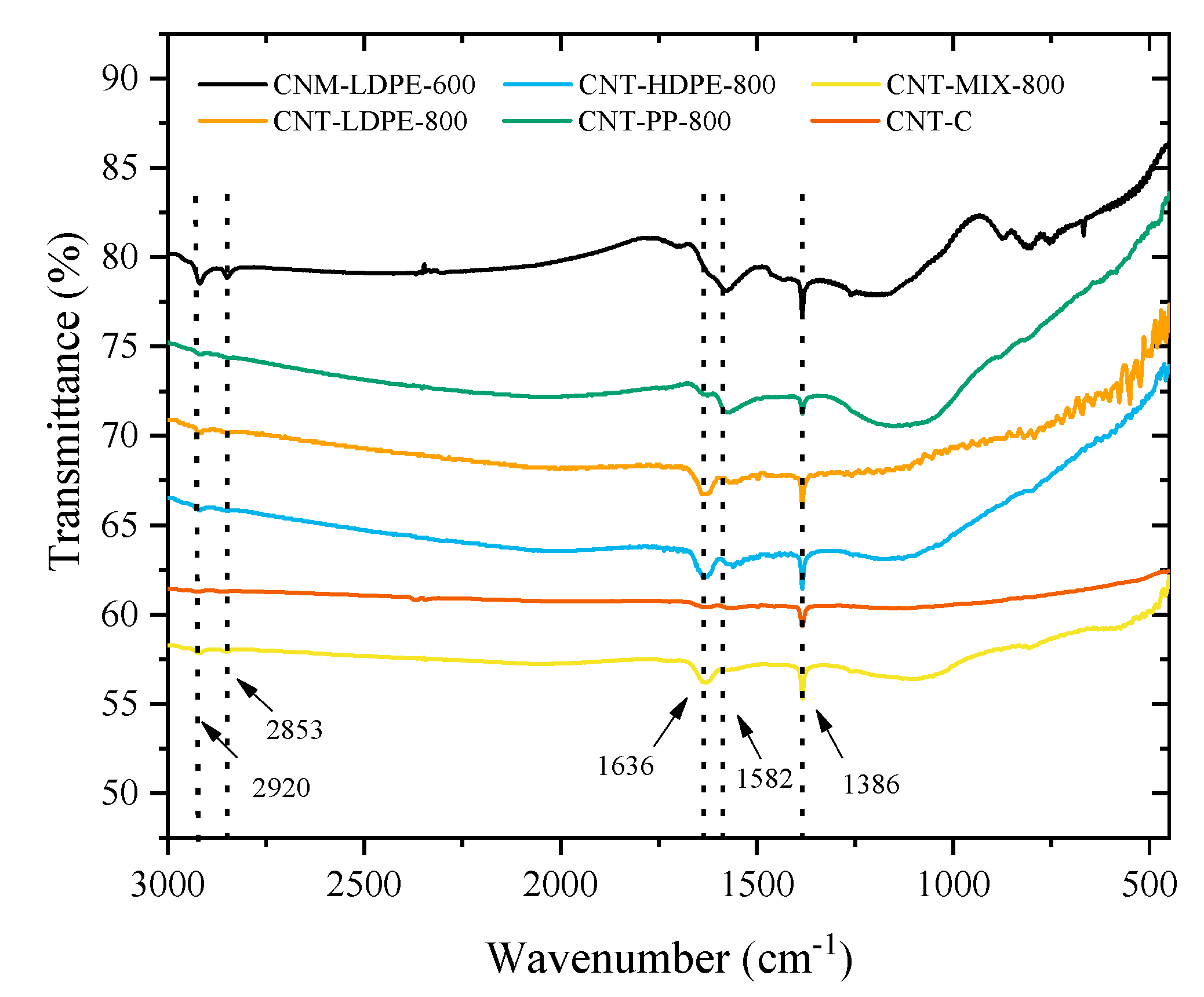
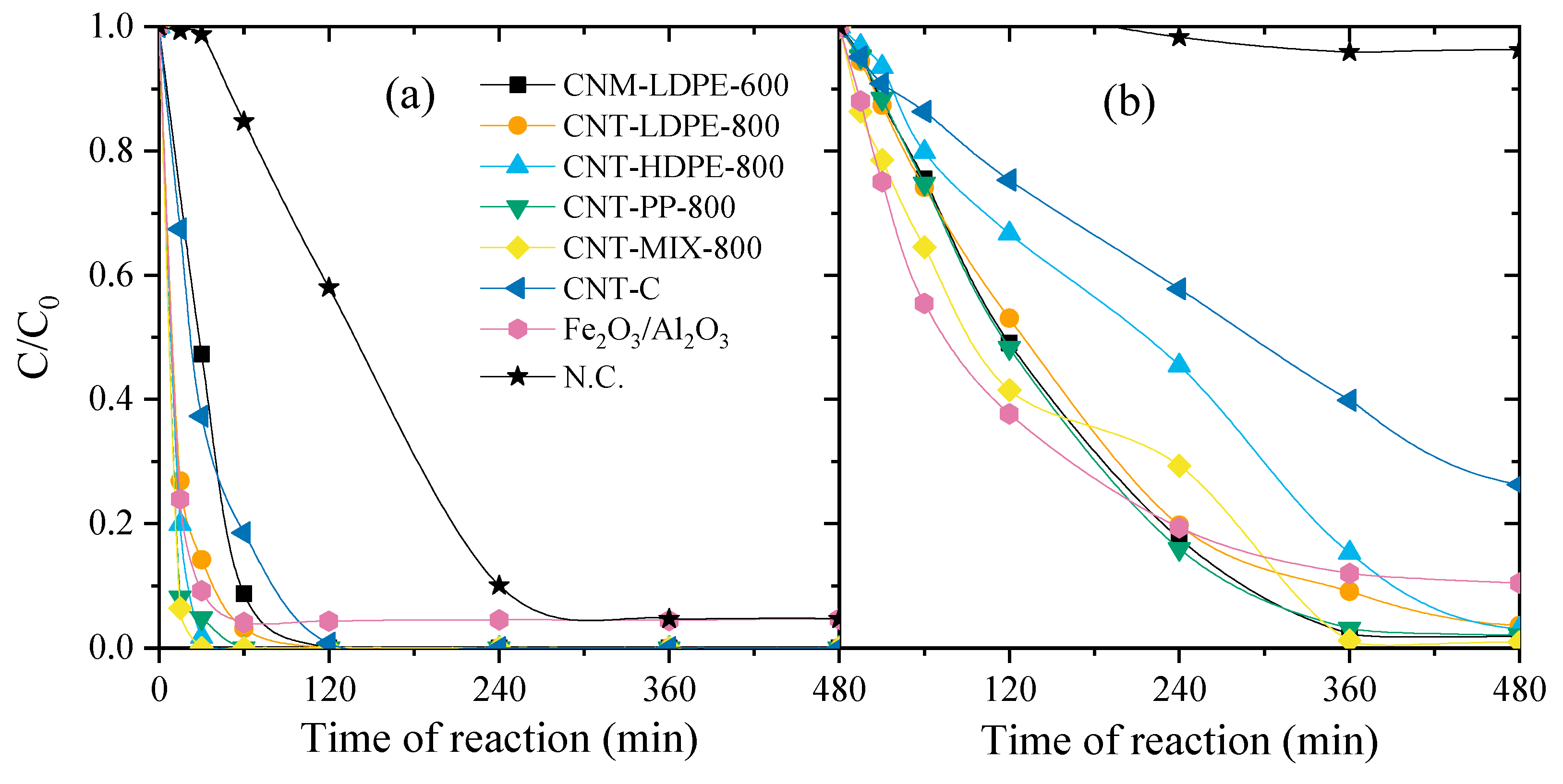

| Material | SBET (m2 g−1) | Vtotal (mm3 g−1) |
|---|---|---|
| CNM-LDPE-600 | 159 | 406 |
| CNT-LDPE-800 | 235 | 594 |
| CNT-HDPE-800 | 189 | 456 |
| CNT-PP-800 | 242 | 595 |
| CNT-MIX-800 | 194 | 496 |
| CNT-C | 254 | 690 |
| Fe2O3/Al2O3 | 98 | 273 |
| Al2O3 | 185 | 437 |
| Material | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) | Remaining (wt.%) | Fe (wt.%) |
|---|---|---|---|---|---|---|---|
| CNM-LDPE-600 | 70.6 ± 2.1 | 1.77 ± <0.01 | 0.07 ± <0.01 | 0.12 ± <0.01 | 4.50 ± 0.01 | 23.0 | 9.7 ± 0.0 |
| CNT-LDPE-800 | 86.2 ± 1.1 | 0.32 ± 0.04 | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.88 ± 0.07 | 12.5 | 11.3 ± 0.2 |
| CNT-HDPE-800 | 85.3 ± 0.5 | 0.27 ± 0.02 | 0.09 ± <0.01 | 0.14 ± 0.01 | 0.83 ± 0.03 | 13.4 | 14.0 ± 0.7 |
| CNT-PP-800 | 84.1 ± 2.6 | 0.41 ± 0.01 | 0.11 ± <0.01 | 0.18 ± 0.03 | 1.18 ± 0.01 | 15.9 | 11.9 ± 0.7 |
| CNT-MIX-800 | 80.5 ± 3.6 | 0.34 ± 0.03 | 0.00 ± <0.01 | 0.36 ± 0.09 | 1.83 ± <0.01 | 17.0 | 14.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, F.F.; De Grande Piccinin, L.; Santos Silva, A.; Diaz de Tuesta, J.L.; Freitas, I.V.K.; Vieira, A.; Gonçalves Lenzi, G.; Manuel Tavares Silva, A.; Faria, J.L.; Gomes, H.T. Carbon Nanomaterials from Polyolefin Waste: Effective Catalysts for Quinoline Degradation through Catalytic Wet Peroxide Oxidation. Catalysts 2023, 13, 1259. https://doi.org/10.3390/catal13091259
Roman FF, De Grande Piccinin L, Santos Silva A, Diaz de Tuesta JL, Freitas IVK, Vieira A, Gonçalves Lenzi G, Manuel Tavares Silva A, Faria JL, Gomes HT. Carbon Nanomaterials from Polyolefin Waste: Effective Catalysts for Quinoline Degradation through Catalytic Wet Peroxide Oxidation. Catalysts. 2023; 13(9):1259. https://doi.org/10.3390/catal13091259
Chicago/Turabian StyleRoman, Fernanda F., Larissa De Grande Piccinin, Adriano Santos Silva, Jose L. Diaz de Tuesta, Isabella V. K. Freitas, Admilson Vieira, Giane Gonçalves Lenzi, Adrián Manuel Tavares Silva, Joaquim Luís Faria, and Helder Teixeira Gomes. 2023. "Carbon Nanomaterials from Polyolefin Waste: Effective Catalysts for Quinoline Degradation through Catalytic Wet Peroxide Oxidation" Catalysts 13, no. 9: 1259. https://doi.org/10.3390/catal13091259
APA StyleRoman, F. F., De Grande Piccinin, L., Santos Silva, A., Diaz de Tuesta, J. L., Freitas, I. V. K., Vieira, A., Gonçalves Lenzi, G., Manuel Tavares Silva, A., Faria, J. L., & Gomes, H. T. (2023). Carbon Nanomaterials from Polyolefin Waste: Effective Catalysts for Quinoline Degradation through Catalytic Wet Peroxide Oxidation. Catalysts, 13(9), 1259. https://doi.org/10.3390/catal13091259













